Abstract
Several studies have suggested that activation of signal transducer and activator of transcription 3 (STAT3) is associated with initiation, progression and metastasis of numerous types of malignancy. However, the role of the Janus kinase-interleukin 6-STAT3 signaling pathway in the pathogenesis and recurrence of meningiomas is unknown. The present study evaluated STAT3 activation by western blotting and immunohistochemistry and assessed its association with Ki-67 labeling in 13 cases of meningioma in which frozen tissue and ≥5.5-year follow-up information were available, and in formalin-fixed meningioma tissues from 14 cases with an 8.4-year follow-up. The results of the western blot analysis indicated that STAT3 phosphorylation was markedly higher in grade II meningiomas compared with that in grade I, with mean densitometric values of 8.6 and 1.7 following normalization to actin, respectively. High STAT3 phosphorylation/activation was identified in 2 of 3 recurrent World Health Organization (WHO) grade I meningiomas and 0 of 3 non-recurrent meningiomas. Strong STAT3 phosphorylation/activation signal was also found in 2 of 4 recurrent grade II meningiomas and 1 of 3 non-recurrent cases. According to the immunohistochemistry results, phospho-STAT3 was not increased in WHO grade II tumors compared with that in grade I tumors, and was not significantly different between recurrent and non-recurrent cases. Ki-67 labeling was significantly increased in grade II vs. grade I tumors, and was also significantly increased in recurrent compared with non-recurrent grade I meningiomas. The results of the current study suggest that, while detection of phosphorylated/activated STAT3 may be useful in isolated cases, identifying activation may be of little value in predicting recurrence.
Keywords: meningiomas, phosphorylated signal transducer and activator of transcription 3, meningioma progression, meningioma recurrence, Ki-67
Introduction
Previous studies by our group and others have identified several key signaling pathways that are activated in meningiomas, including the mitogen-activated protein kinase (MAPK) kinase kinase-MAPK/extracellular signal-regulated kinase (ERK) kinase (MEK1)-p44/42 MAPK cascade and the phosphoinositide 3-kinase (PI3K)-protein kinase B (PKB/Akt)-mammalian target of rapamycin (mTOR) and JAK1-STAT3 pathways, which regulate the proliferation of meningiomas (1,2,3–5) and appear to be activated, in part, by components of the cerebrospinal fluid (6) and/or paracrine effects that activate receptor/kinases, such as the epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) receptor/kinases, in meningiomas (6–10).
Recurrence within 5 years of gross total resection occurs in ~12% of World Health Organization (WHO) grade I meningiomas, while 19% recur within 10 years (11,12). For intracranial WHO grade II tumors at all sites, the overall recurrence rate is 29–40% within 5 years of gross total resection (13). The ability to predict early recurrence of meningiomas would facilitate optimal postoperative management, including frequency of surveillance scanning and possible early radiotherapy (14–16). Tumor location, grade, Ki-67 labeling, genetic mutations and angiogenic factors have predictive value (11,13,17–19). Activation of the growth-regulatory kinase cascade may also be associated with recurrence (8,20). However, the identity and role of these pathways in the early recurrence of WHO and Simpson grade I/II meningiomas have not yet been established. Previous studies have demonstrated that the MAPK kinase (Raf-1)-MEK1-p44/42 MAPK/ERK cascade and the PI3K-PKB/Akt-mTOR and JAK1-STAT3 pathways are variably activated in certain grades of meningiomas (1–3,9,20,21); however, whether this activation of any given pathway is a predictor of recurrence remains unknown. The present study evaluated whether phosphorylation/activation of STAT3 is a predictor of recurrence.
Materials and methods
Meningioma tissue
Frozen tissue from 13 Simpson resection grade I or II meningiomas, including 6 grade I and 7 grade II meningiomas, were collected following intraoperative diagnosis (by Dr Mahlon Johnson) at the University of Rochester Medical Center, with Institutional Review Board approval from April 2007 to September 2009. The mean duration of follow-up was 5.4 years. In addition, formalin-fixed, paraffin-embedded tissues from 14 meningiomas were collected, including 5 WHO grade I and 9 WHO grade II meningiomas, with a mean follow-up for non-recurrences of ≥6 years (mean, 8.4 years) and less for recurrences (mean, 4.9 years). Patient and tumor characteristics are listed in Tables I and II.
