Abstract
Normal immune homeostasis is achieved by several mechanisms, and prominent among them is immunoregulation. While several types of regulatory lymphocyte populations have been described, CD4 T cells expressing the Foxp3 transcription factor (Foxp3+ Tregs) are the best understood. This population of cells is critical for maintaining self-tolerance throughout the life of the organism. Foxp3+ Tregs can develop within the thymus, but also, under selected circumstances, naïve peripheral T cells can be induced to express Foxp3 and become stable Tregs as well. Abundant evidence from animal systems, as well as limited evidence in humans, implicates Tregs in transplant tolerance, although whether these Tregs recognize alloantigens or self-antigens is not clear. New translational approaches to promote immunosuppression minimization and/or actual tolerance are being designed to exploit these observations. These include strategies to boost the generation, maintainence and stability of endogenous Tregs, as well as adoptive cellular therapy with exogenous Tregs.
Keywords: Regulatory T cells, Foxp3+ Tregs, kidney transplant tolerance, immunotherapy, kidney allografts
Introduction
The role of the immune system is to discriminate between self and non-self antigens, protecting the host from foreign pathogens and at the same time maintaining tolerance to self. Immune tolerance is an active process that is characterized by a central and peripheral component (1–7). Central tolerance is a thymic dependent process that involves the deletion of autoreactive clones through the induction of apoptosis. Peripheral tolerance can be subdivided into at least three major categories: clonal deletion, anergy, and suppression. A subset of T cells has been identified that specifically regulate the suppression process. These cells are known as regulatory T cells (Tregs). Even though these cells are frequently grouped under one category, they can be divided into two developmental subsets, thymic-derived (natural) Tregs (tTregs or nTregs), and induced (adaptive) peripheral Tregs (iTregs or pTregs) (8) (Figure 1). Although these cells are predominantly CD4+, CD8+Foxp3+ cells have been identified that are also suppressive in nature. Natural Tregs are produced in the thymus and most express the IL-2 receptor alpha chain (CD25) (9). Their development and functionality depend on the expression of the transcriptional factor forkhead box P3 (Foxp3) (10, 11). Induced Tregs are derived in the periphery from naïve T cells following specific antigenic stimulation. There are also several populations of CD4+Foxp3− T cells that are suppressive, including IL-10 producing T regulatory 1 (Tr1) cells, transforming growth factor beta (TGF-β) T helper 3 (Th3) cells, and T regulatory type 35 (Tr35) cells that produce IL-35, which is related to the IL-12 superfamily (1, 3, 12) (Figure 2). This review will focus on the biology of Tregs, the role that they play in kidney allograft acceptance, and the ways that our knowledge about Tregs is being leveraged in the clinic.
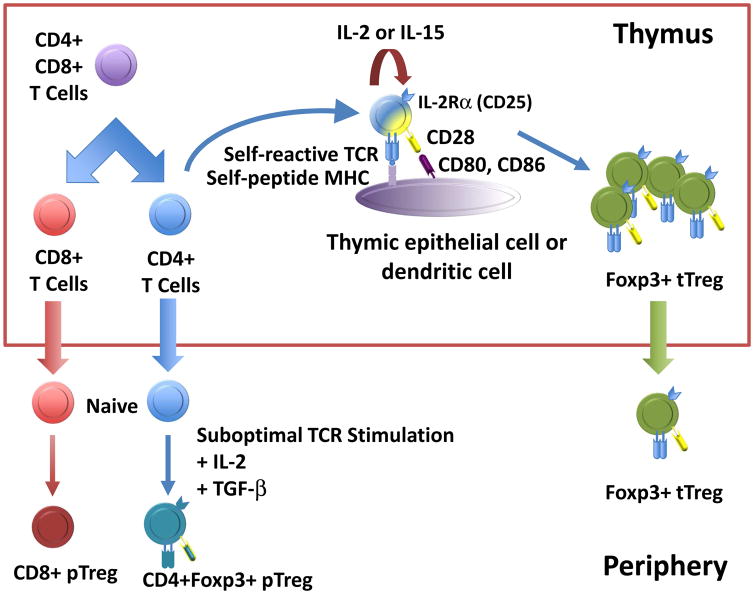
Figure 1.
Origin of Foxp3+ Tregs. During thymic ontogeny, double positive (CD4+CD8+) thymocytes with low affinity for self-peptide + self-MHC undergo positive selection, becoming single positive CD8+ or CD4+ thymocytes which eventually are exported into the peripheral lymphoid compartment as naïve T cells. Under certain conditions (see figure 2 for more details), these naïve T cells can be induced to express Foxp3, and these cells are known as pTregs (for Tregs of peripheral origin). Double positive thymocytes with moderate affinity for self-peptide + self-MHC are induced to express Foxp3, and these cells, known as tTregs because of their thymic origin, are exported into the periphery as “fully formed” Tregs. Abbreviations: Foxp3+ Tregs, Foxp3 positive regulatory T cells; MHC, major histocompatibility complex; tTregs, thymic-derived Tregs; IL, interleukin; TCR, T cell receptor; pTreg, induced peripheral regulatory T cells.
