Abstract
Objective
Approximately 20% of total knee arthroplasty (TKA) recipients have suboptimal pain relief. We evaluated the association between pre-surgical widespread body pain and incomplete pain relief following TKA.
Method
This prospective analysis included 241 patients with knee OA undergoing unilateral TKA who completed questionnaires preoperatively and up to 12 months post-operatively. Questionnaires included the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scale and a body pain diagram. We derived the number of non-index painful body regions from the diagram. We used Poisson regression to determine the association between painful body regions identified preoperatively and both WOMAC pain at follow-up and improvement in pain as defined by the minimal clinically important difference (MCID).
Results
Mean subject age was 66 years (SD 9), and 61% were females. Adjusting for age, sex, co-morbid conditions, baseline pain, pain catastrophizing, and mental health, we found that more widespread body pain was associated with a higher likelihood of reporting 12-month WOMAC pain score >15 (relative risk [RR] per painful body region 1.39, 95% CI 1.18–1.63) and a greater likelihood of failing to achieve the MCID (RR 1.47, 95% CI 1.16–1.86). ). Pain catastrophizing was an independent predictor of persistent pain and failure to improve by the MCID (RR 3.57, 95% CI 1.73–7.31).
Conclusions
Pre-operative widespread pain was associated with greater pain at 12-months and failure to reach the MCID. Widespread pain as captured by the pain diagram, along with the pain catastrophizing score, may help identify persons with suboptimal TKA outcome.
Keywords: widespread pain, total knee arthroplasty, catastrophizing, pain diagram
Introduction
Research on predictors of poor outcome from total knee arthroplasty (TKA) has shown that individuals with greater pre-operative levels of pain, pain catastrophizing, anxiety and depression, more diffuse body pain, lower educational attainment, and other psychological and social factors tend to have worse surgical outcomes.1–6 Prior research on identifying at-risk individuals has relied upon validated measures including the Pain Catastrophizing Scale,7 the Mental Health Inventory-5,8 and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and physical function subscales.9 While such measures are useful for research purposes, they may be difficult to administer in a busy clinic. A simple tool that can identify those patients most at risk of poor TKA outcome may be helpful in clinical settings.
We have begun to examine such a tool – a total body pain diagram that captures patient-reported pain in 19 different body sites.10 In a pre-surgical cohort of subjects with knee osteoarthritis (OA) undergoing elective unilateral TKA, widespread body pain as depicted by the whole body pain diagram was associated cross-sectionally with validated measures of OA-related pain, mental health, and pain catastrophizing.11 These associations between widespread pain and OA-related pain, mental health, and pain catastrophizing persisted after adjustment for age, sex, and comorbid medical conditions. We did not include review of pre-operative knee imaging in our study. It has been established that lower radiographic severity is associated with worse TKR pain outcome.12
Despite the common use of pain diagrams to depict widespread pain in research involving other musculoskeletal conditions (e.g., chronic low back pain, Ehlers-Danlos disease, and carpal tunnel syndrome),13–17 the association between widespread pain as depicted by whole body pain diagrams and TKA outcome has received little study.18 We sought to evaluate the association between pre-surgical pain diagram scores and follow-up WOMAC pain scores up to 12 months post-operatively. Furthermore, we evaluated the association between pre-surgical pain diagram scores and improvement in pain by the estimated minimal clinically important difference (MCID) for WOMAC pain. We hypothesized that those participants who reported more widespread body pain would continue to experience more pain up to 12 months postoperatively and would be less likely to improve by the MCID.
Methods
The Study of Total Knee Arthroplasty Responses (STARs), a prospective cohort study of participants with knee OA undergoing elective unilateral TKA at three clinical sites, was designed to evaluate risk factors for suboptimal TKA outcome. The STARs study enrolled participants at one academic center (NYU Langone Medical Center, New York, NY, USA) and two community orthopedic centers (Orthopaedic Center of the Rockies, Fort Collins, CO, USA, and University of Maryland St. Joseph Medical Center, Towson, MD, USA) from September 2012 to April 2014. Institutional Review Board approval was obtained at all clinical sites and at Brigham and Women’s Hospital, Boston, MA, USA.
Participants
Participants were English-speaking community-dwelling persons, at least 40 years of age at study entry, undergoing TKA with a primary diagnosis of knee OA. Subjects with inflammatory knee arthritis or other underlying diagnoses leading to TKA were excluded. Staff at each center identified potentially eligible subjects and provided the subjects’ contact information to the Brigham and Women’s Hospital research coordinator, who in turn contacted all potential subjects to confirm eligibility, explain the study, and ascertain interest in participation. Study subjects were asked to complete questionnaires pre-operatively and at 6 and 12 months post-operatively to capture changes following surgery. Questionnaires included demographic information, co-morbid medical conditions, the whole body pain diagram, WOMAC pain and function sub-scales,9 Pain Catastrophizing Scale (PCS),7 and the Mental Health Inventory-5 (MHI-5).19 Subjects were reimbursed (USD 25) for returning a questionnaire at each time-point.
While the follow-up data were derived primarily from the one-year questionnaire (n=219), we used data from 6-month questionnaires for 22 subjects in a last value carried forward fashion to maximize sample size and cohort generalizability. To evaluate the validity of the six-month data as a proxy for one year outcomes, we compared mean six-month and 24-month WOMAC scores in 18 subjects who provided both six and 24-month data but no 12-month data. These values were similar (mean [SD] WOMAC pain at 6- and 24-months, respectively: 11 [10.8] and 8 [9.3]), supporting the validity of the substitution.