Table I.
Patient and tumor characteristics for meningiomas assessed by western blotting for activation/phosphorylation of STAT3, and by immunohistochemistry for Ki-67 labeling.
| Patient no. | Age (years)/gender | Location | Simpson grade | Subtype | WHO grade | Recurrence | Approximate follow-up time (years) | Ki-67 index (%) | Relative phospho-STAT3 expression |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/F | R parietal | I | Fibrous | I | No | 6 | 2 | 0.43 |
| 2 | 35/F | R temporal | I | Mixed | I | No | 6 | 5 | 0.29 |
| 3 | 38/F | R temporal | I | Transitional | I | No | 6 | 2 | 0.02 |
| 4 | 73/F | L frontal | I | Meningothelial | I | Yes | 5 | 5 | 5.4 |
| 5 | 63/F | Posterior fossa | I | Transitional | I | Yes | 6 | 6 | 0.25 |
| 6 | 67/F | Posterior fossa | II | Transitional | I | Yes | 6 | 7 | 43.18 |
| 7 | 14/M | Frontal | II | Fibrous | II | Yes | 6 | 8 | 4.6 |
| 8 | 77/F | L frontal | I | Meningothelial | II | Yes | 4 | 13 | 6.26 |
| 9 | 53/M | R frontal | I | Mixed | II | Yes | 5 | 13 | 0.46 |
| 10 | 43/M | Thoracic | II | Transitional | II | No | 6 | 12 | 11.75 |
| 11 | 51/F | R frontal | I | Transitional | II | No | 5 | 15 | 0.35 |
| 12 | 54/F | L spine | II | Fibrous | II | No | 5 | 25 | 1.15 |
| 13 | 91/F | L frontal | I | Meningothelial | II | Yes | 4 | 16 | 1.29 |
STAT3, signal transducer and activator of transcription 3; F, female; M, male; R, right; L, left; WHO, World Health Organization.
Table II.
Patient and tumor characteristics for meningiomas assessed by immunohistochemistry for activation/phosphorylation of STAT3 and Ki-67 labeling.
| Patient no. | Age (years)/gender | Location | Simpson grade | Subtype | WHO grade | Recurrence | Follow-up time | Ki-67 index (%) | Phospho-STAT3 immunoreactivity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/F | R frontal | I | Meningothelial | I | No | 8 yrs 6 mo | 3 | 0 |
| 2 | 74/F | L frontal | I | Meningothelial | I | No | 8 yrs | NA | 0 |
| 3 | 49/F | Post fossa | II | Fibrous | I | No | 8 yrs 4 mo | 5 | 0 |
| 4 | 53/M | Cervical | II | Psammomatous | I | No | 6 yrs | 0 | 1 |
| 5 | 46/F | R temporal | I | Transitional | I | No | 6 yrs | 1 | 0 |
| 6 | 58/F | Convexity | II | Meningothelial | II | Yes | 7 mo | 9 | 1 |
| 7 | 74/M | R frontal | I | Meningothelial | II | Yes | 8 mo | 8 | 0 |
| 8 | 14/M | L hemisphere | II | Fibrous | II | Yes | 30 mo | 14 | 1 |
| 9 | 72/M | R hemisphere | II | Meningothelial | II | Yes | 1 yr | 17 | 0 |
| 10 | 19/M | Sphenoid | II | Transitional | II | No | 11 yrs | 7 | 1 |
| 11 | 66/F | R frontoparietal | I | Mixed | II | No | 5.5 yrs | 14 | 0 |
| 12 | 50/M | R frontal | I | Meningothelial | II | Yes | 3 yrs | 14 | 0 |
| 13 | 59/M | L frontal | II | Meningothelial | II | Yes | 4 yrs | 15 | 1 |
| 14 | 11/M | R frontoparietal | II | Transitional | II | No | 14 yrs | 5 | 0 |
STAT3, signal transducer and activator of transcription 3; F, female; M, male; R, right; L, left; WHO, World Health Organization; mo, months; NA, not assessed; yrs, years.