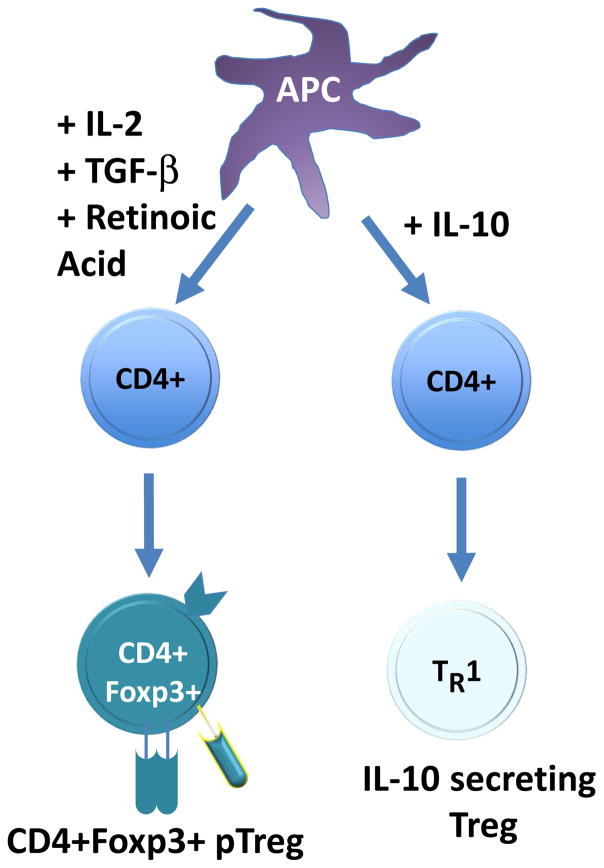
Figure 2.
Induction of Tregs from naïve T cells. In the periphery, conditions which favor the induction of pTregs from naïve T cells include low level continuous antigen exposure, the absence of inflammatory signals (especially IL-6), the presence of cytokines such as IL-2 and TGF-β, and other factors such as retinoic acid. High levels of IL-10 can also induce a regulatory T cell phenotype, however these cells, known as Tr1 cells, do not express Foxp3 and themselves produce IL-10. Abbreviations; Foxp3+ Treg, Foxp3 positive regulatory T cell; pTreg, induced peripheral regulatory T cells; IL, interleukin; TGF- β, transforming growth factor beta; APC, antigen-presenting cell.
Treg biology
Basic Concepts
Foxp3+ Tregs constitute 5 to 10% of peripheral CD4+ T cells in both mice and humans (13) and are critical for maintaining immune homeostasis. Mutations in Foxp3 leading to an absence of functional Tregs is the cause of severe autoimmunity as observed in scurfy mice and in humans with IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) (11, 14). Importantly, Tregs also are critical for maintaining immune homeostasis throughout the lifespan of an animal. This was elegantly demonstrated by Kim and colleagues using a mouse in which the diphtheria toxin receptor was knocked into the Foxp3 locus (Foxp3DTR mouse). In these adult animals, administration of diphtheria toxin leads to the selective ablation/depletion of Tregs, which then results in the induction of a wide variety of autoimmune diseases (15). In addition to their role in preventing autoimmunity, Tregs have also been implicated in the resolution of inflammation and tissue repair (16).
Two types of Foxp3+ Tregs
During thymic ontogeny, developing Tregs are subject to both positive and negative selection processes which together create a repertoire that is self–major histocompatibility complex (MHC) restricted (positive selection) but eliminates many auto-reactive T cells (negative selection). Current data supports a model in which developing thymocytes whose T cell receptors have some modest affinity for self-peptide plus MHC are induced to express Foxp3 and differentiate into Tregs (17). These thymically derived Tregs, termed tTregs (formerly natural or nTregs), are believed to be important in suppressing the response of autoreactive T cells which escape negative selection. Despite having low-level self reactivity, they have a relatively high rate of cycling in vivo, and can be activated by self-antigens in the periphery (18) (19). Once activated, they may be able to suppress in an antigen non-specific manner.
Another population of Tregs, termed pTregs, can be induced from naïve T cells (i.e., resting non-Tregs) in the periphery. In vitro, this population of inducible Tregs is created when T cells are stimulated in the presence of high concentrations of TGF-β (20). Other factors that support Treg induction include vitamin D, retinoic acid, vitamin C, and inhibition of Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI-3 kinase) (21) (22). It has been more difficult to study this population in vivo, but pTreg induction appears to be favored by continual stimulation with low doses of antigen. Conversely, inflammatory signals, particularly IL-6, retard Treg induction (23). We know less about the physiologic role of pTregs, although they appear to be required for maternal-fetal tolerance (24), and thus it has been suggested that they may be more important for tolerance to foreign antigens, with tTregs focusing on self-antigens. It has been difficult to study these two populations of cells due to a lack of easily used molecular markers to distinguish them, although the transcription factor Helios and the surface protein neuropilin1 have been proposed to be selective for tTregs in mice (25). In any event, given the potential role of pTregs in tolerance to alloantigens, there has been a great deal of interest in understanding if there are particular cell types or conditions which, in vivo, can promote their development.
