Abstract
Objective
Maternal age at birth of last child has been associated with maternal longevity. The aim of this study was to determine whether elderly women with a history of late maternal age at last childbirth had a longer leukocyte telomere length compared with those with maternal age at last childbirth of 29 years or less.
Methods
A nested case control study was conducted utilizing data from the Long Life Family Study. Three hundred and eighty-seven women who gave birth to at least 1 child and lived to the top 5th percentile of their birth cohort, or died before the top 5th percentile of their birth cohort died, but were at least 70 years old, were studied. Logistic regression models using generalized estimating equations were used to determine the association between tertiles of telomere length and maternal age at last childbirth, adjusting for covariates.
Results
Age at birth of the last child was significantly associated with leukocyte telomere length. Compared with women who gave birth to their last child before the age of 29, women who were past the age of 33 when they had their last child were 2–3 times more likely to have leukocyte telomere length in the second and third tertiles than in the first tertile.
Conclusion
These findings show an association between longer leukocyte telomere length and a later maternal age at birth of last child, suggesting that extended maternal age at last childbirth may be a marker for longevity.
Keywords: maternal age, menopause, telomere length, familial longevity, aging
Introduction
Several studies have found that late maternal age at last childbirth is positively associated with maternal longevity1–8. Among Amish women born between 1749 and 1912, every year of increase in maternal age at childbirth was associated with a 0.29 year increase in lifespan2. A study of 13,897 once-married couples from the Utah Population Database, married between 1860 and 1899, found that women in the top 95th percentile of maternal age at last birth had a 44% greater chance of reaching age 95 than mothers in the 25th– 75th percentile of maternal age at last child birth5. Extended maternal age has been found among centenarians compared with their birth cohort peers4. Perls and colleagues compared two groups of women born in 1896, and found that women who lived to at least age 100 were four times more likely to have had children while in their forties than women who survived only to age 73, suggesting that the ability to have children in the fifth decade may be a marker for slow aging and subsequent ability to achieve extreme longevity4. A recent study from the Long Life Family Study (LLFS) extended these findings to families with exceptional aging6. This study reported that the odds of living to the top 5th percentile for their birth cohort was two times higher among women who had their last child past the age of 33 years than among those who had their last child prior to the age of 29 years6. This finding suggests that late maternal age at last child birth is a marker for rate of aging and, if heritable, might be associated with genetic variants playing a role in exceptional survival. We hypothesized that factors associated with rate of aging and longevity, such as telomere length, may also be associated with later maternal age at birth of last child9, 10.
Telomeres are the repeated sequence TTAGGG at the end of human chromosomes11. They provide protection to the chromosome during the replication process to prevent the loss of DNA strand nucleotides. Telomeres shorten during DNA replication, and by oxidative stress10, 12, 13. Age is strongly associated with telomere length: telomeres decrease in length as a person ages14, 15. Shorter leukocyte telomere length is an indication of cellular aging and has been shown to be associated with an increased rate of aging and mortality10, 16, 17 and longer telomere length has been associated with better health in centenarians18. In the LLFS cohort, the heritability of telomere length was 0.54 and the odds of being in the highest tertile of telomere length were 2.3 times higher among first-degree relatives of those with exceptional longevity than among spouse controls17. Thus both a later maternal age at birth of last child and a longer telomere length have shown to be associated with longevity, but the relationship between maternal age at birth of last child and telomere length has not been examined. If extended maternal age at last childbirth is associated with longer leukocyte telomere length it may further support evidence for a biological association between reproductive aging and exceptional longevity.