Baseline data
The pre-operative questionnaire included a self-reported body pain diagram completed within 6 weeks of surgery. Subjects were directed to report current pain using checkboxes at nineteen separate pre-defined body sites. Subjects indicated whether they had pain in each of these areas with a ‘check.’ The number of painful body sites is the sum of the number of sites (0 to 19) reported by the study subject on the pain diagram. The index joint was removed from this sum and thus the lowest score would be 0 and the highest would be 18. From the number and location of painful areas reported on the body pain diagram, we derived both the total number of painful sites and the number of painful body regions. Similar to our previously reported technique,11 we established eight different body regions but excluded the surgically-treated or index region so as to best capture widespread pain; thus, possible scores ranged from 0 to 7. The eight body regions were defined as follows: right upper extremity (shoulder girdle, upper arm, and lower arm), left upper extremity (shoulder girdle, upper arm, and lower arm), right lower extremity (upper leg and lower leg), left lower extremity (upper leg and lower leg), right hip (hip and buttock), left hip (hip and buttock), back/neck (upper back, lower back, and neck), and chest (abdomen and chest). In our analyses, the index joint was removed from both the body regions and from the painful sites as we anticipated that the vast majority of study participants would experience pain at the index joint. The extent of widespread body pain was analyzed as both a continuous and a categorical variable (0 versus 1–2 versus ≥3 painful regions). However, the modeling was performed with the continuous form of the widespread body pain regions because models using the categorical form of the variable demonstrated some instability due to small cell sizes. We assessed pre-operative pain catastrophizing utilizing the 13-item PCS7 and pre-operative anxiety and depression with the five-item MHI-5.8,19 Responses to questions were summed and scaled from 0 to 100. PCS scores were dichotomized at 20 with scores of ≥20 indicating high catastrophizing based on an assessment of sample distribution and published clinical studies on knee OA.7,13,17,20 MHI-5 score was dichotomized at 68 with scores <68 indicating poor mental health as previously reported.21 The WOMAC pain score was calculated as the sum of the responses to the five items, each of which had a five-item scale,9 scaled from 0 to 100 with 100 representing the worst possible score.22 Baseline WOMAC pain was categorized as 0–39, 40–69, and ≥70.
Outcome measures
The outcome measures for this analysis included a 1) dichotomized variable for the final WOMAC pain score at follow-up; and 2) the determination of estimated MCID (yes/no) based on pre- and post-surgical WOMAC pain scores. A dichotomous outcome variable for WOMAC pain at follow-up was created using a threshold of 15. This cut-point implies at least mild pain on 3 of 5 items or at least moderate pain on at least one item, representing persistence of pain and suggesting a suboptimal outcome. The MCID was calculated based on the methodology used by Escobar and Riddle22 which takes baseline WOMAC pain into account in determining MCID. Thus, higher or worse baseline scores require a larger gain in pain scores in order to meet the MCID.
Statistical analysis
Descriptive statistics of baseline demographic, clinical, psychosocial measures, and OA-related pain and function measured at both baseline and at follow-up were either summarized as medians (25th and 75th percentiles) or means (standard deviation) for continuous variables based on normality or as percentages for categorical variables. Similar methods were used for WOMAC pain measured at follow-up and for the difference between baseline and follow-up WOMAC pain. Participants were first stratified by follow-up participation status (Appendix Table 1) to examine factors associated with drop-out, and then by number of painful body regions (0, 1–2, and ≥3) among those who provided follow-up data. Differences between proportions for both follow-up participation status and painful body region category groups were assessed by the Chi-square test, and trend was assessed by the Cochran-Mantel-Haenszel statistic. For continuous variables, the difference between two groups was compared by Student’s t-test or Wilcoxon nonparametric test based on assessment of normality, and three group comparisons were made via the Kruskal-Wallis test. The test for trend for continuous variables was assessed using the Jonckheere-Terpstra test.23
Appendix Table 1.