Cellular Mechanisms of Treg Induction
Conventional Dendritic Cells
Dendritic cells are antigen presenting cells that are essential in controlling innate and adaptive immunity and function as key regulators of immune tolerance (26). They capture and process antigens and respond to pathogen- and self-derived danger signals in peripheral tissues. Dendritic cells are a heterogeneous population of leukocytes, consisting of conventional dendritic cells (cDCs), plasmacytoid dendritic cells, and inflammatory dendritic cells. In mice, cDCs can be divided into two groups: CD8α+CD103+ and CD11b+ cells, in which the human counterparts are BDCA-3+ and BDCA-1+, respectively (27). The murine kidney is known to have resident CD11c+ dendritic cells. In healthy mice, the renal CD11c+ dendritic cell population is heterogeneous, expresses MHC II, and has an immature phenotype (i.e. low expression of the co-stimulatory molecules, CD80 and CD86, and very low levels of CD40 expression). These CD11c+MHC II+ renal dendritic cells can be divided into two distinct subsets: CD103+ and CD11b+ dendritic cells. The CD103+ dendritic cells are also CD11bloCX3CR1-F4/80-SIRP-α-, and the CD11b+ dendritic cells are CD103- CX3CR1+F4/80+SIRP-α+ (28). And even though the latter population are F4/80 and CD11b+, markers found on macrophage cells, these renal cells are more similar to dendritic cells based on morphology, expression of co-stimulatory molecules, and functionality (29). Coates and colleagues showed that the isolation of these immature, renal dendritic cells can induce IL-10 producing Tregs in vitro (30).
Plasmacytoid dendritic cells
Plasmacytoid dendritic cells (pDCs) originate in the bone marrow and are B220+, Siglec-H+, PDAC-1+, and in mice, are CD11c low. In humans, pDCs are BDCA-2+, B220+, and CD11c-. While the main function of pDCs is to produce type I interferon in response to viral infections, extensive published studies have shown that pDCs promote regulatory tolerance of cardiac allografts and of hematopoetic stem cell engraftment by converting CD4+ naïve T-cells into induced regulatory T-cells (pTregs) (31, 32), and promote deletional tolerance by eliminating antigen-reactive thymocytes (33, 34). Additionally, pDCs have been shown to mediate oral tolerance (35). In humans, pDCs isolated from blood induce Foxp3+ T cells in vitro in an indoleamine 2,3-dioxygenase and PD-L1-dependent manner (36–38). In contrast, murine pDCs are limited in their ability to induce Tregs (27). Recently, though, Chappell and colleagues have demonstrated that targeting the human BDCA-2 molecule with antigen-conjugated antibody in B6.BDCA2 transgenic mice resulted in antigen-specific tolerance (39). Studies have suggested that the normal kidney contains relatively more pDCs than the heart, and that these have a distinct phenotype in normal and accepted kidneys and in their ability to induce Tregs ex vivo (40). In addition, pDCs have been found in Treg-rich organized lymphoid structures of accepted murine and non-human primate kidney allografts (40).
Role of Tregs in allograft acceptance
It is currently accepted that Tregs play a key role in allotransplant tolerance. Regulatory T cells have been shown to suppress effector T cell activity and function, resulting in allograft tolerance in several mouse model studies involving skin, islets, heart, and kidney allografts (41–48). Functional, intragraft Tregs have been identified in several transplants, such as skin, heart, and kidney, and when adoptively transferred, can confer tolerance to a naïve mouse. Mouse models where Tregs can be specifically and systemically depleted (44, 46) definitively appraised the role of Foxp3+ cells in the tolerance induced spontaneously by transplanted kidneys between certain MHC incompatible mouse strains, without the involvement of hematopoietic cell chimerism (46). In these experiments, the depletion of Tregs resulted in prompt T cell mediated rejection.
