Abstract
Background and aims
Studies evaluating the relationship between dense coronary calcium (DC) and myocardial ischemia have had incongruent results. We sought to clarify whether DC as detected by computed coronary tomographic angiography (CCTA) is an independent predictor of ischemia as measured by invasive fractional flow reserve (FFR).
Methods
In total, 249 (399 lesions) stable patients undergoing CCTA and invasive FFR were enrolled for this post-hoc analysis. DC was defined as plaque with ≥350 HU using quantification software, and ischemia was defined as FFR ≤0.80. We evaluated the relationship of dense calcium volume (DCV), lesion plaque volume (LPV), non-calcified plaque volume (NCV), and area stenosis (AS) with ischemia using logistic regression reporting odds ratios (OR) with 95% confidence intervals (95% CI).
Results
Mean age was 63.0±8.6 years, and 73 (29.3%) were female. Mean DCV was higher in lesions with FFR ≤0.80 (57.0 ± 54.7mm3 vs. 37.6 ± 49.5 mm3, [p <0.001]). DCV and LPV were closely correlated (Pearson’s coefficient = 0.49 [p <0.001]). After adjustment for AS, LPV (OR 1.01, 95% CI 1.00 – 1.04, p<0.001) but not DCV (OR 1.01, 95% CI 0.96 – 1.06, p=0.69) was independently associated with ischemia.
Conclusions
Dense calcium is not an independent predictor of ischemia, but rather a marker of aggregate LPV, which in turn, is predictive of ischemia.
Keywords: Coronary computed tomography angiography, dense calcium, fractional flow reserve, ischemia, plaque volume
Introduction
The interplay between calcific coronary atherosclerosis and myocardial ischemia has not been clearly established. While some non-invasive studies documented an association between coronary calcium and ischemia,1,2 others reported no independent relationship.3 Invasive studies portray a similarly disparate picture, with some investigations using intravascular ultrasound (IVUS) displaying an association between dense calcium and ischemia.4 Conversely, others have found that a larger dense calcium content is more often present in non-ischemic lesions.5 This overall incongruence among both noninvasive and invasive studies indicates that it has yet to be firmly established whether calcium is an independent cause of ischemia, or merely a marker of the atherosclerotic burden.6
Inherently, the extant literature on this topic has several drawbacks. First, computed tomography using derivations of the Agatston score do not factor into account non-calcified plaque burden.7,8 Second, IVUS measurements may not be capable of comprehensively measuring true total dense calcium volume due to poor penetration.9 Third, studies have yet to assess ischemia by fractional-flow reserve (FFR). FFR is considered the current gold standard in the physiological assessment of a lesion, and its use in decision-making for revascularization reduces cardiovascular events and unnecessary revascularization.10–13
Of late, contrast-enhanced computed coronary tomographic angiography (CCTA) has emerged as a noninvasive modality that can evaluate both non-calcified and calcified plaque volume. Further still, plaque imaging characteristics detected by CCTA have been associated with ischemia as measured by FFR.14,15 However, to date, the relationship between calcium volume detected by CCTA and FFR is unknown. We therefore sought to evaluate the relationship between dense calcium and invasive FFR using data from the recent Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study.
Patients and methods
Study population
Details of the DeFACTO study recruitment, design, and procedures have been described elsewhere.16,17 In brief, this was a prospective, multicenter, multinational study. The study population consisted of 252 stable patients (407 coronary lesions). Patients underwent clinically indicated invasive coronary angiography (ICA) following CCTA, with no intervening cardiac event within 60 days. Patients with prior coronary bypass surgery, prior percutaneous coronary intervention with suspected in-stent restenosis, contraindication to adenosine, suspicion of or recent acute coronary syndrome, complex congenital heart disease, prior pacemaker or defibrillator, prosthetic heart valve, significant arrhythmia, serum creatinine level greater than 1.5 mg/dL, allergy to iodinated contrast, pregnant state, body mass index greater than 35 kg/m2, evidence of active clinical instability or life-threatening disease, or inability to adhere to study procedures were excluded. For this post-hoc analysis, a further three patients were excluded due to non-evaluable images during CCTA plaque analysis, resulting in 249 patients (399 coronary lesions) for inclusion.