Simpson grading and postoperative follow-up imaging
Cases were assigned a Simpson grade based on operative notes (22). Follow up magnetic resonance imaging reports by neuroradiologists and clinical notes were reviewed to identify recurrence. Recurrence was considered not present if operative notes identified Simpson grade I or II and available initial postoperative images revealed no tumor (22) (Tables I and II).
Immunohistochemical analysis of phospho-STAT3 and Ki-67 labeling
For immunohistochemistry, a representative formalin-fixed, paraffin-embedded tissue block from each case was analyzed with monoclonal antibodies against phospho-STAT3 phosphorylated at tyrosine 705 (catalog no. 9145; Cell Signaling Technology, Inc., Beverly, MA, USA; dilution, 1:50) and Ki-67 (Dako, Carpinteria, CA, USA; dilution, 1:100; cat. no. M7240), or diluent control followed by pre-diluted MACH4 Universal HRP-polymer (BioCare Medical, Concord, CA, USA). The slides were incubated with primary antibody overnight in humid chambers at room temperature, and then incubated for one hour with the secondary probe at room temperature. Ki-67 immunohistochemistry was performed on a Dako automated immunostainer with a 3 h primary incubation at room temperature. For antigen retrieval, used with both antibodies, tissue sections were incubated in a thermoresistant chamber with 10X Reveal Decloaker (Biocare Medical, Concord, CA, USA) at 120–123°C and pressure of 20–24 psi for 45 min. Immunoreactivity for STAT3 in the nucleus was graded as follows: 0, undetectable; 1, positive in 1–20% of cells; 2, positive in 20–40% of cells; 3, positive in >40% of cells. Ki-67 labeling was determined as a percentage by a manual count.
Western blot analysis of the expression of phosphorylated/activated STAT3 in meningioma tissues
For western blot analysis, meningioma lysates were obtained by mechanical homogenization in Upstate RIPA Lysis Buffer (EMD Millipore, Billerica, MA, USA) using a 1:100 Protease Inhibitor Cocktail (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and then frozen at −85°C. Lysates from the tumors (30 mg protein each) were separated with 7.5% SDS-PAGE and transferred to nitrocellulose membranes, blocked with blotting-grade blocker (Bio-Rad Laboratories, Inc., Hercules, CA, USA), using a semi-dry transfer apparatus (Mini Protean tetra Cell 2 gel system; Bio-Rad Laboratories, Inc.). Western blots were analyzed with monoclonal antibodies against phospho-STAT3 phosphorylated at tyrosine 705 (dilution, 1:4,000; #9145; Cell Signaling Technology, Inc.) incubated whilst rocking at 4°C overnight, followed by secondary goat anti-rabbit HRP (dilution, 1:1,000; #170-6515; Bio-Rad Laboratories, Inc.) for 1 h at room temperature. Loading was assessed with an antibody against β-actin (#4967; Cell Signaling Technology, Inc.). To evaluate the results, the majority of western blots were repeated a second time. Band intensity was normalized relative to actin using the Image Lab for Chemidoc version 5.1 build 8 (Bio-Rad Laboratories, Inc.). Bands were designated as negative, weak if the STAT3:Actin ratio was ≤1 or positive if >1.
Statistical analysis
Normalized values for recurrent vs. non-recurrent WHO grade I or II, recurrent grade I vs. grade II and non-recurrent grades I and II were evaluated by t-tests. Statistical analysis was performed using Microsoft Excel (version 2010; Microsoft Corporation, Redmond, WA, USA). P<0.05 was considered to indicate a significant difference.