Kidney Allografts
Kidney allografts are naturally tolerogenic in mice. Mice develop tolerance of kidney allografts across certain full MHC incompatibilities (e.g., DBA/2 to B6, B10.D2 to B6AF1, and B10.A to B10.BR) without immunosuppression. This tolerance is initially dependent on Foxp3+ cells which are concentrated in the graft in distinctive Treg-rich organized lymphoid structures that contain immature, CD11c+ conventional dendritic cells, as assessed by low surface expression of co-stimulatory molecules (46). Interestingly, recipients of allograft kidneys develop systemic tolerance to skin and heart allografts from the same donor strain. This phenomenon has also been demonstrated in pigs (49–51), non-human primates (52), and to a limited extent in humans (53–55). The advantage of the mouse lies in the opportunity to design definitive experiments using inbred, transgenic, and knock out mouse strains. These studies may reveal that natural tolerogenic mechanisms of the mouse kidney are similar to that in pigs and non-human primates mixed chimerism protocols. If so, this would heighten the general significance of the kidney mechanisms. Alternatively, if the mechanisms in the mouse are different, we will have revealed novel pathway(s) which may have potential clinical application. While these may be unique to the mouse under normal circumstances, analogous mechanisms might be exploitable in humans. Elucidating the cellular and molecular mechanisms of how an accepted kidney allograft confers tolerance of a heart allograft may reveal potential novel regimens applicable clinically.
Besides dendritic cells, other cellular components that may play a role are renal tubular epithelial cells (RTECs). RTECs represent an intriguing candidate population to mediate the systemic tolerogenic nature of kidney transplants, as they are specific to the kidney and proximal RTECs are known to be immunologically active. RTECs can promote T cell unresponsiveness to self- and allo-antigens in mice and humans (56). Additionally, it has been shown that Foxp3+ cells are enriched in the tubules in human kidney allografts (57) as in the mouse (58). Frasca and colleagues have shown that IFNγ-treated human RTEC induce allospecific tolerance via a class II pathway (56). IFNγ induces the T cell inhibitor molecule PD-1 and indoleamine 2, 3-dioxygenase (IDO) on RTEC (59, 60). Importantly, Amarnath and colleagues have recently shown that PD1 signaling results in the conversion of human TH1 cells into Treg cells (61). Finally, RTECs are known to produce and activate TGFβ (62), which is a major inducer of Foxp3+ Treg (63) and tolerogenic pDC (64) generation.
What have we learned from patients?
While studies in mouse transplant models suggest a role for Tregs in allograft acceptance, clinically, this is less clear and is dependent on correlative retrospective, prospective, and cross-sectional studies. Several studies have tried to assess if a direct relationship exists between good kidney allograft outcome versus onset of rejection. The presence of intragraft Tregs has been suggested as a positive predictor of favorable graft outcome in stable patients under an immunosuppression regimen (65–67), especially with subclinical signs of rejection (65). Bestard and colleagues also showed a correlation between Treg infiltrates and a greater Treg/CD3+ T cell ratio with better graft function 2- to 3-year posttransplantation. The presence of Tregs in the graft has also been associated with donor-specific T cell hyporesponsiveness in patients with subclinical signs of rejection (68). However, several studies have shown that a simple correlation with the existence of Foxp3+ cells is not a reliable predictor of favorable kidney graft outcome. Ashton-Chess and colleagues reported that expression of Foxp3 in blood and in the graft could not distinguish between rejecting and non rejecting patients (69). Furthermore, they showed that there is great variability between Foxp3 expression and the patient’s age and the time that samples were taken posttransplantation. Others have shown that the presence of Foxp3+ cells also was a key indicator of acute rejection (57, 70). In fact, transient Foxp3 expression has been observed in antigen-specific T effector cells (71).
Therefore, what better ways could there be to assess Tregs as predictors of favorable kidney allograft outcomes? It has been suggested that perhaps evaluating the ratio of Tregs to effector T (Teff) cells may be more appropriate. In fact, Grimbert and colleagues were able to distinguish kidney allograft borderline rejection from acute rejection by looking at Foxp3-to-granzyme B expression ratios (66). Another approach, which may be more promising, is to look at the level of demethylation of the Treg-specific demethylated region, and the Foxp3 locus, as a demethylated Treg-specific demethylated region correlates with the Treg function and stability (72, 73). This approach was used by Bestard and colleagues and they demonstrated that sub-clinical signs of rejection patients with demethylation of Treg-specific demethylated region exhibited greater kidney graft function five years post transplantation (74).
To date there have been only limited studies on Tregs in tolerant patients. In large part this stems from the fact that, simply put, there are few patients with operational kidney allograft tolerance. These small numbers of patients fall into two categories: those who were enrolled in protocols designed to create a tolerant state and who achieved that endpoint, and those discovered to be tolerant when their medications were discontinued (either due to life-threatening complications of immunosuppression or as a result of non-adherence). The vast majority, perhaps all, of the HLA mismatched patients who achieved sustained operational tolerance as part of a deliberate protocol received donor bone marrow to induce either transient-mixed, or long-term, hematopoietic chimerism (75) (76). In patients enrolled in transient-mixed chimerism studies within roughly two weeks after transplantation, donor derived cells are no longer present, meaning that all lymphocytes are of recipient origin. Based upon this observation, it has become possible to ask whether there is evidence of recipient anti-donor Tregs. Using an assay that combines proliferative responses to donor cells with high throughput T cell receptor sequencing, Morris et al. demonstrated a marked reduction in the frequency of donor-reactive cells in the peripheral blood in tolerant recipients (77). However, while reduced in number, many alloreactive cells remained, suggesting either that their ability to cause graft damage is being constrained by Tregs and/or that many of these cells are themselves Tregs (i.e., donor-reactive Tregs). In trials where tolerance has been induced by the use of full hematopoietic chimerism, the question of regulation becomes, in a sense, moot, as there are no detectable recipient T cells. Finally, it should be noted that the studies above were all done using HLA mismatched donor-recipient pairs. A number of studies have created tolerance in HLA-identical kidney allograft recipients, however functional studies of suppression are extremely difficult in this setting due to the inability to induce a reliable proliferative response in vitro when there are only minor mismatches.