CCTA acquisition and analysis
CCTA was performed using 64-slice or higher detector row scanners with prospective or retrospective electrocardiographic gating. Subjects received 80–100 mL of contrast through the antecubital vein via power injector followed by 50–80 mL of normal saline at 5 mL/s. Images were reconstructed using 0.5-to 0.75-mm slice thickness in 0.3mm slice increments, 160-to 250-mm field of view, 512×512 matrix, and a standard kernel. Images were interpreted in an intention-to-diagnose fashion using standard protocols at a core laboratory as previously described.16,18 Atherosclerosis was defined as structures ≥1mm2 within or adjacent to the coronary artery lumen that could be distinguished from surrounding pericardial tissue, fat or lumen. For the present analysis, quantitative and qualitative atherosclerotic plaque analysis was performed using previously validated semi-automated plaque analysis software (QAngio CT Research Edition v2.02, Medis Medical Imaging Systems, Leiden, the Netherlands). This automatically detects the vessel and the contours of the vessel wall and lumen. A reference frame using a linear slope drawn between reference normal, non-bifurcated, non-diseased segments before and after the diseased vessel was used. This frame represented the progressive tapering of the presumed non-diseased vessel. Automated plaque quantitation was then calculated using the contours compared to this reference vessel.19 This software also allowed for manual adjustment by the observer if required. In this scenario, the proximal and distal end of a lesion was identified as the section before and after maximal stenosis from atherosclerotic plaque, with normal reference segments of the vessel being immediately adjacent to the lesion, with no plaque. Lesion plaque volume (LPV) was defined as the total volume of plaque (calcified and/or non-calcified) within a coronary lesion. Dense calcium (DC) was defined as calcification intensity of 350 Hounsfield Units (HU) or higher, as previously proposed. Dense calcium volume (DCV) was defined as the volume of dense calcium within a particular lesion. The proportion of dense calcium (%DC) for a particular lesion was calculated as %DC = DCV ÷ LPV × 100 (Fig. 1). Non-calcified plaque volume (NCV) was defined as LPV – DCV. Lumen area stenosis (AS) was measured using cross-sectional images, using proximal and distal reference segments as previously defined. Additionally, the presence of previously reported adverse plaque characteristics14 was noted. These included low attenuation plaque (LAP, defined as any plaque with attenuation of < 30 Hounsfield units), positive remodeling (PR, defined as a remodeling index ≥1.1), and spotty calcification (SC, defined as an intra-lesion calcific plaque <3 mm in length that comprised <90° of the lesion circumference) (Fig. 2).
Fig. 1. Analysis of atherosclerotic plaque on CCTA.
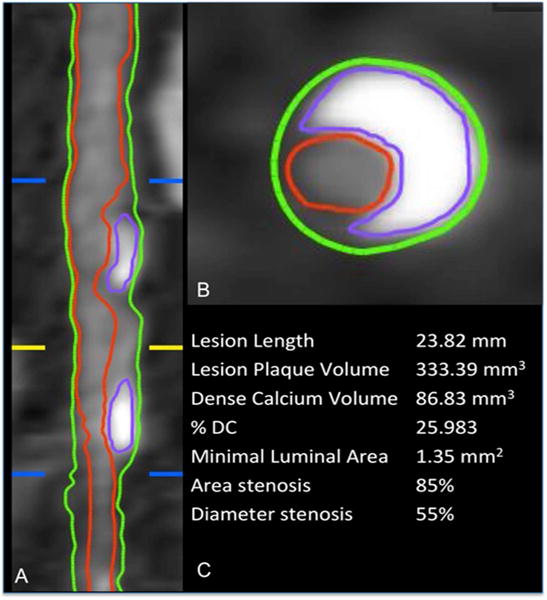
The proximal and distal borders of the lesion identified (blue lines), is the section of maximal stenosis (yellow line). The vessel (green outline) and luminal (red outline) borders are identified, and the volume in between represents the lesion plaque volume (LPV). In luminal and cross-sectional view, the various plaque components are identified. Dense calcium is identified (purple outline) (DCV). This, along with all other plaque components comprises LPV. %DC = DCV ÷ LPV × 100. In this example, DCV = 86.8 mm3, LPV = 333.4 mm3, and %DC = 26.0%.
Fig. 2. Direct comparison between CCTA plaque analysis and FFR.