Results
Analysis of phospho-STAT3 in meningioma tissues
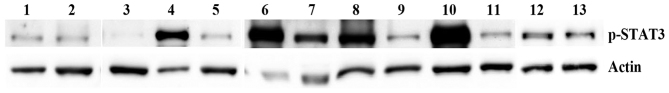
The results of the western blot analysis are summarized in Table I and presented in Fig. 1. In the 7 grade I meningiomas, phospho-STAT3 was weak in 4 cases and strong in 3 cases. In the 6 grade II meningiomas, phopsho-STAT3 was weak in 2, moderate in 2 and strong in 2 cases. The mean value of phospho-STAT3 in non-recurrent tumors (n=7) was 2.65 band intensity relative to actin. For recurrent tumors (n=6), the mean value was 9.49. Grade I tumors had a mean Ki-67 labeling index of 4.5 overall and 3 or 6 in non-recurrent or recurrent tumors respectively. In grade II, the Ki-67 index was 14.6 overall, with 17.3 and 12.8 in non-recurrent and recurrent cases, respectively. Overall the grade II tumors had a significantly higher Ki-67 labeling index percentage compared with grade I tissues, according to analysis using t-tests.
Figure 1.
Western blot analysis of p-STAT3 in non-recurrent and recurrent meningiomas. World Health Organization grade I (lanes 1–6) and grade II (lanes 7–13) meningiomas were analyzed for the expression of p-STAT3. Meningiomas 4–9 and 13 were recurrent. Actin was used as a loading control. The mean value for phospho-STAT3 was higher, but variable, in recurrent tumors as compared with non-recurrent tumors, and consequently not statistically significant. p-STAT3, phosphorylated/activated signal transducer and activator of transcription 3.
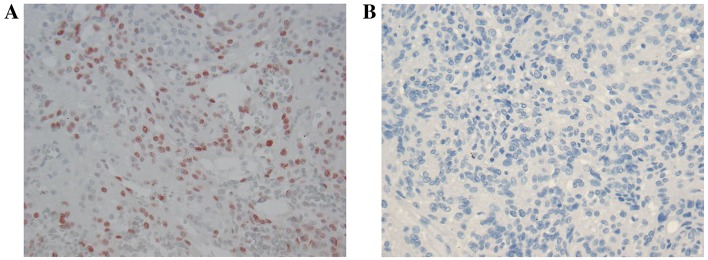
In cases used only for immunohistochemistry, none of the WHO grade I meningiomas were recurrent. Limited phospho-STAT3 immunoreactivity was identified in 1/4 cases. In the grade II tumors, phospho-STAT3 immunoreactivity was 0.33 in non-recurrent cases and 0.5 for recurrent tumors (Table II; Fig. 2); however, this was not significantly different.
Figure 2.
Phospho-STAT3 immunoreactivity in WHO grade II meningioma. (A) Phospho-STAT3 immunoreactivity in a WHO grade II meningioma exhibited limited nuclear staining (red) compared with (B) the negative control. Original magnification, ×400; hematoxylin counterstain. WHO, world health organization.
For formalin-fixed tissue, including those tumors with frozen tissue analyzed by western blotting, and those with only formalin-fixed tissue, WHO grade I meningiomas without and with recurrence had mean Ki-67 labeling indexes of 2.87 and 6.00, respectively, which were significantly different (P=0.007). WHO grade II meningiomas without and with recurrence had mean Ki-67 labeling indexes of 13.00 and 12.70%, respectively; this difference was not significant.
Discussion
As STAT3 regulates cell proliferation and apoptosis, it was hypothesized that it may be more selectively activated in recurrent, as compared with in non-recurrent, WHO grade I and/or II meningiomas. Furthermore, the phosphorylation/activation of STAT3 may serve as a marker for recurrence, particularly in postoperative years 1–6. In the present study, intense phosphorylation/activation of STAT3 was more common in recurrent meningiomas compared with non-recurrent cases; this may have been underestimated due to the short follow-up time in some of the non-recurrent meningiomas with weak activation. By immunohistochemistry, activation/phosphorylation of STAT3 was identified to be significantly higher in recurrent compared with non-recurrent meningiomas. Thus, although preliminary, these findings suggest the possibility that high levels of STAT3 combined with other clinical and molecular markers may reliably predict meningioma recurrence.
