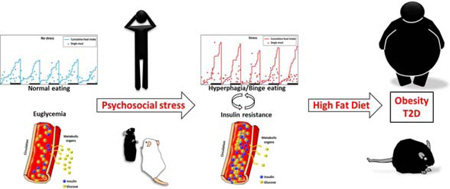
Abstract
Eating disorders and obesity have become predominant in human society. Their association to modern lifestyle, encompassing calorie-rich diets, psychological stress, and comorbidity with major diseases are well documented. Unfortunately the biological basis remains elusive and the pharmacological treatment inadequate, in part due to the limited availability of valid animal models. Human research on binge eating disorder (BED) proves a strong link between stress exposure and bingeing: state-levels of stress and negative affect are linked to binge eating in individuals with BED both in laboratory settings and the natural environment. Similarly, classical animal models of BED reveal an association between acute exposure to stressors and binging but they are often associated with unchanged or decreased body weight, thus reflecting a negative energy balance, which is uncommon in humans where most commonly BED is associated with excessive or unstable body weight gain. Recent mouse models of subordination stress induce spontaneous binging and hyperphagia, altogether more closely mimicking the behavioral and metabolic features of human BED. Therefore the translational relevance of subordination stress models could facilitate the identification of the neurobiological basis of BED and obesity-associated disease and inform on the development of innovative therapies.
Keywords: animal model, chronic subordination stress, social stress, stress, negative affect, Ecological Momentary Assessment
Graphical abstract
Introduction
The etiology and therapy of eating disorders have been receiving increasing scientific attention in light of the current epidemic of metabolic diseases, for which Western diet, sedentary life style and environmental stress are considered predisposing factors (GBD 2013 Risk Factors Collaborators, 2015; Polivy and Herman, 2002). Food overconsumption can be conducive to the excessive body weight and adiposity that define obesity, a condition that is pervasively damaging of health and is now included among the high-burden chronic conditions such as diabetes, hypercholesterolemia, and hypertension (Bauer et al. 2014; National Task Force on the Prevention and Treatment of Obesity, 2000; O’Connor et al. 2008; Whiteford et al. 2013; Wilfley et al. 2003).
Obesity is fundamentally dependent on the disruption of complex neuromolecular mechanisms receiving inputs from brain regions codifying autonomic responses, memory, arousal, cognition, reward processing, and emotional reactivity. The physiologic regulation of metabolism indeed relies on the communication between brain, gut, and adipose tissue (reviewed in Berthoud and Morrison, 2008; Cummings and Overduin, 2007; Lee and Abizaid, 2014) and its complexity embodies its essentiality for metabolic disease. Malfunctioning at the central pathways level can result in abnormal elevation of sympathetic outflow or, in case of defective or weakened signal feedback by the brain in response to satiety and nutrients from the gut, can cause overfeeding and disinhibition of liver glucose production, and thereby promote metabolic disease (Berthoud, 2002). The inherent complexity of metabolic regulation is further magnified in the interaction with stress. Stress imposes on metabolic responses the influence of higher levels of glucocorticoids as well as many other stress-related pathways, including the sympathetic nervous system / sympatho-adreno-medullary axis (Cacho et al. 2003; Pankevich et al. 2010; Razzoli and Bartolomucci, 2016).
Stress and negative affect are increasingly recognized as risk factors for obesity and binge eating disorder (BED; Goldschmidt et al. 2008; O’Connor et al. 2008; Stein et al. 2007; Striegel-Moore et al. 2007). BED is the most common eating disorder and is currently included in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5, 5th ed.; American Psychiatric Association, 2013). BED is characterized by recurrent binge-eating episodes, which are defined as ingestion of large amounts of food in discrete periods of time accompanied by feelings of loss of control and marked distress. This occurs in the absence of compensatory behaviors such as purging, fasting, or excessive exercise. BED has been shown to impact quality of life, to be associated with other psychopathologies, and to confer an increased risk for components of the metabolic syndrome (MetS), in particular for glucose dysregulation and diabetes, as seen in BED patients compared to BMI matched controls (see Mitchell, 2016 for a review). Not all BED patients present obesity. Nevertheless, excessive or unstable body weights are consequences of significant binging and have been associated to BED since its first description (Spitzer et al., 1992, 1993), making BED the eating disorder with the higher prevalence of obesity (Villarejo et al. 2012).
Overall, the parallel increase in incidence of seemingly independent psychiatric and metabolic diseases has long been suspected to be due to common predispositions. The difficulty to identify their causal factors and underlying molecular mechanisms in humans makes animal models very valuable and needed to address mechanistic questions. Nevertheless, current animal models present several limitations. Data from human clinical studies as well as from classical and recently developed animal models of BED will be reviewed hereafter to highlight possible mechanisms of a stress-bingeing-obesity pathway for obesity and obesity-associated metabolic disease.
Binge Eating and Obesity
Binge eating is one of the most commonly reported disordered eating behaviors among individuals with obesity, and similarly full-syndrome BED, which is one of the most commonly occurring eating disorders (American Psychiatric Association, 2013; Hudson, et al., 2007; Swanson et al., 2011). BED has been identified among children and adolescents in epidemiologic samples such as the National Comorbidity Survey – Adolescent Supplements (Swanson et al., 2011); however, diagnosing binge eating in this age group can be complex, and thus “loss of control eating” is often studied instead (Matherne, et al., 2015; Matheson, et al., 2012; Tanofsky-Kraff, et al., 2011). Further complicating the diagnosis of BED, many obese individuals who initially deny binge eating during face-to-face interviews later endorse this behavior during real-time, naturalistic assessments (i.e., ecological momentary assessment [EMA]), suggesting the utility and significance of studying binge eating in individuals with obesity (Greeno et al., 2000; Le Grange et al., 2001). Binge eating is considerably more common among adults with obesity than in the general population, and individuals who binge eat are more likely to become obese than individuals without disordered eating (Hudson et al., 2007). This is particularly concerning because the co-occurrence of binge eating and obesity is linked to more problematic eating-, activity-, and weight-related patterns, poorer quality of life, and more severe psychopathology (de Zwaan et al., 1994; Goldschmidt et al., 2011; Hsu et al., 2002; Perez and Warren, 2012; Striegel-Moore et al., 1998; Wadden et al., 1992; Yanovski et al., 1992). In order to improve existing treatments and develop more effective interventions, it is necessary to understand the complex interplay of precipitating factors that promote binge eating among individuals with obesity.
Stress and binge-eating in the clinical population
Research in humans examining stress and binge-eating has proceeded along three main lines. First, brief manipulations of stress or inductions of negative affect have been used to study the stress and binge-eating relationship. Second, the relationship of stress occurring in the natural daily environment and its link to binge eating have been examined using ecological momentary assessment, a technique ideally suited to this purpose. Last, a very small literature has examined biochemical or physiologic correlates of binge eating and stress. Each of these areas will be examined in turn.