The association between baseline demographic and clinical characteristics and participation up to 1-year
| Baseline characteristics | No N=26 |
Yes N=241 |
P-value | ||
|---|---|---|---|---|---|
|
| |||||
| N | % or Mean (SD) | N | % or Mean (SD) | ||
|
| |||||
| Age (years) | 26 | 62.8 (8.3) | 240 | 66.2 (8.7) | 0.10 |
|
| |||||
| Female | 0.02 | ||||
| No | 4 | 15.4 | 95 | 39.4 | |
| Yes | 22 | 84.6 | 146 | 60.6 | |
|
| |||||
| Body mass index (kg/m2) | 25 | 33.1 (7.3) | 232 | 30.5 (6.6) | 0.07 |
|
| |||||
| White race | <0.001 | ||||
| No | 9 | 34.6 | 22 | 9.1 | |
| Yes | 17 | 65.4 | 219 | 90.9 | |
|
| |||||
| Education | 0.26 | ||||
| High school or less | 9 | 34.6 | 49 | 20.6 | |
| Some college, vocational, or technical education | 6 | 23.1 | 62 | 26.0 | |
| Undergraduate or technical school degree | 11 | 42.3 | 127 | 53.4 | |
|
| |||||
| Surgical site | 0.08 | ||||
| Maryland | 10 | 38.5 | 84 | 34.8 | |
| Colorado | 6 | 23.1 | 104 | 43.1 | |
| NYU-Langone | 10 | 38.5 | 53 | 22.0 | |
|
| |||||
| Living arrangement | 0.31 | ||||
| Alone | 8 | 30.8 | 51 | 21.4 | |
| Married or with partner | 15 | 57.7 | 171 | 71.8 | |
| Other | 3 | 11.5 | 16 | 6.7 | |
|
| |||||
| Number of co-morbid conditions† | 0.09 | ||||
| 0 | 4 | 16.0 | 62 | 25.9 | |
| 1 | 12 | 48.0 | 84 | 35.1 | |
| 2 | 9 | 36.0 | 60 | 25.1 | |
| ≥3 | 0 | 0 | 33 | 13.8 | |
|
| |||||
| WOMAC function categories | <0.001 | ||||
| 0–39 | 7 | 28.0 | 98 | 40.8 | |
| 40–69 | 11 | 44.0 | 129 | 53.7 | |
| ≥70 | 7 | 28.0 | 13 | 5.4 | |
|
| |||||
| WOMAC pain categories | 0.002 | ||||
| 0–39 | 6 | 23.1 | 102 | 42.7 | |
| 40–69 | 14 | 53.8 | 124 | 51.9 | |
| ≥70 | 6 | 23.1 | 13 | 5.4 | |
|
| |||||
| Number of painful body regions | 0.13 | ||||
| 0 | 2 | 7.7 | 53 | 22.0 | |
| 1–2 | 13 | 50.0 | 121 | 50.2 | |
| ≥3 | 11 | 42.3 | 67 | 27.8 | |
|
| |||||
| Number pain sites§ | 26 | 3.3 (2.6) | 241 | 2.5 (2.5) | 0.05 |
| Median (25th, 75th percentiles) | 3 (1, 5) | 2 (1, 4) | |||
| Range | 0–10 | 0–15 | |||
Co-morbid conditions are comprised of diabetes mellitus, depression, stomach ulcers, cancer, chronic kidney disease, liver disease, cardiovascular disease, and hypertension
Index leg sites have been removed
We utilized Poisson regression to estimate the adjusted relative risk for each covariate.24 We used this approach rather than logistic regression because odds ratios are often an overestimation of the risk ratio for common outcomes. Our regression models were used to (1) determine the crude associations between baseline measures (of pain, widespread pain depicted by a whole body pain diagram, pain catastrophizing, and MHI-5) and the dichotomous outcome for WOMAC pain at follow-up; and (2) to evaluate the independent association between baseline painful body regions and dichotomous WOMAC pain at follow-up after adjustment for age, body mass index, number of co-morbid conditions, and baseline levels of WOMAC pain, MHI-5, and pain catastrophizing. The final model is the most parsimonious model, inclusive of age, sex, and co-morbid conditions, as determined by a p-value approach.
Similar methods as above were employed for assessing the associations with attainment of estimated MCID. Poisson regression methods were used to evaluate the crude associations between number of painful body regions, WOMAC pain, pain catastrophizing, and MHI-5 measured at baseline and attainment of the estimated MCID for WOMAC pain. The independent association between body pain regions and attainment of estimated MCID was assessed again after adjusting for select baseline measures including age, body mass index, number of co-morbid conditions, WOMAC pain, MHI-5, and pain catastrophizing (full model). The final model included all covariates that were statistically significant in the full model. The final model again is the most parsimonious model as determined by assessing p-values and collinearity among psychosocial variables.
Only missing WOMAC pain data at 12-months were imputed. Our approach to addressing missing one year WOMAC pain outcome data was guided by the observation that the majority of subjects who were missing outcome data at one-year provided data at 6 and 24 months and there was no appreciable difference between 6-month and 24-month outcome. Consequently, we opted to carry the 6-month data forward rather than perform a more complex imputation. To assess the impact of this approach to missing data on our analyses, we performed several sensitivity analyses. Specifically, we excluded patients with missing outcome data at one year in one analysis. In another analysis, we used 24-month data (instead of 6-month data) in cases where 12-month data were missing. These sensitivity analyses yielded very similar results and identical interpretation as the primary analysis.
Analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC). All reported p-values are two-sided, and p-values less than 0.05 were considered to be statistically significant.
Results
Cohort characteristics
At three clinical enrollment sites, 385 patients were eligible and agreed to participate. Of these, 267 subjects returned questionnaires prior to TKA surgery. The median number of days from data collection to surgery was 5 days (25th and 75th percentiles: 2 and 9 days prior to surgery). Follow-up questionnaire data were completed by 241 subjects (90%); this sample was the basis for this analysis. The 26 individuals who did not return questionnaires beyond 3 months were more likely to be female, non-white, and have more co-morbid medical conditions (Appendix Table 1). In addition, participants with higher baseline WOMAC pain scores (p=0.002) were less likely to complete follow-up surveys.