The present study identified extensive STAT3 phosphorylation/activation more frequently in recurrent meningiomas. These findings are consistent with the known effects of STAT3 activation on cell proliferation, apoptosis and angiogenesis (20,23–25). In a previous study, we demonstrated that cerebrospinal fluid stimulated the phosphorylation/activation of STAT3 in grade I/II primary meningioma cell cultures (6). Furthermore, our previous study revealed that the mitogenic effects of cerebrospinal fluid on WHO grade I and II meningioma cells appear to be exerted, in part, via the activation of STAT3 (6). In addition, we have identified activation of STAT3 in grade I and II meningiomas, with increased phosphorylation/activation in grade II tumors (9). Similar findings were subsequently reported by Zhang et al (26), and Magrassi et al (2) reported that the levels of JAK1-2 and STAT1-6 proteins were increased in 17 WHO grade I meningiomas as compared with control dural tissue (15). STAT3 activation has been implicated in the initiation and progression of various solid malignancies, including breast, ovarian, pulmonary, colonic, pancreatic, renal and prostatic carcinomas (27). Involvement in gliomas has also been reported (27).
STAT3 may be activated by the MEK1-MAPK and PI3K-Akt-mTOR pathways and also by a number of different cytokine/growth factor receptors (20,24). Growth factor or cytokine receptor activation leads to the phosphorylation of JAK1 in receptor complexes, resulting in latent cytoplasmic STAT dimerization and translocation into the nucleus (20,24,25). Latent STAT3 in the cytoplasm can be activated by various growth factors, including PDGF and EGF, as well as cytokines, such as IL-6 (20,23–25). Although the role of STAT3 in meningioma recurrence is not known, inhibition of STAT3 reduces tumor formation by breast carcinoma cells and reduces recurrence in treated xenografts of human breast cancer (28).
A number of previous studies have found that the Ki-67 labeling index is associated with meningioma grade and possibly with the risk of recurrence, whereas others have failed to identify such a correlation (29–31). In WHO grade I meningiomas, a Ki-67 labeling index of >4% may portend relapse (29). In the present limited study, Ki-67 labeling was revealed to be significantly higher in recurrent compared with non-recurrent grade I meningiomas. Ki-67 labeling was also significantly higher in grade II than in grade I meningiomas; however, the index was similar between non-recurrent and recurrent cases among grade II tumors. A previous study identified a strong correlation between Ki-67 or MIB-1 labeling and the risk of recurrence but this is influenced by the means of calculating the percentage or labeling index, which may vary amongst institutions; in addition, it suggests the possibility that other biological markers may be at least as useful as predictors of recurrence, and less variable, compared with the determination of Ki-67 labeling indexes in laboratories without automated counting (32). In the present study, Ki-67 labeling was associated with the phosphorylation/activation of STAT3, supporting this contention.
Tissue samples used in the current study were promptly frozen to avoid degradation. The rate of dephosphorylation and the phosphatases that are involved in the dephosphorylation of phospho-p44/42 MAPK, -Akt or -STAT3 in meningioma tissue collected intraoperatively have not been established. However, in tissue collected routinely following surgery and placed in formalin, preservation of phosphorylation of p44/42 MAPK and mTOR has been demonstrated to be less stable with cold ischemia than certain other phosphoproteins (33). Nonetheless, in our previous studies, cerebrospinal fluid, which activates STAT3 and stimulates meningioma cell proliferation, promoted STAT3 phosphorylation that could be detected in meningioma cells at 72 h following treatment suggesting phospho-STAT3 maintenance or stability in at least some in vitro conditions. Furthermore, extensive phosphorylation of p44/42 MAPK and Akt was detected in frozen tissue (1,3,6). Thus, in cases of extensive cellular activation, phospho-STAT3 may be stable and reliably detected in tissue frozen relatively promptly following surgical extirpation.
In conclusion, the present study suggests that the activation/phosphorylation of STAT3 may be important to the biology of meningioma cell proliferation but not a sensitive predictor of meningioma recurrence itself. If additional studies implicate STAT3 activation in meningioma recurrence, the detection of phospho-STAT3 by western blot analysis or immunohistochemistry may guide therapeutic efforts to prevent recurrence. Identification of phospho-STAT3 may be performed in clinical laboratories to create a meningioma assessment that is more reliable than that based on clinical features and WHO grade alone. At present, a number of STAT3 inhibitors are under development or in clinical trials (21,28).