Therapeutic approaches to augment Tregs
Given our understanding of the role of Tregs in promoting long-term engraftment and tolerance, there is a great deal of interest in promoting Tregs in transplant recipients as a complement to pharmacologic ways to disarm effector T cells. These efforts take two forms.
Sparing/expanding endogenous Tregs
The mainstay of standard immunosuppressive therapy, calcineurin inhibition, non-selectively inhibits T cell activation and expansion, including Tregs. In contrast, mTOR inhibitors such as rapamycin, although somewhat weaker in their immunosuppressive effects, also exhibit some specificity for effector T cells, as Tregs are less dependent on mTOR and its downstream pathways to meet their energy needs (78). In addition to using agents which exhibit relative selectivity for effector T cells, there have been a number of studies recently utilizing low dose interleukin 2 (IL-2) as a means to expand Tregs in vivo. The IL-2 receptor is a three chain heterotrimer (α,β, and γ chain). Unique among T cells, Tregs constitutively express all three chains of the receptor, which enable them to respond to low concentrations of IL-2, compared with resting T cells or NK cells which express only the β and γ chains, and therefore require higher IL-2 concentrations. Low dose IL-2 has not yet been studied in solid organ transplantation, but has shown great promise in chronic graft versus host disease and vasculitis, where it induces a dramatic and relatively selective Treg expansion, induces Treg activation, and suppresses disease.
Tregs as cellular therapy
Another means to increase Tregs in patients is via cellular therapy. A recent review by Hutchinson and Geissler outlines the rationale for such an approach (79) A number of early stage clinical trials have been performed, most of which use poly-clonal (i.e., non-specific) Tregs. In this approach, recipient Tregs are isolated, purified, expanded using mitogenic anti-CD3 and anti-CD28 antibodies plus IL-2, and then re-infused. A number of phase I studies have been performed in graft-versus-host disease and type I diabetes, and studies have been initiated in kidney transplantation. These clearly show that polyclonal T regs are safe, and perhaps also show hints of efficacy, but a more definitive determination will require Phase II studies, which are still underway.
While polyclonal Tregs are relatively easy to manufacture, antigen-specific Tregs are theoretically much more attractive. In animal models of transplantation, they are ~10 times as potent in preventing rejection and inducing tolerance. As well, they should have only a very minimal risk of non-specific immunosuppression, though a previous study has shown that the infusion of Tregs, either in single or multiple doses, in conditions where host T cells were depleted, resulted in enhanced memory T cell and antidonor alloantibodies, leading to decreased allograft function (80).Three groups are using antigen-specific regulatory cells in solid organ transplantation studies. Based on a concept pioneered by Guinan et al (81, 82), the Kyoto group (83) generated regulatory cells by culturing recipient peripheral blood mononuclear cells (PBMCs) with irradiated donor cells in the presence of anti-CD80 and anti-CD86 Abs (to block CD28 costimulation). They used the unpurified product as a source of enriched Tregs. In a pilot study in liver transplantation, 7 of 10 patients have been successfully weaned from immunosuppression, including 4 patients who have been off drugs for over 2 years. As part of the ONE Study (83A), the Massachusetts General Hospital (MGH) and University of California San Francisco (UCSF) groups are using antigen-specific Tregs in kidney transplantation. The MGH group is employing a procedure similar to the Kyoto group, except that belatacept is substituted for the anti-CD80/86 Abs, and highly purified Tregs are isolated from the cultures prior to infusion. Thus, recipient PBMCs are cultured with irradiated donor PBMCs in the presence of belatcept for 72 hours after which Tregs are isolated by the sequential use of magnetic bead-based depletion of CD8+ and CD19+ cells, followed by magnetic bead-based enrichment for CD25+ cells. At UCSF, recipient Tregs are first isolated using GMP flow-based sorting for CD4+CD25hiCD127lo cells. These cells are then expanded using donor B cells which were stimulated with CD154-expressing K562 cells. These different culture/manufacturing conditions both yield a population of highly purified Foxp3+ cells (with Treg-specific demethylated region demethylation) that are polyclonal and exhibit high specificity for donor cells in an in vitro suppression assay,
Of course the idea of promoting endogenous Treg proliferation and administering exogenously administered Tregs are not mutually exclusive. Studies combining Tregs with low dose IL-2 are likely to be initiated in the near future. Tregs have also been used experimentally to enhance the effectiveness of other tolerance approaches, e.g., to enable the achievement of mixed chimerism without the use of cytoreductive conditioning (84)
Conclusion
The importance of Tregs in kidney allograft acceptance is well documented. Their potential use as therapeutic agents in conferring transplant tolerance is very promising but more has yet to be done, especially with regards to their stability and long-term effects. In addition, understanding the unique role the kidney plays in the induction of Treg-dependent systemic tolerance – identifying the cellular and molecular components – will prove to be important in the development of transplant regimens that will result in the long-term survival of allografts.
