Abstract
Tourette syndrome (TS) is a neurodevelopmental condition characterized by multiple, recurring motor and phonic tics. Rich empirical evidence shows that the severity of tics and associated manifestations is increased by several stressors and contextual triggers; however, the neurobiological mechanisms responsible for symptom exacerbation in TS remain poorly understood. This conceptual gap partially reflects the high phenotypic variability in tics, as well as the existing difficulties in operationalizing and standardizing stress and its effects in a clinical setting. Animal models of TS may be highly informative tools to overcome some of these limitations; these experimental preparations have already provided critical insights on key aspects of TS pathophysiology, and may prove useful to identify the neurochemical alterations induced by different stressful contingencies. In particular, emerging knowledge on the role of contextual triggers in animal models of TS may inform the development of novel pharmacological interventions to reduce tic fluctuations in this disorder.
Keywords: Tourette syndrome, tics, premonitory sensory phenomena, animal models, stress
1. Introduction
Tics are sudden, non-rhythmic, repetitive movements and vocalizations, corresponding to the activation of a discrete group of muscles. Both motor and phonic manifestations are characterized by a wide range of phenotypic variability and complexity (Jankovic, 1992): simple tics are brief and repetitive actions, such as nose wrinkling, eye blinking, sniffing or throat-clearing noises; conversely, complex tics engage multiple muscle groups in coordinated patterns, including touching objects or uttering phrases (Jankovic, 1992).
Another key diagnostic feature of tics is their partial controllability. Indeed, unlike other dyskinetic phenomena, tics can be delayed and camouflaged, even though they cannot be fully suppressed. Although the occasional presentation of tics should not be regarded as pathological, their recurrent and intense display can negatively impact social, educational and occupational functioning (Stokes et al., 1991; Conelea et al., 2013; Gutierrez-Colina et al., 2015), and is therefore classified as a tic disorder. The most severe among these nosological entities, Tourette syndrome (TS), is defined by the presence of multiple motor tics and at least one phonic tic, lasting for longer than one year and with an onset prior to 18 years of age. Once regarded as a rare disorder, TS is observed in ~0.5% of the pediatric population according to a recent meta-analysis (Scharf et al., 2015), even though previous studies estimated the international prevalence of the disorder at 1% (Robertson, 2008). About 86% of TS patients exhibit at least one comorbid neuropsychiatric condition (Hirschtritt et al., 2015); in particular, most TS patients are also diagnosed with attention-deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) (Ghanizadeh and Mosallaei, 2009). One of the most prominent features of TS is the high degree of variability in tic severity across different temporal stages of the disorder. Tics emerge in childhood (most typically around 6–7 years of age) and undergo a gradual escalation in intensity and frequency, often followed by a full or partial remission during puberty (Leckman et al., 1998); it is estimated, however, that approximately 24% of TS patients retain moderate and severe tics in adulthood (Goetz et al., 1992). In addition to these diachronic fluctuations, the baseline intensity and frequency of tics oscillate widely, reflecting the impact of multiple contextual and emotional factors. In particular, rich empirical evidence indicates that the severity of tics and associated symptoms increases in the presence of physical and psychological stress (Robertson et al., 2002; O’Connor et al., 2003; Eapen et al., 2004). Indeed, multiple surveys have shown an association between stressful life events and greater severity/earlier onset of tic disorders (Bornstein et al., 1990; Steinberg et al., 2013). This relationship was confirmed by longitudinal studies, which documented that cumulative psychosocial stress predicts for future severity of tics (Lin et al., 2007). On a shorter timescale, several studies have indicated a direct correlation between tic severity and self-report ratings of daily life stress (Findley et al., 2003), as well as negative small events in the same or preceding week (Hoekstra et al., 2004). For such a well-documented relationship, little is surprisingly known about the neurobiological and neurochemical mechanisms whereby tics are exacerbated by stress, and the specific pharmacological and behavioral interventions that may help prevent stress-induced aggravation of tics. An optimal, yet often undervalued tool to overcome these critical barriers is afforded by animal models of TS. The unique advantage of these preparations lies in the possibility to manipulate, standardize and analyze the neurobiological impact of specific stressors in the presence of experimental controls for each of the potential variables involved. In the present review, we will summarize the current knowledge on the effects of stress in TS, and indicate how animal models may help identify the neurobiological mechanisms whereby distinct modalities of contextual stress increase tic severity. In addition, we will overview potential experimental directions that may inform new therapies for the reduction of the impact of stress on TS.
2. Phenomenology and neurobiology of tics
2.1. Sensory antecedents of tics
Although the first clinical description of TS dates back to 1825, a more systematic characterization of the phenomenology and neurobiological basis of tics has been achieved only in the past few years. In particular, while these manifestations were long regarded as neurological entities, recent research has shown that they are the epiphenomenon of a much more complex behavioral sequence, rooted in premonitory sensory phenomena (PSP) (Houghton et al., 2014). PSP are uncomfortable sensations, typically characterized by a feeling of oversensitivity and aversion for specific interoceptive and exteroceptive stimuli, with a hyperattentive focus on somatic input (Cohen and Leckman, 1992; Houghton et al., 2014). The source of PSP is not traced back to alterations in sensory transmission efficiency (Belluscio et al., 2011), but may instead depend on perceptual processing dysfunctions. In particular, TS patients exhibit deficits in gating functions, which enable the filtering of irrelevant or redundant information (Castellanos et al., 1996; Sutherland-Owens et al., 2011). One of the best-described PSP is the premonitory urge, an intrusive somatic sensation of inner tension and discomfort typically relieved by the execution of tics (Cohen and Leckman, 1992; Leckman et al., 1993; Evers and van de Wetering, 1994). Although these phenomena are not consistently reported in childhood (Banaschewski et al., 2003), they are likely the main negative reinforcers of tics (Woods et al., 2008); indeed, most patients report that tics are primarily executed to alleviate the distress associated with premonitory urges (Lang, 1991; Kwak et al, 2003), and the temporary suppression of tics magnifies the intensity of premonitory urges (Himle et al., 2007).
2.2. Neurobiology of PSP and tics
Recent neuroimaging studies have revealed that tics and their sensory/psychological antecedents result from a sequence of activation patterns across the cortico-striatal-thalamic-cortical (CSTC) loop, a key pathway serving the integration of sensory and motor functions (for a full discussion on the neuroanatomical bases of TS, see Ganos et al., 2013). The functional regulation of the CSTC pathway is based on the integration of topographically segregated networks; in particular, the generation of saliency maps in these sub-circuits is based on “center-on, surround-off” contrasts, in which the stimulation of a network is paralleled by the silencing of competing responses from adjacent tracts via neighboring interneurons (for an overview of the neuronal correlates of these mechanisms in the cortex, see Helmstaedter et al., 2009).
Building on this framework, current interpretations explain PSP and tics as the result of an insufficient inhibitory tone (or excessive excitatory output) in both cortical and striatal areas of the CSTC loop, respectively. Specifically, oversensitivity to stimuli has been shown to reflect the excessive stimulation of the sensorimotor cortex (Wang et al., 2011; Biermann-Ruben et al., 2012); conversely, premonitory urges are rooted in the hyperactivity of the supplementary motor area (SMA), anterior cingulate cortex, insula and parietal operculum (Bohlhalter et al., 2006; Jackson et al., 2011; Wang et al., 2011; Neuner et al., 2014; Worbe et al., 2015). Finally, the negative emotional components associated with the urges appear to be underpinned by the activation of the amygdala (Wang et al., 2011). Some of these mechanisms have been recently confirmed by the finding that transcranial magnetic stimulation of the SMA produces urges and motor manifestations akin to tics (Finis et al., 2013). While the specific mechanisms underpinning the overactivation of these regions are still unclear, preliminary reports have documented reductions in GABAergic interneurons in the insula of TS patients (Vaccarino et al., 2013). Although these findings await confirmation with larger number of samples and different regions of the CSTC loop, they are in line with other evidence documenting that the GABA content in the somatosensory cortex of TS patients is low and negatively correlated with motor tic severity (Tinaz et al., 2014; Puts et al., 2015). Furthermore, intracortical inhibition has been found to be deficient in TS (Ziemann et al., 1997).
Along similar lines, several studies have documented the reduction and altered distribution of parvalbumin-positive GABAergic and cholinergic interneurons in the dorsal striatum of TS patients (Kalanithi et al., 2005; Kataoka et al., 2010), as well as decreased binding of GABA-A receptors in the striatum, globus pallidus, thalamus, amygdala and insula (Lerner et al., 2012). These changes may partially reflect metabolic dysfunctions of striatal interneurons, as suggested by transcriptomic alterations (Lennington et al., 2016). Deficits in interneurons and GABA-A receptors are posited to result in the formation of ectopic foci in the dorsal striatum (Mink, 2001; Albin and Mink, 2006). An excessive cortical and/or amygdalar output (corresponding to intense and discomforting PSP) would lead to the transient activation of these neuronal clusters, engendering tics through the stimulation of downstream pathways in the thalamus and primary motor cortex (Neuner et al., 2014). Recent findings indicate that the stimulation of the primary motor cortex may reduce the excitability of the somatosensory cortex (Enomoto et al., 2001; Seyal et al., 2005), providing a tentative mechanism accounting for the reduction in PSP following tic execution.
2.3. The role of dopamine in tics
Activation of the striatum is directed by dopamine, a key neurotransmitter in the regulation of motor output in the CSTC. The role of dopamine in TS is indicated by the anti-tic effects of dopamine receptor antagonists, such as haloperidol and pimozide (Roessner et al., 2013); furthermore, dopaminergic agonists have been shown to increase TS symptom severity (Shale et al., 1986). Differences in dopaminergic functions between TS patients and non-affected individuals have been shown by Steeves and colleagues (2010), who reported that the dopamine releaser amphetamine led to a significantly more widespread activation in several CSTC areas of TS patients. Although the exact contribution of dopamine in tic ontogeny is partially unclear, several authors have hypothesized that tics may reflect an excessive phasic release and reduced tonic transmission of dopamine in the basal ganglia (Wong et al., 2008; Buse et al., 2013), which could result in the activation of ectopic foci by virtue of volume-transmission mechanisms and activation of extrasynaptic dopamine receptors. This mechanism would imply a primary role of D1 receptors in TS pathophysiology, given that they are posited to mediate phasic dopamine signaling in the dorsal striatum and are predominantly extrasynaptic (Caillé et al., 1996; Dreyer et al., 2010). From this perspective, it is worth noting that D1 receptor antagonism has been recently found to have therapeutic efficacy in TS (Gilbert et al., 2014). Several other lines of research point to the involvement of D1 receptors: first, these receptors have been recently shown to regulate recurrent efferent feedback from the somatosensory cortex and facilitate detection of behavioral stimuli (Happel et al., 2014); second, activation of D1 receptors in the striatum is instrumental in the induction of striatal synaptic plasticity processes, such as long-term potentiation (Kerr and Wickens, 2001; Centonze et al., 2003), and has been shown to have a widespread facilitatory effect on the connectivity between thalamus and somatosensory cortex (Steiner and Kitai, 2001).