METHODS
Study Population
Study participants were selected from the Long Life Family Study (LLFS). LLFS is a longitudinal, family-based study to determine the genetic and phenotypic traits that increase the probability of survival to extreme ages and to determine the sub-phenotypes of exceptional survival19. Recruitment for the LLFS study took place from 2005 until 2009, at four different field centers: Boston University, Columbia University, University of Pittsburgh, and the University of Southern Denmark. In the U.S., initial eligibility criteria included probands were over 80 years of age, lived less than a three hour drive from one of the study sites, did not have end stage renal disease, and were not in hospice care19. A pilot mailing tested the yield of families recruited from mailing to individuals in their 80’s and higher age strata. Based on these yields, subsequent mailings targeted those age 89 and older19. Individuals 90 years and older were identified in Denmark by using the Danish National Registry. The family was included in the study if the proband had at least 1 sibling, and if the proband, at least one sibling and at least one offspring or member of the offspring generation (nieces, nephews) was willing and able to participate in an in- person interview, provide a blood sample for DNA extraction and was able to provide informed consent. Spouses in both generations were also recruited to participate as controls. Eligible families had to have a Family Longevity Selection Score (FLoSS) of at least 7. The FLoSS is a metric of the degree of familial longevity that is based on sex and birth-year cohort survival probabilities of the proband and their siblings20. Participants have been followed since recruitment. There are 4,559 participants from 551 different families. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of all participating sites.
Study Design
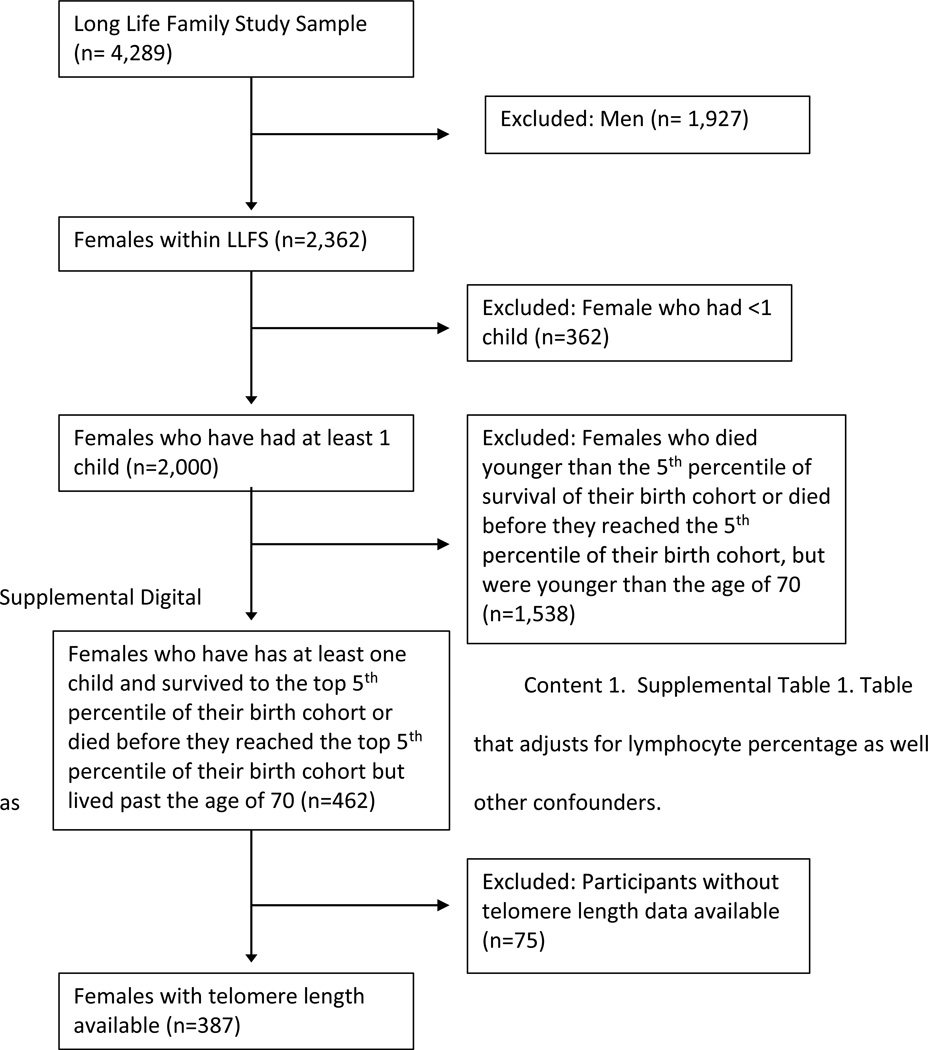
This study extends the study by Sun and colleagues on the association between maternal age at birth of last child and women’s longevity within the LLFS cohort to include examination of telomere length6, using the same study sample as used in the previous analysis. LLFS women were included in the study if they lived to or beyond the 5th percentile of survival for their birth cohorts or if they died at an age younger than the 5th percentile of their birth cohort, but lived past the age of 706. Participants younger than 70 years were excluded from the analysis to avoid comparing cases and controls from substantially different birth cohorts and introduce unmeasured confounders due to secular trends in child bearing. The 5th percentile of survival was chosen to focus attention to a heritable and hence genetically regulated definition of longevity since we have shown that the heritability of human longevity increases as we look at older and older ages beyond the 5th percentile of survival21. To determine the 5th percentile of survival, cohort life tables from the US Social Security Administration were used6. The study was limited to participants above the age of 70 to control for confounding by secular trends in child bearing: 97.7% of participants were from the