Summary
The phosphatidylinositol 3-kinase (PI3K)/mammalian (or mechanistic) target of rapamycin (mTOR) signalling pathway is commonly dysregulated in acute lymphoblastic leukaemia (ALL). A phase 1 trial of the mTOR inhibitor temsirolimus in combination with UKALL R3 re-induction chemotherapy was conducted in children and adolescents with second or greater relapse of ALL. The initial temsirolimus dose level (DL1) was 10 mg/m2 weekly × three doses. Subsequent patient cohorts received temsirolimus 7.5 mg/m2 weekly × three doses (DL0) or, secondary to toxicity, 7.5 mg/m2 weekly × two doses (DL-1). Sixteen patients were enrolled, 15 were evaluable for toxicity. Dose-limiting toxicity (DLT) occurred at all three dose levels and included hypertriglyceridaemia, mucositis, ulceration, hypertension with reversible posterior leucoencephalopathy, elevated gamma-glutamyltransferase or alkaline phosphatase and sepsis. The addition of temsirolimus to UKALL R3 re-induction therapy resulted in excessive toxicity and was not tolerable in children with relapsed ALL. However, this regimen induced remission in seven of fifteen patients. Three patients had minimal residual disease levels <0.01%. Inhibition of PI3K signalling was detected in patients treated at all dose levels of temsirolimus, but inhibition at an early time point did not appear to correlate with clinical responses at the end of re-induction therapy.
Keywords: acute lymphoblastic leukaemia, mTOR inhibitor, clinical trials, pharmacodynamics, relapse
Introduction
Mammalian (or mechanistic) target of rapamycin (mTOR)/phosphatidylinositol 3-kinase (PI3K) signalling, a critical pathway in cell proliferation, metabolism and apoptosis, is commonly dysregulated in acute lymphoblastic leukaemia (ALL), which may confer chemotherapy resistance (Tasian, et al 2014). While mutations in the MTOR gene, also known as FRAP (FKBP-12-rapamycin associated protein), itself are less common, constitutive activation of PI3K/mTOR signal transduction proteins or loss of negative regulation of mTOR occurs in many haematological malignancies (Bjornsti and Houghton 2004, Grabiner, et al 2014, Smolewski 2006, Tasian, et al 2014). Constitutive signalling activation may result from increased surface expression of growth factor receptors on leukaemia cells or from mutation of intracellular downstream effectors, including PI3K/mTOR pathway proteins (Chapuis, et al 2010). Additionally, loss of tumor suppressors that normally regulate PI3K signalling, such as PTEN (phosphatase and tensin homolog), can induce dysregulation of normal cellular equilibrium and facilitate aberrant signalling activation (Gutierrez, et al 2009).
Targeting constitutive PI3K/mTOR activation with small molecular inhibitors has been studied in ALL (Tasian, et al 2016). mTOR inhibitors (MTIs), such as sirolimus (rapamycin), inhibit growth of B-ALL and T-ALL cell lines in vitro and induce synergistic cell death in combination with anthracyclines, L-asparaginase and dexamethasone (Houghton, et al 2008, Teachey, et al 2006, Teachey, et al 2008). Further, mTOR inhibition can reverse glucocorticoid resistance in ALL, partly due to modulation of anti-apoptotic proteins, such as MCL1 (Wei, et al 2006). In murine models of human ALL, MTIs result in inhibition of leukaemia proliferation and prolong animal survival compared to vehicle-treated controls (Crazzolara, et al 2009, Houghton, et al 2008, Teachey, et al 2006, Teachey, et al 2008).
Despite aggressive retrieval strategies, the prognosis for children with refractory ALL is poor (Chessells 1998, Gaynon, et al 1998, Nguyen, et al 2008, Parker, et al 2010). Molecularly-targeted agents, including kinase inhibitors such as MTIs, have shown promise in treating some patients with haematological malignancies (Tasian, et al 2014). We conducted a trial to define the maximum tolerated dose (MTD) of temsirolimus in combination with intensive re-induction chemotherapy in children or adolescents with second or greater relapse of ALL. Additional exploratory aims included preliminary assessment of treatment response within the context of a phase 1 trial and pharmacodynamic measurement of PI3K/mTOR pathway inhibition.
Methods
Patient eligibility
Patients ≥1 and <22 years of age in second or greater relapse of ALL were eligible. Relapsed leukaemia was defined as >25% blasts in bone marrow (M3) or 5–25% blasts in bone marrow (M2) with evidence of extramedullary disease. Patients with an isolated central nervous system relapse or extramedullary disease requiring immediate radiation therapy were not eligible.