These mechanisms may be instrumental in the pathophysiology of TS by facilitating habit formation, a core adaptive process that appears to be hyperactive in TS (Delorme et al., 2016) and is also accelerated by D1 receptor activation (Nelson and Kilcross, 2013). In this respect, it should be noted that activity-dependent dopaminergic feedback appears to play a major role in the reinforcement of tics (Albin and Mink, 2006). Although these premises may apparently suggest that the neurobiological bases of TS are well-defined, it should be noted that this disorder may encompass multiple distinct entities with only partial mechanistic overlap. Initial attempts to qualify different subtypes of TS are ongoing (Grados et al., 2008; Eapen and Robertson, 2015), but little to no neurobiological information about the neurobiological underpinnings of phenotypic differences is currently available.
3. Animal models of TS
Rodent models of TS offer an interesting complementary experimental platform to investigate the neurobiological basis of tics. The main advantage of these preparations lies in the possibility to test for specific mechanistic hypotheses that may emerge from correlational clinical data, and identify the neurochemical bases of different symptoms. At the same time, due to the obvious neurobiological differences, animal models can only approximate the dynamic complexity of human pathophysiology. Furthermore, subjective phenomena cannot be interrogated in animals, and can only be investigated by means of indirect proxy endophenotypes based on overlapping neurobiological mechanisms.
Over the past few years, one of the most common approaches to model TS in rodents has been based on the development of genetic mutations found in TS patients. The first notable example of this strategy was the generation of mice with a nonsense mutation for the gene Slitrk1 (Katayama et al., 2010), following the identification of an association between the human gene and rare, familial forms of TS (Abelson et al., 2005). While these mouse mutants did not exhibit tic-like manifestations, they showed increased anxiety- and depression-like phenotypes (Katayama et al., 2010), which may be linked to the comorbidity between TS and these psychiatric conditions (Coffey et al., 2000; Hirschtritt et al., 2015). A similar approach was followed after the discovery of a rare mutation for the gene encoding histidine decarboxylase (HDC) in a TS pedigree (Ercan-Sencicek et al., 2010). Hdc-deficient mice exhibit elevated levels of dopamine in the striatum, as well as information-processing deficits akin to those observed in TS patients (Castellan-Baldan et al., 2014). While tic-like repetitive responses are not spontaneously exhibited by these mutants, they can be elicited by a challenge with the dopaminergic indirect agonist d-amphetamine or an acute stress (Castellan-Baldan et al., 2014; Xu et al., 2015a).
In this section, we will briefly examine how animal models are strengthening our neurobiological views on the mechanisms underpinning PSP and tics. For comprehensive reviews on animal models of TS and tic disorders, the interested reader is referred to Bronfeld et al. (2013a), Macrì et al. (2013) and Godar et al. (2014).
3.1. Model-based approaches to PSP
Animal models cannot properly reproduce PSP, given their subjective nature; thus, the most common approach to study these phenomena is based on indices of perceptual efficiency that may share common neurobiological substrates with PSP. In particular, great emphasis has been placed on sensorimotor gating, given the deficits in this domain documented in TS patients. The best-validated, cross-species paradigm for gating measurement is the prepulse inhibition (PPI) of the startle reflex, which represents the reduction of a startle response enabled by a weak signal immediately preceding the startling stimulus (Hoffman and Ison, 1980; Braff et al., 1992; Braff et al., 2001). Clinical reports have documented PPI disruptions in TS patients throughout different paradigms (Castellanos et al., 1996; Swerdlow et al., 2001). While PPI cannot be considered a direct index of PSP severity, it may serve as a phenomenological proxy for the information-processing impairments that underpin sensory antecedents of tics (Swerdlow, 2013).
Converging lines of research on the anatomical and psychophysiological bases of PPI have revealed that the substrates of this endophenotype largely overlap with those of TS (Swerdlow, 2013): first, in TS patients, PPI is correlated with activity of the superior parietal cortex and associated with deficits in the dorsal striatum (caudate) and other regions responsible for integration of somatosensory stimuli, such as the prefrontal cortex (Buse et al., 2016a); second, neuroimaging studies have shown that PPI and PSP are based on overlapping neuroanatomical circuits in the somatosensory cortex and caudate nucleus (Neuner et al., 2010; Wang et al., 2011; Zebardast et al., 2013); third, clinical and preclinical reports have documented that PPI is impaired by dopaminergic agonists (Braff et al., 2001; Geyer et al., 2001; Swerdlow et al., 2008), whereas PPI deficits are improved by dopaminergic antagonists (Braff et al., 2001).
An alternative strategy to study the neurobiology of PSP lies in the employment of animal models that reproduce the neurobiological correlates of these manifestations, such as the over-activation (or under-inhibition) of the somatosensory, insular and prefrontal cortex. Along these lines, a recent study showed that local infusion of low-dose picrotoxin (a GABA-A receptor antagonist) in the sensorimotor cortex of mice led to tic-like responses, associated with increased exploration, sniffing and paw-licking behaviors. These phenotypes underwent sensitization with repeated interventions, and were abrogated by local activation of GABA-A receptors in the dorsolateral striatum (Pogorelov et al., 2015). While these results cannot ultimately verify whether activation of the sensorimotor cortex is directly responsible for premonitory urges, the tight association with tic-like responses and generalized motor phenomena suggests that local disinhibition of the somatosensory cortex may indeed underlie PSP. Given that this region has inhibitory processes overlapping with PPI (Nakagawa et al., 2014), it would be interesting to verify whether pharmacological disinhibition of the somatosensory cortex would impair sensorimotor gating. It should be noted, however, that tactile, rather than acoustic PPI, may be best suited to capture these impairments, given the nature of the sensory information processed in this area. The notion that insufficient GABAergic inhibition in cortical areas may impair information processing is supported by multiple lines of preclinical evidence: for example, recent findings have demonstrated that maturation of GABAergic circuits in the insula are essential for sensory integration (Gogolla et al., 2014); furthermore, previous research has indicated that lesions of the medial prefrontal and entorhinal cortex disrupt acoustic PPI (Yee, 2000; Goto et al. 2002).
Some initial studies have taken advantage of optogenetic tools to show the behavioral consequences of the activation of corticostriatal connections. In particular, repeated hyperactivation of the connectivity between the orbitofrontal cortex and the ventromedial striatum produced a progressive, long-lasting increase in grooming compulsions, potentially indicating that repetitive motor manifestations may be the result of a chronic enhancement in the activity of select cortical regions (Ahmari et al., 2013). Of the currently available TS animal models, the one that perhaps best recapitulates the relevance of cortical hyperactivation in PSP is the D1CT-7 transgenic mouse, which was generated by the insertion of a cholera toxin subunit A1 gene behind the promoter of dopamine D1 receptor (Campbell et al., 1999; Nordstrom and Burton, 2002). This construct leads to the constitutive activation of Gs proteins in a subset of D1-positive neurons in the layers II and III of the somatosensory cortex, layer II of the piriform cortex, as well as the intercalated nucleus of the amygdala (Campbell et al., 1999). The activation of output neurons in these two regions is thought to reproduce some of the neural events that occur during premonitory urges and sensory phenomena (Nordstrom et al., 2015). Accordingly, D1CT-7 mice exhibit spontaneous tic-like manifestations, consisting in sudden axial jerks, which emerge during early neurodevelopmental stages and appear with a sexual dimorphism reminiscent of that observed in TS (Nordstrom and Burton, 2002). In addition to their high face validity, D1CT-7 mice show a high predictive validity for anti-tic therapies, including clonidine and haloperidol (Nordstrom and Burton, 2002). Further validation that the phenotypes of D1CT-7 may be relevant to the link between PSP and tics is provided by our recent results that these animals (but not their wild-type littermates) exhibited PPI deficits and exacerbations of their tic-like manifestations in response to a mild acute stress (Godar et al., 2016). These results indicate that the neuropotentiation of somatosensory cortex and amygdala may increase the predisposition to respond to stressful contextual triggers with tics.
3.2 Model-based approaches to tics
Given the differences in motor pattern organization between humans and rodents, it is extremely difficult to define the phenotypic equivalences of tics in non-primate animal models. In general, one of the most common experimental proxies for tics in experimental animals is provided by the repetitive motor responses induced by dopaminergic agonists. Activation of dopamine receptors in the dorsal striatum has been shown to result in repetitive responses, which are facilitated by deficits of cholinergic interneurons in the same area (Arnt, 1985; Arnt et al., 1988; Conti et al., 1997; Aliane et al., 2009; 2011; 2012). Notably, these phenomena have been shown to be responsive to most TS therapies across multiple species (Rotrosen et al, 1972; Asper et al., 1973; Dewey and Fibiger, 1983).
These behaviors have been traditionally described as “stereotypies”, in relation to their repetitive nature; however, this definition may be poorly suitable to a translational framework, given that, in humans, this term refers to a very specific type of behavioral phenomenon, characterized by invariant, highly rhythmic presentation, as well as lack of premonitory urges (Crosland et al., 2005; Singer, 2013). The clinical distinction between tics and stereotypies is so important that the DSM-5 has included the latter manifestations in the differential diagnostic criteria for tics.
The ambiguity in the current nomenclature is also problematic as it fails to distinguish between spontaneous, drug-induced and stress-induced repetitive behaviors, even though their neurophysiological mechanisms may be at least partially divergent (for a more detailed analysis of this issue, see Lewis and Kim, 2009). Taken together, these premises highlight the need for a more precise classification of repeated behaviors in animal models, also in consideration of the potentially relevance of each of these responses to multiple neuropsychiatric conditions in humans.