References
- 1.Johnson MD, Okediji E, Woodard A, Toms SA, Allen GS. Evidence for phosphatidylinositol 3-Kinase Akt-p70S6K pathway activation and transduction of mitogenic signals by platelet derived growth factor in human meningioma cells. J Neurosurg. 2002;97:668–675. doi: 10.3171/jns.2002.97.3.0668. [DOI] [PubMed] [Google Scholar]
- 2.Magrassi L, De-Fraja C, Conti L, Butti G, Infuso L, Govoni S, Cattaneo E. Expression of the JAK and STAT superfamilies in human meningiomas. J Neurosurg. 1999;91:440–446. doi: 10.3171/jns.1999.91.3.0440. [DOI] [PubMed] [Google Scholar]
- 3.Johnson MD, Woodard A, Kim P, Frexes-Steed M. Evidence for mitogen-associated protein kinase activation and transduction of mitogenic signals from platelet-derived growth factor in human meningioma cells. J Neurosurg. 2001;94:293–300. doi: 10.3171/jns.2001.94.2.0293. [DOI] [PubMed] [Google Scholar]
- 4.Mawrin C, Sasse T, Kirches E, Kropf S, Schneider T, Grimm C, Pambor C, Vorwerk CK, Firsching R, Lendeckel U, Dietzmann K. Different activation of mitogen activated protein kinase and Akt signalling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res. 2005;11:4074–4082. doi: 10.1158/1078-0432.CCR-04-2550. [DOI] [PubMed] [Google Scholar]
- 5.Schrell UM, Koch HU, Marschlaek R, Schrauzer T, Anders M, Adams E, Fahlbusch R. Formation of autocrine loops in human cerebral meningioma tissue by leukemia inhibitor factor, interleukin-6 and oncostatin Minhibition of meningioma cell growth in vitro by recombinant oncostatin M. J Neurosurg. 1998;88:541–548. doi: 10.3171/jns.1998.88.3.0541. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MD, O'Connell M, Facik M, Maurer P, Jahromi B, Pilcher W. Cerebrospinal fluid stimulates leptomeningeal and meningioma cell proliferation and activation of STAT3. J Neurooncol. 2012;107:121–133. doi: 10.1007/s11060-011-0736-9. [DOI] [PubMed] [Google Scholar]
- 7.Carroll RS, Black PM, Zhang J, Kirsch M, Percec I, Lau N, Guha A. Expression and activation of epidermal growth factor receptors in meningiomas. J Neurosurg. 1997;87:315–323. doi: 10.3171/jns.1997.87.2.0315. [DOI] [PubMed] [Google Scholar]
- 8.Johnson M, Toms S. Mitogenic signal transduction pathways in meningiomas: Novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol. 2006;64:1029–1036. doi: 10.1097/01.jnen.0000189834.63951.81. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MD, O'Connell M, Vito F, Bakos RS. Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J Neuro-Oncol. 2009;92:129–135. doi: 10.1007/s11060-008-9746-7. [DOI] [PubMed] [Google Scholar]
- 10.Shamah SM, Alberta JA, Giannobile WV, Guha A, Kwon YK, Carroll RS, Black PM, Stiles CD. Detection of activated platelet-derived growth factor receptors in human meningioma. Cancer Res. 1997;57:4141–4147. [PubMed] [Google Scholar]
- 11.Jääskeläinen J. Seemingly complete removal of histologically benign intracranial meningioma: Late recurrence rate and factors predicting recurrence in 637 patients. A multivariate analysis. Surg Neurol. 1986;26:461–469. doi: 10.1016/0090-3019(86)90259-4. [DOI] [PubMed] [Google Scholar]
- 12.Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: A review. J Neurooncol. 1996;29:197–205. doi: 10.1007/BF00165649. [DOI] [PubMed] [Google Scholar]
- 13.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: An analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455–1465. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP. Gamma knife surgery for skull base meningiomas. J Neurosurg. 2012;116:588–597. doi: 10.3171/2011.11.JNS11530. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan JP, Williams BJ, Yen CP. Stereotactic radiosurgery for WHO grade I meningiomas. J Neurooncol. 2010;99:407–416. doi: 10.1007/s11060-010-0363-x. [DOI] [PubMed] [Google Scholar]