Experimental Stress Manipulations and Binge-Eating
Existing theories (e.g., affect regulation model: Polivy and Herman, 1993; escape theory: Heatherton and Baumeister, 1991) suggest that individuals may binge eat in the presence of stress or negative affect, perhaps in an effort to alleviate or distract from that state (Dallman et al. 2003). Indeed, research to date supports this notion that negative affect and stress are important state-level risk factors for binge eating in BED (e.g., Cardi et al. 2015; Haedt-Matt and Keel, 2011; Leehr et al. 2015; Nicholls et al. 2016). Two types of methodologies have been employed to enhance our understanding of the link between state-levels of stress and binge eating in BED: (a) laboratory studies in which negative mood or stress is induced followed by a test meal of some kind and (b) ecological momentary assessment (EMA) in which negative affect and stress are measured in one’s natural environment in the hours leading up to a binge eating episode.
Laboratory Mood Induction Studies
Although the affect-focused theories of binge eating have existed for some time, researchers have only begun to study the link between BED and stress in the laboratory. These studies tend to employ various valid procedures, ranging from watching sad videos (e.g., Svaldi et al. 2014) to undergoing a stressful performance task (e.g., tier social stress test, TSST: Kirschbaum et al. 1993; Laessle and Schultz 2009), to elicit a negative mood which is supposed to produce a stress-response. The overall objective of these studies is to create a negative mood state in a controlled environment and to then assess the impact of that state on food consumption, thus directly assessing the link between negative mood and binge eating in BED. Overall, five studies using this design with adult BED samples found that when individuals with BED are in a negative mood or stressed, they display a higher amount of food intake compared (a) to when they are in a relaxed state and (b) to non-BED individuals in a negative mood state (Rosenberg et al. 2013; Schultz and Laessle, 2010, 2012; Svaldi et al. 2014; Zeeck et al. 2011). Furthermore, two of these studies also identified a higher loss of control in the BED samples following the mood induction compared to both the relaxed state and to non-BED controls following the mood induction (Rosenberg et al. 2013; Schultz and Laessle, 2012). Individuals with BED appear to not only eat more when stressed, but may also eat at greater rates of speed when stressed compared to not-stressed and controls (Schultz and Laessle, 2012), suggesting that they are perhaps eating in such a way to distract from that stressful state. That is, the rapid eating may be “consuming” their thoughts and behaviors, thereby allowing them to “forget” about their stress and negative affect. Moreover, it appears that when the specific feelings of sadness, guilt, and disappointment are induced in the laboratory, individuals with BED show an increased urge to binge (Zeeck et al. 2011). Therefore, it may be the case that these feelings in particular are ones that link general negative affect and stress to binge eating.
Overall, laboratory studies have enhanced our understanding of the relationship between stress and binge eating behavior in BED. In particular, the controlled environment is incredibly beneficial for directly investigating this link; however, one downfall of this is the potential lack of ecological validity. That is, it is difficult to know if these laboratory findings generalize to the daily lives of individuals with BED.
Ecological Momentary Assessment Studies and Binge-Eating
Ecological Momentary Assessment (EMA) is an important technique that has been used to examine the relationship of stress to disordered eating. EMA involves the use of palm computers or smartphones to collect data about behaviors of interest and their correlates in the natural environment. Participants provide self-report data in response to device prompts, at the time of behaviors of interest (such as binge eating), and at the end of the day. The typical recording window is the one to two weeks. There are clear advantages to EMA, as compared to typical retrospective recall techniques. First, the data are collected in, more or less, real time (that is, they are “momentary”). Furthermore, the behaviors are assessed in the natural living environment, as compared to the laboratory (that is, the data are “ecological”).
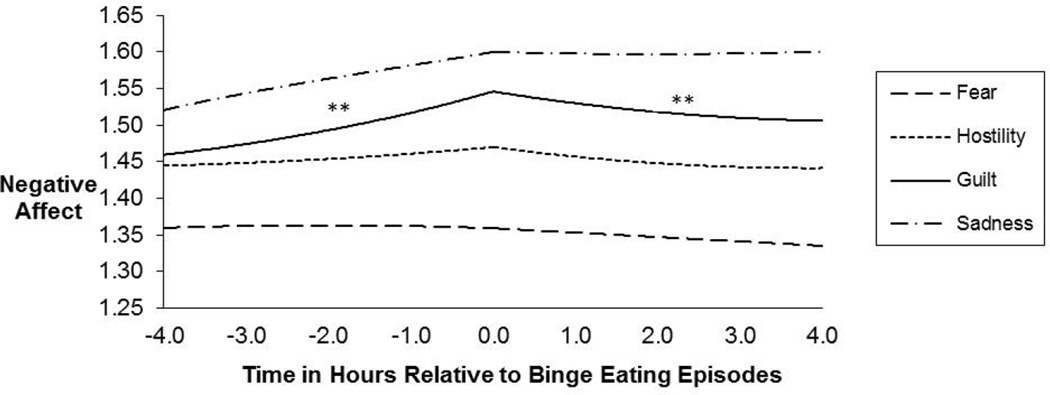
Although less controlled than a laboratory study of mood induction, EMA studies have the advantage of gathering data as individuals go about their days in their natural environment. Thus, considering the findings from both methodologies is important for understanding the relationship between stress and binge eating in BED. EMA findings suggest that binge eating episodes tend to occur on days when negative affect is high (Berg et al. 2014). Unlike healthy controls, individuals with BED report significantly worse moods prior to binge eating episodes compared to normal eating episodes (Hilbert and Tuschen Caffier, 2007). Furthermore, negative affect and tension tend to increase in the hours leading up to a binge eating episode while positive affect tends to decrease (Berg et al. 2015; Munsch et al. 2012). In fact, EMA findings mimic that of laboratory mood induction studies in that the specific emotion of guilt tends to increase prior to binge eating episodes (Berg et al. 2015) (Figure 1). It also seems to be the case that momentary distress is linked to not only binge eating episodes in BED, but loss of control episodes as well (Goldschmidt et al. 2012). Thus, it appears that findings from both laboratory and EMA studies suggest that state-levels of stress and negative affect, and particularly guilt, are significantly linked to binge eating in individuals with BED.
Figure 1. Temporal association between lower-order negative affect (NA) subscales and binge eating episodes.
The figure shows the momentary levels and trajectories of the facets of negative affect associated with binge eating episodes. The pre- and post-event trajectories of each negative affect subscale were modeled separately using piecewise linear, quadratic, and cubic functions centered on the time at which each of the eating episodes occurred. Momentary observations (Level 1) were nested within subjects (Level 2). With regard to Guilt, there were significant findings for both the pre- and post-event linear components of the binge eating model. The scaling of the y-axis ranged from 1.0 to 5.0. **p<.01. Modified from [Berg et al., 2015].
Biologic Markers, Stress, and Binge-Eating
Very limited work has investigated changes in biological markers of stress in the context of binge eating. The results have been quite mixed in terms of identifying biological stress markers.
First, Radin et al. (2015) described a sample of 178 adolescents with or without loss-of-control eating who underwent a mood induction and subsequently consumed a test meal. Throughout, salivary cortisol was measured. The results of the study showed that depressive symptoms correlated with cortisol levels, but loss-of-control eating was not correlated in any way with cortisol results (Radin et al. 2015). Second, Klatzkin et al. (2015) reported the results of a small study examining obese individuals with BED (n=9), obese, non-BED individuals (n=15), and 15 normal-weight, non-binge-eating participants (n=15). After baseline assessment, a mental stress task was used. In the BED group, increases in blood pressure in response to the mental health stressor were greater than those seen in the other two samples. However, while heart rate response was blunted in the BED group, the BED group did not differ from the other two groups after controlling for depression. Finally, Gluck and colleagues (Gluck et al. 2014, 2004) have reported in two studies on findings of biochemical markers after a cold pressor test in 10 non-BED and 11 individuals with BED. In this study, baseline ghrelin was equivalent in the two groups and they also did not differ significantly after a cold pressor test. In a second analysis, the BED group had higher basal cortisol levels than the non-BED group, and the area under curve after the cold pressor test was greater in the BED group.