A different approach to model tics in animals is based on the generation of artificial “ectopic foci” in the striatum, resulting from either excess activation, or functional deficits in the inhibitory components, of this region. In particular, GABA-A receptor blockade in the dorsal striatum of rodents and primates has been found to elicit tic-like manifestations (McCairn et al., 2009; Bronfeld et al., 2013b). Bronfeld and colleagues (2013b) showed that the injection of the GABA-A receptor antagonist bicuculline in the striatum of rats; this experiment led to a somatotopically organized pattern of movements, which began as a jerk-like phenomenon, became gradually more pronounced, and finally decreased in intensity until reaching complete cessation. Notably, the temporal properties of tic expression in this model of focal disinhibition were recently found to be influenced by the frequency and amplitude of the stimulation of the motor cortex and the ensuing striatal activation (Israelashvili and Bar-Gad, 2015).
Overall, these findings lend strong support to the idea that local deficits in GABAergic interneurons may lead to tics through activation of ectopic foci, but also qualifies that fluctuations of tics in frequency and intensity may be under direct cortical control. These mechanisms have been implemented in the development of novel mouse models of TS based on the selective ablation of cholinergic and parvalbumin-positive, GABA-ergic interneurons (Xu et al., 2015b; Xu et al., 2016). These preparations aimed at studying the functional relevance of the lack of striatal interneurons in TS, as documented by post-mortem findings (Kalanithi et al., 2005; Kataoka et al., 2010). In both models, targeted deletion of either subpopulations of interneurons in the dorsal striatum predisposed to repetitive sniffing and grooming and sensitization to stress following short-term stress (Xu et al., 2015b; 2016).
Taken collectively, the evidence in animal models of TS indicate that local disinhibition in the somatosensory cortex and in the dorsal striatum predisposes to phenomena directly related to PSP and tics; however, these responses are directly triggered by acute stress, highlighting the critical role of this factor in eliciting and modulating the expression of TS symptoms. In the next section, we will explore this relationship in greater detail, and review the main lines of translational evidence from clinical and preclinical studies indicating the role and mechanistic bases of specific stressors in symptom fluctuations in TS.
4. Contextual and emotional triggers as exacerbating factors in TS
As noted in the previous sections, tics and PSP are exacerbated by various stressors, defined as environmental factors that force the organism to transiently operate beyond its physiological capacity. Similarly, psychological stressors increase the perceived gap between a challenging environmental contingency and the ability to successfully react to it (Lazarus, 1966). Irrespective of the nature of the stressor, the brain orchestrates an allostatic response, i.e., a complex series of autonomic, neuroendocrine, neuroimmune and behavioral changes aimed at re-establishing a homeostatic balance (McEwen, 2007; McEwen and Wingfield, 2010). In this context, homeostasis refers to the ability to maintain the organism within a narrow range of survival-promoting functional conditions. The phenomenological link between acute stress and exacerbation of PSP and tics has been the object of several studies. In particular, a number of recent meta-analyses have reviewed the current knowledge on the specific environmental triggers associated with tic exacerbation (Conelea and Woods, 2008; Caurin et al., 2014; Hoekstra et al., 2013), and generally confirmed that TS symptoms are worsened by several (but not all) modalities of acute stress. Nevertheless, the analysis of this relationship is complicated by a number of methodological and theoretical restrictions: first, the lack of an operationalized definition of acute stress in TS pathophysiology limits our ability to recognize which stressors are specifically able to exacerbate urges and trigger tics; second, the standardization of the role of stressors in TS is complicated by the vast heterogeneity of clinical presentations, socio-cultural background and experiential factors in patients; finally, while the execution of tics often reduces the stress associated with PSP, it can also compound the sensation of psychological stress, depending on the context. These limitations notwithstanding, certain generalizations can be made on the main contextual factors associated with rapid variations in TS symptom severity. The bulk of available clinical evidence indicates that tic fluctuations predominantly occur in relation to four types of partially overlapping scenarios: 1) high or low sensory stimulation; 2) anxiogenic situations; 3) frustrating and anger-inducing contingencies; 4) fatigue and sleep loss. The allostatic mechanisms enacted to withstand these stressors are likely to interact with the neurobiological mechanisms of TS. A synoptic diagram of the key areas that may be activated by different contextual trigger is presented in Table 1 (for details, see below). Strikingly, manipulations related to these contextual factors have also been shown to trigger or intensify TS-related phenotypes in animal models.
Table 1.
Synoptic table of the major hypothesized contextual triggers activating the key areas of the functional circuit responsible for Tourette Syndrome.
| Primary neuroanatomical substrate | Major hypothesized contextual triggers |
|---|---|
| Sensorimotor cortex/SMA | Overstimulation/Understimulation/Fatigue (?) |
| Prefrontal cortex and insula | Anxiety Fatigue/Sleep loss/Frustration |
| Amygdala | Fear/Anxiety |
| Striatum | Frustration/Sleep loss/Overstimulation (?) |
| Thalamus | Overstimulation/Understimulation (?)/Sleep loss |
4.1. Sensory over- or understimulation
Tics are generally exacerbated by conditions that entail either too much or too little sensory stimulation (generally classified as “boredom” by most patients). The most common scenarios associated with overstimulation include watching TV and playing videogames (Silva et al., 1995; Caurin et al., 2014; Barnea et al., 2016). Interestingly, engaging in multitasking activity has also been linked to tic exacerbation (O’Connor et al., 1994); this phenomenon may be interpreted as a consequence of overstimulation, but also reflect the greater difficulties encountered by TS and TS+ADHD patients in executive functions (Channon et al., 2003). The mechanisms underpinning tic exacerbation in response to overstimulation are still elusive but likely reflect an information overload contributed by gating deficits. From this perspective, it is worth noting that PPI responses elicited by monaural prepulses are greater than their binaural counterparts (Marsh et al., 1976; Hoffman and Ison, 1980; Kumari et al., 2005), probably indicating that sensory integration lowers filtering ability.
Low environmental stimulation is also likely to distort information processing, possibly by shifting attentive focus towards interoceptive stimuli and the pursuit of alternative goals (Bench and Lench, 2013). In keeping with this idea, boredom is associated with negative self-awareness (Seib and Vodanovich, 1998) and a greater risk for impulsivity (Watt and Vodanovich, 1992). While the association between boredom and tics has been shown in a small subset of TS patients in several studies (Robertson et al., 2002; Eapen et al., 2004), the neurobiological underpinnings of this link remain elusive. In agreement with clinical data, a moderate spatial confinement has been shown to worsen TS-related behaviors in animal models, particularly in the presence of other predisposing factors. For example, spatial restriction of the testing arena has been shown to increase repetitive responses induced by d-amphetamine (Fowler and Mahoney, 2010). Furthermore, our group recently showed that spatial confinement within a familiar environment (home cage) greatly exacerbates tic-like responses and causes PPI deficits in D1CT-7 mice (Godar et al., 2016). Interestingly, these phenomena were reduced by dopaminergic antagonists, as well as by clonidine, supporting that these behavioral manifestations are likely supported by enhancements in catecholamine neurotransmission (Godar et al., 2016).
From this perspective, it is worth noting that one of the best-validated stressors in the lab setting is physical restraint, an extreme situation of spatial restriction. This manipulation has been shown to induce PPI deficits in mice and rats (Conti and Printz, 2003; Guercio et al., 2014) as well as an initial increase of dopamine release in the ventral striatum (Puglisi-Allegra et al., 1991).
4.2. Anxiety
A host of studies have documented that feelings of anxiety increased the severity of tics (Bornstein et al., 1990; O’Connor et al., 1994; Silva et al., 1995) and premonitory urges (Rozenman et al., 2015) in TS patients. In particular, anxiety has been recently shown to trigger “tic attacks”, episodic bouts of severe, continuous and non-suppressible tics, in a subset of TS patients (Robinson and Hedderly, 2016). Although the detrimental impact of anxiety on TS pathophysiology is well supported, recent evidence indicates that this relationship may not be generalized to all anxiogenic contexts. For example, a recent experimental study has documented that the Trier social stress test reduces tic frequency, even though it enhances neuroendocrine and physiological indices of the stress response (Buse et al., 2016b). On the other hand, other studies reported that socialization and anticipation of social events are associated with tic exacerbation (Steinberg et al., 2013; Caurin et al., 2014; Himle et al., 2014). The relationship between anxiety-like responses and TS-related endophenotypes is also not consistently straightforward in animals. On one hand, fear-inducing experimental stimuli in the lab setting, such as a mild footshock, have failed to induce PPI deficits in experimental animals (Guercio et al., 2014). On the other hand, anxiogenic stimuli (such as sudden acoustic bursts or fear-conditioning procedures) were sufficient to trigger tic-like behaviors in animal models with reductions of striatal interneurons (Xu et al., 2015b; 2016).
These contrasting findings may signify that, while anxiety may be intrinsically insufficient to trigger TS-related deficits, it may synergize with other predisposing factors to increase these phenotypes. In particular, anxiety may trigger tics only in a subset of individuals with a greater proclivity to engage in externalizing, rather than internalizing responses (such as those with a comorbid diagnosis of ADHD or disruptive behavior disorder). A similar possibility has been examined to interpret the complex interplay existing between anxiety and reactive/defensive aggression (Bubier and Drabick, 2009; Neumann et al, 2010). Indeed, these two manifestations are often associated in children and share common risk factors, neuroendocrine correlates and temperamental styles (Bubier and Drabick, 2009). An alternative/complementary explanation may be that the association between anxiety and tics may be particularly relevant in situations of intense emotional tension. Indeed, Franklin and colleagues (2009) found that anxiety-spectrum constructs are negatively correlated with PPI. Furthermore, PPI deficits were found in relation to high-trait anxiety in college students (Duley et al, 2007) and panic disorder patients (Ludewig et al, 2002). Considering that the prevalence of anxiety disorders is high in TS patients (Coffey et al., 2000; Hirschtritt et al., 2015), it is possible that a subset of TS patients may over-react to specific stimuli, and that the ensuing exaggerated responses may lead to the exacerbation of PSP and tics. Irrespective of the specific contingencies, most available evidence supports the possibility that the contribution of anxiety to the severity of TS manifestations may be primarily contributed by the amygdala, the key region for the regulation of fear response. Indeed, TS patients display morphological and functional alterations of this region, including an increased volume (Peterson et al., 2007), as well as an elevated activity during tics (Wang et al., 2011) and exposure to emotional face expression (Neuner et al., 2010).
4.3. Frustration
Frustration can be conceptualized as the emotional response to the omission of a desired goal (Abler et al., 2005) or the absence of reinforcement to an expected reward (Amsel, 1992). Psychological experiences of frustration may generate anger and aggressive behaviors (Pawliczek et al., 2013). Aggression and conduct disorder has been frequently associated with TS (Comings and Comings, 1987; Wand et al., 1993; Dehning et al., 2015; Kano et al., 2015).