Acknowledgments
Support: Supported by National Institutes of Health grants P01-HL018646 and P01-AI123086.
Peer Review: Evaluated by 2 external peer reviewers, a Handling Editor, Education Editor Gilbert, and Editor-in-Chief Levey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7(4):305–10. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 3.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10(7):689–95. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 4.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19(2):176–85. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13(3):108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Rudensky AY, Gavin M, Zheng Y. FOXP3 and NFAT: partners in tolerance. Cell. 2006;126(2):253–6. doi: 10.1016/j.cell.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road. J Cell Physiol. 2007;211(3):590–7. doi: 10.1002/jcp.21001. [DOI] [PubMed] [Google Scholar]
- 8.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25(2):195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4(+)CD25(+) T regulatory cells. Nat Immunol. 2003;4(4):337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12.Newell KA, Phippard D, Turka LA. Regulatory cells and cell signatures in clinical transplantation tolerance. Curr Opin Immunol. 2011;23(5):655–9. doi: 10.1016/j.coi.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non–self. Nat Immunol. 2005;6(4):345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 16.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162(5):1078–89. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435(7042):598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, van der Veeken J, Shugay M, Putintseva EV, Osmanbeyoglu HU, Dikiy S, et al. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature. 2015;528(7580):132–6. doi: 10.1038/nature16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, et al. Continuous Activation of Autoreactive CD4+ CD25+ Regulatory T Cells in the Steady State. J Exp Med. 2003;198(5):737–46. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of Peripheral CD4+CD25- Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-{beta} Induction of Transcription Factor Foxp3. J Exp Med. 2003;198(12):1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16(2):188–96. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue X, Trifari S, Aijo T, Tsagaratou A, Pastor WA, Zepeda-Martinez JA, et al. Control of Foxp3 stability through modulation of TET activity. J Exp Med. 2016;213(3):377–97. doi: 10.1084/jem.20151438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(47):18460–5. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188(3):976–80. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 26.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 27.Osorio F, Fuentes C, Lopez MN, Salazar-Onfray F, Gonzalez FE. Role of Dendritic Cells in the Induction of Lymphocyte Tolerance. Front Immunol. 2015;6:535. doi: 10.3389/fimmu.2015.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206(13):3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, et al. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol. 2004;15(3):613–21. doi: 10.1097/01.asn.0000114553.36258.91. [DOI] [PubMed] [Google Scholar]
- 30.Coates PT, Duncan FJ, Colvin BL, Wang Z, Zahorchak AF, Shufesky WJ, et al. In vivo-mobilized kidney dendritic cells are functionally immature, subvert alloreactive T-cell responses, and prolong organ allograft survival. Transplantation. 2004;77(7):1080–9. doi: 10.1097/01.tp.0000122183.60680.c9. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Bozulic LD, Miller T, Xu H, Hussain LR, Ildstad ST. CD8α+ plasmacytoid precursor DCs induce antigen-specific regulatory T cells that enhance HSC engraftment in vivo. Blood. 2011;117(8):2494–505. doi: 10.1182/blood-2010-06-291187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 33.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36(3):438–50. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9(11):1253–60. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29(3):464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173(7):4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 37.Puccetti P, Fallarino F. Generation of T cell regulatory activity by plasmacytoid dendritic cells and tryptophan catabolism. Blood Cells Mol Dis. 2008;40(1):101–5. doi: 10.1016/j.bcmd.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Conrad C, Gregorio J, Wang YH, Ito T, Meller S, Hanabuchi S, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012;72(20):5240–9. doi: 10.1158/0008-5472.CAN-12-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chappell CP, Giltiay NV, Draves KE, Chen C, Hayden-Ledbetter MS, Shlomchik MJ, et al. Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance. J Immunol. 2014;192(12):5789–801. doi: 10.4049/jimmunol.1303259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh NYC, Tonsho M, Ndishabandi D, Russell P, Colvin R, Madsen J, Alessandrini A. Distinct Phenotype and Function of Plasmacytoid Dendritic Cells from Kidney Allografts in Tolerant Recipients. Am J Transplant. 2016;16(suppl 3) [Google Scholar]