proband generation and 2.3% were from the offspring generation. Of the 462 women included in the previous study by Sun and colleagues6, 387 had telomere length data and therefore were included in this analysis (Figure 1). The distribution of age at last birth among the reduced set of 387 women was similar to the distribution of age at last birth in the original set of 462 women (mean 33.9 years vs 34.01 years, respectively). All women included in the analysis were non-Hispanic Whites.
Figure 1.
Sample Selection Diagram. Females who have had at least one child and survived to the top 5th percentile of their birth cohort or died before they reached the top 5th percentile of their birth cohort but lived past the age of 70 and who had telomere length data available were included.
Participant Assessment
A comprehensive set of interviews and examinations were conducted for all participants19. Date of birth was validated with any government issued document at the in-person visit. Participants self-reported their race, sex, years of education, number of children, age at birth of last child, and age at menopause. Medical history was collected by self-report or proxy report. During the in-person interview, the research assistant collected data about a participant’s physical activity levels, smoking history, and history of alcohol consumption. The physical examination collected data on a participant’s height, weight, vital signs, force vital capacity, ankle arm index, and tests of physical and cognitive functions. Blood was collected from participants for DNA and biomarker measures19.
Telomere Length Measurement
Blood samples that were drawn during the baseline visit were used to determine telomere length as previously described for the LLFS cohort.17 To obtain DNA from the white blood cells (WBC) from frozen packaged cells, a salt precipitation method (Gentra Puregene, Qiagen INC., Germantown, MD) was used17. The WBCs were extracted from fasting blood from EDTA anticoagulated whole blood samples. DNA samples were then stored at −80°C. The DNA was used and processed to determine a telomere length measurement blinded to the study participant’s characteristics17 To determine the average telomere length the method of Cawthon and colleagues was used22. The method employed real time PCR to determine the telomere/single gene ratio (T/S ratio), which is proportional to the average telomere length. A CFX394 thermocycler (Biorad, Richmond, CA) was used to preform PCR. The same 384-well plate was used for both the telomere (T) and single gene copy (S), along with a standard DNA reference sample on each plate17. The standard DNA reference sample is a purified DNA equal mixture of 5 different individuals, and small aliquots of this reference sample are used as a reference in all telomere studies. Telomere length was categorized into tertiles with tertile 1 as the shortest tertile and tertile 3 as the longest tertile.
Maternal Age at Birth of Last Child Measurement
Maternal age at birth of last child was assessed by self-report during the baseline survey. The variable was collected as a continuous variable and was categorized into quartiles (≤29 years, 30–33 years, 34–37 years, and ≥38 years).