Although very few studies have examined the neural bases of frustration, a recent report has shown that brain activity patterns during frustration closely mirrored those reported in acute stress experiments, including the prefrontal cortex, insula, dorsal striatum and cingulate cortex (Bierzynska et al., 2016). In another neuroimaging study, frustration increased activity along the prefrontal cortex, insula, amygdala and midbrain (Yu et al., 2014), a network that closely aligns with rage circuitry (Panksepp, 2005). Although only a few studies have studied frustration in experimental animals, novel paradigms have been recently developed to study this construct in reference to operant tasks (Burokas et al., 2012). Notably, changes in the predictability of food delivery can increase frustration (Bassett and Buchanan-Smith, 2007) and have been linked to an increase in spontaneous repeated behaviors (Waitt and Buchanan-Smith, 2001). In addition, preliminary observations in our lab indicate that the frustration of a conditioned reward exacerbated tic-like responses in D1CT-7 mice. Future studies will be needed to verify how the application of operant paradigms to reproduce frustrating conditions may lead to stereotypies or PPI deficits in TS models.
4.4. Fatigue and sleep loss
Fatigue may be defined as exhaustion due to physical or mental exertion. Ample evidence has shown that fatigue and sleep loss (a key correlate of fatigue) increase tic severity in patients (Bornstein et al., 1990; Silva et al., 1995; Cohrs et al., 2001; Robertson et al., 2002; Eapen et al., 2004). In keeping with this possibility, several clinical reports have documented sleep abnormalities in TS patients (Kostanecka-Endress et al., 2003; Ghosh et al., 2014; Modafferi et al., 2016). While the mechanism linking sleep disturbances (and fatigue) to tics is still largely unclear, total sleep deprivation has been shown to disrupt PPI in both humans and rats (Frau et al., 2008; Petrovsky et al., 2014), and to increase repetitive responses induced by dopamine agonists in comparison with controls (Ferguson and Dement, 1969; Tufik et al., 1978). The main effect of fatigue on tics may be related to a reduced tolerance for some of the other contextual/emotional triggers. Accordingly, fatigue has been shown to reduce tolerance to frustration (Anitei et al., 2013).
The neurobiological bases of the exacerbation of PSP and tics in sleep-deprived patients likely reflects the disinhibition/enhancement of cortical excitability and alterations of thalamic feedback mechanisms (Gorgoni et al., 2014; Killgore et al., 2015), as well as the enhanced expression, binding and activity of dopamine receptors in the dorsal striatum (Tufik et al., 1978; Demontis et al., 1990; Nunes-Junior et al., 1994).
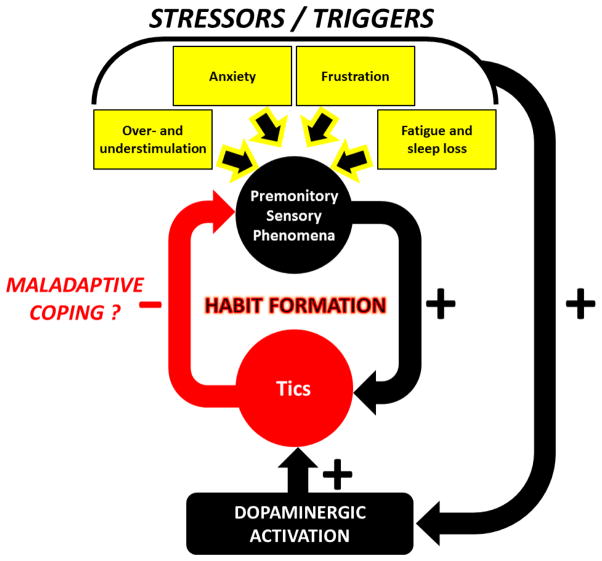
Taken together, clinical and animal findings support that urges and tics are increased by a number of specific stressors through different, yet inter-related mechanisms. Irrespective of the specific process, however, all these modalities lead to alterations of sensory processing in the cortex and greater likelihood of information overload, by either increasing the degree of stimulation or reducing the ability to filter sensory input. Emotional responses to certain types of anxiety, frustration and perhaps boredom also appear to increase the activation of specific areas in the CSTC circuitry, and ultimately facilitate the stimulation of the striatum. This mechanism may be specifically facilitated by dopamine release; the enhanced efflux of this neurotransmitter, which can be also contributed by acute stress, may help reduce the discomfort of the urges and facilitate the formation of maladaptive habits (Fig. 1). In the next section, we will discuss how neuroendocrine mechanisms of stress response may play a direct role in this process, and outline potential future directions to research this link and develop therapies that may reduce the impact of stress in TS pathophysiology.
Figure 1. Synoptic diagram of the hypothesized link between premonitory sensory phenomena and tics.
Stressors and contextual triggers increase the intensity of premonitory sensory phenomena and dopaminergic activity, ultimately leading to tic exacerbation. In turn, tic execution mitigates the intensity of premonitory sensory phenomena; this hypothetical coping process may potentially result in the formation of a maladaptive habit. See text for details.
5. Conclusions: neurobiological mechanisms and therapeutic perspectives
The physiological response to acute stress is largely mediated by the hypothalamic-pituitary-adrenal (HPA) axis, which enables the utilization of additional cognitive and energetic resources to mount an adaptive response to the stressor and regain homeostasis (McEwen, 2002; McEwen, 2007). The neuroendocrine effects of the HPA axis are initiated with the secretion of corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus, which in turn promotes the concatenated release of adrenocorticotropic hormone (ACTH) from the pituitary gland, and cortisol from the adrenal cortex. The rapid surge of these hormones is essential for stress coping; one of the mechanisms that mediate this action may be the increase in dopamine release following acute release of CRH and cortisol (Lemos et al., 2012; Vaessen et al., 2015). This increased dopamine efflux has been primarily documented in key regions of the mesocorticolimbic system, including the prefrontal cortex, nucleus accumbens and dorsal striatum (Abercrombie et al., 1989; Lavicky and Dunn, 1993; Hermans et al., 2014), and appears to be essential for stress resilience and coping (Cabib and Puglisi-Allegra, 2012; Pfau and Russo, 2015).
Once the stressor is no longer present, a fast recovery to baseline activity is ensured by feedback inhibition of CRH release from cortisol. Other endocrine factors are involved in the modulation of stress response, including neuropeptides (such as endogenous opioids, vasopressin and neuropeptide Y), neurosteroids and endocannabinoids. These neuromediators help balance the effects of the HPA axis to help counteract negative emotional consequences of negative emotional consequences that may be triggered by excess or chronic stress. For opioids, the interactions between CRH with enkephalins and certain neurosteroids (such as allopregnanolone or tetrahydrocorticosterone) are critical for the modulation of stress response (Gunn et al., 2011; Valentino and van Bockstaele, 2015).
5.1. What mechanisms may underpin the role of stress in tic fluctuations?
A number of studies have shown that TS patients exhibit greater HPA axis activation in response to acute stressors, with higher levels of CRH, ACTH and cortisol (Chappell et al., 1994; Chappell et al., 1996; Corbett et al., 2008). Notably, however, a strong, negative correlation was found between evening cortisol levels and tic severity (Corbett et al., 2008), leading to the possible interpretation that tics may have anxiolytic properties in TS patients. To contextualize these findings, it is worth noting that, particularly in adolescent and adult patients, tics are regarded as a response aimed at mitigating the discomfort associated with premonitory urges (Kwak et al., 2003).
Applying these concepts to our knowledge of the neurobiological machinery serving acute stress response and TS pathophysiology, the release of CRH and dopamine is likely to be transiently enacted with the goal of mitigating the stressful sensations associated with PSP. The discomforting effect of PSP appears to be compounded by specific contextual factors that could either increase their psychological burden (with a greater degree of cortical and amygdalar activation, such as in the case of overstimulation or high anxiety) or reduce the subjective ability to tolerate urges (as in the case of fatigue). Irrespective of the cause, the increase in cortical output and dopamine release in the striatum may lead to the activation of ectopic foci and the production of tics. From this perspective, tics may first occur as a by-product of these mechanisms, and then be progressively consolidated as a maladaptive coping response throughout the developmental trajectory of the disorder. This plastic adaptation would be enabled by negative-reinforcement mechanisms facilitated by D1 receptor stimulation in corticostriatal areas.
The involvement of CRH and dopamine in the enactment of defensive reactions may be also the distinguishing feature that may link only certain anxiogenic stimuli with tic exacerbation (see above). Indeed, dopamine has been shown to be instrumental for the enactment of defensive responses to acute stress (Cabib and Puglisi-Allegra, 2012). From a psychological standpoint, these premises suggest that anxiety and other negative emotional states may either aggravate or reduce tic severity in TS patients, depending on their coping style. Building on this notion, different subtypes of TS may lead to different reactions to contextual triggers, contingent on their profile of comorbid neuropsychiatric entities. For example, it is likely that patients with high levels of reactive aggression and impulsivity may exhibit a greater proclivity to display tic exacerbations in response to anxiogenic and frustrating stimuli; conversely, concurrent depressive/dysthymic symptoms (in which CRH and dopamine responses are blunted) may lead to tic reduction in the presence of certain contextual factors. Future studies will be necessary to verify how different coping modalities may predict for different fluctuations of tic severity in response to various contextual triggers. Furthermore, further research is warranted to ascertain how tic-like responses and information-processing impairments (including PPI deficits) in animal models of TS may be modified by environmental manipulations that increase either defensive reactivity (such as exposure to predator cues) or depression-like responses (such as chronic mild stress).
5.2. How can the role of stress in TS inform the development of novel therapies?
Another highly intriguing corollary of this conceptual framework is that, if tics are a component of stress-coping mechanisms in TS, these manifestations should be attenuated by therapies that reduce, rather than increase, the resilience to acute stress. In line with this concept, the main pharmacological strategy in TS therapy consists of dopaminergic antagonists, which are also known to suppress active defensive responses aimed at countering stress (Cabib and Puglisi-Allegra, 2012). Along the same lines, opioid antagonists have been generally shown to reduce the severity of tics (Sandyk and Awerbuch, 1989; Kurlan et al., 1991; van Wattum et al., 2000; but see Erenberg and Lederman, 1992 for contrasting findings). Finally, our group has shown that inhibition of neurosteroid synthesis by the 5α-reductase inhibitor finasteride leads to a marked reduction of tic severity in TS patients (Bortolato et al., 2007; Muroni et al., 2011); these findings were paralleled by the discovery that finasteride reduces PPI deficits and stereotypies induced by dopaminergic agonists in rats and mice, likely through the attenuation of D1 receptor signaling (Bortolato et al., 2008; Frau et al., 2013; 2016).