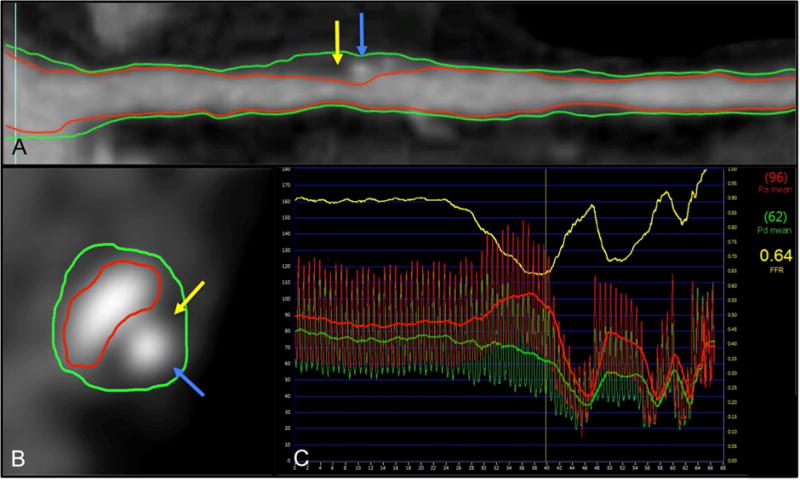
(A) CCTA luminal multiplanar reconstruction (MPR); (B) CCTA cross-section of a lesion. The vessel wall is demarcated (green outline), and the luminal boundaries outlined (red line). Atherosclerotic plaque characteristics (APCs) of spotty calcification (blue arrows) and low attenuation plaque (yellow arrows) can be observed; (C) integration with fractional flow reserve showed the lesion to be ischemic
ICA acquisition and FFR measurement
ICA was performed selectively in accordance with standard protocol, with at least 2 projections acquired per vessel distribution.20 ICA images were transferred and analyzed at a core laboratory (University of British Columbia, Vancouver, Canada) for masked quantitative coronary angiography of all vessels using commercially available software (Discovery Quinton). FFR (PressureWire Certus, St June Medical Systems; ComboWire, Volcano Corp.) was performed at the time of ICA in clinically indicated vessels, with stenoses between 30% and 90%. Vessels deemed not clinically indicated for FFR were not interrogated. After administration of nitroglycerin, a pressure-monitoring guide wire was advanced distal to a stenosis. Hyperemia was induced by administration of intravenous adenosine at a rate of 140 μg/kg per minute. FFR was calculated by dividing the mean distal coronary pressure by the mean aortic pressure during hyperemia. An FFR of ≤0.80 was considered diagnostic of ischemia.12
Integration of CCTA and FFR
Direct comparison between CCTA plaque analysis and FFR was performed at the precise location of the wire transducer at the time of FFR (Fig. 2). To maintain masking, this procedure was performed at an integration core laboratory (Minneapolis Heart Institute, Minneapolis, MN). The location on CCTA that corresponded to the point where FFR was measured was identified. The location was communicated to the CCTA images by an arrow on a 3-dimensional volume-rendered CCTA image of the coronary arteries.
Statistical methods
The outcome of interest was coronary lesion-specific ischemia, defined as an FFR ≤0.80.13 Measures of interest (DCV, %DC, AS, LPV) were analyzed as continuous variables. Continuous variables were described as mean ± standard deviations (SD), or medians with interquartile ranges (IQR) where appropriate. Categorical variables were displayed as frequencies and percentages. Continuous data was compared across two groups using the two-sample t-test or Mann-Whitney test for non-normally distributed data, and categorical data were compared using Pearson’s Chi-squared test. Continuous variables displaying a non-normal distribution were log-transformed for normality, as necessary. Multivariable linear and logistic regression techniques using clustered robust standard errors to account for within patient correlations within a random effects model were used to assess the association between variables. Forward and backwards model selections were used to aid in retaining the most appropriate covariates for model adjustment, wherein candidate predictors with p values <0.05 were allowed entry into these models. In the event two parameters were correlated with a Pearson’s coefficient >0.30, the candidate with the lower predictive value was omitted, unless relevant to the clinical question. Candidate variables included all baseline demographic variables and plaque measures. Analyses were conducted using STATA version 14 (StataCorp LP, College Station, Texas) and SAS version 9.2 (SAS Institute Inc., Cary, NC). A two-tailed p value <0.05 was considered statistically significant for all instances.
Results
Baseline characteristics and ischemia
A total 249 patients were included, of whom 128 (51.4%) had at least one lesion with FFR ≤0.80. Mean age was 63.0±8.6 years, and there was no significant difference in mean age between those with FFR >0.80 and those with FFR ≤0.80 (p = 0.84). In Table 1, no differences were observed for traditional risk factors including diabetes, hypertension, hyperlipidemia, smoking status, and family history of CAD between those with FFR >0.80 and FFR ≤0.80. Though, women were less likely to present with an FFR ≤0.80 as compared with men (p = 0.04).