Potential Confounders
The potential confounders included in the analysis were: age at blood draw, history of tobacco use (ever vs never), length of education (classified as less than or equal and greater than 12 years), family longevity (yes vs no), Field center (US or Denmark), parity (.(≥3 children vs <3 children). history of oophorectomy (yes vs no), heart disease, stroke, hypertension, diabetes, and cancer (yes vs no). Age of the woman at the time of the blood collection was used to control for the association between telomere length and age. Field center was included to account for potential social differences in child bearing patterns between the United States and Denmark (Danish or US).
Statistical Analysis
Means and standard deviations were determined for continuous measures, and frequencies and proportions were determined for categorical variables. Characteristics were summarized for all of the women and then by tertiles of telomere length. Tertiles of telomere length were employed to account for potential threshold effects in the relationship of telomere length to late maternal age. Univariate analyses used Pearson chi-squared test and ANOVA to describe the relation of study variables to tertiles of telomere length. To determine the association between maternal age at birth of last child and telomere length, logistic regression analysis using Generalized Estimating Equations (GEE) which accounts for familial clustering, was used to relate quartiles of maternal age at last childbirth to tertiles of telomere length, adjusting for age at blood collection, history of tobacco use (ever vs never), length of education (< 12 years vs ≥12 years), study center (New York, NY; Boston, MA; Pittsburgh, PA; or Denmark), familial longevity (yes or no: blood relative or spouse), and parity (<3 children or ≥3 children), history of oophorectomy (yes vs no), heart disease, stroke, hypertension, diabetes, and cancer (yes vs no). Telomere tertile 2 versus 1 and telomere tertile 3 versus 1 were modeled in separate analyses. Since different leukocyte subpopulations have different replicative histories, and may thus differ in telomere length, we performed an additional subanalysis adjusting for differences in leukocyte differential counts. Lymphocytes are generally longer-lived than neutrophils, eosinophils, basophils, or monocytes; The lymphocyte percentage varied from 5% to 90%, with mean (SD) of 28% (11%) and did not differ across tertiles of telomere length (Table 1). Our subanalysis adjusted for the percentage of lymphocytes as a fraction of the total white blood cell (leukocyte) counts. These data were available for 362 of the 387 women (93.5%).
Table 1.
Study group characteristics by telomere length tertile
| Characteristic | Total |
Telomere Tertile 1 (4484.6– 4947.61 bp |
Telomere Tertile2 (4947.62– 5217.28 bp) |
Telomere Tertile 3 (5217.29– 7715.65 bp) |
|---|---|---|---|---|
| Group Size, n | 387 | 129 | 129 | 129 |
| Age at blood draw, M (SD) | 93.21 (5.45) | 94.05 (4.9) | 92.15 (5.8) | 93.43 (5.4) |
|
Maternal Age at birth of Last Child ≤ 29 years (n, %) |
87 (22.5) | 38 (43.7) | 23 (26.4) | 26 (29.9) |
|
Maternal Age at birth of Last Child 30–33 years (n, %) |
83 (21.4) | 27 (32.5) | 32 (38.6) | 24 (28.9) |
|
Maternal Age at birth of Last Child 34–37 years (n, %) |
106 (27.4) | 32 (30.2) | 41 (38.7) | 33 (31.1) |
|
Maternal Age at birth of Last Child ≥38 years (n, %) |
111 (28.7) | 32 (28.8) | 33 (29.7) | 46 (41.4) |
| History of tobacco use (ever), n (%) | 106 (27.4) | 34 (32.1) | 38 (35.8) | 34 (32.1) |
| Education (≥12 y), n (%) | 104 (26.9) | 38 (36.5) | 38 (36.5) | 28 (26.9) |