The development of pharmacological agents that target the interface between stressors and their neurobiological targets may be critical for the improvement of life quality in TS patients. The employment of animal models of TS may prove essential to enable the pursuit of this goal. In this respect, a particularly attractive direction may be to test the response of these models to behavioral paradigms based on relevant triggers, such as sleep deprivation, frustration of expected rewards, and sensory over- or understimulation. The refinement of these paradigms and experimental protocols may enable us to study the behavioral and neurobiological impact of key neuromodulators, as well as its relevance to multiple components of tic phenomenology. This approach may ultimately help tailor different pharmacological and behavioral therapeutic strategies for specific subtypes of TS patients.
Highlights.
Tourette syndrome (TS) patients exhibit stress-response abnormalities
Tics and premonitory sensory phenomena are worsened by several contextual trigger
Animal models can be employed to study the impact of stress on TS symptoms
The main categories of triggers in TS and their preclinical models are reviewed
Tics may be maladaptive coping responses to reduce the negative effects of stress
Acknowledgments
The present manuscript was partially supported by was supported by grants from the National Institute of Mental Health (NIH R01 MH104603) National Institute of General Medical Sciences (NIH P20 GM103638) and Tourette Syndrome Association (to MB). We are grateful to Dr. Simona Scheggi for her valuable assistance with bibliography compilation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, Mathews CA, Pauls DL, Rasin MR, Gunel M, Davis NR, Ercan-Sencicek AG, Guez DH, Spertus JA, Leckman JF, Dure LS, 4th, Kurlan R, Singer HS, Gilbert DL, Farhi A, Louvi A, Lifton RP, Sestan N, State MW. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. Journal of neurochemistry. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Abler B, Walter H, Erk S. Neural correlates of frustration. Neuroreport. 2005;16:669–672. doi: 10.1097/00001756-200505120-00003. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends in neurosciences. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Aliane V, Perez S, Bohren Y, Deniau JM, Kemel ML. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134:110–118. doi: 10.1093/brain/awq285. [DOI] [PubMed] [Google Scholar]
- Aliane V, Perez S, Deniau JM, Kemel ML. Raclopride or high-frequency stimulation of the subthalamic nucleus stops cocaine-induced motor stereotypy and restores related alterations in prefrontal basal ganglia circuits. The European journal of neuroscience. 2012;36:3235–3245. doi: 10.1111/j.1460-9568.2012.08245.x. [DOI] [PubMed] [Google Scholar]
- Aliane V, Perez S, Nieoullon A, Deniau JM, Kemel ML. Cocaine-induced stereotypy is linked to an imbalance between the medial prefrontal and sensorimotor circuits of the basal ganglia. The European journal of neuroscience. 2009;30:1269–1279. doi: 10.1111/j.1460-9568.2009.06907.x. [DOI] [PubMed] [Google Scholar]
- Amsel A. Frustration Theory: An Analysis of Dispositional Learning and Memory. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- Anitei M, Chraif M, Liliana M. Influence of fatigue on impulsiveness, aspiration level, performance motivation and frustration tolerance among young Romanian psychological students. Procedia - Social and behavioral sciences. 2013;78:630–634. [Google Scholar]
- Arnt J. Antistereotypic effects of dopamine D-1 and D-2 antagonists after intrastriatal injection in rats. Pharmacological and regional specificity. Naunyn-Schmiedeberg's archives of pharmacology. 1985;330:97–104. doi: 10.1007/BF00499901. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Meier E. Inactivation of dopamine D-1 or D-2 receptors differentially inhibits stereotypies induced by dopamine agonists in rats. European journal of pharmacology. 1988;155:37–47. doi: 10.1016/0014-2999(88)90400-1. [DOI] [PubMed] [Google Scholar]
- Asper H, Baggiolini M, Burki HR, Lauener H, Ruch W, Stille G. Tolerance phenomena with neuroleptics catalepsy, apomorphine stereotypies and striatal dopamine metabolism in the rat after single and repeated administration of loxapine and haloperidol. European journal of pharmacology. 1973;22:287–94. doi: 10.1016/0014-2999(73)90028-9. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Woerner W, Rothenberger A. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: Developmental aspects in children and adolescents. Developmental medicine and child neurology. 2003;45:700–703. doi: 10.1017/s0012162203001294. [DOI] [PubMed] [Google Scholar]
- Barnea M, Benaroya-Milshtein N, Gilboa-Sechtman E, Woods DW, Piacentini J, Fennig S, Apter A, Steinberg T. Subjective versus objective measures of tic severity in Tourette syndrome - The influence of environment. Psychiatry research. 2016;242:204–209. doi: 10.1016/j.psychres.2016.05.047. [DOI] [PubMed] [Google Scholar]
- Belluscio BA, Jin L, Watters V, Lee TH, Hallett M. Sensory sensitivity to external stimuli in Tourette syndrome patients. Movement disorders. 2011;26:2538–2543. doi: 10.1002/mds.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bench SW, Lench HC. On the function of boredom. Behavioral sciences. 2013;3:459–472. doi: 10.3390/bs3030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann-Ruben K, Miller A, Franzkowiak S, Finis J, Pollok B, Wach C, Sudmeyer M, Jonas M, Thomalla G, Muller-Vahl K, Munchau A, Schnitzler A. Increased sensory feedback in Tourette syndrome. NeuroImage. 2012;63:119–125. doi: 10.1016/j.neuroimage.2012.06.059. [DOI] [PubMed] [Google Scholar]
- Bierzynska M, Bielecki M, Marchewka A, Debowska W, Duszyk A, Zajkowski W, Falkiewicz M, Nowicka A, Strelau J, Kossut M. Effect of Frustration on Brain Activation Pattern in Subjects with Different Temperament. Frontiers in psychology. 2016;6:1989. doi: 10.3389/fpsyg.2015.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzman R, Hallett M. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Stefl ME, Hammond L. A survey of Tourette syndrome patients and their families: the 1987 Ohio Tourette Survey. The Journal of neuropsychiatry and clinical neurosciences. 1990;2:275–281. doi: 10.1176/jnp.2.3.275. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orru M, Bourov Y, Marrosu F, Mereu G, Devoto P, Gessa GL. Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology. 2008;33:3146–3156. doi: 10.1038/npp.2008.39. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Muroni A, Marrosu F. Treatment of Tourette's syndrome with finasteride. The American journal of psychiatry. 2007;164:1914–1915. doi: 10.1176/appi.ajp.2007.07060978. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of general psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Israelashvili M, Bar-Gad I. Pharmacological animal models of Tourette syndrome. Neuroscience and biobehavioral reviews. 2013a;37:1101–1119. doi: 10.1016/j.neubiorev.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Frontiers in systems neuroscience. 2013b;7:50. doi: 10.3389/fnsys.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubier JL, Drabick DA. Co-occurring anxiety and disruptive behavior disorders: the roles of anxious symptoms, reactive aggression, and shared risk processes. Clinical psychology review. 2009;29:658–669. doi: 10.1016/j.cpr.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A, Gutierrez-Cuesta J, Martin-Garcia E, Maldonado R. Operant model of frustrated expected reward in mice. Addiction biology. 2012;17:770–782. doi: 10.1111/j.1369-1600.2011.00423.x. [DOI] [PubMed] [Google Scholar]
- Buse J, Beste C, Herrmann E, Roessner V. Neural correlates of altered sensorimotor gating in boys with Tourette Syndrome: A combined EMG/fMRI study. World journal of biological psychiatry. 2016a;17:187–197. doi: 10.3109/15622975.2015.1112033. [DOI] [PubMed] [Google Scholar]
- Buse J, Enghardt S, Kirschbaum C, Ehrlich S, Roessner V. Tic Frequency Decreases during Short-term Psychosocial Stress - An Experimental Study on Children with Tic Disorders. Frontiers in psychiatry. 2016b;7:84. doi: 10.3389/fpsyt.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse J, Schoenefeld K, Munchau A, Roessner V. Neuromodulation in Tourette syndrome: dopamine and beyond. Neuroscience and biobehavioral reviews. 2013;37:1069–1084. doi: 10.1016/j.neubiorev.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neuroscience and biobehavioral reviews. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Campbell KM, de Lecea L, Severynse DM, Caron MG, McGrath MJ, Sparber SB, Sun LY, Burton FH. OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons. The Journal of neuroscience. 1999;19:5044–5053. doi: 10.1523/JNEUROSCI.19-12-05044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan-Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, Anderson GM, Loring E, Gorczyca R, Billingslea E, Wasylink S, Panza KE, Ercan-Sencicek AG, Krusong K, Leventhal BL, Ohtsu H, Bloch MH, Hughes ZA, Krystal JH, Mayes L, de Araujo I, Ding YS, State MW, Pittenger C. Histidine decarboxylase deficiency causes Tourette syndrome: parallel findings in humans and mice. Neuron. 2014 Jan 8;81(1):77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biological psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Caurin B, Serrano M, Fernandez-Alvarez E, Campistol J, Perez-Duenas B. Environmental circumstances influencing tic expression in children. European journal of paediatric neurology. 2014;18:157–162. doi: 10.1016/j.ejpn.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. The Journal of neuroscience. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S, Pratt P, Robertson MM. Executive function, memory, and learning in Tourette's syndrome. Neuropsychology. 2003;17:247–254. doi: 10.1037/0894-4105.17.2.247. [DOI] [PubMed] [Google Scholar]
- Chappell P, Leckman J, Goodman W, Bissette G, Pauls D, Anderson G, Riddle M, Scahill L, McDougle C, Cohen D. Elevated cerebrospinal fluid corticotropin-releasing factor in Tourette's syndrome: comparison to obsessive compulsive disorder and normal controls. Biological psychiatry. 1996;39:776–783. doi: 10.1016/0006-3223(95)00221-9. [DOI] [PubMed] [Google Scholar]
- Chappell P, Riddle M, Anderson G, Scahill L, Hardin M, Walker D, Cohen D, Leckman J. Enhanced stress responsivity of Tourette syndrome patients undergoing lumbar puncture. Biological psychiatry. 1994;36:35–43. doi: 10.1016/0006-3223(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Coffey BJ, Biederman J, Smoller JW, Geller DA, Sarin P, Schwartz S, Kim GS. Anxiety disorders and tic severity in juveniles with Tourette's disorder. Journal of the american academy of child and adolescent psychiatry. 2000;39:562–568. doi: 10.1097/00004583-200005000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette's syndrome. The journal of clinical psychiatry. 1992;53:319–323. [PubMed] [Google Scholar]