Overall these studies provide a mixed picture, with inconsistent support for biological stress marker elevations in BED.
Classical rodent models of BED
Available animal models of eating disorders have been classified to evaluate their relevance to human disease and are ranked according to four criteria: etiologic, isomorphic, mechanistic, and predictive (Smith 1989) (Table 1). These criteria closely resemble the ones established for models of psychiatric disorders (McKinney 1974) in that they capture their causal, symptomatic, mechanistic, and therapeutic valence.
Table 1.
Rodent models associated with the development of binge-eating like behaviors.
| Model | Species | Procedure | Eating Outcome | Obesity outcome |
Reference |
|---|---|---|---|---|---|
| Sham feeding | Rat | Animals are fitted with chronic fistulas to drain liquid food before entering the gastrointestin al tract |
Hyperphagia in brief periods of time |
No body weight gain |
Smith et al. 1989 |
| Restriction/Refeedi ng |
Rat | access to palatable diet is followed by food deprivation cycle |
Rebound hyperphagia of highly palatable food upon refeeding |
No body weight gain |
Hagan and Moss, 1996 |
| Tail pinch | Rat | Pinching a rat’s tail while measuring the rat’s food intake |
Increased intake during tail pinching and shortly afterwards |
No body weight gain |
Rowland and Antelman, 1976 |
| Shock stress | Rat | Measurement of food intake after application of an acute 0.6 mA electric shock. |
Increased daily consumption of high palatable diet when associated with food restriction |
No body weight gain |
Hagan et al. 2002 |
| Limited access | Rat/mous e |
Limited availability of optional source of dietary fat under non- food-deprived conditions. |
Increased high sucrose/fat/mix ed diet at onset of access, and larger fewer sugary meals throughout the access period |
Body weight gain/ No body weight gain |
Avena et al. 2009; King et al., 2016; Wojnicki et al. 2008. |
| Visible burrow system |
Rat | Single housing after 2 weeks exposure to subordination |
Hyperphagia from longer meals with longer inter- meal intervals |
Body weight gain |
Melhorn et al. 2010 |
| in mixed-sex colonies |
occurring in the recovery post- stress phase |
||||
| Chronic social defeat |
Mouse | Single housing after 10 days subordination and protected co-housing with 10 different aggressor mice. |
Increased daily food intake, unknown meal pattern occurring in the recovery post- stress phase |
Body weight gain |
Lutter et al. 2008 |
| Chronic psychosocial stress |
Mouse | 4 weeks subordination and protected co-housing with the same aggressor mouse. |
Hyperphagia from higher consumption rate and reduced satiety intervals |
Body weight gain |
Razzoli et al. 2015 |
The sham feeding model is achieved by equipping the animals with chronic fistulas to drain liquid food before entering the gastrointestinal tract therefore producing a defect in satiation. This allows to separate positive oro-sensory feedback, stimulating feeding, from negative intestinal feedback, inhibiting feeding (Corwin and Buda-Levin, 2004; Smith, 2004). While this model allows for the exploration of basic mechanisms relevant for binging, it presents limitations for most validity criteria, not reproducing the voluntary elements of food restriction and since animals maintain weight by being fed outside the sham-feeding tests.
Several models have been based upon cycles of dieting and overeating. Restriction/refeeding cycles consist of several days of limited food access followed by a few days of ad libitum access (Corwin and Buda-Levin, 2004; Virts et al. 1992). Most commonly body weight diminishes during these protocols, and the increased food intake accomplished with this procedure does not seem to be greater than the amount of food than most animals would eat during a similar time, due to compensatory eating. Nevertheless, some of these studies highlight neural and biological changes that might have importance to binging related disorders (Colantuoni et al. 2002, 2001; Rada et al. 2005).
Another common methodology is based on limited access to fatty food, in particular to an optional source of dietary fat under non-food-deprived conditions over extended period of time. As access to the fat decreases, consumption of the fat increases when it is provided (Avena et al. 2009; Czyzyk et al. 2010). Nevertheless, a compensatory eating pattern emerges, as animals overeat on binge days and under eat on non-binge days. This response is very robust as it does not seem to habituate however doesn’t lead to excess body weight or adiposity (Czyzyk et al. 2010). With these limitations in mind, this model presents the advantage of voluntary behaviors that are reliably expressed and of good isomorphic validity, although the binging and non-binging phases seem to correspond to different neurobiological substrates.
Stress and binge eating disorders
Stress (Koolhaas et al. 2011; Harris 2015 for a review) has been often applied in developing eating disorder models. During chronic stress and the corresponding hyperactivation of the hypothalamus-pituitary-adrenal (HPA) axis, glucocorticoids and insulin increase craving for calorie-reach meals, a phenomenon explained by the “comfort food” hypothesis (Dallman et al. 2003; Dallman, 2010). According to this hypothesis, the preference for palatable food ingestion reduces the negative effects of stress via downregulation of corticotropin releasing factor (CRF) in the amygdala as well as through stimulation the anterior part of the pleasure-associated anterior nucleus accumbens shell that outweighs the contribution of the stress-stimulated posterior, defensive part of this nucleus (Dallman 2004, 2003; La-Fleur, 2004; Pecoraro, 2004; Rebuffe'-Scrive, 1992). Additionally, the consumption of palatable foods downregulates the stress response by activating reward pathways through reward-based structural plasticity (Avena and Bocarsly, 2012; Teegarden and Bale, 2008). Insulin level, elevated by food overconsumption, are thought to contribute to dampening adrenocorticotropic hormone (ACTH) and glucocorticoid responses to stress, as supported by the negative correlation between the hypothalamic para ventricular nucleus (PVN) CRF mRNA expression and plasma insulin levels (Dallman et al. 2007). As a result, the stress-induced increase in consumption of palatable foods is also associated with reduced behavioral stress indices, such as anxiety-related behaviors, stress-induced learned helplessness, and reactions of pain and distress (Ulrich-Lai et al. 2015).
A variety of stress models have been used to stimulate food intake, differing in nature (physical or psychological), severity, and time of application.
The tail-pinch model has been used to induce hyperphagia, but the effect is short lived and does not set the stage for an increased body weight (Rowland and Antelman, 1977). Furthermore, results from the application of several pharmacological compounds have provided conflicting results, limiting the predictive validity of the model (Morley et al. 1983).