Among the 241 individuals with follow-up data, age, sex, body mass index, race, and clinical site were similar across body pain region groups (0, 1–2, and ≥3 painful regions) (Table 1). Study subjects reporting higher levels of preoperative widespread pain were more likely to report more co-morbid medical conditions, use of supportive devices, and worse pre-operative ability to straighten their knees. Median values for WOMAC pain at baseline were significantly higher among subjects with a higher number of painful body regions. There was a significant inverse association between MHI-5 scores and preoperative number of painful body regions (Table 1).
Table 1.
Baseline demographic, clinical, and resource utilization characteristics by regional body pain groups in STARS participants with one-year data
| Regional Body Pain Diagram Groups | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristic | 0 N=53 |
1–2 N=121 |
3–6 N=67 |
Overall | Trend | |||
|
| ||||||||
| N | %, mean (SD) | N | %, mean (SD) | N | %, mean (SD) | |||
|
| ||||||||
| Age – yrs | 53 | 66.5 (7.8) | 120 | 65.6 (9.1) | 67 | 67.2 (8.7) | 0.49 | 0.64 |
|
| ||||||||
| Female | 0.24 | 0.64 | ||||||
| No | 19 | 35.8 | 54 | 44.6 | 22 | 32.8 | ||
| Yes | 34 | 64.1 | 67 | 55.4 | 45 | 67.2 | ||
|
| ||||||||
| BMI (kg/m2) | 52 | 30.5 (7.5) | 116 | 30.3 (6.2) | 64 | 30.8 (6.6) | 0.80 | 0.51 |
|
| ||||||||
| White race | 0.81 | 0.68 | ||||||
| No | 6 | 11.3 | 10 | 8.3 | 6 | 9.0 | ||
| Yes | 47 | 88.7 | 111 | 91.7 | 61 | 91.0 | ||
|
| ||||||||
| Surgical site | 0.10 | 0.23 | ||||||
| Maryland | 25 | 47.2 | 41 | 33.9 | 18 | 26.9 | ||
| Colorado | 15 | 28.3 | 54 | 44.6 | 35 | 52.2 | ||
| NYU-Langone | 13 | 24.5 | 26 | 21.5 | 14 | 20.9 | ||
|
| ||||||||
| Number of co-morbid conditions† | 0.06 | 0.001 | ||||||
| 0 | 18 | 35.3 | 31 | 25.6 | 13 | 19.4 | ||
| 1 | 19 | 37.2 | 47 | 38.8 | 18 | 26.9 | ||
| 2 | 11 | 21.6 | 28 | 23.1 | 21 | 31.4 | ||
| ≥3 | 3 | 5.9 | 15 | 12.4 | 15 | 22.4 | ||
|
| ||||||||
| Use of supportive device | 0.005 | 0.002 | ||||||
| No | 41 | 78.8 | 85 | 72.6 | 31 | 52.5 | ||
| Yes | 11 | 21.1 | 32 | 27.3 | 28 | 47.5 | ||
|
| ||||||||
| Limp without support device | 0.60 | 0.06 | ||||||
| None | 7 | 13.2 | 15 | 12.6 | 6 | 9.0 | ||
| Slight | 22 | 41.5 | 39 | 32.8 | 21 | 31.3 | ||
| Moderate | 18 | 34.0 | 45 | 37.8 | 24 | 35.8 | ||
| Severe/unable to walk without support | 6 | 11.3 | 20 | 16.8 | 16 | 23.9 | ||
|
| ||||||||
| Ability to straighten knee | 0.01 | 0.002 | ||||||
| Completely straight | 28 | 52.8 | 56 | 46.7 | 27 | 40.9 | ||
| Between 5–10° from straight | 20 | 37.7 | 36 | 30.0 | 17 | 25.8 | ||
| Between 11–20° from straight | 5 | 9.4 | 20 | 16.7 | 10 | 15.1 | ||
| More than 20° from straight | 0 | 0 | 8 | 6.7 | 12 | 18.2 | ||
|
| ||||||||
| Number of pain sites§‡ | 53 | 0 (0, 0) | 121 | 2 (1, 2) | 67 | 5 (4, 7) | <0.001 | <0.001 |
| Range | 0 – 1 | 1 – 6 | 3 – 15 | |||||
|
| ||||||||
| Median WOMAC pain‡ | 53 | 35 (25, 45) | 121 | 40 (30, 55) | 65 | 50 (35, 60) | 0.04 | 0.009 |
|
| ||||||||
| Pain catastrophizing categories | 0.40 | 0.28 | ||||||
| <20 | 38 | 82.6 | 84 | 83.2 | 41 | 74.6 | ||
| ≥20 | 8 | 17.4 | 17 | 16.8 | 14 | 25.5 | ||
|
| ||||||||
| MHI-5 score categories* | 0.01 | 0.003 | ||||||
| 0–67 | 10 | 19.2 | 34 | 28.3 | 29 | 43.9 | ||
| 68–100 | 42 | 80.8 | 86 | 71.7 | 37 | 56.1 | ||
Unlike WOMAC pain and Pain Catastrophizing Scale where higher values are indicative for worse pain and catastrophizing, respectively, MHI-5 scores of lower value indicate poorer mental health
Co-morbid conditions are comprised of diabetes mellitus, depression, stomach ulcers, cancer, chronic kidney disease, liver disease, cardiovascular disease, and hypertension
Index leg sites have been removed.
Median reported (25th and 75th percentiles).