| Field Center (Danish), n (%) | 86 (22.2) | 29 (33.7) | 28 (32.6) | 29 (33.7) |
| Family longevity (yes), n (%) | 360 (93.0) | 119 (33.1) | 119 (33.1) | 122 (33.9) |
| Parity (≥ 3 children), n (%) | 210 (54.3) | 72 (34.3) | 72 (34.3) | 66 (31.4) |
| Oophorectomy (yes), n (%) | 67 (17.9) | 24 (19.4) | 24 (19.4) | 19 (15.0) |
| Heart disease (yes), n (%) | 59 (15.2( | 22 (17.1) | 19 (14.7) | 18 (14.0) |
| Stroke (yes), n (%) | 70 (18.1) | 27 (20.9) | 28 (21.7) | 15 (11.6) |
| Hypertension (yes), n (%) | 275 (71.1) | 95 (34.5) | 93 (72.1) | 87 (67.4) |
| Diabetes (yes), n (%) | 36 (9.3) | 11 (8.5) | 11 (8.5) | 14 (10.9) |
| Cancer (yes), n (%) | 132 (34.1) | 42 (32.6) | 51 (39.5) | 39 (30.2) |
| Lymphocyte Percentage M (SD) | 0.28 (.11) | 0.29 (.13) | 0.27 (.11) | 0.28 (.09) |
Results
Table 1 shows the study group characteristics for the total group (n=387) and by telomere tertiles (n=129 per tertile). Mean age at blood collection was younger for the second telomere length tertile than for the first telomere length tertile (p <.05). The proportion of women in the longest telomere tertile, the 3rd tertile, was higher for women in the fourth quartile of maternal age at birth of their last child than in the first quartile (35.7 % vs. 20.2% p = .023). A history of tobacco use, length of education, study center, family longevity, parity, history of oophorectomy, heart disease, hypertension, diabetes and cancer were not significantly different among the telomere length tertiles while the frequency of a history of stroke was lower among women in the longest tertile (tertile 3) of telomere length (p = .053). Table 2 shows the odds of being in tertile 2 or tertile 3 of telomere length compared with tertile 1 by quartile of maternal age at birth of the last child. Compared with women who had their last child at age 29 or less, women with older ages at last childbirth were 2.7–3.4 times more likely to have telomeres in the 2nd tertile than in the first tertile. Similarly, women who had their last child at ages older than age 29 were 1.6 to 3.7 times more likely to have telomeres in the third quartile than women who had their last child at age 29 or less, adjusting for covariates (Table 2). Inclusion of the lymphocyte fraction did not change the relationship between maternal age at last birth and leukocyte telomere length (Supplemental Digital Content. Table 1).
Table 2.
Relation of maternal age at last birth with telomere length: Odds of having telomere lengths in the second and third tertiles compared with the first tertile by quartile of maternal age at last birth.
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Telomere tertile 2 compared with telomere tertile 1 | |||
| Maternal Age at birth of Last Child ≤ 29 years |
1.0 | ____ | reference |
| Maternal Age at Birth of Last Child: 30–33 years |
2.71 | (1.17– 6.24) | 0.019 |
| Maternal Age at Birth of Last Child: 34–37 years |
3.36 | (1.42– 7.93 | 0.006 |
| Maternal Age at Birth of Last Child ≥38 years |
2.69 | (1.09– 6.63) | 0.032 |
| Telomere tertile 3 compared with telomere tertile 1 | |||
|
Maternal Age at birth of Last Child ≤ 29 years |
1.0 | _____ | reference |
|
Maternal Age at Birth of Last Child 30–33 years |
1.59 | (0.69–3.63) | 0.28 |
|
Maternal Age at Birth of Last Child 34–37 years |
2.31 | (1.02–5.23) | 0.044 |
|
Maternal Age at Birth of Last Child ≥38 years |
3.70 | (1.64–8.35) | 0.002 |
Covariates include: family longevity (yes vs no), length of education (≥12 years vs <12 years), age at blood draw, history of tobacco use (ever vs never), field center (Denmark vs United States), parity (≥3 children vs <3 children), oophorectomy heart disease, stroke, hypertension, diabetes, and cancer.