While a similar case can be made for the application of shock stress per se, when combined with food restricting/refeeding protocols shock stress induces binge eating of palatable food (Hagan et al. 2002). Although again the model doesn’t facilitate the development of obesity and has been used to induce bingeing during short durations only, it seems to possess good construct validity and to prove useful for identifying mechanistic predispositions to bingeing. The ability of chronic psychological stress to increase food preferences towards highly palatable food has been related to the intensity of the stress experienced. A milder version of the traditional chronic variable stress in terms of severity and duration/frequency of stress application, resulted in a more modest and short-lived increased preference for palatable foods than in rats stressed in the traditional chronic variable stress (Thompson et al. 2015). These data would suggest that higher intensity paradigms correspond to more pronounced and sustained preference for high palatable foods (Thompson et al. 2015). While the stress relief properties of the consumption of high palatable food may be transitory/temporary during continuous stress exposure, the unresolved hyperactivation of the HPA axis over time can continue to sustain food overconsumption and facilitate obesity, due for example to the permissive effect of glucocorticoids on lipogenesis and fat deposition (de Guia and Herzig, 2015). Nevertheless, palatable food intake blunts acute stress responses both in human and rodent studies, although the mechanisms involved still remain elusive (Ulrich-Lai et al. 2015).
Validity of classical models of binge eating disorders
Most binge eating in humans seems not to be driven by hunger or metabolic demands and most patients are overweight or obese (DSM5; Mathes et al. 2009; Mussell et al. 1995; Waters et al. 2001). Therefore the fact that experimental animals from classical BED models as highlighted above do not show significant increases in body weight/adiposity (Cao et al. 2014; Czyzyk et al. 2010) limits their isomorphic validity. Similarly, most stress encountered by human and corresponding with the development of BED are of psychological nature (Heatherton et al. 1991; O’Connor et al., 2008), with onset of binge eating being preceded by elevated perceived stress and increased incidence of life stressors (Pike et al. 2006; Striegel-Moore et al. 2007).
This again limits the construct validity of some of the traditional models of human BED, particularly if based upon a physical stressor and may only partially target the interested physiology (mechanistic and predictive validity) (Rospond, 2016; add here some of the cits of Rev #3).
Aside from these traditional models of BED recent experimental evidences reviewed below strongly suggest that rodent models of chronic social stress elicit robust changes in eating behavior with high degree of face validity for BED and translational relevance for the human condition (Table 1).
Binge eating, hyperphagia and metabolic disorders in rodent models of social stress
Metabolic disorders due to psychosocial stress have now been well documented in humans (Bose, 2009; Dallman, 2006), non-human primates (Shively, 2009), and rodents (Bartolomucci et al. 2009, 2004; Coccurello et al. 2009; Finger, 2011; Kuo et al. 2007). Social subordination stress has long been considered ideal to mimic the impact of psychosocial stress on human pathologies (Bartolomucci et al. 2005; Koolhaas et al. 2011; Sapolsky, 2005; Scott, 2012; Razzoli and Bartolomucci, 2016) and has been recently presented as a major risk factor for insulin resistance and T2D (Kelly and Ismail, 2015).
Nevertheless limited work addresses the role of subordination stress on meal pattern. In the visible burrow system (VBS) laboratory rats are housed together in a mixed-sex colony for several weeks during which males form a dominance hierarchy through agonistic interactions. Subordinate males present reproducible behavioral, endocrine, physiological, and neurochemical changes consistent with a severe stress phenotype, that is also associated to a profound weight loss due to reduced food intake (Blanchard et al. 1995; Nguyen et al., 2007). During recovery from the VBS, subordinate rats immediately become hyperphagic and quickly regain the lost weight primarily as fat, resulting in greater overall and visceral adiposity than dominant and control rats. This effect is further enhanced in rats exposed to a second cycle of VBS stress and recovery. Consistent with increased adiposity, subordinates have elevated plasma leptin and insulin levels (Tamashiro et al. 2011, 2006). Meal pattern alterations have been observed during VBS housing and correspond to reduced meal size and frequency amounting to a hypophagic response that reflects the behavioral and neuroethological adaptations to the VBS environment. On the other hand, the hyperphagia observed during the VBS recovery is the outcome of larger and longer meals with longer inter-meal intervals (Melhorn et al. 2010).
Similarly to subordinate rats recovering from the VBS, a hyperphagic response has been observed in mice recovering from the chronic sensory contact or social defeat stress (CSD) model (Kumar et al. 2013). In this paradigm a link has been demonstrated between increased hypothalamic expression of the orexigenic neuropeptides NPY and AgRP in subordinate mice. This activation of NPY/AgRP neurons can contribute to the observed increased food intake and body weight and promote the use of carbohydrates as fuel while sparing fat (Chuang et al. 2010a, 2010b; Kumar et al. 2013). At the endocrine level, leptin production remains suppressed, and ghrelin secretion is increased to induce a potent feeding response that increases available energy stores (Lutter et al. 2008; Chuang et al. 2011). Similar to the behavior shown by subordinate in the VBS, in the CSD model hyperphagia results from larger but less frequent meals, considered indicative of a deficit in satiation.
In the chronic psychosocial stress model, subordinate mice develop a hyperphagia-dependent insulin resistance which, associated to high fat diet feeding, leads to MetS and obesity (Bartolomucci et al. 2009, 2005; Dadomo et al. 2011; Razzoli et al. 2015a; Sanghez et al. 2016, 2013). Similarly to the CSD, the chronic psychosocial stress model is also based on the exploitation of a naturalistic valid construct of social disparity, the difference between the two being the instability (the former) versus the stability (the latter) of the social hierarchy (Razzoli and Bartolomucci, 2016). In the chronic psychosocial stress model, the subordinate animals exhibit a complex behavioral and metabolic syndrome characterized by up-regulated HPA axis functioning, behavioral depression-like disorders and autonomic and immune-endocrine changes (Bartolomucci, 2007; Bartolomucci et al. 2010, 2005; Dadomo et al. 2011). Hyperphagia arises and persists spontaneously in subordinate mice (Bartolomucci et al., 2010, 2009, 2004; Dadomo et al. 2011; Patterson et al. 2013; Sanghez et al. 2013). Furthermore, hyperphagia is not a compensatory response since, in mice housed at room temperature, it develops in absence of increased energy expenditure (Moles et al. 2006; Sanghez et al. 2013) and in presence of depression of locomotor activity (Bartolomucci et al. 2010, 2009; Dadomo et al. 2011; Razzoli et al. 2014). The overstimulation of food intake that leads to obesity occurs in concert with stress induced elevations of circulating corticosterone, glucose, and ghrelin, and hypothalamic expression of AGRP and NPY mRNA (Bartolomucci 2007; Bartolomucci et al. 2010, 2005; Dadomo et al. 2011; Razzoli et al. 2015, 2014; Patterson et al. 2013). In the subordinate mouse, the presence of an altered inhibitory feedback of the HPA axis suggested by elevated corticosterone levels, downregulated expression of hippocampal GR and dexametasone resistance (Bartolomucci et al. 2004, 2003; Razzoli et al. 2014) could contribute over time to sustain hyperphagia, while it is known that interruption of ghrelin signaling can blunt hyperphagia and its anabolic consequences (Patterson et al. 2013). On the other hand, lower levels of ghrelin receptors in the hypothalamic PVN have been associated with increased consumption of fat rich foods (Patterson and Abizaid, 2013).