- Cohrs S, Rasch T, Altmeyer S, Kinkelbur J, Kostanecka T, Rothenberger A, Ruther E, Hajak G. Decreased sleep quality and increased sleep related movements in patients with Tourette's syndrome. Journal of neurology, neurosurgery, and psychiatry. 2001;70:192–197. doi: 10.1136/jnnp.70.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Comings BG. A controlled study of Tourette syndrome. II. Conduct. American journal of human genetics. 1987;41:742–760. [PMC free article] [PubMed] [Google Scholar]
- Conelea CA, Woods DW. The influence of contextual factors on tic expression in Tourette's syndrome: a review. Journal of psychosomatic research. 2008;65:487–496. doi: 10.1016/j.jpsychores.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Conelea CA, Woods DW, Zinner SH, Budman CL, Murphy TK, Scahill LD, Compton SN, Walkup JT. The impact of Tourette Syndrome in adults: results from the Tourette Syndrome impact survey. Community mental health journal. 2013;49:110–120. doi: 10.1007/s10597-011-9465-y. [DOI] [PubMed] [Google Scholar]
- Conti LH, Printz MP. Rat strain-dependent effects of repeated stress on the acoustic startle response. Behavioural brain research. 2003;144:11–18. doi: 10.1016/s0166-4328(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Conti LH, Segal DS, Kuczenski R. Maintenance of amphetamine-induced stereotypy and locomotion requires ongoing dopamine receptor activation. Psychopharmacology. 1997;130:183–188. doi: 10.1007/s002130050227. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza SP, Baym CL, Bunge SA, Levine S. Examining cortisol rhythmicity and responsivity to stress in children with Tourette syndrome. Psychoneuroendocrinology. 2008;33:810–820. doi: 10.1016/j.psyneuen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosland KA, Zarcone JR, Schroeder S, Zarcone T, Fowler S. Use of an antecedent analysis and a force sensitive platform to compare stereotyped movements and motor tics. American journal of mental retardation. 2005;110:181–192. doi: 10.1352/0895-8017(2005)110<181:UOAAAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dehning S, Burger MB, Krause D, Jobst A, Yundina E, Muller N, Meyer S, Zill P, Buchheim A. Tourette syndrome is associated with insecure attachment and higher aggression. The International journal of neuroscience. 2015;125:521–525. doi: 10.3109/00207454.2014.951040. [DOI] [PubMed] [Google Scholar]
- Delorme C, Salvador A, Valabregue R, Roze E, Palminteri S, Vidailhet M, de Wit S, Robbins T, Hartmann A, Worbe Y. Enhanced habit formation in Gilles de la Tourette syndrome. Brain. 2016;139:605–615. doi: 10.1093/brain/awv307. [DOI] [PubMed] [Google Scholar]
- Demontis MG, Fadda P, Devoto P, Martellotta MC, Fratta W. Sleep deprivation increases dopamine D1 receptor antagonist [3H]SCH 23390 binding and dopamine-stimulated adenylate cyclase in the rat limbic system. Neuroscience letters. 1990;117:224–227. doi: 10.1016/0304-3940(90)90148-3. [DOI] [PubMed] [Google Scholar]
- Dewey KJ, FIbiger HC. The effects of dose and duration of chronic pimozde administration on dopamine receptor supersensitivity. Naunyn Schmiedeberg's archives of pharmacology. 1983;322:261–70. doi: 10.1007/BF00508341. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. The journal of neuroscience. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen V, Fox-Hiley P, Banerjee S, Robertson M. Clinical features and associated psychopathology in a Tourette syndrome cohort. Acta neurologica Scandinavica. 2004;109:255–260. doi: 10.1046/j.1600-0404.2003.00228.x. [DOI] [PubMed] [Google Scholar]
- Eapen V, Robertson MM. Are there distinct subtypes in Tourette syndrome? Pure-Tourette syndrome versus Tourette syndrome-plus, and simple versus complex tics. Neuropsychiatric disease and treatment. 2015;11:1431–1436. doi: 10.2147/NDT.S72284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Ugawa Y, Hanajima R, Yuasa K, Mochizuki H, Terao Y, Shiio Y, Furubayashi T, Iwata NK, Kanazawa I. Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clinical Neurophysiology. 2001;112:2154–2158. doi: 10.1016/s1388-2457(01)00667-8. [DOI] [PubMed] [Google Scholar]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, Abbott T, Gupta A, King RA, Pauls DL, Tischfield JA, Heiman GA, Singer HS, Gilbert DL, Hoekstra PJ, Morgan TM, Loring E, Yasuno K, Fernandez T, Sanders S, Louvi A, Cho JH, Mane S, Colangelo CM, Biederer T, Lifton RP, Gunel M, State MW. L-histidine decarboxylase and Tourette's syndrome. New england journal of medicine. 2010;362:1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erenberg G, Lederman RJ. Naltrexone and Tourette's syndrome. Annals of neurology. 1992;31:574. doi: 10.1002/ana.410310521. [DOI] [PubMed] [Google Scholar]
- Evers RA, van de Wetering BJ. A treatment model for motor tics based on a specific tension-reduction technique. Journal of behavior therapy and experimental psychiatry. 1994;25:255–260. doi: 10.1016/0005-7916(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Ferguson J, Dement W. The behavioral effects of amphetamine on REM deprived rats. Journal of psychiatric research. 1969;7:111–118. doi: 10.1016/0022-3956(69)90016-8. [DOI] [PubMed] [Google Scholar]
- Findley DB, Leckman JF, Katsovich L, Lin H, Zhang H, Grantz H, Otka J, Lombroso PJ, King RA. Development of the Yale Children's Global Stress Index (YCGSI) and its application in children and adolescents ith Tourette's syndrome and obsessive-compulsive disorder. Journal of the american academy of child and adolescent psychiatry. 2003;42:450–457. doi: 10.1097/01.CHI.0000046816.95464.EF. [DOI] [PubMed] [Google Scholar]
- Finis J, Enticott PG, Pollok B, Munchau A, Schnitzler A, Fitzgerald PB. Repetitive transcranial magnetic stimulation of the supplementary motor area induces echophenomena. Cortex. 2013;49:1978–1982. doi: 10.1016/j.cortex.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Mahoney LP. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2010. The likelihood of expression of amphetamine-induced focused stereotypy in rats is higher in a smaller than in a larger observation arena. Program No. 270.13. 2010. Online. [Google Scholar]
- Franklin JC, Bowker KB, Blumenthal TD. Anxiety and prepulse inhibition of acoustic startle in a normative sample: the importance of a signal-to-noise ratio. Personality and individual differences. 2009;46:369–373. [Google Scholar]
- Frau R, Mosher LJ, Bini V, Pillolla G, Pes R, Saba P, Fanni S, Devoto P, Bortolato M. The neurosteroidogenic enzyme 5α-reductase modulates the role of D1 dopamine receptors in rat sensorimotor gating. Psychoneuroendocrinology. 2016;63:59–67. doi: 10.1016/j.psyneuen.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Orru M, Puligheddu M, Gessa GL, Mereu G, Marrosu F, Bortolato M. Sleep deprivation disrupts prepulse inhibition of the startle reflex: reversal by antipsychotic drugs. The international journal of neuropsychopharmacology. 2008;11:947–955. doi: 10.1017/S1461145708008900. [DOI] [PubMed] [Google Scholar]
- Frau R, Pillolla G, Bini V, Tambaro S, Devoto P, Bortolato M. Inhibition of 5α-reductase attenuates behavioral effects of D1-, but not D2-like receptor agonists in C57BL/6 mice. Psychoneuroendocrinology. 2013;38:542–551. doi: 10.1016/j.psyneuen.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganos C, Roessner V, Münchau A. The functional anatomy of Gilles de la Tourette syndrome. Neuroscience and biobehavioral reviews. 2013;37:1050–1062. doi: 10.1016/j.neubiorev.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Ghanizadeh A, Mosallaei S. Psychiatric disorders and behavioral problems in children and adolescents with Tourette syndrome. Brain & development. 2009;31:15–19. doi: 10.1016/j.braindev.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Rajan PV, Das D, Datta P, Rothner AD, Erenberg G. Sleep disorders in children with Tourette syndrome. Pediatric neurology. 2014;51:31–5. doi: 10.1016/j.pediatrneurol.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Budman CL, Singer HS, Kurlan R, Chipkin RE. A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome. Clinical neuropharmacology. 2014;37:26–30. doi: 10.1097/WNF.0000000000000017. [DOI] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Di Giovanni G, Bortolato M. Animal models of tic disorders: a translational perspective. Journal of neuroscience methods. 2014;238:54–69. doi: 10.1016/j.jneumeth.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Strathman HJ, Gochi AM, Jones CM, Fowler SC, Bortolato M. The D1CT-7 mouse model of Tourette syndrome displays sensorimotor gating deficits in response to spatial confinement. British journal of pharmacology. 2016;173:2111–2121. doi: 10.1111/bph.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tanner CM, Stebbins GT, Leipzig G, Carr WC. Adult tics in Gilles de la Tourette's syndrome: description and risk factors. Neurology. 1992;42:784–788. doi: 10.1212/wnl.42.4.784. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoni M, Ferlazzo F, Moroni F, D'Atri A, Donarelli S, Fanelli S, Gizzi Torriglia I, Lauri G, Ferrara M, Marzano C, Rossini PM, Bramanti P, De Gennaro L. Sleep deprivation affects somatosensory cortex excitability as tested through median nerve stimulation. Brain stimulation. 2014;7:732–739. doi: 10.1016/j.brs.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Goto K, Ueki A, Iso H, Morita Y. Reduced prepulse inhibition in rats with entorhinal cortex lesions. Behavioural brain research. 2002;134:201–207. doi: 10.1016/s0166-4328(02)00039-6. [DOI] [PubMed] [Google Scholar]
- Grados MA, Mathews CA Tourette Syndrome Association International Consortium for G. Latent class analysis of gilles de la tourette syndrome using comorbidities: clinical and genetic implications. Biological psychiatry. 2008;64:219–225. doi: 10.1016/j.biopsych.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guercio GD, Bevictori L, Vargas-Lopes C, Madeira C, Oliveira A, Carvalho VF, d'Avila JC, Panizzutti R. D-serine prevents cognitive defict induced by acute stress. Neuropharmacology. 2014;86:1–8. doi: 10.1016/j.neuropharm.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress. Frontiers in neuroscience. 2011;5:131. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Colina AM, Eaton CK, Lee JL, LaMotte J, Blount RL. Health-related quality of life and psychosocial functioning in children with Tourette syndrome: parent-child agreement and comparison to healthy norms. Journal of child neurology. 2015;30:326–332. doi: 10.1177/0883073814538507. [DOI] [PubMed] [Google Scholar]