Eligibility criteria included a Lansky/Karnofsky performance score ≥50, recovery from acute toxic effects of prior therapy and lack of active infections. Patients had to be ≥2 weeks from prior cytotoxic therapy with the exception of maintenance-type ALL therapy for which there was no washout period or a single intrathecal methotrexate within 72 h of systemic therapy initiation. Patients had to be ≥7 days from short-acting growth factor therapy or ≥14 days if long-acting, ≥7 days from biological therapy, >42 days from immunotherapy and > 3 half-lives from prior therapy with a monoclonal antibody. Patients had to be ≥3 months from prior HSCT and without evidence of graft-versus-host disease. At least two weeks from local radiation had to have elapsed. Patients receiving corticosteroids must have been taking a stable or decreasing dose for 7 days prior to enrolment. Hydroxycarbamide (hydroxyurea) use was permitted until 24 h prior to the first dose of study chemotherapy.
Other eligibility requirements included a normal age-adjusted serum creatinine or glomerular filtration rate (GFR) ≥70 ml/min/1.73 m2, normal cardiac function defined by shortening fraction ≥27% or ejection fraction ≥50%, adequate pulmonary function with a baseline oxygen saturation >94% on room air, and adequate liver function defined as total bilirubin ≤1.5× the institutional upper limit of normal for age, normal gamma-glutamyltransferase (GGT) for age, alanine aminotransferase ≤225 u/l and albumin ≥20 g/l. Serum triglyceride and cholesterol were required to be ≤3.89 mmol/l and 7.77 mmol/l, respectively, and a fasting glucose had to be within normal limits for age.
This clinical trial was registered at www.clinicaltrials.gov (NCT01403415). Local institutional review board approval of the protocol was required. Written informed consent from patients ≥18 years or from parents/legal guardians of children age <18 years was obtained (and assent as appropriate) according to institutional policies. All authors had access to primary clinical trial data.
Drug administration and study design
The primary objectives of the study were 1) to estimate the MTD and/or recommended phase 2 dose of temsirolimus administered in combination with UKALL R3 re-induction chemotherapy (Parker, et al 2010) in children with relapsed ALL and 2) to define and describe the toxicities of temsirolimus in combination with intensive re-induction chemotherapy. The secondary objectives were 1) to determine the complete response rate (CR), 2) to determine minimal residual disease (MRD) levels at end induction and 3) to evaluate the responsiveness of ALL cells to mTOR inhibition.
A rolling six dose escalation trial design was used (Skolnik, et al 2008) in which two to six patients can be concurrently enrolled onto a dose level, dependent upon (1) the number of patients enrolled at the current dose level, (2) the number of patients who have experienced DLT at the current dose level, and (3) the number of patients entered but with tolerability data pending at the current dose level. The MTD is the maximum dose at which fewer than one-third of patients experience DLT, therefore, if two or more of a cohort of up to six patients experience DLT at a given dose level, then the MTD has been exceeded and dose escalation is stopped. Temsirolimus was supplied by the Cancer Therapy Evaluation Program (National Cancer Institute; Bethesda, MD).
Each temsirolimus dose was administered intravenously 1 h post-mitoxantrone on day 1 and in proximity to vincristine on days 8 and 15. In DL-1 the day 15 temsirolimus dose was excluded. The starting dose of temsirolimus at dose level 1 (DL1) was 10 mg/m2/dose, two-thirds of the Federal Drug Administration-approved monotherapy dose in adults (Hudes, et al 2007) and well below the single agent dose of 150 mg/m2/dose identified in children with relapsed/refractory solid tumours (Spunt, et al 2011). Dose escalation to 15 mg/m2/dose (DL2) and 20 mg/m2/dose (DL3) was planned with one dose de-escalation to 7.5 mg/m2/dose (DL0) if DL1 was deemed too toxic.
No intra-patient dose escalation was allowed. Each patient was enrolled for a single 36-day cycle of reinduction therapy and was monitored for toxicity to at least day 42. Due to DLTs at both DL1 and DL0, the protocol was amended to add a DL-1 cohort that received weekly temsirolimus 7.5 mg/m2/dose for two doses (in lieu of the three doses administered at DL0 and DL1).
Toxicities were graded according to the Common Terminology Criteria for Adverse Events version 4.0 (http://ctep.cancer.gov). International Consensus Conference on Toxicity Assessment guidelines were utilized to identify expected toxicities of multi-agent chemotherapy backbones and to define DLTs of combination therapy for the current study (Horton, et al 2010). Per these guidelines, specific non-haematological toxicities occurring in >6% of patients with similar chemotherapy were not considered DLTs if they returned to grade ≤2 by Day 42 of protocol therapy. These Grade 3 and 4 laboratory abnormalities included electrolyte abnormalities, elevated hepatic function tests and hypofibrinogenaemia. In addition, grade 3 fasting hyperglyceamia, hypercholesterolaemia, and hypertriglyceridaemia were not considered DLTs and use of medications to decrease hyperglycaemia or hyperlipidaemia was permitted. Other toxicities not considered DLTs included grades 3 or 4 nausea, vomiting, fatigue, anorexia, malaise or weight loss, hypertension, fever, infection and febrile neutropenia that were anticipated sequelae of the chemotherapy regimen. Any toxicity resulting in temsirolimus dose omission was considered a DLT. Haematological DLT for patients with ALL was defined as bone marrow aplasia at ≥day 42 not attributable to leukaemia progression.