Table 1.
Baseline characteristics of the study population
| Overall (n=249) |
FFR >0.80 (n=121) |
FFR ≤0.80 (n=128) |
p value | |
|---|---|---|---|---|
| Age (mean, SD) | 63.0±8.6 | 62.9±8.8 | 63.1±8.5 | 0.84 |
| Female (%) | 73 (29.3) | 43 (58.9) | 30(41.1) | 0.04 |
| Diabetes (%) | 52 (20.9) | 20 (38.5) | 32 (61.5) | 0.12 |
| Hypertension (%) | 175 (70.3) | 82 (46.9) | 93 (53.1) | 0.30 |
| Hyperlipidemia (%) | 199 (79.9) | 95 (47.7) | 104 (52.3) | 0.59 |
| Active smoker (%) | 44 (17.7) | 21 (47.7) | 23 (52.3) | 0.90 |
| Family history of CAD (%) | 50 (20.1) | 23(46.0) | 27 (54.0) | 0.66 |
FFR, fractional flow reserve; CAD, coronary artery disease.
Lesion characteristics and ischemia
There were a total 399 coronary lesions, of which 150 (37.6%) had an FFR ≤0.80. Lesion characteristics are summarized in Table 2. The presence of dense calcium, as well as particular measures of dense calcium such as DCV and %DC, appeared to be more prevalent among ischemic lesions. Other measures of atherosclerosis including LPV, NCV and lumen AS ≥70% also appeared more prevalent in ischemic lesions. Likewise, the individual APCs were significantly more prevalent in ischemic lesions.
Table 2.
Lesion characteristics.
| Overall (n=399) |
FFR >0.80 n=249 |
FFR ≤0.80 n=150 |
p value | |
|---|---|---|---|---|
| Dense calcium present (%) | 367 (92.0%) | 222 (89.1%) | 145 (96.7%) | 0.01 |
| DCV, mm3 (mean, SD) | 44.9 ± 52.3 | 37.6 ± 49.5 | 57.0 ± 54.7 | <0.001 |
| %DC (mean, SD) | 19.4 ± 17.1 | 18.2 ± 17.1 | 21.5 ± 16.9 | 0.02 |
| LPV, mm3 (mean, SD) | 251.9 ± 9.2 | 207.1 ± 9.4 | 326.2 ± 17.2 | <0.001 |
| NCV, mm3 (mean, SD) | 207.7 ± 163.5 | 170.5 ± 8.4 | 269.3 ± 15.5 | <0.001 |
| LAP volume, mm3 (mean, SD) | 31.13 ± 43.7 | 23.03 ± 30.4 | 44.55 ± 57.1 | <0.001 |
| Area stenosis, %(mean, SD) | 62.72 ± 17.0 | 57.69 ± 16.9 | 71.07 ± 13.5 | <0.001 |
| Area stenosis ≥70% (%) | 147 (36.8%) | 61 (24.5%) | 86 (57.3%) | <0.001 |
| Low attenuation plaque (%) | 90 (22.6%) | 24 (9.6%) | 66 (44.0%) | <0.001 |
| Positive remodeling (%) | 191 (47.9%) | 70 (28.1%) | 121 (80.7%) | <0.001 |
| Spotty calcification (%) | 67 (16.8%) | 25 (10.0%) | 42 (28.0%) | <0.001 |
| Lesion length, mm (mean, SD) | 25.11 ± 12.1 | 22.07 ± 10.9 | 30.17 ± 12.3 | <0.001 |
FFR, fractional flow reserve; DCV, dense calcium volume; %DC, percentage dense calcium; LAP, low attenuation plaque; LPV, lesion plaque volume; NCV, non calcified volume.
Lesion characteristics and dense calcium
The relationship between broader anatomical measures of atherosclerosis and dense calcium were statistically significant and concordant. DCV and LPV were closely correlated (Pearson’s coefficient = 0.49, p <0.001), such that each 10mm3 increment in DCV was associated with a 17.2mm3 (95% CI, 13.4 – 21.0) increment in LPV (p <0.001). Similarly, a 10mm3 increment in DCV was associated with a 0.7% (95% CI, 0.4 – 1.0, p <0.001) increment in AS (Pearson coefficient = 0.22, p <0.001). Relatedly, %DC was significantly associated with LPV and AS (p<0.001 for both). AS was also correlated with LPV (Pearson coefficient = 0.38, p <0.001).