- 41.Aluvihare VR, Betz AG. The role of regulatory T cells in alloantigen tolerance. Immunol Rev. 2006;212:330–43. doi: 10.1111/j.0105-2896.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 42.Benghiat FS, Graca L, Braun MY, Detienne S, Moore F, Buonocore S, et al. Critical influence of natural regulatory CD25+ T cells on the fate of allografts in the absence of immunosuppression. Transplantation. 2005;79(6):648–54. doi: 10.1097/01.tp.0000155179.61445.78. [DOI] [PubMed] [Google Scholar]
- 43.Graca L, Silva-Santos B, Coutinho A. The blind-spot of regulatory T cells. Eur J Immunol. 2006;36(4):802–5. doi: 10.1002/eji.200635967. [DOI] [PubMed] [Google Scholar]
- 44.Hu M, Wang C, Zhang GY, Saito M, Wang YM, Fernandez MA, et al. Infiltrating Foxp3(+) regulatory T cells from spontaneously tolerant kidney allografts demonstrate donor-specific tolerance. Am J Transplant. 2013;13(11):2819–30. doi: 10.1111/ajt.12445. [DOI] [PubMed] [Google Scholar]
- 45.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178(4):1635–45. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller YD, Mai G, Morel P, Serre-Beinier V, Gonelle-Gispert C, Yung GP, et al. Anti-CD154 mAb and rapamycin induce T regulatory cell mediated tolerance in rat-to-mouse islet transplantation. PLoS One. 2010;5(4):e10352. doi: 10.1371/journal.pone.0010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol. 2009 doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 49.Madariaga ML, Michel SG, La Muraglia GM, 2nd, Sekijima M, Villani V, Leonard DA, et al. Kidney-Induced Cardiac Allograft Tolerance in Miniature Swine is Dependent on MHC-Matching of Donor Cardiac and Renal Parenchyma. Am J Transplant. 2015;15(6):1580–90. doi: 10.1111/ajt.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mezrich JD, Yamada K, Lee RS, Mawulawde K, Houser SL, Schwarze ML, et al. Mechanisms of tolerance induction in the heart/kidney model in miniature swine. J Heart Lung Transplant. 2001;20(2):172–3. doi: 10.1016/s1053-2498(00)00343-0. [DOI] [PubMed] [Google Scholar]
- 51.Yamada K, Menard MT, Mawulawde K, Slisz JK, Choo JK, Erhorn AE, et al. The effects of heart/kidney versus double heart transplantation on tolerance induction and prevention of cardiac allograft vasculopathy. Transplantation Proceedings. 1999;31(1–2):108. doi: 10.1016/s0041-1345(98)01462-6. [DOI] [PubMed] [Google Scholar]
- 52.Tonsho M, Benichou G, Boskovic S, Cappetta K, Nadazdin O, Smith RN, et al. Effect of kidney cotransplantation on induction of heart graft tolerance in non-human primates. 24th Int Cong Transpl Soc. 2012 Abstr Mon.PO04.004. [Google Scholar]
- 53.Narula J, Bennett LE, DiSalvo T, Hosenpud JD, Semigran MJ, Dec GW. Outcomes in recipients of combined heart-kidney transplantation: multiorgan, same-donor transplant study of the International Society of Heart and Lung Transplantation/United Network for Organ Sharing Scientific Registry. Transplantation. 1997;63(6):861–7. doi: 10.1097/00007890-199703270-00012. [DOI] [PubMed] [Google Scholar]
- 54.Vermes E, Grimbert P, Sebbag L, Barrou B, Pouteil-Noble C, Pavie A, et al. Long-term results of combined heart and kidney transplantation: a French multicenter study. J Heart Lung Transplant. 2009;28(5):440–5. doi: 10.1016/j.healun.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Vermes E, Kirsch M, Houel R, Legouvelo S, Benvenuti C, Aptecar E, et al. Immunologic events and long-term survival after combined heart and kidney transplantation: a 12-year single-center experience. J Heart Lung Transplant. 2001;20(10):1084–91. doi: 10.1016/s1053-2498(01)00308-4. [DOI] [PubMed] [Google Scholar]
- 56.Frasca L, Marelli-Berg F, Imami N, Potolicchio I, Carmichael P, Lombardi G, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53(3):679–89. doi: 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 57.Veronese F, Rotman S, Smith RN, Pelle TD, Farrell ML, Kawai T, et al. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant. 2007;7(4):914–22. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 58.Brown K, Moxham V, Karegli J, Phillips R, Sacks SH, Wong W. Ultra-Localization of Foxp3+ T Cells within Renal Allografts Shows Infiltration of Tubules Mimicking Rejection. Am J Pathol. 2007;171(6):1915–22. doi: 10.2353/ajpath.2007.070396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoop R, Wahl P, Le Hir M, Heemann U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant. 2004;19(11):2713–20. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 60.Mohib K, Guan Q, Diao H, Du C, Jevnikar AM. Proapoptotic activity of indoleamine 2,3-dioxygenase expressed in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293(3):F801–12. doi: 10.1152/ajprenal.00044.2007. [DOI] [PubMed] [Google Scholar]