An in depth analysis of subordinate meal pattern assigned a strong validity for modeling human BED associated with obesity (Razzoli et al. 2015): subordinate mice ingest more food at higher rate and with reduced satiety ratio than control mice, suggesting an impaired satiety (Strubbe and Woods, 2004), and a heightened stress reactivity (Harb et al. 1985; Krebs et al. 1997). Interestingly, this phenomenon does not reach a ceiling effect, since hyperphagia can increase further in response to an acute stress (Razzoli et al. 2015). The microstructure of subordinate hyperphagia changes over time, evolving from longer and more frequent meals to a paroxystic eating, consisting of an exaggerated amount of food consumed over much shorter meal times. Altogether, these features make the case for considering chronic subordination stress a valid model of BED since the majority of the diagnostic criteria are captured (Corwin and Buda-Levin, 2004) (Table 1): 1) The behavior occurs repeatedly over an extended period of time; 2) Bingeing animals consume more food in brief, discrete, periods of time than controls do under similar circumstances. 3) If compensatory behavior is present, it should be initiated by the animal rather than imposed by the investigator.
The association of subordination induced bingeing with the propensity to obesity and MetS as part of the subordinate metabolic phenotype is also very remarkable (Sanghez et al. 2016, 2013). When subordinate mice are fed standard diet, their hyperphagia is associated with dyslipidemia but normal glucose tolerance in presence of increased body weight (Sanghez et al. 2013). Conversely, glucose intolerance, insulin resistance, and obesity develop due to the synergistic effect of high fat diet and subordination stress induced hyperphagia (Bartolomucci et al. 2009; Dadomo et al. 2011; Sanghez et al. 2013). Among the endocrine and metabolic changes induced in subordinate mice, increased glucocorticoid and free fatty acid levels might be considered crucial contributors for the development of insulin resistance through the downregulation of the insulin signaling pathway (Kahn, 1994; Sanghez et al. 2013; Shpilberg et al. 2012; Stumvoll, 2005; Taniguchi et al. 2006). Subordination stress is able to induce a molecular signature of insulin resistance in skeletal muscle and liver which is larger in magnitude to the effect of high fat diet per se (Sanghez et al. 2016). Decreased IRS1/2 appears to be the key molecular node critically downregulated in the signaling pathway of subordinate mice. Interestingly, the adipose tissue is found to remain insulin sensitive possibly to help repartitioning of nutrients to the fat organ (Sanghez et al. 2016).
Because obesity is a major risk factor for the development of the MetS and type 2 diabetes (Björntorp and Rosmond, 2000; Després and Lemieux, 2006; Eckel et al. 2005; Haslam and James, 2005; Zimmet et al. 2001) a food restriction regimen promoting weight loss should normalize the MetS as well (Aude et al. 2004; Kastorini et al. 2011). Preventing hyperphagia using a pair feeding protocol in subordinate mice abrogated body weight gain and visceral adiposity compared to subordinate mice that were fed ad libitum, without normalizing glucose homeostasis, potentially due to an exaggerated hunger stress sensitivity as demonstrated by the exacerbated hypercorticosteronemia induced by fasting (Razzoli et al. 2015). Additional studies are warranted to evaluate if the limited food availability in pair fed animals could have the potential to stimulate food intake as suggested by increased craving induced by deprivation protocols in humans as well as bingeing as a consequence of limited access models in rodents (Berner et al., 2008; Polivy et al. 2005). Still, the prevention of subordination-induced obesity by precluding hyperphagia maintains clear translational implications for therapeutic interventions (McElroy et al. 2004) offering the potential of treating obesity with non-pharmacological interventions effective for binge eating and acting in a faster way (drugs are often effective after several weeks of treatment).
Concluding Remarks and Future Perspectives
The treatment of eating and metabolic disorders has proven challenging. While obesity, T2D, and metabolic disorders have become pandemic, there are still unmet needs in spite of the available pharmacological, surgical, and behavioral therapeutic strategies. One critical issue is that obesity is typically viewed as a homogenous condition, but there may be important and discrete etiopathologies – including, for example, binge-eating. This indirectly underscores the role played by external factors such as low socio-economic status and psychosocial stress as predispositions for the development of a broad spectrum of eating and obesity-associated conditions. So far, an increasing scientific scrutiny has contributed to identify several endocrine players in the dynamic regulation of metabolic function in response to stress, both at the central and peripheral level. A strong need remains to develop valid preclinical models of stress-related metabolic disorders. Evidences reviewed here suggest that the development and validation of a preclinical model in which binge-eating is associated with obesity is critical to disentangling underlying molecular mechanisms, generating testable predictions of innovative drug candidates, and translating preclinical observations into clinical settings. This is particularly poignant since the available classical models of BED do not recapitulate most of the diagnostic criteria and lacki construct validity. Conversely, chronic subordination stress models offer several advantages in linking stress to binging and obesity to ultimately facilitate the identification of the neurobiological basis of BED-related obesity.
Highlights.
Eating disorders and obesity are prevalent and can associate with psychological stress.
Human studies link bingeing and stress exposure.
Most classic preclinical models of binge eating involve food deprivation and acute stress.
Mouse subordination stress causes bingeing and metabolic disorder similarly to humans.
Acknowledgments
AB is supported by NIH/NIDDK R01DK102496, NIH/NIA R01AG043972. SC is supported by NIDDK/NIH P30DK50456, and CP is supported by NIMH/NIHT32082761
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-5. American Psychiatric Association; 2013. [Google Scholar]
- Aude YW, Mego P, Mehta JL. Metabolic syndrome: dietary interventions. Curr. Opin. Cardiol. 2004;19(5):473–479. doi: 10.1097/01.hco.0000134610.68815.05. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology. 2012;63(1):87–96. doi: 10.1016/j.neuropharm.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J. Nutr. 2009;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A. Social stress, immune functions and disease in rodents. Front. Neuroendocrinol. 2007;28:28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell’Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One. 2009;4(1):e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Carola V, Pascucci T, Puglisi-Allegra S, Cabib S, Lesch KP, Parmigiani S, Palanza P, Gross C. Increased vulnerability to psychosocial stress in heterozygous serotonin transporter knockout mice. Dis. Model Mech. 2010;3:459–470. doi: 10.1242/dmm.004614. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Parmigiani S, Pederzani T, Merlot E, Neveu PJ, Dantzer R. Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res. Bull. 2003;62:173–178. doi: 10.1016/j.brainresbull.