Predictors of ischemia
Univariable and multivariable analyses between atherosclerotic variables and ischemia are shown in Table 3. In univariable analysis, DCV, AS, NCV and LPV were significantly associated with an increased likelihood of ischemia. %DC was only modestly associated with ischemia in univariable analysis, and as such, not entered into the multivariable model. For the multivariable analysis, none of the clinical characteristics were retained, with the model therefore only evaluating DCV, AS, NCV and LPV. Of the latter parameters, AS was associated with a greater likelihood of ischemia after adjustment (Table 3). Also, LPV remained independently predictive (adjusted OR: 1.04, 95% CI: 1.00 – 1.04, p <0.001) of ischemia. Conversely, DCV did not remain predictive of ischemia following adjustment.
Table 3.
Univariable and multivariable analysis for predictors of ischemia
| Univariable OR (95% CI) |
p value | Multivariable* OR (95% CI) |
p value | |
|---|---|---|---|---|
| DCV (10 mm3 per increment) | 1.07 (1.03 – 1.12) | 0.002 | 1.01 (0.96 – 1.06) | 0.69 |
| %DC | 1.12 (1.00 – 1.26) | 0.06 | N/A | N/A |
| Area stenosis (10% per increment) | 1.79(1.52 – 2.12) | <0.001 | 1.65 (1.38 – 1.97) | <0.001 |
| LPV (10 mm3 per increment) | 1.04(1.03 – 1.05) | <0.001 | 1.01(1.00 – 1.04) | <0.001 |
| NCV (10 mm3 per increment) | 1.04(1.03 – 1.05) | <0.001 | 1.01 (0.99 – 1.02) | 0.26 |
| Lesion length (10 mm per increment) | 1.06(1.04 – 1.08) | <0.001 | 1.05 (1.01 – 1.09) | 0.02 |
OR, odds ratio; CI, confidence interval; DCV, dense calcium volume; LPV, lesion plaque volume.
Adjusted for clinical risk factors and APCs with backward stepwise regression.
Exploratory analyses
Similarly, NCV was associated with an increased likelihood of ischemia in univariate analysis, (OR: 1.04, 95% CI: 1.03 – 1.05, p <0.001) but not in multivariate analysis (adjusted OR: 1.01, 95% CI: 0.99 – 1.02, p = 0.26). As noncalcified plaque volume is highly collinear with total and calcified plaque volume by definition (NCV + DCV = LPV, variance inflation factor = 13.33), the incremental predictive value of NCV above and beyond DCV and TPV cannot be determined. Lesion length was associated with an increased likelihood of ischemia both in univariate (OR: 1.06, 95% CI: 1.04 – 1.08, p <0.001) and multivariate analysis (adjusted OR: 1.05, 95% CI: 1.01 – 1.09, p = 0.02). We performed a sensitivity analysis for the multivariate model using >70% diameter stenosis cut-off rather than area stenosis and found a similar odds ratio for DCV as a predictor (adjusted OR: 1.02, 95% CI: 0.96 – 1.08, p = 0.47). Using LPV in addition to AS did not significantly improve the ROC curve for the prediction of ischemia (AUC of 0.74 versus 0.73, p = 0.45). Although APCs were included in the multivariate model as previously described, an exploratory analysis was done using the number of APCs within a lesion as a variable in a multivariate model. The presence of ≥ 1 APC was an independent predictor of ischemia (adjusted OR: 5.06, 95% CI: 2.77 – 9.25, p <0.001).
Discussion
The present analysis derived from a multicenter, prospective study cohort, who underwent CCTA and invasive FFR, sheds further light on the relationship between coronary calcific atherosclerosis and myocardial ischemia. A chief finding of this study was that DCV did not independently predict ischemia after accounting for LPV based on a model that incorporated broader measures of plaque area stenosis and total plaque volume. The close correlation between DCV and LPV suggests that dense calcium only reflects a surrogate of overall plaque volume and extent, and is not independently associated with ischemia.