Discussion
Compared with women who had their last child by the age of 29, women with a later age at birth of last child (34–37 years or ≥38 years old) were found to have increased odds of being in the longest tertile of telomere length versus the shortest tertile of telomere length when adjusting for covariates. The strength of the association with the longest telomere length increased as the maternal age at birth of last child became later in life. Only a few studies have examined the relationship between telomere length and reproductive aging. Analysis of the relationship between telomere length and age at menopause was examined among 799 post-menopausal women participating in the NHANES survey23. Increased telomere length was associated with later age at menopause among non-Hispanic White women, with earlier age at menopause among Hispanic women and was not associated with age at menopause among African American women23. Mean telomere length was shorter among women with a history of recurrent miscarriage compared with women from the general population or those who had a healthy pregnancy after age 3724. This is the first published study that we know of studying the potential association between maternal age at last birth and telomere length. Besides the phenotype, another important difference between this current study and others is the inclusion of subjects with a familial predisposition for exceptional longevity.
There are several limitations to this study. The study was limited to participants older than 70 years to avoid comparing cases and controls from substantially different birth cohorts and introduce unmeasured confounders due to secular trends in child bearing: 97.7% of participants were from the proband generation and 2.3% were from the offspring generation6. By restricting the analysis to this population, the number of women available to participate in the study decreased and it is possible that these findings may not generalize to women in current birth cohorts who have tended to initiate child bearing at older ages than the women analyzed here and who are likely to experience a different set of environmental and social influences affecting child bearing decisions. All participants included in the analysis were non-Hispanic white women. Prior research has found differences in age- and sex-adjusted telomere length and rate of telomere shortening with age and stressful life experiences between non-Hispanic Whites, Hispanics and African Americans25–28, limiting the generalizability of our findings. Additionally, it is likely that there are numerous personal and social factors which influence when a woman stops having children that may be independent of age-associated fertility. These can include economic factors, such as income and occupational status, the number of previous children, a special desire for a male or female child, the nature of the personal relationship with a spouse or partner, and the effect of other stressors. We included educational level as a covariate, which is generally most reliably reported for both men and women and also predicts income and occupational status29, but did not have the data for the more personal and social factors which might influence child bearing decisions, limiting generalizability of our findings.
Conclusion
The phenotypes of longevity and prolonged ability to bear children appear to have a common association with longer telomeres, suggesting that extended maternal age at last childbirth may be a marker for healthy aging. These findings suggest a potential genetic basis for the relationship between reproductive lifespan, longevity and an underlying mechanism related to biological aging. Further studies to understand the bases of these associations are needed.
Supplementary Material
Acknowledgments
Sources of Funding. Sponsored by the National Institute on Aging (NIA cooperative agreements U01-AG023712, U01AG23744, U01-AG023746, U01-AG023749, and U01-AG023755). The Danish 1905-cohort is funded by NIH/ NIA, P01 AG08761. The Danish Aging Research Center is funded by the VELUX Foundation.
Footnotes
Conflicts of Interest
Disclosure Statement: The authors have no conflicts of interest to declare.
Supplemental Digital Content.