- Happel MF, Deliano M, Handschuh J, Ohl FW. Dopamine-modulated recurrent corticoefferent feedback in primary sensory cortex promotes detection of behaviorally relevant stimuli. Journal of neuroscience. 2014;34:1234–1247. doi: 10.1523/JNEUROSCI.1990-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Sakmann B, Feldmeyer D. Neuronal correlates of local, lateral, and translaminar inhibition with reference to cortical columns. Cerebral cortex. 2009;19:926–937. doi: 10.1093/cercor/bhn141. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJ, Joels M, Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in neurosciences. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Himle MB, Capriotti MR, Hayes LP, Ramanujam K, Scahill L, Sukhodolsky DG, Wilhelm S, Deckersbach T, Peterson AL, Specht MW, Walkup JT, Chang S, Piacentini J. Variables Associated With Tic Exacerbation in Children With Chronic Tic Disorders. Behavior modification. 2014;38:163–183. doi: 10.1177/0145445514531016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himle MB, Woods DW, Conelea CA, Bauer CC, Rice KA. Investigating the effects of tic suppression on premonitory urge ratings in children and adolescents with Tourette's syndrome. Behaviour research and therapy. 2007;45:2964–2976. doi: 10.1016/j.brat.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, King RA, Sandor P, McMahon WM, Lyon GJ, Cath DC, Kurlan R, Robertson MM, Osiecki L, Scharf JM, Mathews CA Tourette Syndrome Association International Consortium for G. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA psychiatry. 2015;72:325–333. doi: 10.1001/jamapsychiatry.2014.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra PJ, Lundervold AJ, Lie SA, Gillberg C, Plessen KJ. Emotional development in children with tics: a longitudinal population-based study. European child & adolescent psychiatry. 2013;22:185–192. doi: 10.1007/s00787-012-0337-y. [DOI] [PubMed] [Google Scholar]
- Hoekstra PJ, Steenhuis MP, Kallenberg CG, Minderaa RB. Association of small life events with self reports of tic severity in pediatric and adult tic disorder patients: a prospective longitudinal study. The journal of clinical psychiatry. 2004;65:426–431. doi: 10.4088/jcp.v65n0320. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological review. 1980;87:175–189. [PubMed] [Google Scholar]
- Houghton DC, Capriotti MR, Conelea CA, Woods DW. Sensory Phenomena in Tourette Syndrome: Their Role in Symptom Formation and Treatment. Current developmental disorders reports. 2014;1:245–251. doi: 10.1007/s40474-014-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelashvili M, Bar-Gad I. Corticostriatal Divergent Function in Determining the Temporal and Spatial Properties of Motor Tics. The journal of neuroscience. 2015;35:16340–16351. doi: 10.1523/JNEUROSCI.2770-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SR, Parkinson A, Kim SY, Schuermann M, Eickhoff SB. Resolving confusions about urges and intentions. Cognitive neuroscience. 2011;2:252–257. doi: 10.1080/17588928.2011.618628. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Diagnosis and classification of tics and Tourette syndrome. Advances in neurology. 1992;58:7–14. [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proceedings of the national academy of sciences of the united states of america. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y, Kono T, Matsuda N, Nonaka M, Kuwabara H, Shimada T, Shishikura K, Konno C, Ohta M. The impact of tics, obsessive-compulsive symptoms, and impulsivity on global functioning in Tourette syndrome. Psychiatry research. 2015;226:156–161. doi: 10.1016/j.psychres.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. The journal of comparative neurology. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Yamada K, Ornthanalai VG, Inoue T, Ota M, Murphy NP, Aruga J. Slitrk1-deficient mice display elevated anxiety-like behavior and noradrenergic abnormalities. Molecular Psychiatry. 2010;15:177–184. doi: 10.1038/mp.2008.97. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. Journal of neurophysiology. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Vanuk JR, Knight SA, Markowski SM, Pisner D, Shane B, Fridman A, Alkozei A. Daytime sleepiness is associated with altered resting thalamocortical connectivity. Neuroreport. 2015;26:779–784. doi: 10.1097/WNR.0000000000000418. [DOI] [PubMed] [Google Scholar]
- Kostanecka-Endress T, Banaschewski T, Kinkelbur J, Wüllner I, Lichtblau S, Cohrs S, Rüther E, Woerner W, Hajak G, Rothenberger A. Disturbed sleep in children with Tourette syndrome: a polysomnographic study. Journal of psychosomatic Research. 2003;55:23–29. doi: 10.1016/s0022-3999(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Kumari V, Das M, Hodgins S, Zachariah E, Barkataki I, Howlett M, Sharma T. Association between violent behaviour and impaired prepulse inhibition of the startle response in antisocial personality disorder and schizophrenia. Behavioural brain research. 2005;158:159–166. doi: 10.1016/j.bbr.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Majumdar L, Deeley C, Mudholkar GS, Plumb S, Como PG. A controlled trial of propoxyphene and naltrexone in patients with Tourette's syndrome. Annals of neurology. 1991;30:19–23. doi: 10.1002/ana.410300105. [DOI] [PubMed] [Google Scholar]
- Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette's syndrome. Movement disorders. 2003;18:1530–1533. doi: 10.1002/mds.10618. [DOI] [PubMed] [Google Scholar]
- Lang A. Patient perception of tics and other movement disorders. Neurology. 1991;41:223–228. doi: 10.1212/wnl.41.2_part_1.223. [DOI] [PubMed] [Google Scholar]
- Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. Journal of neurochemistry. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Some principles of psychological stress and their relation to dentistry. Journal of dental research. 1966;45:1620–1626. doi: 10.1177/00220345660450060901. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. The american journal of psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim YS, Peterson BS. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, Huttner A, Pletikos M, Sestan N, Leckman JF, Vaccarino FM. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biological psychiatry. 2016;79:372–382. doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Simmons JM, Mari Z, Bonne O, Xu B, Kazuba D, Herscovitch P, Carson RE, Murphy DL, Drevets WC, Hallett M. Widespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndrome. Brain. 2012;135:1926–1936. doi: 10.1093/brain/aws104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Kim SJ. The pathophysiology of restricted repetitive behavior. Journal of neurodevelopmental disorders. 2009;1:114–32. doi: 10.1007/s11689-009-9019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Wang HS, Wong MC, Wu CT, Lin KL. Tourette's syndrome with cervical disc herniation. Brain & development. 2007;29:61–63. doi: 10.1016/j.braindev.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. Depression and anxiety. 2002;15:55–60. doi: 10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- Macrì S, Proietti Onori M, Laviola G. Theoretical and practical considerations behind the use of laboratory animals for the study of Tourette syndrome. Neuroscience and biobehavioral reviews. 2013;37:1085–1100. doi: 10.1016/j.neubiorev.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Marsh RR, Hoffman HS, Stitt CL. Eyeblink inhibition by monaural and binaural stimulation: one ear is better than two. Science. 1976;192:390–391. doi: 10.1126/science.1257776. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. The psychiatric clinics of north america. 2002;25:469–494. ix. doi: 10.1016/s0193-953x(01)00009-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. What is in a name? Integrating homeostasis, allostasis and stress. Hormones and behavior. 2010;57:105–11. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatric neurology. 2001;25:190–198. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- Modafferi S, Stornelli M, Chiarotti F, Cardona F, Bruni O. Sleep, anxiety and psychiatric symptoms in children with Tourette syndrome and tic disorders. European journal of paediatric neurology. 2016 doi: 10.1016/j.ejpn.2016.05.003. in press. [DOI] [PubMed] [Google Scholar]
- Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Movement disorders. 2011;26:2146–2147. doi: 10.1002/mds.23810. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Inui K, Yuge L, Kakigi R. Inhibition of somatosensory-evoked cortical responses by a weak leading stimulus. Neuroimage. 2014;101:416–424. doi: 10.1016/j.neuroimage.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Killcross S. Accelerated habit formation following amphetamine exposure is reversed by D1, but enhanced by D2, receptor antagonists. Frontiers in neuroscience. 2013;7:76. doi: 10.3389/fnins.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Frontiers in behavioral neuroscience. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I, Kellermann T, Stocker T, Kircher T, Habel U, Shah JN, Schneider F. Amygdala hypersensitivity in response to emotional faces in Tourette's patients. The world journal of biological psychiatry. 2010;11:858–872. doi: 10.3109/15622975.2010.480984. [DOI] [PubMed] [Google Scholar]
- Neuner I, Werner CJ, Arrubla J, Stocker T, Ehlen C, Wegener HP, Schneider F, Shah NJ. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Frontiers in human neuroscience. 2014;8:362. doi: 10.3389/fnhum.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom EJ, Bittner KC, McGrath MJ, Parks CR, 3rd, Burton FH. "Hyperglutamatergic cortico-striato-thalamo-cortical circuit" breaker drugs alleviate tics in a transgenic circuit model of Tourettes syndrome. Brain research. 2015;1629:38–53. doi: 10.1016/j.brainres.2015.09.032. [DOI] [PubMed] [Google Scholar]
- Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette's syndrome and obsessive-compulsive disorder circuitry. Molecular psychiatry. 2002;7:617–625. 524. doi: 10.1038/sj.mp.4001144. [DOI] [PubMed] [Google Scholar]
- Nunes-Júnior GP, Tufik S, Nobrega JN. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain research bulletin. 1994;34:453–456. doi: 10.1016/0361-9230(94)90018-3. [DOI] [PubMed] [Google Scholar]
- O'Connor KP, Gareau D, Blowers GH. Personal constructs associated with tics. The British journal of clinical psychology. 1994;33( Pt 2):151–158. doi: 10.1111/j.2044-8260.1994.tb01106.x. [DOI] [PubMed] [Google Scholar]