Complete response (CR) for patients with ALL was defined as bone marrow morphology with <5% blasts (M1), no evidence of extramedullary disease, and recovery of peripheral blood counts (absolute neutrophil count (ANC) > 0.5 × 109/l and a platelet count > 50 × 109/l). A CR with incomplete blood count recovery (CRi) was a CR without normalization of ANC and/or platelet count by Day 43 of re-induction. Partial response (PR) was defined as clearance of peripheral blasts with 5–25% blasts in bone marrow (M2) or as an M1 bone marrow without complete eradication of extramedullary disease. Patients who failed to qualify as a CR, CRi or PR were defined as stable or progressive disease (SD/PD). Disease evaluations were obtained at baseline and at the end of the 36-day cycle of therapy.
Pharmacodynamic analyses
In consenting patients, peripheral blood specimens were obtained to assess in vivo inhibition of PI3K pathway targets using phosphoflow cytometry as previously described (Loh, et al 2015). Samples (3–5 ml) were obtained at three time points: immediately prior to temsirolimus therapy (baseline, day 0), at day 3–5 of therapy (after the first dose of temsirolimus) and at day 36 (at end of re-induction).
Results
Patient Characteristics
Sixteen patients, aged 1–21 years, were enrolled between February 2012 and August 2014 (Table I). One patient did not initiate protocol therapy due to patient choice and was thus not evaluable. One evaluable patient had T-ALL, 14 evaluable patients had B-lineage ALL including infant KMT2A (MLL)-rearranged ALL (n=3), or BCR-ABL1-rearranged (Ph+) ALL (n=2) (Table II). Twelve patients were in second relapse, and three were in third relapse. Patients had previously undergone a mean of three prior salvage chemotherapy regimens prior to study entry (range 2–5), and 7 of the 15 patients had relapsed after a bone marrow transplant. All evaluable patients completed the full cycle of re-induction therapy.
Table I.
Characteristics of patients enrolled on ADVL1114.
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median | 9 |
| Range | 1 – 21 |
| Sex | |
| Male | 8 (50) |
| Female | 8 (50) |
| Race | |
| White | 13 (81) |
| Asian | 0 (0) |
| American Indian or Alaska Native | 0 (0) |
| Black or African American | 2 (13) |
| Unknown | 1 (6) |
| Ethnicity | |
| Non-Hispanic | 12 (75) |
| Hispanic | 4 (25) |
| Prior Chemotherapy Regimens | |
| Median | 3 |
| Range | 2 – 5 |
Table II.
Dose-limiting toxicities and clinical responses of patients treated on ADVL1114.
| Dose | Patient | Dx | Response | Dose-Limiting Toxicity |
|---|---|---|---|---|
|
DL1 10 mg/m2 × 3 |
1 | KMT2A | PD | |
| 2 | B-ALL | Inevaluable | ||
| 3 | KMT2A | PD | Hypertension, mucositis, and GGT not resolved by day 42 |
|
| 4* | B-ALL | CRi3 | ||
| 5 | B-ALL | PD | Hypertriglyceridaemia | |
|
DL0 7.5 mg/m2 × 3 |
6* | B-ALL | CR4 | |
| 7 | B-ALL | PD | ||
| 8 | B-ALL | died | Sepsis, mucositis, acidosis, RPLE | |
| 9 | B-ALL | PD | Perianal ulcer not resolved by day 42 | |
| 10* | B-ALL | CRi3 | ||
| 11* | T-ALL | PD | GGT elevation not resolved by day 42 | |
|
DL-1 7.5 mg/m2 × 2 |
12* | Ph+ | CR3 | |
| 13 | KMT2A | PD | ||
| 14* | Ph+ | CRi3 | Anorexia, GGT elevation, alkaline phosphatase elevation not resolved by day 42 |
|
| 15* | B-ALL | CR3 | Hypertriglyceridaemia | |
| 16 | B-ALL | CR3 | Hypertriglyceridaemia |
= undergone prior haematopoetic stem cell transplantation; B-ALL = B cell acute lymphoblastic leukaemia; CR = complete response; CR3 = 3rd complete response; CR4= 4th complete response; CRi3 = 3rd complete response with incomplete blood count recovery; DL = dose level; Dx = diagnosis; GGT= gamma glutamyltransferase; KMT2A = KMT2A (MLL)-rearranged acute lymphoblastic leukaemia (infant)PD = progressive diseasePh+ = BCR-ABL1-rearranged acute lymphoblastic leukaemia; RPLE = reversible posterior leukoencephalopathy; T-ALL = T cell acute lymphoblastic leukaemia.