Body regions include the following painful body sites as follows:
Upper extremity=shoulder girdle, upper arm and lower arm
Lower extremity= upper leg and lower leg
Hip = hip and buttock Back/neck=upper back, lower back, and neck
Abdomen/chest=abdomen and chest
Upper extremity, lower extremity, and hip body regions include both left and right sides.
WOMAC pain at 12 months
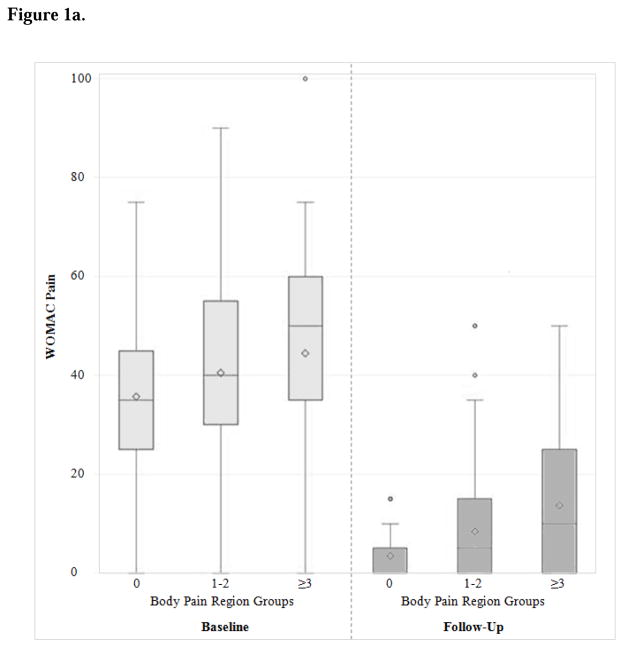
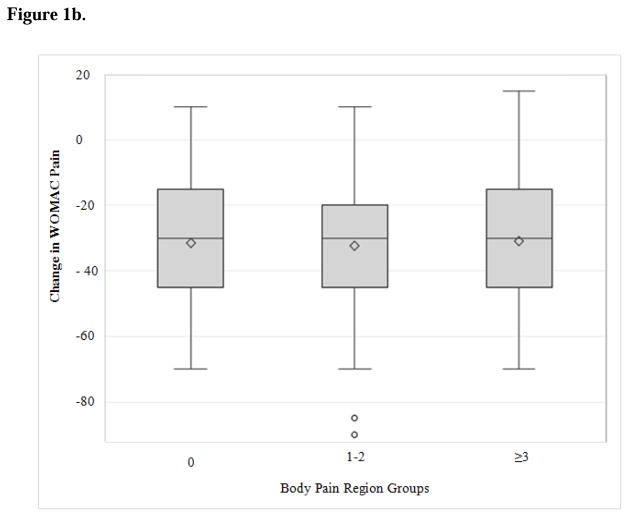
At follow-up, participants in all three levels of painful body regions benefited considerably from TKA, with improvement in median WOMAC pain scores of approximately 30 points (Figure 1a, Table 2). The subjects reporting more painful body regions at baseline experienced similar change in WOMAC pain scores from baseline to follow-up (Figure 1b) -- but more pain at follow-up -- compared to those reporting pain in fewer body regions (Table 2). Participants with pre-operative pain in 3–6 body regions reported higher WOMAC scores at follow-up compared to study subjects with no painful body regions (median 10 versus 0) and were also less likely to achieve MCID (77% versus 98%).
Figure 1.
Figure 1a. WOMAC pain scores at baseline and at 12-months (Follow-up) by body pain region group
Figure 1b. Change in WOMAC pain scores by body pain region group
Table 2.
WOMAC pain outcomes by regional body pain groups in STARS participants
| Regional Body Pain Diagram Groups | P-value | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcome | 0 N=53 |
1–2 N=121 |
3–6 N=67 |
Trend | |||
|
| |||||||
| N | median (25th, 75th %ile) | N | median (25th, 75th %ile) | N | median (25th, 75th %ile) | ||
|
| |||||||
| WOMAC pain Score | 51 | 0 (0, 5) | 120 | 5 (0, 15) | 66 | 10 (0, 25) | <0.001 |
|
| |||||||
| WOMAC pain categories | <0.001 | ||||||
| 0–14 | 47 | 92.2 | 84 | 70.0 | 37 | 56.1 | |
| 15–39 | 4 | 7.8 | 33 | 27.5 | 23 | 34.8 | |
| ≥40 | 0 | 0 | 3 | 2.5 | 6 | 9.1 | |
|
| |||||||
| WOMAC pain change* | 51 | −30 (−45, −15) | 120 | −30 (−45, −20) | 64 | −30 (−45, −15) | 0.82 |
|
| |||||||
| % change in WOMAC pain | 51 | −99.5% (−99.9%, −76.9%) | 120 | −89.3% (−99.8%, −59.9%) | 64 | −69.6% (−99.6%, −50%) | 0.008 |
|
| |||||||
| Achieved MCID | <0.001 | ||||||
| No | 1 | 2.0 | 10 | 8.3 | 15 | 23.4 | |
| Yes | 50 | 98.0 | 110 | 91.7 | 49 | 76.6 | |
Change score calculated as follow-up – baseline
Percent change in WOMAC pain contains two outliers. Two subjects reported no pain at baseline but pain at the follow-up visit. Their change in pain has been truncated to 100%.