Supplemental Digital Content. SDC 1. Table that adds adjustment for lymphocyte fraction. docx
REFERENCES
- 1.Grundy E, Kravdal Ø. Reproductive history and mortality in late middle age among Norwegian men and women. American Journal of Epidemiology. 2008;167(3):271–279. doi: 10.1093/aje/kwm295. [DOI] [PubMed] [Google Scholar]
- 2.McArdle PF, Pollin TI, O'Connell JR, et al. Does having children extend life span? A genealogical study of parity and longevity in the Amish. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(2):190–195. doi: 10.1093/gerona/61.2.190. [DOI] [PubMed] [Google Scholar]
- 3.Muller HG, Chiou JM, Carey JR, Wang JL. Fertility and life span: late children enhance female longevity. J Gerontol A Biol Sci Med Sci. 2002;57(5):B202–B206. doi: 10.1093/gerona/57.5.b202. [DOI] [PubMed] [Google Scholar]
- 4.Perls TT, Alpert L, Fretts RC. Middle-aged mothers live longer. Nature. 1997;389(6647):133-. doi: 10.1038/38148. [DOI] [PubMed] [Google Scholar]
- 5.Smith KR, Mineau GP, Bean LL. Fertility and post-reproductive longevity. Biodemography and Social Biology. 2002;49(3–4):185–205. [PubMed] [Google Scholar]
- 6.Sun F, Sebastiani P, Schupf N, et al. Extended maternal age at birth of last child and women’s longevity in the Long Life Family Study. Menopause. 2015;22(1):26–31. doi: 10.1097/GME.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wainer-Katsir K, Zou JY, Linial M. Extended fertility and longevity: the genetic and epigenetic link. Fertil Steril. 2015;103(5):1117–1124. doi: 10.1016/j.fertnstert.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon A. Natural fertility and longevity. Fertil Steril. 2015;103(5):1109–1116. doi: 10.1016/j.fertnstert.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Harley CB, Vaziri H, Counter CM, Allsopp RC. The telomere hypothesis of cellular aging. Experimental gerontology. 1992;27(4):375–382. doi: 10.1016/0531-5565(92)90068-b. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiologic reviews. 2013;35(1):112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence,(TTAGGG) n, present at the telomeres of human chromosomes. Proceedings of the National Academy of Sciences. 1988;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Zglinicki T. Oxidative stress shortens telomeres. Trends in biochemical sciences. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 13.Zakian VA. Structure and function of telomeres. Annual review of genetics. 1989;23(1):579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- 14.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing research reviews. 2013;12(2):509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. The Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 17.Honig LS, Kang MS, Cheng R, et al. Heritability of telomere length in a study of long-lived families. Neurobiology of aging. 2015;36(10):2785–2790. doi: 10.1016/j.neurobiolaging.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry DF, Nolan VG, Andersen SL, Perls TT, Cawthon R. Association of longer telomeres with better health in centenarians. J Gerontol A Biol Sci Med Sci. 2008;63(8):809–812. doi: 10.1093/gerona/63.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman AB, Glynn NW, Taylor CA, et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany NY) 2011;3(1):63. doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebastiani P, Hadley EC, Province M, et al. A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009;170(12):1555–1562. doi: 10.1093/aje/kwp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastiani P, Nussbaum L, Andersen SL, Black MJ, Perls TT. Increasing Sibling Relative Risk of Survival to Older and Older Ages and the Importance of Precise Definitions of "Aging," "Life Span," and "Longevity". J Gerontol A Biol Sci Med Sci. 71(3):340–346. doi: 10.1093/gerona/glv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic acids research. 2002;30(10):e47-e. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenassa ED, Rossen LM. Telomere length and age-at-menopause in the US. Maturitas. 2015;82(2):215–221. doi: 10.1016/j.maturitas.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Hum Reprod. 2009;24(5):1206–1211. doi: 10.1093/humrep/dep007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown L, Needham B, Ailshire J. Telomere Length Among Older U.S. Adults: Differences by Race/Ethnicity, Gender, and Age. J Aging Health. 2016 doi: 10.1177/0898264316661390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzpatrick AL, Kronmal RA, Kimura M, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US Black Women Experience Stress-Related Accelerated Biological Aging?: A Novel Theory and First Population-Based Test of Black-White Differences in Telomere Length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamad R, Tuljapurkar S, Rehkopf DH. Racial and Socioeconomic Variation in Genetic Markers of Telomere Length: A Cross-Sectional Study of U.S. Older Adults. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elo IT. Social Class Differentials in Health and Mortality: Patterns and Explanations in Comparative Perspective. Annual Review of Sociology. 2009;35:553–572. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.