Toxicity
Four patients were enrolled at DL1, two of whom experienced DLTs. One patient had persistence of Grade 3 hypertension, elevated GGT and mucositis at Day 42. The other patient had grade 4 fasting hypertriglyceridaemia (Table II). Per protocol design, the dose for the subsequent cohort was reduced to DL0. Of the six patients enrolled at DL0, three experienced DLTs. One patient had reversible posterior leucoencephalopathy syndrome, acidosis and mucositis, and died of sepsis at Day 33 prior to end of reinduction bone marrow evaluation. Another patient had persistently elevated GGT, and a third patient had grade 2 perirectal ulceration that did not resolve by day 42. The study was temporarily closed while clinical and correlative biology data were evaluated. Pharmacodynamic assays demonstrated inhibition of PI3K signalling at a temsirolimus dose of 7.5 mg/m2, confirming that this dose had clinical potential for benefit, and the trial was amended to include DL-1 as outlined in the Methods.
Three of five patients treated at DL-1 experienced DLTs. Two patients had asymptomatic grade 4 hypertriglyceridemia, one of whom required omission of the second dose of temsirolimus. A third patient had persistent elevation in GGT and alkaline phosphatase and persistent grade 2 anorexia. DLTs occurred in more than one third of patients at all dose levels including temsirolimus 7.5 mg/m2/dose weekly × 2 doses (DL-1). Thus the MTD was exceeded at all evaluated dose levels and the study was closed due to the demonstrated toxicity of temsirolimus in combination with the UKALL R3 backbone.
Table III delineates the non-haematological non-dose limiting toxicities at least possibly attributed to temsirolimus that occurred in >10% of patients. The most common non-haematological toxicities were electrolyte abnormalities, transaminitis and/or elevated GGT levels, and fever. Hypertriglyceridaemia and hyperglycaemia were more commonly reported than hypercholesterolaemia. Renal and pulmonary toxicities were rare.
Table III.
Non-dose-limiting non-haematological toxicities related to protocol therapy and observed in >10% of evaluable patients (n=15).
| Toxicity | Maximum Grade of Toxicity | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Abdominal pain | 1 | 1 | ||
| Alanine aminotransferase increased | 2 | 7 | 4 | |
| Alkaline phosphatase increased | 3 | 1 | ||
| Anorexia | 3 | 2 | ||
| Aspartate aminotransferase increased | 3 | 3 | 2 | |
| Blood bilirubin increased | 3 | 2 | 1 | |
| Catheter-related infection | 4 | |||
| Cholesterol high | 2 | |||
| Diarrhoea | 4 | 3 | 1 | |
| Fatigue | 2 | 3 | ||
| Febrile neutropenia | 7 | 1 | ||
| Fever | 2 | 1 | ||
| GGT increased | 1 | 2 | 1 | |
| Hyperglycaemia | 1 | 4 | 3 | |
| Hypertension | 3 | 2 | ||
| Hypertriglyceridaemia | 1 | 3 | 4 | 4 |
| Hypoalbuminaemia | 1 | 4 | 1 | |
| Hypocalcaemia | 2 | 1 | 1 | 1 |
| Hypokalaemia | 2 | 2 | 1 | |
| Hyponatraemia | 3 | 2 | ||
| Hypophosphataemia | 2 | 4 | 2 | |
| Infections and infestations - other | 1 | 1 | ||
| Mucositis oral | 3 | 2 | 1 | |
| Nausea | 2 | 2 | 1 | |
| Oral pain | 1 | 1 | ||
| Pain | 1 | 2 | ||
| Peripheral sensory neuropathy | 1 | 1 | ||
| Sepsis | 1 | |||
| Vomiting | 4 | |||
A grade 5 sepsis event was the only infection-related toxicity that met the protocol definition of DLT. Other infectious toxicity was observed in this highly immunocompromised patient population including 11 of 15 patients (73%) with febrile neutropenia. Of the ten evaluable patients treated at DL1 or DL0, six had microbiologically-documented bacteraemia at day 10–12 of therapy or grade 3 catheter-related infections. Infectious organisms included gram-negative rods (Klebsiella, E. coli, Citrobacter, Pseudomonas), gram-positive cocci (S. epidermis or S. aureus, Enterococcus) and fungus (Candida). Four patients had >1 documented infection during their time on study. Three patients had grade 3 bacterial colitis/enterocolitis. The protocol amendment to decrease temsirolimus administration to two doses per cycle (DL-1) also included recommendations for hospitalization and broad-spectrum anti-bacterial and anti-fungal prophylaxis in the cohort of patients treated at DL-1.