Associations between pain diagram scores and 12-month WOMAC pain score
We found bivariate associations between baseline measures of widespread body pain (painful body regions), WOMAC pain, pain catastrophizing, and mental health with follow-up WOMAC pain score dichotomized at 15 (Table 3a).. After adjusting for baseline WOMAC pain, pain catastrophizing, MHI-5, age, sex, and number of co-morbid conditions, widespread pain (measured by number of painful body regions per 1 region) remained associated with follow-up WOMAC pain in multivariable models (Table 3a; full model). In the final model, subjects reporting pain in 1 additional body region were 1.37 times more likely (95% CI: 1.16–1.61) to continue to experience pain at follow-up (WOMAC pain score >15).
Table 3a.
Crude and adjusted models for 12-month WOMAC pain >15
| Crude Models* | Fully Adjusted† | Final Model† | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pain and psychosocial measures | RR | 95% CI | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Painful body regions, per 1 region | 1.40 | 1.21–1.62 | <0.001 | 1.39 | 1.18–1.63 | <0.001 | 1.37 | 1.16–1.61 | <0.001 |
| Baseline WOMAC pain | |||||||||
| 40–69 vs 0–39 | 2.13 | 1.08–4.21 | 0.03 | 2.66 | 1.15–6.15 | 0.02 | 2.57 | 1.12–5.89 | 0.03 |
| 70+ vs 0–39 | 3.85 | 1.56–9.50 | 0.003 | 3.28 | 1.17–9.23 | 0.02 | 2.91 | 1.06–7.94 | 0.04 |
| Pain catastrophizing >20 | 2.81 | 1.58–5.00 | <0.001 | 2.37 | 1.28–4.39 | 0.006 | 2.04 | 1.15–3.61 | 0.01 |
| MHI-5 <68 | 1.76 | 1.02–3.05 | 0.04 | 0.69 | 0.36–1.31 | 0.26 | -- | -- | -- |
The crude models columns presents separate bivariate models with follow-up WOMAC Pain > 15 as the outcome and each row variable (eg painful body regions; baseline WOMAC pain) as the independent variable
The fully adjusted and final model columns present a single model; each is adjusted for age, sex, and number of co-morbid conditions
Association between pain diagram score and estimated MCID
Bivariate analyses of baseline pain and psychosocial measures and attainment of estimated MCID for WOMAC pain revealed significant associations with number of painful body regions, pain catastrophizing, and MHI-5 (Table 3b). A statistically significant association between painful body regions and estimated MCID persisted after adjusting for all covariates. In the final model, each additional painful body region was associated with a higher likelihood of not achieving an estimated MCID for WOMAC pain (RR 1.50, 95% CI 1.19–1.88).
Table 3b.
Crude and adjusted models for not achieving MCID for WOMAC pain
| Crude Models* | Fully Adjusted† | Final Model† | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pain and psychosocial measures | RR | 95% CI | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Painful body regions, per 1 region | 1.49 | 1.22–1.82 | <0.001 | 1.47 | 1.16–1.86 | 0.001 | 1.50 | 1.19–1.88 | <0.001 |
| WOMAC pain | |||||||||
| 40–69 vs 0–39 | 1.28 | 0.58–2.82 | 0.55 | 1.64 | 0.57–4.74 | 0.36 | -- | -- | -- |
| 70+ vs 0–39 | 2.56 | 0.79–8.28 | 0.12 | 1.76 | 0.43–7.26 | 0.43 | -- | -- | -- |
| Pain catastrophizing >20 | 4.16 | 1.86–9.27 | <0.001 | 2.76 | 1.16–6.59 | 0.02 | 3.57 | 1.73–7.36 | <0.001 |
| MHI-5 <68 | 2.83 | 1.35–5.92 | 0.006 | 1.36 | 0.58–3.21 | 0.48 | -- | -- | |
The crude models columns presents separate bivariate models with follow-up WOMAC Pain > 15 as the outcome and each row variable (eg painful body regions; baseline WOMAC pain) as the independent variable
The fully adjusted and final model columns present a single model; each is adjusted for age, sex, and number of co-morbid conditions
Discussion
In our initial study on the association of widespread body pain with known predictors of TKA outcome,11 we found that widespread preoperative body pain as measured by a whole body pain diagram was associated cross-sectionally with preoperative pain catastrophizing. In this paper, we found that measures of widespread preoperative body pain were associated prospectively with a WOMAC pain score > 15 at 12-months follow-up as well as with failure to achieve the estimated MCID. These associations persisted after adjustment for baseline WOMAC pain, pain catastrophizing, and mental health (including anxiety and depression).
A large body of research has suggested that up to 20% of persons undergoing TKA have persistent pain.6,25,26 We represented the outcome of persistent pain with a WOMAC pain score > 15. These participants have, on average, mild to moderate pain, suggesting a suboptimal outcome. While participants in all three painful body region groups had significant improvement in WOMAC pain pre- and post-operatively, our findings suggest that widespread pain is a risk factor for suboptimal outcome following TKA. Similarly, 88% of individuals in the cohort achieved the MCID, suggesting a highly favorable outcome overall. However, the proportion reaching the MCID ranged from 98% in those subjects with no reported areas of preoperative pain other than the index joint to 77% in those subjects with 3 or more painful areas.