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Sacerdote P, Panerai AE, Sgoifo A, Dantzer R, Parmigiani S. Social factors and individual vulnerability to chronic stress exposure. Neurosci. Biobehav. Rev. 2005;29:67–81. doi: 10.1016/j.neubiorev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- Berg KC, Crosby RD, Cao L, Crow SJ, Engel SG, Wonderlich SA, Peterson CB. Negative affect prior to and following overeating-only, loss of control eating-only, and binge eating episodes in obese adults. Int. J. Eat. Disorder. 2015;48:641–653. doi: 10.1002/eat.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Crosby RD, Cao L, Crow SJ, Engel SG, Wonderlich SA. Relationship between daily affect and overeating-only, loss of control eating-only, and binge eating episodes in obese adults. Psychiatry Res. 2014;215:185–191. doi: 10.1016/j.psychres.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity. 2008;16:1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. The metabolic syndrome--a neuroendocrine disorder? Br. J. Nutr. 2000;(83 Suppl 1):S49–S57. doi: 10.1017/s0007114500000957. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Bose M, Oliván B, Laferrère B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009;16(5):340–346. doi: 10.1097/MED.0b013e32832fa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacho R, Fano E, Areso P, Garmendia L, Vegas O, Brain PF, Azp roz A. Endocrine and lymphoproliferative response changes produced by social stress in mice. Phys. Behav. 2003;78(3):505–512. doi: 10.1016/s0031-9384(03)00018-0. [DOI] [PubMed] [Google Scholar]
- Cao X, Xu P, Oyola MG, Xia Y, Yan X, Saito K, Zou F, Wang C, Yang Y, Hinton AJr, Yan C, Ding H, Zhu L, Yu L, Yang B, Feng Y, Clegg DJ, Khan S, DiMarchi R, Mani SK, Tong Q, Xu Y. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J. Clin. Invest. 2014;124(10):4351–4362. doi: 10.1172/JCI74726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardi V, Leppanen J, Treasure J. The effects of negative and positive mood induction on eating behavior: A meta-analysis of laboratory studies in the healthy population and eating and weight disorders. Neur. Biobehav. Rev. 2015;57:299–309. doi: 10.1016/j.neubiorev.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Chuang CJ, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, Zigman JM, Elmquist JK, Nestler EJ, Lutter M. A β-3-Adrenergic-Leptin-Melanocortin Circuit Regulates Behavioral and Metabolic Changes Induced by Chronic Stress. Biol. Psy. 2010a;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Cui H, Mason BL, Mahgoub M, Bookout AL, Yu HG, Perello M, Elmquist JK, Repa JJ, Zigman JM, Lutter M. Chronic social defeat stress disrupts regulation of lipid synthesis. J. Lipid Res. 2010b;51(6):1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Invest. 2011;121(7):2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R, D’Amato FR, Moles A. Chronic social stress, hedonism and vulnerability to obesity: lessons from rodents. Neurosci. Biobehav. Rev. 2009;33(4):537–550. doi: 10.1016/j.neubiorev.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence intermittent, excessive sugar intake causes endogenous opioid dependence. Obes. Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol. Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J. Clin. Invest. 2007 Jan;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk TA, Sahr AE, Statnick MA. A model of binge-like eating behavior in mice that does not require food deprivation or stress. Obesity. 2010;18:1710–1717. doi: 10.1038/oby.2010.46. [DOI] [PubMed] [Google Scholar]
- Dadomo H, Sanghez V, Di Cristo L, Lori A, Ceresini G, Malinge I, Parmigiani S, Palanza P, Sheardown M, Bartolomucci A. Vulnerability to chronic subordination stress-induced depression-like disorders in adult 129SvEv male mice. Progr. Neuro-Psychopharmacol. Biol. Psy. 2011;35(6):1461–1471. doi: 10.1016/j.pnpbp.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinol. 2004;145(6):2633–26338. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc. Nat. Acad. Sc.USA. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, Akana SF, Laugero KC, Houshyar H, Strack AM, Bhatnagar S, Bell ME. Glucocorticoids, chronic stress, and obesity. Prog. Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Warne JP, Foster MT, Pecoraro NC. Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J. Physiol. 2007;583(Pt 2):431–436. doi: 10.1113/jphysiol.2007.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guia RM, Herzig S. How do glucocorticoids regulate lipid metabolism? Adv. Exp. Med. Biol. 2015;872:127–144. doi: 10.1007/978-1-4939-2895-8_6. [DOI] [PubMed] [Google Scholar]
- De Zwaan M, Mitchell JE, Seim HC, Specker SM, Pyle RL, Raymond NC, Crosby RB. Eating related and general psychopathology in obese females with binge eating disorder. Int. J. Eat. Disord. 1994;15:43–52. doi: 10.1002/1098-108x(199401)15:1<43::aid-eat2260150106>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;29(192):351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Sep 10; doi: 10.1016/S0140-6736(15)00128-2. pii: S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck M, Geliebter A, Hung J, Yahav E. Cortisol, Huunger, and Desire to Binge Eat Following a Cold Stress Test in Obese Women with Binge Eating Disorder. Psychosom. Med. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- Gluck M, Yahav E, Hashim S, Geliebter A. Ghrelin Levels After a Cold Pressor Stress Test in Obese Women With Binge Eating Disorder. Psychosom. Med. 2014;76:74–79. doi: 10.1097/PSY.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Engel SG, Wonderlich SA, Crosby RD, Peterson CB, Le Grange D, Tanofsky-Kraff M, Cao L, Mitchell JE. Momentary affect surrounding loss of control and overeating in obese adults with and without binge eating disorder. Obesity. 2012;20:1206–1211. doi: 10.1038/oby.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Le Grange D, Powers P, Crow SJ, Hill LL, Peterson CB, Mitchell JE. Eating disorder symptomatology in normal-weight vs. obese individuals with binge eating disorder. Obesity. 2011;19:1515–1518. doi: 10.1038/oby.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Yanovski SZ, Braet C, Marcus MD, Wilfley DE, Yanovski JA. Subtyping children and adolescents with loss of control eating by negative affect and dietary restraint. Behav. Res. Ther. 2008;46:777–787. doi: 10.1016/j.brat.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeno CG, Wing RR, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. J. Consult. Clin. Psychol. 2000;68:95–102. [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psych. Bull. 2011;137:660–681. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol. Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Harb MY, Reynolds VS, Campling RC. Eating behaviour, social dominance and voluntary intake of silage in group-fed milking cattle. Grass Forage Sci. 1985;40:113–118. [Google Scholar]
- Harris RB. Chronic and acute effects of stress on energy balance: are there appropriate animal models? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R250–R265. doi: 10.1152/ajpregu.00361.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psych. Bull. 1991;110:86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Herman CP, Polivy J. Effects of physical threat and ego threat on eating behavior. J. Personal. Soc. Psychol. 1991;60:138–143. doi: 10.1037//0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Tuschen Caffier B. Maintenance of binge eating through negative mood: a naturalistic comparison of binge eating disorder and bulimia nervosa. Int. J. Eat. Disorder. 2007;406:521–530. doi: 10.1002/eat.20401. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hirpi E, Pope HG, Kessler RC. The Prevalence and Correlates of Eating Disorders in the National Comorbidty Survey Replication. Biol. Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LK, Mulliken B, McDonagh B, Krupa Das S, Rand W, Fairburn CG, Rolls B, McCrory MA, Saltzman E, Shikora S, Dwyer J, Roberts S. Binge eating disorder in extreme obesity. Int. J. Obes Relat. Metab. Disord. 2002;26:1398–1403. doi: 10.1038/sj.ijo.0802081. [DOI] [PubMed] [Google Scholar]