Response
Clinical responses were observed in patients treated at each dose level (Table II). Seven of the 15 evaluable patients had a CR or CRi. Three of these had flow cytometric MRD <0.01% and two others had MRD 0.01–0.03%, thereby allowing potential eligibility for subsequent bone marrow transplantation beyond the scope of this trial (Pulsipher, et al 2014). Six of the seven responders had undergone a prior bone marrow transplant and 1 was in 3rd relapse at time of study entry.
Pharmacodynamic assessments
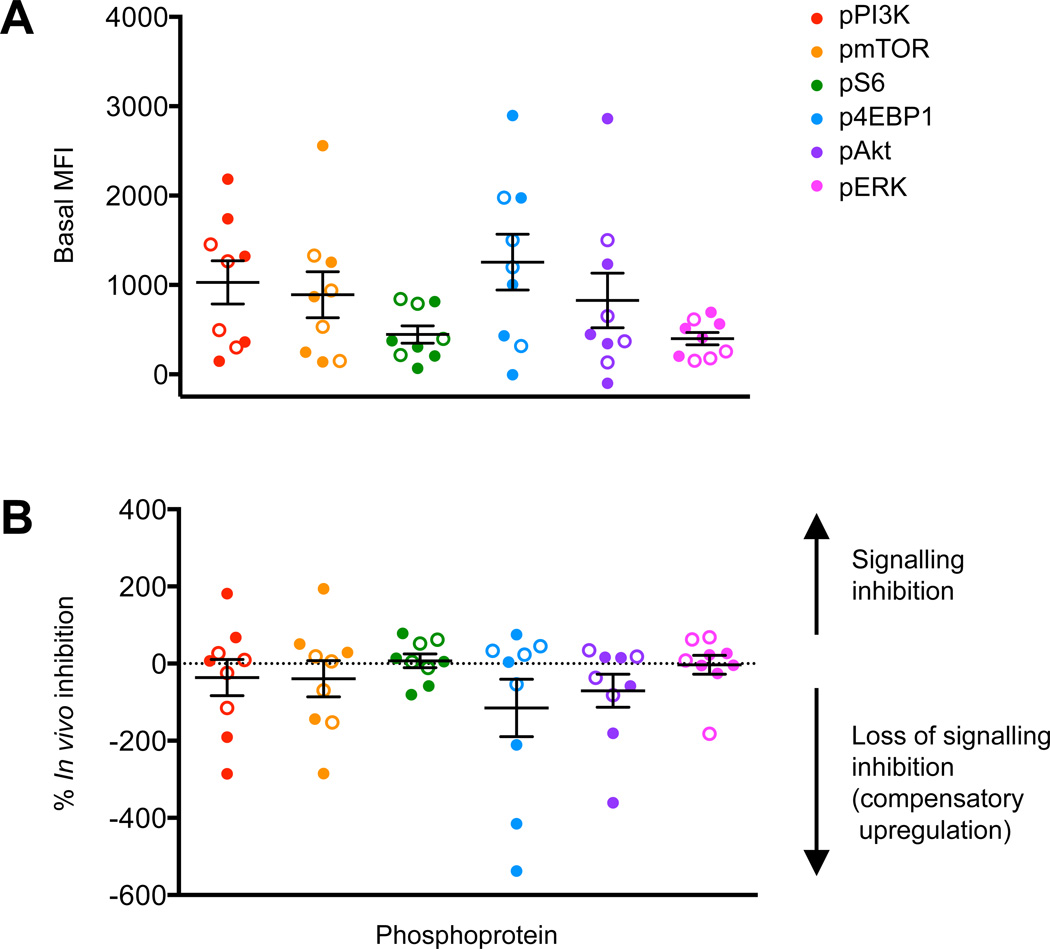
Peripheral blood samples from patients with B-ALL (n=8) or T-ALL (n=1) were obtained from patients treated at DL0 (n=5) or DL-1 (n=4) and analysed by phosphoflow cytometry. Two patients without constitutive activation of PI3K/mTOR signalling did not demonstrate signalling inhibition post-treatment. Seven of nine patients demonstrated elevated pre-treatment (Day 0) levels of phosphorylated (p) PI3K pathway phosphoproteins (pPI3K, pmTOR, pS6, p4EBP1, pAkt, and/or pERK) in gated ALL blasts in comparison to controls (Figure 1A) (Tung, et al 2007). Compared to baseline, the mean inhibition of one or more PI3K phosphoproteins decreased in all Day 3–5 post-treatment blood specimens tested (Figure 1B). Insufficient numbers of residual ALL cells were present in most Day 36 peripheral blood samples for reliable measurement of leukaemia-specific signal transduction (data not shown). No differences in signalling inhibition were observed between patients treated at DL0 vs. DL-1. In vivo inhibition of PI3K/mTOR proteins at the Day 3–5 time point after temsirolimus and cytotoxic chemotherapy initiation was not predictive of clinical responses at Day 36.