The pain diagram score and pain catastrophizing score each contributed independently to suboptimal outcome in this study. This finding suggests that the effect of the pain diagram score on outcome is not entirely explained by its documented association with pain catastrophizing.11 Participants with widespread body pain might have additional musculoskeletal conditions aside from knee OA that do not respond to TKA (such as contralateral knee OA, hip OA, spinal stenosis, foot problems, etc.). A recent carefully-done study by Brummett and colleagues assessed the association between outcomes from knee and hip arthroplasty with pre-operative scores on a fibromyalgia survey.18 The researchers reported that worse pre-operative survey scores were associated with worse post-TKA and post-hip arthroplasty WOMAC pain subscale outcomes six months after surgery. The Brummett study has many similarities to ours in both design and in study findings, reinforcing the robustness of the conclusion that total body pain is associated with worse pain outcomes following joint replacement. Our study has important distinctions and thus builds upon these findings. We conducted our study in three different settings across the US, including both academic and community practices. The pain diagram used in our study differed somewhat from that used by Brummett and colleagues. We had access to extensive information on medical co-morbidities in our patient population. The fact that our findings were similar despite these differences in design, measures and setting speaks to the robustness of the association between widespread pain and TKA outcome. Further, the 19 sites in the Michigan Body Map are validated for fibromyalgia and are commonly referred to clinically as tender points.27 Although these sites differ from the body sites in our whole body pain diagram, the similarity in the results suggest that the pain diagram score may reflect, in at least some individuals, fibromyalgia symptoms and severity.
In our study, it is also noteworthy that the association between pre-operative widespread pain and TKA outcome was observed both with an outcome expressed as the pain score achieved within one year (the ‘destination’) and with an outcome expressed as the improvement in pain score (‘the journey’).28 This observation speaks to the robustness of the association. We chose an estimate of the MCID as the measure of improvement because the MCID is generally regarded as a clinically relevant metric.29 We note, though, that the MCID was derived using data from Escobar and Riddle22 and not from our subjects. Thus, we cannot state with certainty that the individual subjects who achieved the MCID in our cohort regarded their improvement as clinically important.
An important strength of our study was the minimal (~10%) overall loss to follow-up. We note, however, that the small proportion lost to follow up at 12 months had more pre-surgical widespread body pain. As such, we might be overestimating post-surgical improvement. Other limitations of our study include that the body regions delineated on our body pain diagram do not directly correspond to joints. It is unclear whether the association between widespread pain in muscles (perhaps more representative of fibromyalgia) and TKA outcome differs from the association between widespread pain in joints (perhaps more indicative of multi-site OA) and TKA outcome. Additionally, there might be underreporting of joint pain. Prior research has shown that lower radiographic severity of osteoarthritis is an independent predictor of greater postsurgical pain outcome.12 We did not have access to pre-operative imaging for all subjects and thus could not include radiographic osteoarthritis severity to our models. Further, participants were primarily white (Appendix Table 1) and were not allowed to record out-of-body or external sites of pain on the pain diagrams.
Research on the role of pain sensitization in the development of persistent knee osteoarthritis-related pain may provide further avenues for treatment of widespread pain. Widespread pain and pain sensitization appear to arise from a constellation of factors, including muscle nociceptor communication with neurons and neuronal sensitization by persistent nociceptor activation, impaired descending modulation by inhibitory neurons in the development of chronic pain, pressure hyperalgesia, thermal hyperalgesia, and spinal hyperexcitability.30,31 The roles of specific cytokines, nerve growth factor, and sodium channel blockers in osteoarthritis-related pain are additionally being actively investigated32 and may yield further insight into which patients are more likely to have successful outcomes from knee replacement. Translational research that bridges these pathways with the clinical phenotyping of widespread pain on a pain diagram would help to advance the field.
Our data suggest that a pre-operative whole body pain diagram, which can be readily and easily completed by participants, as well as a measure of catastrophizing, might be used to identify persons at risk for suboptimal outcome following TKA. Clinicians might consider using these tools in their practices. Further research should determine whether or not patients with both high pain diagram scores and high catastrophizing scores might benefit from additional pre-surgical care. This care could include detailed discussions about likely surgical outcomes in order to align expectations, as well as psychological interventions such as cognitive behavioral therapy for patients with catastrophizing.28
Acknowledgments
Support: The authors have no potential conflicts of interest relevant to this manuscript and have not received support or benefits from commercial sources for the work reported. The work was supported in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases: T32 AR055885 and T32AR007530
Footnotes
Competing Interests:
None
Author contributions
Conception and design: AJD, EL, JNK
Analysis: FS, EL, JEC
Interpretation of the data: All authors
Drafting of the article: AJD, FS, YCL
Critical revision of the article for important intellectual content: All authors
Final approval of the article: All authors
Provision of study materials or patients: PB, DFD, RI, KK
Statistical expertise: FS, EL, JEC
Obtaining of funding: JNK, EL
Administrative, technical, or logistic support: IU
Collection and assembly of data: FS, JEC, IU
Role of Funding Source:
The work was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The funding agency had no role in the design, implementation, interpretation, or reporting of this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2009;14(4):307–11. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Judge A, Arden NK, Cooper C, Kassim Javaid M, Carr AJ, Field RE, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology (Oxford) 2012;51(10):1804–13. doi: 10.1093/rheumatology/kes075. [DOI] [PubMed] [Google Scholar]
- 3.Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. British journal of anaesthesia. 2015;114(4):551–61. doi: 10.1093/bja/aeu441. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Lewallen DG. Depression in primary TKA and higher medical comorbidities in revision TKA are associated with suboptimal subjective improvement in knee function. BMC musculoskeletal disorders. 2014;15:127. doi: 10.1186/1471-2474-15-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan M, Tanzer M, Stanish W, Fallaha M, Keefe FJ, Simmonds M, et al. Psychological determinants of problematic outcomes following Total Knee Arthroplasty. Pain. 2009;143(1–2):123–9. doi: 10.1016/j.pain.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Wylde V, Dieppe P, Hewlett S, Learmonth ID. Total knee replacement: is it really an effective procedure for all? The Knee. 2007;14(6):417–23. doi: 10.1016/j.knee.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychological Assessment. 1995;7(4):524–32. [Google Scholar]
- 8.Berwick DM, Murphy JM, Goldman PA, Ware JE, Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Medical care. 1991;29(2):169–76. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 10.Fibromialgia. [Accessed 11 November];Nuevos criterios para el diagnostico de la fibromialgia. 2010 http://www.fibromialgia.nom.es/fibromialgia-sindrome-de-fatiga-cronica-sindrome-quimico-mulltiple-Noticias-2010/nuevo-criterios-para-el-diagnostico-de-la%20fibromialgia.html.