- Kahn C. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011;57(11):1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Ismail M. Stress and type 2 diabetes: a review of how stress contributes to the development of type 2 diabetes. Annu. Rev. Public Health. 2015;36:441–462. doi: 10.1146/annurev-publhealth-031914-122921. [DOI] [PubMed] [Google Scholar]
- King SJ, Rodrigues T, Watts A, Murray E, Wilson A, Abizaid A. Investigation of a role for ghrelin signaling in binge-like feeding in mice under limited access to high-fat diet. Neuroscience. 2016;319:233–45. doi: 10.1016/j.neuroscience.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Tier Social Stress Test’. A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiol. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Gaffney S, Cyrus K, Bigus E, Brownley KA. Binge eating disorder and obesity: Preliminary evidence for distinct cardiovascular and psychological phenotypes. Phys. Behav. 2015;142:20–27. doi: 10.1016/j.physbeh.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neur. Biobehav. Rev. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Krebs H, Weyers P, Macht M, Weijers HG, Janke W. Scanning behavior of rats during eating under stressful noise. Physiol. Behav. 1997;62(1):151–154. doi: 10.1016/s0031-9384(97)00026-7. [DOI] [PubMed] [Google Scholar]
- Kumar J, Chuang JC, Na ES, Kuperman A, Gillman AG, Mukherjee S, Zigman JM, McClung CA, Lutter M. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite. 2013;64:81–88. doi: 10.1016/j.appet.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress induced obesity and metabolic syndrome. Nat. Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Laessle RG, Schultz S. Stress-induced laboratory eating behavior in obese women with binge eating disorder. Int. J. Eat. Disorder. 2009;42:505–510. doi: 10.1002/eat.20648. [DOI] [PubMed] [Google Scholar]
- Lee CY, Abizaid A. The gut-brain-axis as a target to treat stress-induced obesity. Front Endocrinol (Lausanne) 2014;5:117. doi: 10.3389/fendo.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, Giel KE. Emotion regulation model in binge eating disorder and obesity - a systematic review. Neurosc. Biobehav. Rev. 2015;49:125–134. doi: 10.1016/j.neubiorev.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Le Grange D, Gorin A, Catley D, Stone AA. Does momentary assessment detect binge eating in overweight women that is denied at interview? Eur. Eat Disord. Rev. 2001;9:309–324. [Google Scholar]
- Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11(7):752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherne CE, Tanofsky-Kraff M, Altschul AM, Shank LM, Schvey NA, Brady SM, Galescu O, Demidowich AP, Yanovski SZ, Yanovski JA. A preliminary examination of Loss of Control Eating Disorder (LOC-ED) in middle childhood. Eat. Behav. 2015;18:57–61. doi: 10.1016/j.eatbeh.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes WF, Brownley KA, Mo X, Bulik CL. The biology of binge eating. Appetite. 2009;52:545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson BE, Tanofsky-Kraff M, Shafer-Berger S, Sedaka NM, Mooreville M, Reina SA, Vannucci A, Shomaker LB, Yanovski SZ, Yanovski JA. Binge eating disorder: the next generation of research. Int. J. Eat. Disord. 2012;46:193–207. doi: 10.1002/eat.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J. Clin. Psychiatry. 2004;65:634–651. doi: 10.4088/jcp.v65n0507. [DOI] [PubMed] [Google Scholar]
- McKinney WT. Animal models in psychiatry. Perspect. Biol. Med. 1974;17:529–542. [PubMed] [Google Scholar]
- Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299(3):R813–R822. doi: 10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE. Medical comorbidity and medical complications associated with binge-eating disorder. Int. J. Eat. Disord. 2016;49(3):319–323. doi: 10.1002/eat.22452. [DOI] [PubMed] [Google Scholar]
- Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, Rizzi R, Ciani B, D’Amato FR. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology. 2006;31:623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Rowland NE. Stress induced eating. Life Sci. 1983;32:2169–2182. doi: 10.1016/0024-3205(83)90415-0. [DOI] [PubMed] [Google Scholar]
- Munsch S, Meyer AH, Wuartier V, Wilhelm FH. Binge eating in binge eating disorder: a breakdown of emotion regulatory process? Psychiatry Res. 2012;1953:118–124. doi: 10.1016/j.psychres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Mussell MP, Mitchell JE, Weller CL, Raymond NC, Crow SJ, Crosby RD. Onset of binge eating, dieting, obesity, and mood disorders among subjects seeking treatment for binge eating disorder. Int. J. Eat. Disord. 1995;17:395–401. doi: 10.1002/1098-108x(199505)17:4<395::aid-eat2260170412>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch. Int. Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- Nguyen MM, Tamashiro KL, Melhorn SJ, Ma LY, Gardner SR, Sakai RR. Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology. 2007;148(12):6145–6156. doi: 10.1210/en.2007-0471. [DOI] [PubMed] [Google Scholar]
- Nicholls W, Devonport TJ, Blake M. The associations between emotions and eating behavior in an obese population with binge eating disorder. Obesity Rev. 2016;17:30–42. doi: 10.1111/obr.12329. [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Jones F, Conner M, McMillan B, Ferguson E. Effects of daily hassles and eating style on eating behavior. Health Psychol. 2008;27(Suppl 1):S20–S31. doi: 10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J. Neurosci. 2010;30(48):16399–407. doi: 10.1523/JNEUROSCI.1955-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson ZR, Abizaid A. Stress induced obesity: lessons from rodent models of stress. Front. Neurosci. 2013;7:130. doi: 10.3389/fnins.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson ZR, Khazall R, Mackay H, Anisman H, Abizaid A. Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology. 2013a;154(3):1080–1091. doi: 10.1210/en.2012-1834. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinol. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Perez M, Warren CS. The relationship between quality of life, binge-eating disorder, and obesity status in an ethnically diverse sample. Obesity. 2012;20:879–885. doi: 10.1038/oby.2011.89. [DOI] [PubMed] [Google Scholar]
- Pike KM, Wilfley D, Hilbert A, Fairburn CG, Dohm FA, Striegel-Moore RH. Antecedent life events of binge-eating disorder. Psychiatry Res. 2006;142:19–29. doi: 10.1016/j.psychres.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, Herman C. Etiology of binge eating: psychological mechanisms. In: Fairburn C, Wilson G, editors. Binge Eating- Nature, Assessment, and Treatment. New York: The Guilford Press; 1993. pp. 173–205. [Google Scholar]
- Polivy J, Herman CP. Causes of eating disorders. Annu. Rev. Psychol. 2002;53:187–213. doi: 10.1146/annurev.psych.53.100901.135103. [DOI] [PubMed] [Google Scholar]