Figure 1. Phosphoflow cytometric analyses of in vivo signalling effects of temsirolimus and UKALLR3 chemotherapy.
(A) Basal (pre-treatment) levels of PI3K pathway phosphoproteins were measured in gated ALL cells in peripheral blood specimens from ADVL1114 patients treated at DL0 or DL-1. (B) Phosphoprotein inhibition at Day 3–5 of therapy after first dose of temsirolimus in comparison to basal phosphoprotein levels for each patient. Solid symbols = patients with complete response / complete response with incomplete blood count recovery, open symbols = stable disease/progressive disease. MFI = median fluorescence intensity. Data are displayed with means (central horizontal lines) and standard errors of the mean (whiskers).
Discussion
Therapy for children with relapsed ALL is hampered by low remission rates and high toxicity, especially in second and subsequent relapses. Many promising molecularly targeted agents are in development, but probably need to be combined with cytotoxic chemotherapy to improve cure rates and overall survival. This trial was conducted to evaluate the safety and tolerability of the MTI temsirolimus in combination with re-induction chemotherapy.
In this trial, the addition of temsirolimus to UKALL R3 re-induction therapy resulted in excessive toxicity and was deemed not tolerable in children with relapsed ALL. Dose limiting toxicity occurred at all dose levels. Most DLTs, such as hypertriglyceridaemia, mucositis and poor wound healing, were predictable based upon prior reports of mTOR inhibition in other cancers (Bagatell, et al 2014, Hudes, et al 2007, Spunt, et al 2011), as well as expected toxicities of similar intensive ALL re-induction chemotherapy backbones (Horton, et al 2010, Raetz, et al 2008, Sun, et al 2014). The use of high-dose dexamethasone pulses and asparaginase probably exacerbated the hypertriglyceridaemia often reported with MTI monotherapy. Similarly, a recent phase 1 trial of temsirolimus combined with irinotecan and temozolomide in children with relapsed solid tumours required modification to exclude patients on chronic steroids due to hyperlipidaemia (Bagatell, et al 2014). Temporary hypertriglyceridaemia may be considered an acceptable therapy-associated sequela in patients with multiply relapsed leukaemia. However, combination of MTIs with alternative salvage chemotherapy backbones that evoke fewer overlapping metabolic toxicities may increase successful delivery of this therapeutic strategy and reinduction of leukaemia remission. For example, in patients with first ALL relapse, preliminary data from a phase 1 trial combining the oral MTI everolimus with a 4-drug ALL induction has reported CR2 rates approaching 90% with less severe mucosal and gastrointestinal toxicity than was observed in our trial (Place, et al 2015).
Children and adolescents with multiply relapsed ALL have a significant risk of morbidity and mortality with intensive salvage therapy. Several recent relapsed ALL trials have reported a 4–5% induction mortality rate and 45–50% rate of grade 3 or 4 infections during re-induction therapy (Messinger, et al 2012, Raetz, et al 2008). Data from this study are highly concordant with reported infectious toxicity. We observed a 73% febrile neutropenia rate (11/15) and a 53% (8/15 patients) grade 3 or 4 documented infection rate and one grade 5 bacterial sepsis. The Therapeutic Advances in Childhood Leukaemia (TACL) consortium reported Grade ≥ 3 infections in 92% of patients with relapsed/refractory ALL treated with the R3 re-induction platform and 31% of patients had polymicrobial infection (Sun, et al 2014). More stringent hospitalization recommendations and infectious prophylaxis guidelines may abrogate some infectious complications (Messinger, et al 2012). Our study indicates that the addition of temsirolimus may increase infection risk of the UKALL R3 backbone, however, the presence or magnitude of increased risk cannot be fully assessed due to the infectious complication rate of the UKALL R3 backbone and risk of infection in this patient population.
Despite the excessive toxicity of combination therapy, 7 of the 15 evaluable patients (47%) were in CR/CRi at the end of re-induction therapy with five of these patients having MRD < 0.03%. Six of the 12 patients with a second relapse achieved a CR3, and 1 of the 3 patients with a 3rd relapse achieved a CR4. Reismuller et al (2013) reported 30–40% CR3 and CR4 rates in a similar patient population. While remission rates of patients in second or greater ALL relapse treated with UKALL R3 therapy alone are currently unknown, our study with combination therapy demonstrates that a third or greater CR is achievable in nearly half of patients.
Pharmacodynamic assays performed during our study demonstrated baseline PI3K pathway signalling activation in most patients treated on this trial and in vivo target inhibition is measurable after first doses of temsirolimus and chemotherapy.