- 11.Dave AJ, Selzer F, Losina E, Klara KM, Collins JE, Usiskin I, et al. Is There an Association Between Whole-body Pain With Osteoarthritis-related Knee Pain, Pain Catastrophizing, and Mental Health? Clinical orthopaedics and related research. 2015;473(12):3894–902. doi: 10.1007/s11999-015-4575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valdes AM, Doherty SA, Zhang W, Muir KR, Maciewicz RA, Doherty M. Inverse relationship between preoperative radiographic severity and postoperative pain in patients with osteoarthritis who have undergone total joint arthroplasty. Seminars in arthritis and rheumatism. 2012;41(4):568–75. doi: 10.1016/j.semarthrit.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Khanna V, Caragianis A, Diprimio G, Rakhra K, Beaule PE. Incidence of hip pain in a prospective cohort of asymptomatic volunteers: is the cam deformity a risk factor for hip pain? The American journal of sports medicine. 2014;42(4):793–7. doi: 10.1177/0363546513518417. [DOI] [PubMed] [Google Scholar]
- 14.Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24(1):57–65. doi: 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 1994;10(6):618–23. doi: 10.1016/s0749-8063(05)80058-1. [DOI] [PubMed] [Google Scholar]
- 16.Rombaut L, Scheper M, De Wandele I, De Vries J, Meeus M, Malfait F, et al. Chronic pain in patients with the hypermobility type of Ehlers-Danlos syndrome: evidence for generalized hyperalgesia. Clinical rheumatology. 2014 doi: 10.1007/s10067-014-2499-0. [DOI] [PubMed] [Google Scholar]
- 17.Southerst D, Cote P, Stupar M, Stern P, Mior S. The reliability of body pain diagrams in the quantitative measurement of pain distribution and location in patients with musculoskeletal pain: a systematic review. Journal of manipulative and physiological therapeutics. 2013;36(7):450–9. doi: 10.1016/j.jmpt.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67(5):1386–94. doi: 10.1002/art.39051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rumpf HJ, Meyer C, Hapke U, John U. Screening for mental health: validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry research. 2001;105(3):243–53. doi: 10.1016/s0165-1781(01)00329-8. [DOI] [PubMed] [Google Scholar]
- 20.Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134–43. doi: 10.1002/art.38685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly MJ, Dunstan FD, Lloyd K, Fone DL. Evaluating cutpoints for the MHI-5 and MCS using the GHQ-12: a comparison of five different methods. BMC psychiatry. 2008;8:10. doi: 10.1186/1471-244X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar A, Riddle DL. Concordance between important change and acceptable symptom state following knee arthroplasty: the role of baseline scores. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014;22(8):1107–10. doi: 10.1016/j.joca.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Jonckheere A. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–45. [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ open. 2012;2(1):e000435. doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–72. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Bennett RM, Goldenberg DL. Fibromyalgia, myofascial pain, tender points and trigger points: splitting or lumping? Arthritis research & therapy. 2011;13(3):117. doi: 10.1186/ar3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz JN. Editorial: appropriateness of total knee arthroplasty. Arthritis Rheumatol. 2014;66(8):1979–81. doi: 10.1002/art.38688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh JA, Luo R, Landon GC, Suarez-Almazor M. Reliability and clinically important improvement thresholds for osteoarthritis pain and function scales: a multicenter study. The Journal of rheumatology. 2014;41(3):509–15. doi: 10.3899/jrheum.130609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arendt-Nielsen L, Graven-Nielsen T. Translational musculoskeletal pain research. Best practice & research. Clinical rheumatology. 2011;25(2):209–26. doi: 10.1016/j.berh.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(7):1043–56. doi: 10.1016/j.joca.2015.02.163. [DOI] [PubMed] [Google Scholar]
- 32.Schaible HG. Mechanisms of chronic pain in osteoarthritis. Current rheumatology reports. 2012;14(6):549–56. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]