- Polivy J, Coleman J, Herman CP. The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. Int. J. Eat. Disord. 2005;38(4):301–309. doi: 10.1002/eat.20195. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel G. Daily Bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Radin RM, Shomaker LB, Kelly NR, Pickworth CK, Thompson KA, Brady SM, Demidowich A, Galescu O, Altschul AM, Shank LM, Yanovski SZ, Tanofsky-Kraff M, Yanovski JA. Cortisol response to an induction of negative affect among adolescents with and without loss of control eating. Pediatric Obesity. 2015 doi: 10.1111/ijpo.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Bartolomucci A. The Dichotomous Effect of Chronic Stress on Obesity. Trends Endocrinol. Metab. 2016 doi: 10.1016/j.tem.2016.04.007. pii: S1043-2760(16)30023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Karsten C, Yoder JM, Bartolomucci A, Engeland WC. Chronic subordination stress phase advances adrenal and anterior pituitary clock gene rhythms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307(2):R198–R205. doi: 10.1152/ajpregu.00101.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Sanghez V, Bartolomucci A. sChronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front. Nutr. 2015a;1(30) doi: 10.3389/fnut.2014.00030. pii:00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuffé-Scrive M, Walsh UA, McEwen B, Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Phys. Behav. 1992;52(3):583–590. doi: 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg N, Bloch M, Avi IB, Rouach V, Schreiber S, Stern N, Greenman Y. Cortisol response and desire to binge following psychological stress: comparison between obese subjects with and without binge eating disorder. Psy. Res. 2013;208:156–161. doi: 10.1016/j.psychres.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Rospond B, Szpigiel J, Sadakierska-Chudy A, Filip M. Binge eating in pre-clinical models. Pharmacol. Rep. 2015;67(3):504–512. doi: 10.1016/j.pharep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Antelman SM. Stress-induced hyperphagia and obesity in rats: a possible model for understanding human obesity. Science. 1977;191:310–312. doi: 10.1126/science.1246617. [DOI] [PubMed] [Google Scholar]
- Sanghez V, Cubuk C, Sebastián-Leon P, Carobbio S, Dopazo J, Vidal-Puig A, Bartolomucci A. Chronic subordination stress selectively downregulates the insulin signaling pathway in liver and skeletal muscle but not in adipose tissue of male mice. Stress. 2016;19(2):214–224. doi: 10.3109/10253890.2016.1151491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghez V, Razzoli M, Carobbio S, Campbell M, McCallum J, Cero C, Ceresini G, Cabassi A, Govoni P, Franceschini P, de Santis V, Gurney A, Ninkovic I, Parmigiani S, Palanza P, Vidal-Puig A, Bartolomucci A. Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the Metabolic Syndrome. Psychoneuroendocrinology. 2013;38(12):2933–2942. doi: 10.1016/j.psyneuen.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schultz S, Laessle RG. Associations of negative affect and eating behavior in obese women with and without binge eating disorder. Eat. Weight Disord. 2010;15:e287–e293. doi: 10.1007/BF03325311. [DOI] [PubMed] [Google Scholar]
- Schultz S, Laessle RG. Stress-induced laboratory eating behavior in obese women with binge eating disorder. Appetite. 2012;582:457–461. doi: 10.1016/j.appet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Scott KA, Melhorn SJ, Sakai RR. Effects of Chronic Social Stress on Obesity. Curr. Obes. Rep. 2012;1(1):16–25. doi: 10.1007/s13679-011-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am. J. Primatol. 2009;71(9):742–751. doi: 10.1002/ajp.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilberg Y, Beaudry JL, D’Souza A, Campbell JE, Peckett A, Riddell MC. A rodent model of rapid-onset diabetes induced by glucocorticoids and high-fat feeding. Dis. Model Mech. 2012;5(5):671–680. doi: 10.1242/dmm.008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite. 2004;43:11–13. doi: 10.1016/j.appet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Devlin M, Walsh BT, Hasin D, Wing R, Marcus MD, Stunkard A, Wadden T, Yanovski S, Agras S, Mitchell J, Cathy Nonas RD. Binge eating disorder: A multiside field trial of the diagnostic criteria. Int. J. Eating Dis. 1992;11:191–203. [Google Scholar]
- Spitzer RL, Yanovski S, Wadden T, Wing R, Marcus MD, Stunkard A, Devlin M, Mitchell J, Hasin D, Horne RL. Binge eating disorder: Its further validation in a multisite study. Int. J. Eating Dis. 1993;13(2):137–153. [PubMed] [Google Scholar]
- Stein RI, Kenardy J, Wiseman CV, Dounchis JZ, Arnow BA, Wilfley DE. What’s driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. Int. J. Eat. Disord. 2007;40:195–203. doi: 10.1002/eat.20352. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Dohm FA, Kraemer HC, Schreiber GB, Taylor CB, Daniels SR. Risk factors for binge-eating disorders: an exploratory study. Int. J. Eat. Disord. 2007;40:481–487. doi: 10.1002/eat.20400. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Wilson GT, Wilfley DE, Elder KA, Brownell KD. Binge eating in an obese community sample. Int. J. Eat. Disord. 1998;23:27–37. doi: 10.1002/(sici)1098-108x(199801)23:1<27::aid-eat4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC. The timing of meals. Psychol. Rev. 2004;111(1):128–41. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Tuschen-Caffier B, Treatowska M, Caffier D, Naumann E. Differential caloric intake in overweight females with and without binge eating: effects of a laboratory-based emotion-regulation training. Behav. Res. Therapy. 2014;56:39–46. doi: 10.1016/j.brat.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and Correlates of Eating Disorders and Adolescents. Arch. Gen. Psychiatry. 2011;68:714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol. Behav. 2006;89(4):536–542. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14(5):468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell. Biol. 2006;7(2):85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, Raciti G, Zocca JM, Wilfley DE, Yanovsky SZ, Yanovsky JA. A prospective study of pediatric loss of control eating and psychological outcomes. J. Abnorm. Psychol. 2011;120:108–118. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol. Behav. 2008;93(4–5):713–23. doi: 10.1016/j.physbeh.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Fourman S, Packard AE, Egan AE, Ryan KK, Ulrich-Lai YM. Metabolic consequences of chronic intermittent mild stress exposure. Physiol. Behav. 2015;150:24–30. doi: 10.1016/j.physbeh.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure, food intake and emotional state. Stress. 2015;18(4):381–399. doi: 10.3109/10253890.2015.1062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarejo C, Fernández-Aranda F, Jiménez-Murcia S, Peñas-Lledó E, Granero R, Penelo E, Tinahones FJ, Sancho C, Vilarrasa N, Montserrat-Gil de Bernabé M, Casanueva FF, Fernández-Real JM, Frühbeck G, De la Torre R, Treasure J, Botella C, Menchón JM. Lifetime obesity in patients with eating disorders: increasing prevalence, clinical and personality correlates. Eur. Eat. Disord. Rev. 2012. 2012;20(3):250–254. doi: 10.1002/erv.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virts KL, Schlundt DG, Katsenes KM, Hill JO. Physiological effects of dieting and bingeing in rats. Physiol. Behav. 1992;51:151–155. doi: 10.1016/0031-9384(92)90217-p. [DOI] [PubMed] [Google Scholar]
- Wadden T, Yanovski S, Agras S, Mitchell J, Cathy Nonas RD. Binge eating disorder: A multiside field trial of the diagnostic criteria. Int. J. Eating Dis. 1992;11:191–203. [Google Scholar]
- Waters A, Hill A, Waller G. Internal and external antecedents of binge eating episodes in a group of women with bulimia nervosa. Int. J. Eat. Disord. 2001;29:17–22. doi: 10.1002/1098-108x(200101)29:1<17::aid-eat3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Wilson GT, Agras WS. The clinical significance of binge eating disorder. Int. J. Eat. Disord. 2003;34:S96–S106. doi: 10.1002/eat.10209. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ, Leet M, Yanovski JA, Flood M, Gold PW, Kissileff HR, Walsh BT. Food selection and intake of obese women with binge-eating disorder. Am. J. Clin. Nutr. 1992;56:975–980. doi: 10.1093/ajcn/56.6.975. [DOI] [PubMed] [Google Scholar]
- Zeeck A, Stelzer N, Linster HW, Joos A, Hartmann A. Emotion and eating in binge eating disorder and obesity. Europ. Eat. Disord. Rev. 2011;195:426–437. doi: 10.1002/erv.1066. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]