- 16.Aboukais R, Baroncini M, Zairi F, Reyns N, Lejeune JP. Early postoperative radiotherapy improves progression free survival in patients with grade 2 meningiomas. Acta Neurochir (Wien) 2013;155:1385–1390. doi: 10.1007/s00701-013-1775-0. [DOI] [PubMed] [Google Scholar]
- 17.Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Reccurent spheno-orbital meningioma. J Neurosurg. 1994;80:202–208. doi: 10.3171/jns.1994.80.2.0202. [DOI] [PubMed] [Google Scholar]
- 18.Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, Parsa AT, Yang I. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.2.FOCUS1116. [DOI] [PubMed] [Google Scholar]
- 19.Abry E, Thomassen IØ, Salvesen ØO, Torp SH. The significance of Ki-67 /MIB-1 labeling index in human meningiomas: A literature study. Pathol Res Pract. 2010;206:810–815. doi: 10.1016/j.prp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Klampfer L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drugs and Targets. 2006;6:107–121. doi: 10.2174/156800906776056491. [DOI] [PubMed] [Google Scholar]
- 21.Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: A patent review. Expert Opin Ther Pat. 2011;21:65–83. doi: 10.1517/13543776.2011.539205. [DOI] [PubMed] [Google Scholar]
- 22.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al. The IL-6/JAK/STAT3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 25.Kortylewski M, Yu H. STAT3 as a potential target for cancer immunotherapy. J Immunother. 2007;30:131–139. doi: 10.1097/01.cji.0000211327.76266.65. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang A, Wang Y, Liu N, You Y, Kang C, Pu P. New insights into the roles of ncRNA in the STAT3 pathway. Future Oncol. 2012;8:723–730. doi: 10.2217/fon.12.52. [DOI] [PubMed] [Google Scholar]
- 27.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Dave B, Landis MD, Tweardy DJ, Chang JC, Dobrolecki LE, Wu MF, Zhang X, Westbrook TF, Hilsenbeck SG, Liu D, Lewis MT. Selective small molecule Stat3 inhibitor reduces breast cancer tumor-initiating cells and improves recurrence free survival in a human-xenograft model. PLoS One. 2012;8:e30207. doi: 10.1371/journal.pone.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vankalakunti M, Vasishata RK, Das Radotra B, Khosla VK. MIB-1 immunolabeling: A valuable marker in prediction of benign recurring meningiomas. Neuropathology. 2007;27:407–412. doi: 10.1111/j.1440-1789.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi A, Chakrabarty A, Franks A. MIB1 proliferation index in meningiomas: Does it predict recurrence? A clinicopathologiccal study. Br J Neurosurg. 2004;18:357–361. doi: 10.1080/02688690400005008. [DOI] [PubMed] [Google Scholar]
- 31.Abramovich CM, Prayson RA. MIB-1 labeling indices in benign, aggressive malignant meningiomas: A study of 90 tumors. Human Pathol. 1998;29:1420–1417. doi: 10.1016/S0046-8177(98)90010-7. [DOI] [PubMed] [Google Scholar]
- 32.Markiewicz T, Grala B, Kolowski W, Osowski S. Computer system for cell counting in selected brain tumors at Ki-67 immunohistochemical staining. Anal Quant Cytol Histol. 2010;32:323–332. [PubMed] [Google Scholar]
- 33.Vassilakopoulou M, Parisi F, Siddiqui S, England AM, Zarella ER, Anagnostou V, Kluger Y, Hicks DG, Rimm DL, Neumeister VM. Preanalytic variables and phosphepitope expression in FFPE tissue: A quantative epitope assessment after variable cold ischemic time. Lab Invest. 2015;95:334–341. doi: 10.1038/labinvest.2014.139. [DOI] [PubMed] [Google Scholar]