The association between coronary calcium burden and ischemia has previously been studied noninvasively using several approaches.1,2 In a study of 1,195 patients who underwent stress/rest dual isotope myocardial perfusion single-photon emission computed tomography (SPECT) and coronary artery calcium (CAC) scoring, multivariable analysis found that calcium burden as defined by log CAC score was the most potent predictor of ischemia, when compared with other traditional cardiovascular risk factors.2 Albeit, it bears mentioning that this study was unable to measure noncalcified plaque or individual plaque characteristics. This is in contrast to a study of 84 patients with stable chest pain who underwent CCTA and myocardial computed tomography perfusion (CTP). In this study, DCV, along with area stenosis, lesion length and LPV, was significantly more prevalent in ischemia-related lesions in univariate analysis. However, only area stenosis and lesion length were independently correlated with perfusion defects.3 This is congruent with the current study. It is worth noting that CTP has a per-vessel sensitivity of 76% when compared with FFR.21 Foremost, DCV as quantified by IVUS has been shown to be correlated with low FFR in ischemic lesions (i.e., those with FFR ≤0.75) in patients with acute coronary syndrome.4 The latter study was limited by being performed in a clinically unstable population, which may differ from a stable population who are more likely to undergo CCTA. A study of 484 vessels in 254 stable patients found that DCV, NCV and LAP volumes were all inversely related to FFR.22 In concordance with our study, DCV and NCV were not independent predictors of ischemia. Similarly, SC was not an independent predictor of ischemia. In that study, lesion length was not an independent predictor, unlike the current study. It is worth noting that lesion length was measured using a binary cutoff, whereas the present study uses a continuous measurement. Overall, the current findings extend upon the prior literature by demonstrating the lack of a relationship between DCV and FFR in stable patients, after accounting for broader measures of coronary atherosclerosis.
The observed correlation between DCV and LPV in the present study concurs with prior IVUS and histological studies that have found a strong relationship between plaque calcium and aggregate plaque burden. In a prospective, multicenter, non-randomized IVUS registry of 990 patients with either stable symptoms or acute coronary syndrome, the absolute amount of dense calcium as measured by cross sectional area was positively correlated to the total plaque area. In a larger IVUS study of 1,442 patients, multivariable linear regression analysis demonstrated that atherosclerotic plaque burden (as measured by cross-sectional area) was an independent predictor of the arc of target lesion calcium.23 The latter IVUS studies are concordant with previous histological findings, wherein a study by Sangiorgi and co-workers comprising 723 coronary artery sections found a significant correlation between the square root of coronary calcium area and the square root of plaque area (r=0.52, p<0.0001).24
When considered in light of the present study’s findings, this literature adds weight to the notion that coronary calcium is a marker of atheroma at both the lesion and aggregate level. Further still, at the plaque level, measures of volume appear to have a lower predictive effect on ischemia than area stenosis, although it should be borne in mind that AS alone may not adequately identify ischemic lesions that require revascularization.11,25 In contrast, other APCs detected by CCTA have been shown to be strongly associated with ischemia in coronary lesions independent of AS and aggregate plaque volume. In general, PR was found to be an independent predictor of ischemia.14,26,27 In a study with 49 stable patients undergoing either SPECT or positron emission tomography (PET), both PR and LAP were independent predictors of myocardial hypoperfusion, whereas SC was not.27 The present study differed in that we did not find LAP to be an independent predictor of ischemia. The present study did not have longitudinal follow-up to address the previously established link between the APCs studied and acute coronary syndrome (ACS).28 The findings of the present study indicate that DCV is not an APC, though it may still be clinically valuable for other uses (e.g., risk stratification).
There are some limitations to the present study that need to be emphasized. The definition of DC has varied in prior studies, and is modality-and methodology-dependent. We used a cutoff of ≥350 HU, which has been previously employed by others given that it effectively distinguishes calcium from luminal contrast.29,30 Nevertheless, this definition of DC is not identical to that measured in non-contrast studies using the Agatston score, or that used in IVUS.4,23 The present study findings may be more accurate given that IVUS may not be capable of clearly measuring total calcium volume due to poor penetration of the ultrasound beam,9 whereas CCTA directly measures the DCV. Nevertheless, as the results of our study concur with prior literature performed in other modalities, it can be surmised that DCV detected by CCTA in general is representative of overall calcium volume. The current study was cross-sectional in nature, and as a consequence, examining the progression in DCV, ischemia, or LPV with time or risk factor modification was beyond the scope of this investigation. Indeed, these matters warrant further study. Importantly, to the best of our knowledge, this is the first and largest prospective study that investigated the relationship between CCTA measures of DC, plaque volume, and ischemia as measured by invasive FFR on a lesion-specific basis.
In stable patients undergoing CCTA and invasive FFR, neither DCV nor the proportion of DC was an independent predictor of ischemia after adjustment for other plaque characteristics. Instead, DCV appears to reflect a marker of total plaque volume, which in turn, is predictive of ischemia.