- 61.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111ra20. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson H, Wong WK, Talbot D, Burt AD, Kirby JA. Tubulitis after renal transplantation: demonstration of an association between CD103+ T cells, transforming growth factor beta1 expression and rejection grade. Transplantation. 2001;71(2):306–13. doi: 10.1097/00007890-200101270-00024. [DOI] [PubMed] [Google Scholar]
- 63.Zheng SG. The Critical Role of TGF-beta1 in the Development of Induced Foxp3+ Regulatory T Cells. Int J Clin Exp Med. 2008;1(3):192–202. [PMC free article] [PubMed] [Google Scholar]
- 64.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–8. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 65.Bestard O, Cruzado JM, Rama I, Torras J, Goma M, Seron D, et al. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol. 2008;19(10):2020–6. doi: 10.1681/ASN.2007111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimbert P, Mansour H, Desvaux D, Roudot-Thoraval F, Audard V, Dahan K, et al. The regulatory/cytotoxic graft-infiltrating T cells differentiate renal allograft borderline change from acute rejection. Transplantation. 2007;83(3):341–6. doi: 10.1097/01.tp.0000248884.71946.19. [DOI] [PubMed] [Google Scholar]
- 67.Mansour H, Homs S, Desvaux D, Badoual C, Dahan K, Matignon M, et al. Intragraft levels of Foxp3 mRNA predict progression in renal transplants with borderline change. J Am Soc Nephrol. 2008;19(12):2277–81. doi: 10.1681/ASN.2008030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bestard O, Cruzado JM, Mestre M, Caldes A, Bas J, Carrera M, et al. Achieving donor-specific hyporesponsiveness is associated with FOXP3+ regulatory T cell recruitment in human renal allograft infiltrates. J Immunol. 2007;179(7):4901–9. doi: 10.4049/jimmunol.179.7.4901. [DOI] [PubMed] [Google Scholar]
- 69.Ashton-Chess J, Giral M, Soulillou JP, Brouard S. Using biomarkers of tolerance and rejection to identify high- and low-risk patients following kidney transplantation. Transplantation. 2009;87(9 Suppl):S95–9. doi: 10.1097/TP.0b013e3181a2e295. [DOI] [PubMed] [Google Scholar]
- 70.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353(22):2342–51. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 71.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112(9):1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37(9):2378–89. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 73.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bestard O, Cunetti L, Cruzado JM, Lucia M, Valdez R, Olek S, et al. Intragraft regulatory T cells in protocol biopsies retain foxp3 demethylation and are protective biomarkers for kidney graft outcome. Am J Transplant. 2011;11(10):2162–72. doi: 10.1111/j.1600-6143.2011.03633.x. [DOI] [PubMed] [Google Scholar]
- 75.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4(124):124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morris H, DeWolf S, Robins H, Sprangers B, LoCascio SA, Shonts BA, et al. Tracking donor-reactive T cells: Evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med. 2015;7(272):272ra10. doi: 10.1126/scitranslmed.3010760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newton R, Priyadharshini B, Turka LA. Immunometabolism of regulatory T cells. Nat Immunol. 2016;17(6):618–25. doi: 10.1038/ni.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hutchinson JA, Geissler EK. Now or never? The case for cell-based immunosuppression in kidney transplantation. Kidney Int. 2015;87(6):1116–24. doi: 10.1038/ki.2015.50. [DOI] [PubMed] [Google Scholar]
- 80.Ezzelarab MB, Zhang H, Guo H, Lu L, Zahorchak AF, Wiseman RW, et al. Regulatory T Cell Infusion Can Enhance Memory T Cell and Alloantibody Responses in Lymphodepleted Nonhuman Primate Heart Allograft Recipients. Am J Transplant. 2016;16(7):1999–2015. doi: 10.1111/ajt.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davies JK, Gribben JG, Brennan LL, Yuk D, Nadler LM, Guinan EC. Outcome of alloanergized haploidentical bone marrow transplantation after ex vivo costimulatory blockade: results of 2 phase 1 studies. Blood. 2008;112(6):2232–41. doi: 10.1182/blood-2008-03-143636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guinan EC, Boussiotis VA, Neuberg D, Brennan LL, Hirano N, Nadler LM, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340(22):1704–14. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 83.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64(2):632–43. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 84.Pilat N, Baranyi U, Klaus C, Jaeckel E, Mpofu N, Wrba F, et al. Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transplant. 2010;10(4):751–62. doi: 10.1111/j.1600-6143.2010.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83A.The One Study. [date accessed: Nov 20, 2016];A Unified Approach to Evaluating Cellular Immunotherapy in Solid Organ Transplantation. www.Onestudy.org.