Temsirolimus with UKALL R3 re-induction chemotherapy led to unacceptable toxicity in this trial, however, alternative chemotherapy backbones in combination with other MTIs have demonstrated safety and tolerability (Daver, et al 2015, Tasian, et al 2014). A phase 1 trial (NCT01614197) evaluating the safety of temsirolimus in combination with 5 days of etoposide and cyclophosphamide is currently accruing. The study is based on the hypothesis that the combination of temsirolimus, etoposide and cyclophosphamide will result in less additive toxicity, such as hyperglycaemia, hypertriglyceridaemia and/or poor wound healing. In addition, oral formulations of MTIs, such as everolimus (NCT01523977) are being evaluated (Place, et al 2015). Furthermore, targeting of more proximal or multiple proteins in the PI3K/mTOR pathway with alternative tyrosine kinase inhibitors may result in greater sustained signalling inhibition and, possibly, to less dependence on conventional cytotoxic chemotherapy to achieve cure (Reismuller, et al 2013, Tasian, et al 2016).
Acknowledgments
We would like to thank Charlotte Ahern for her contributions to this work. Research reported in this publication was supported by National Institutes of Health/National Cancer Institute awards U01 CA97452 and UM1 CA097452 to the Children’s Oncology Group and K12CA076931 and K08CA18441, the Rally Foundation for Childhood Cancer Research, the Leukemia & Lymphoma Society, the Cookies for Kids’ Cancer Foundation and the Children's Oncology Group Foundation.
DTT has a consulting role with Janssen. DTT and JAW receive research funding from Novartis.
Footnotes
Presented in part at the American Society of Clinical Oncology, Chicago, IL, USA June 2015
Authorship
SRR and SKT designed research, performed research, analysed data and wrote the paper, JAW, DTT and SMB designed research, analysed data and edited the paper, XL and CGM analysed data and performed statistical analyses. EF designed research, analysed data and wrote the paper. MJB and BJW performed research, analysed data and edited the paper.
Conflict of interest
All other authors declare no competing financial interest.
References
- Bagatell R, Norris R, Ingle AM, Ahern C, Voss S, Fox E, Little AR, Weigel BJ, Adamson PC, Blaney S. Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: a Children's Oncology Group Study. Pediatr Blood Cancer. 2014;61:833–839. doi: 10.1002/pbc.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Chapuis N, Tamburini J, Cornillet-Lefebvre P, Gillot L, Bardet V, Willems L, Park S, Green AS, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: therapeutic value of neutralizing anti-IGF-1R antibody. Haematologica. 2010;95:415–423. doi: 10.3324/haematol.2009.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessells JM. Relapsed lymphoblastic leukaemia in children: a continuing challenge. Br J Haematol. 1998;102:423–438. doi: 10.1046/j.1365-2141.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- Crazzolara R, Cisterne A, Thien M, Hewson J, Baraz R, Bradstock KF, Bendall LJ. Potentiating effects of RAD001 (Everolimus) on vincristine therapy in childhood acute lymphoblastic leukemia. Blood. 2009;113:3297–3306. doi: 10.1182/blood-2008-02-137752. [DOI] [PubMed] [Google Scholar]
- Daver N, Boumber Y, Kantarjian H, Ravandi F, Cortes J, Rytting ME, Kawedia JD, Basnett J, Culotta KS, Zeng Z, Lu H, Richie MA, Garris R, Xiao L, Liu W, Baggerly KA, Jabbour E, O'Brien S, Burger J, Bendall LJ, Thomas D, Konopleva M. A Phase I/II Study of the mTOR Inhibitor Everolimus in Combination with HyperCVAD Chemotherapy in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia. Clin Cancer Res. 2015;21:2704–2714. doi: 10.1158/1078-0432.CCR-14-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynon PS, Qu RP, Chappell RJ, Willoughby ML, Tubergen DG, Steinherz PG, Trigg ME. Survival after relapse in childhood acute lymphoblastic leukemia: impact of site and time to first relapse--the Children's Cancer Group Experience. Cancer. 1998;82:1387–1395. doi: 10.1002/(sici)1097-0142(19980401)82:7<1387::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Grabiner BC, Nardi V, Birsoy K, Possemato R, Shen K, Sinha S, Jordan A, Beck AH, Sabatini DM. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4:554–563. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Sanda T, Grebliunaite R, Carracedo A, Salmena L, Ahn Y, Dahlberg S, Neuberg D, Moreau LA, Winter SS, Larson R, Zhang J, Protopopov A, Chin L, Pandolfi PP, Silverman LB, Hunger SP, Sallan SE, Look AT. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114:647–650. doi: 10.1182/blood-2009-02-206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton TM, Sposto R, Brown P, Reynolds CP, Hunger SP, Winick NJ, Raetz EA, Carroll WL, Arceci RJ, Borowitz MJ, Gaynon PS, Gore L, Jeha S, Maurer BJ, Siegel SE, Biondi A, Kearns PR, Narendran A, Silverman LB, Smith MA, Zwaan CM, Whitlock JA. Toxicity assessment of molecularly targeted drugs incorporated into multiagent chemotherapy regimens for pediatric acute lymphocytic leukemia (ALL): review from an international consensus conference. Pediatr Blood Cancer. 2010;54:872–878. doi: 10.1002/pbc.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton PJ, Morton CL, Kolb EA, Gorlick R, Lock R, Carol H, Reynolds CP, Maris JM, Keir ST, Billups CA, Smith MA. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- Loh ML, Tasian SK, Rabin KR, Brown P, Magoon D, Reid JM, Chen X, Ahern CH, Weigel BJ, Blaney SM. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: A Children's Oncology Group phase 1 consortium study (ADVL1011) Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger YH, Gaynon PS, Sposto R, van der Giessen J, Eckroth E, Malvar J, Bostrom BC. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood. 2012;120:285–290. doi: 10.1182/blood-2012-04-418640. [DOI] [PubMed] [Google Scholar]
- Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, Winick NJ, Hunger SP, Gaynon PS, Loh ML. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, Ancliff P, Morgan M, Masurekar A, Goulden N, Green N, Revesz T, Darbyshire P, Love S, Saha V. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376:2009–2017. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place AE, Pikman Y, Stevenson KE, Cooper TM, Gore L, Hijiya N, Loh ML, Pauly M, Sulis ML, Neuberg DS, Stegmaier K, Sallan SE, Silverman LB. Phase I Trial of the mTOR Inhibitor Everolimus Given in Combination with Multiagent Chemotherapy in Relapsed Childhood Acute Lymphoblastic Leukemia. Blood. 2015;126 doi: 10.1002/pbc.27062. abstract 3765. [DOI] [PubMed] [Google Scholar]
- Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, Raetz E, Gardner S, Gastier-Foster JM, Howrie D, Goyal RK, Douglas JG, Borowitz M, Barnes Y, Teachey DT, Taylor C, Grupp SA. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014;123:2017–2025. doi: 10.1182/blood-2013-10-534297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, Carroll WL. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected] Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reismuller B, Peters C, Dworzak MN, Potschger U, Urban C, Meister B, Schmitt K, Dieckmann K, Gadner H, Attarbaschi A, Mann G for the Austrian, ALL-BFM Study Group. Outcome of children and adolescents with a second or third relapse of acute lymphoblastic leukemia (ALL): a population-based analysis of the Austrian ALL-BFM (Berlin-Frankfurt-Munster) study group. J Pediatr Hematol Oncol. 2013;35:e200–e204. doi: 10.1097/MPH.0b013e318290c3d6. [DOI] [PubMed] [Google Scholar]
- Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- Smolewski P. Investigating mammalian target of rapamycin inhibitors for their anticancer properties. Expert Opin Investig Drugs. 2006;15:1201–1227. doi: 10.1517/13543784.15.10.1201. [DOI] [PubMed] [Google Scholar]
- Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, Berkenblit A, Krygowski M, Ananthakrishnan R, Boni JP, Gilbertson RJ. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol. 2011;29:2933–2940. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Smith AM, Etan O, Sposto R, Wilkes JJ, Gardner RA, Minjun H, Pineros V, Olson E, Tan Y, Rheingold SR, Burke MJ, Wayne AS. The UK ALLR3 Chemotherapy Regimen for Relapsed/Refractory Acute Lymphoblastic Leukemia of Childhood: A Multi-Institutional Retrospective Study of Treatment Related Adverse Events. Blood. 2014;124 abstract 3647. [Google Scholar]
- Tasian SK, Teachey DT, Rheingold SR. Targeting the PI3K/mTOR Pathway in Pediatric Hematologic Malignancies. Front Oncol. 2014;4:108. doi: 10.3389/fonc.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasian SK, Teachey DT, Li Y, Shen F, Harvey RC, Chen IM, Ryan T, Vincent TL, Willman CL, Perl AE, Hunger SP, Loh ML, Grupp SA. Potent Efficacy of Combined PI3K/mTOR and JAK or ABL Inhibition in Murine Xenograft Models of Ph-like Acute Lymphoblastic Leukemia. Blood. 2016 doi: 10.1182/blood-2016-05-707653. in press, doi: blood-2016-05-707653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, Houghton PJ, Brown VI, Grupp SA. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107:1149–1155. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J, Reid GS, Seif AE, Norris R, Chang YJ, Carroll M, Grupp SA. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112:2020–2023. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JW, Heydari K, Tirouvanziam R, Sahaf B, Parks DR, Herzenberg LA. Modern flow cytometry: a practical approach. Clin Lab Med. 2007;27:453–468. doi: 10.1016/j.cll.2007.05.001. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Pieters R, Golub TR, Armstrong SA. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]