Supplementary Material
Highlights.
The association between dense coronary calcium and ischemia is unclear
We evaluated dense coronary calcium with coronary computed tomography angiography (CCTA) and ischemia with fractional flow reserve (FFR)
Dense coronary calcium is not an independent predictor of ischemia, but a surrogate
Lesion plaque volume is an independent predictor of ischemia
Acknowledgments
Financial support
This study was funded by the National Institute of Health (Bethesda, MD, USA) under award numbers R01 HL111141, R01 HL115150, and R01 HL118019. This study was also supported, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging (New York, NY, USA) and the Michael Wolk Foundation (New York, NY, USA).
Abbreviations
- %DC
percentage dense calcium
- APC
atherosclerotic plaque characteristic
- AS
area stenosis
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- DC
dense calcium
- DCV
dense calcium volume
- FFR
fractional flow reserve
- HU
Hounsfield Units
- ICA
invasive coronary angiography
- LAP
low attenuation plaque
- LPV
lesion plaque volume
- NCV
noncalcified calcium volume
- PR
positive remodeling
- SC
spotty calcification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
James Min serves as a consultant to HeartFlow. All other authors have reported no disclosures, conflicts of interests, or relationships with industry.
Author contributions
Lohendran Baskaran conceived and wrote the majority of the manuscript. Bríain Ó Hartaigh reviewed and revised the manuscript. Joshua Schulman-Marcus MD reviewed and revised the manuscript. Heidi Gransar performed the majority of the statistical analysis. Fay Lin conceived part, reviewed and revised the manuscript. James K. Min conceived the clinical question.
References
- 1.He ZX, Hedrick TD, Pratt CM, Verani MS, Aquino V, Roberts R, Mahmarian JJ. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244–251. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 2.Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, Friedman JD, Kang X, Polk D, Hachamovitch R, Shaw L, Rozanski A. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–930. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 3.Van Rosendael AR, Kroft LJ, Broersen A, Dijkstra J, van den Hoogen IJ, van Zwet EW, Bax JJ, de Graaf MA, Scholte AJ. Relation between quantitative coronary CTA and myocardial ischemia by adenosine stress myocardial CT perfusion. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2016 doi: 10.1007/s12350-016-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hüseyinova G, Aslanger E, Çakir O, Atici A, Panç C, Demirkiran A, Sürmen S, Sarikaya R, Erdoğan O, Gölcük E, Umman S, Sezer M. Potential contribution of virtual histology plaque composition to hemodynamic-morphologic dissociation in patients with non-ST elevation acute coronary syndrome. Int J Cardiol. 2015;187:33–38. doi: 10.1016/j.ijcard.2015.03.316. [DOI] [PubMed] [Google Scholar]
- 5.Chung J-H, Ann SH, Singh GB, Nam C-W, Doh J-H, Kim HI, Koo B-K, Shin ES. Segmental assessments of coronary plaque morphology and composition by virtual histology intravascular ultrasound and fractional flow reserve. Int J Cardiovasc Imaging. 2015 doi: 10.1007/s10554-015-0794-8. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Narula J, Chandrashekhar Y. The never-ending story on coronary calcium: is it predictive, punitive, or protective? J Am Coll Cardiol. 2015;65:1283–1285. doi: 10.1016/j.jacc.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 8.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8:461–471. doi: 10.1016/j.jcmg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, el Gamal MI. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92:3183–3193. doi: 10.1161/01.cir.92.11.3183. [DOI] [PubMed] [Google Scholar]
- 11.Pijls NHJ, van Schaardenburgh P, Manoharan G, Boersma E, Bech J-W, van’t Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 12.Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, FAME Study Investigators Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 13.De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, Jagic N, Möbius-Winkler S, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF, FAME 2 Trial Investigators Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 14.Park H-B, Heo R, ó Hartaigh B, Cho I, Gransar H, Nakazato R, Leipsic J, Mancini GBJ, Koo B-K, Otake H, Budoff MJ, Berman DS, Erglis A, Chang H-J, Min JK. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging. 2015;8:1–10. doi: 10.1016/j.jcmg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Zhang J, Pan J, Lu Z. Coronary stenosis: Morphologic index characterized by using CT angiography correlates with fractional flow reserve and is associated with hemodynamic status. Radiology. 2013;269:713–721. doi: 10.1148/radiol.13122550. [DOI] [PubMed] [Google Scholar]