- O'Connor K, Brisebois H, Brault M, Robillard S, Loiselle J. Behavioral activity associated with onset in chronic tic and habit disorder. Behavior research and therapy. 2003;41:241–9. doi: 10.1016/s0005-7967(02)00051-7. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Consciousness and cognition. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Pawliczek CM, Derntl B, Kellermann T, Gur RC, Schneider F, Habel U. Anger under control: neural correlates of frustration as a function of trait aggression. PloS one. 2013;8:e78503. doi: 10.1371/journal.pone.0078503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, Liu J, Xu D, Bansal R. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Archives of general psychiatry. 2007;64:1281–1291. doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, Ettinger U, Hill A, Frenzel L, Meyhofer I, Wagner M, Backhaus J, Kumari V. Sleep deprivation disrupts prepulse inhibition and induces psychosis-like symptoms in healthy humans. Journal of neuroscience. 2014;34:9134–9140. doi: 10.1523/JNEUROSCI.0904-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau ML, Russo SJ. Peripheral and Central Mechanisms of Stress Resilience. Neurobiology of stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelov V, Xu M, Smith HR, Buchanan GF, Pittenger C. Corticostriatal interactions in the generation of tic-like behaviors after local striatal disinhibition. Experimental neurology. 2015;265:122–128. doi: 10.1016/j.expneurol.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain research. 1991;554:217–222. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- Puts NA, Harris AD, Crocetti D, Nettles C, Singer HS, Tommerdahl M, Edden RA, Mostofsky SH. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. Journal of neurophysiology. 2015;114:808–817. doi: 10.1152/jn.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. Journal of psychosomatic research. 2008;65:461–72. doi: 10.1016/j.jpsychores.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Banerjee S, Eapen V, Fox-Hiley P. Obsessive compulsive behaviour and depressive symptoms in young people with Tourette syndrome. A controlled study. European child & adolescent psychiatry. 2002;11:261–265. doi: 10.1007/s00787-002-0301-3. [DOI] [PubMed] [Google Scholar]
- Robinson S, Hedderly T. Novel Psychological Formulation and Treatment of "Tic Attacks" in Tourette Syndrome. Frontiers in pediatrics. 2016;4:46. doi: 10.3389/fped.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner V, Schoenefeld K, Buse J, Bender S, Ehrlich S, Munchau A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology. 2013;68:143–149. doi: 10.1016/j.neuropharm.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Rotrosen J, Wallach MB, Angrist B, Gershon S. Antagonism of apomorphine-induced stereotypy and emesis in dogs by thioridazine, haloperidol, and pimozide. Psychopharmacologia. 1972;26:185–94. doi: 10.1007/BF00422105. [DOI] [PubMed] [Google Scholar]
- Rozenman M, Johnson OE, Chang SW, Woods DW, Walkup JT, Wilhelm S, Peterson A, Scahill L, Piacentini J. Relationships between Premonitory Urge and Anxiety in Youth with Chronic Tic Disorders. Children's health care. 2015;44:235–248. doi: 10.1080/02739615.2014.986328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandyk R, Awerbuch G. Recurrence of complex motor and vocal tics in an elderly woman responsive to opiates. The international journal of neuroscience. 1989;44:317–320. doi: 10.3109/00207458908986209. [DOI] [PubMed] [Google Scholar]
- Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Movement disorders. 2015;30:221–228. doi: 10.1002/mds.26089. [DOI] [PubMed] [Google Scholar]
- Seib HM, Vodanovich SJ. Cognitive correlates of boredom proneness: the role of private self-consciousness and absorption. The journal of psychology. 1998;132:642–652. doi: 10.1080/00223989809599295. [DOI] [PubMed] [Google Scholar]
- Seyal M, Shatzel AJ, Richardson SP. Crossed inhibition of sensory cortex by 0.3 Hz transcranial magnetic stimulation of motor cortex. Journal of Clinical Neurophysiology. 2005;22:418–421. doi: 10.1097/01.WNP.0000176198.11079.AA. [DOI] [PubMed] [Google Scholar]
- Shale H, Fahn S, Mayeux R. Tics in a patient with Parkinson's disease. Movement disorders. 1986;1:79–83. doi: 10.1002/mds.870010111. [DOI] [PubMed] [Google Scholar]
- Silva RR, Munoz DM, Barickman J, Friedhoff AJ. Environmental factors and related fluctuation of symptoms in children and adolescents with Tourette's disorder. Journal of child psychology and psychiatry, and allied disciplines. 1995;36:305–312. doi: 10.1111/j.1469-7610.1995.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Singer HS. Motor control, habits, complex motor stereotypies, and Tourette syndrome. Annals of the new york academy of sciences. 2013;1304:22–31. doi: 10.1111/nyas.12281. [DOI] [PubMed] [Google Scholar]
- Steeves TD, Ko JH, Kideckel DM, Rusjan P, Houle S, Sandor P, Lang AE, Strafella AP. Extrastriatal dopaminergic dysfunction in tourette syndrome. Annals of neurology. 2010;67:170–181. doi: 10.1002/ana.21809. [DOI] [PubMed] [Google Scholar]
- Steinberg T, Shmuel-Baruch S, Horesh N, Apter A. Life events and Tourette syndrome. Comprehensive psychiatry. 2013;54:467–473. doi: 10.1016/j.comppsych.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Steiner H, Kitai ST. Unilateral striatal dopamine depletion: time-dependent effects on cortical function and behavioural correlates. The e uropean journal of neuroscience. 2001;14:1390–1404. doi: 10.1046/j.0953-816x.2001.01756.x. [DOI] [PubMed] [Google Scholar]
- Stokes A, Bawden HN, Camfield PR, Backman JE, Dooley JM. Peer problems in Tourette's disorder. Pediatrics. 1991;87:936–942. [PubMed] [Google Scholar]
- Sutherland-Owens AN, Miguel EC, Swerdlow NR. Sensory gating scales and premonitory urges in Tourette syndrome. The scientific world journal. 2011;11:736–741. doi: 10.1100/tsw.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR. Update: studies of prepulse inhibition of startle, with particular relevance to the pathophysiology or treatment of Tourette Syndrome. Neuroscience and biobehavioral reviews. 2013;37:1150–1156. doi: 10.1016/j.neubiorev.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Breier M, Mora AB, Ko D, Shoemaker JM. A novel rat strain with enhanced sensitivity to the effects of dopamine agonists on startle gating. Pharmacology, biochemistry, and behavior. 2008;88:280–290. doi: 10.1016/j.pbb.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette's syndrome: in search of an "fMRI-friendly" startle paradigm. Biological psychiatry. 2001;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Belluscio BA, Malone P, van der Veen JW, Hallett M, Horovitz SG. Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging. Human brain mapping. 2014;35:5834–5846. doi: 10.1002/hbm.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufik S, Lindsey CJ, Carlini EA. Does REM sleep deprivation induce a supersensitivity of dopaminergic receptors in the rat brain? Pharmacology. 1978;16:98–105. doi: 10.1159/000136753. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Kataoka-Sasaki Y, Lennington JB. Cellular and molecular pathology in Tourette syndrome. In: Martino D, Leckman JF, editors. Tourette Syndrome. Oxford University Press; 2013. pp. 205–220. [Google Scholar]
- Vaessen T, Hernaus D, Myin-Germeys I, van Amelsvoort T. The dopaminergic response to acute stress in health and psychopathology: A systematic review. Neuroscience and biobehavioral reviews. 2015;56:241–251. doi: 10.1016/j.neubiorev.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Endogenous Opioids: The Downside of Opposing Stress. Neurobiology of stress. 2015;1:23–32. doi: 10.1016/j.ynstr.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wattum PJ, Chappell PB, Zelterman D, Scahill LD, Leckman JF. Patterns of response to acute naloxone infusion in Tourette's syndrome. Movement disorders. 2000;15:1252–1254. doi: 10.1002/1531-8257(200011)15:6<1252::aid-mds1030>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. The American journal of psychiatry. 2011;168:1326–1337. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt JD, Vodanovich SJ. Relationship between boredom proneness and impulsivity. Psychological reports. 1992;70:688–690. doi: 10.2466/pr0.1992.70.3.688. [DOI] [PubMed] [Google Scholar]
- Wong TT, Chen YW, Guo WY, Chang KP, Ho DM, Yen SH. Germinoma involving the basal ganglia in children. Child's nervous system. 2008;24:71–78. doi: 10.1007/s00381-007-0495-2. [DOI] [PubMed] [Google Scholar]
- Woods DW, Piacentini JC, Chang S, Deckersbach T, Ginsburg GS, Peterson AL, Scahill LD, Walkup JT, Wilhelm S. Managing Tourette syndrome: A behavioral intervention for children and adults. New York, NY: Oxford University Press; 2008. [Google Scholar]
- Worbe Y, Marrakchi-Kacem L, Lecomte S, Valabregue R, Poupon F, Guevara P, Tucholka A, Mangin JF, Vidailhet M, Lehericy S, Hartmann A, Poupon C. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain. 2015;138:472–482. doi: 10.1093/brain/awu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kobets A, Du JC, Lennington J, Li L, Banasr M, Duman RS, Vaccarino FM, DiLeone RJ, Pittenger C. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proceedings of the national academy of sciences of the united states of america. 2015b;112:893–898. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Ohtsu H, Pittenger C. Histidine decarboxylase knockout mice, a genetic model of Tourette syndrome, show repetitive grooming after induced fear. Neuroscience letters. 2015a;595:50–53. doi: 10.1016/j.neulet.2015.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–329. doi: 10.1016/j.neuroscience.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK. Cytotoxic lesion of the medial prefrontal cortex abolishes the partial reinforcement extinction effect, attenuates prepulse inhibition of the acoustic startle reflex and induces transient hyperlocomotion, while sparing spontaneous object recognition memory in the rat. Neuroscience. 2000;95:675–689. doi: 10.1016/s0306-4522(99)00441-8. [DOI] [PubMed] [Google Scholar]
- Yu R, Mobbs D, Seymour B, Rowe JB, Calder AJ. The neural signature of escalating frustration in humans. Cortex. 2014;54:165–178. doi: 10.1016/j.cortex.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Zebardast N, Crowley MJ, Bloch MH, Mayes LC, Wyk BV, Leckman JF, Pelphrey KA, Swain JE. Brain mechanisms for prepulse inhibition in adults with Tourette syndrome: initial findings. Psychiatry research. 2013;214:33–41. doi: 10.1016/j.pscychresns.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: evidence from transcranial magnetic stimulation. The american journal of psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]