- 16.Min JK, Berman DS, Budoff MJ, Jaffer FA, Leipsic J, Leon MB, Mancini GBJ, Mauri L, Schwartz RS, Shaw LJ. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J Cardiovasc Comput Tomogr. 2011;5:301–309. doi: 10.1016/j.jcct.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Min JK, Leipsic J, Pencina MJ, Berman DS, Koo B-K, van Mieghem C, Erglis A, Lin FY, Dunning AM, Apruzzese P, Budoff MJ, Cole JH, Jaffer FA, Leon MB, Malpeso J, Mancini GBJ, Park S-J, Schwartz RS, Shaw LJ, Mauri L. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308:1237–1245. doi: 10.1001/2012.jama.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, Weigold WG. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Boogers MJ, Broersen A, van Velzen JE, de Graaf FR, El-Naggar HM, Kitslaar PH, Dijkstra J, Delgado V, Boersma E, de Roos A, Schuijf JD, Schalij MJ, Reiber JHC, Bax JJ, Jukema JW. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J. 2012;33:1007–1016. doi: 10.1093/eurheartj/ehr465. [DOI] [PubMed] [Google Scholar]
- 20.Naidu SS, Rao SV, Blankenship J, Cavendish JJ, Farah T, Moussa I, Rihal CS, Srinivas VS, Yakubov SJ, Society for Cardiovascular Angiography and Interventions Clinical expert consensus statement on best practices in the cardiac catheterization laboratory: Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2012;80:456–464. doi: 10.1002/ccd.24311. [DOI] [PubMed] [Google Scholar]
- 21.Ko BS, Cameron JD, Meredith IT, Leung M, Antonis PR, Nasis A, Crossett M, Hope SA, Lehman SJ, Troupis J, DeFrance T, Seneviratne SK. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur Heart J. 2012;33:67–77. doi: 10.1093/eurheartj/ehr268. [DOI] [PubMed] [Google Scholar]
- 22.Gaur S, Øvrehus KA, Dey D, Leipsic J, Bøtker HE, Jensen JM, Narula J, Ahmadi A, Achenbach S, Ko BS, Christiansen EH, Kaltoft AK, Berman DS, Bezerra H, Lassen JF, Nørgaard BL. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J. 2016:ehv690. doi: 10.1093/eurheartj/ehv690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mintz GS, Pichard AD, Popma JJ, Kent KM, Satler LF, Bucher TA, Leon MB. Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol. 1997;29:268–274. doi: 10.1016/s0735-1097(96)00479-2. [DOI] [PubMed] [Google Scholar]
- 24.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 25.Tonino PAL, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van’t Veer M, Pijls NHJ. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 26.Nakazato R, Shalev A, Doh J-H, Koo B-K, Gransar H, Gomez MJ, Leipsic J, Park HB, Berman DS, Min JK. Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol. 2013;62:460–467. doi: 10.1016/j.jacc.2013.04.062. [DOI] [PubMed] [Google Scholar]
- 27.Shmilovich H, Cheng VY, Tamarappoo BK, Dey D, Nakazato R, Gransar H, Thomson LEJ, Hayes SW, Friedman JD, Germano G, Slomka PJ, Berman DS. Vulnerable plaque features on coronary CT angiography as markers of inducible regional myocardial hypoperfusion from severe coronary artery stenoses. Atherosclerosis. 2011;219:588–595. doi: 10.1016/j.atherosclerosis.2011.07.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motoyama S, Sarai M, Narula J, Ozaki Y. Coronary CT angiography and high-risk plaque morphology. Cardiovasc Interv Ther. 2013;28:1–8. doi: 10.1007/s12928-012-0140-1. [DOI] [PubMed] [Google Scholar]
- 29.Hong C, Becker CR, Schoepf UJ, Ohnesorge B, Bruening R, Reiser MF. Coronary artery calcium: absolute quantification in nonenhanced and contrast-enhanced multi-detector row CT studies. Radiology. 2002;223:474–480. doi: 10.1148/radiol.2232010919. [DOI] [PubMed] [Google Scholar]
- 30.Mühlenbruch G, Wildberger JE, Koos R, Das M, Flohr TG, Niethammer M, Weiss C, Günther RW, Mahnken AH. Coronary calcium scoring using 16-row multislice computed tomography: nonenhanced versus contrast-enhanced studies in vitro and in vivo. Invest Radiol. 2005;40:148–154. doi: 10.1097/01.rli.0000153024.12712.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


