Abstract
Extracytoplasmic function (ECF) sigma factors are a subfamily of σ70 sigma factors that activate genes involved in stress-response functions. In many bacteria, ECF sigma factors regulate resistance to antimicrobial compounds. This review will summarize the ECF sigma factors that regulate antimicrobial resistance in model organisms and clinically relevant pathogens.
Keywords: extracytoplasmic function sigma factors, ECF sigma factors, antimicrobial resistance, regulation
1. Introduction
The Centers for Disease Control and Prevention report, “Antibacterial Resistance Threats in the United States, 2013,” estimated that nearly two million infections and 23,000 deaths occur each year in the United States due to antibiotic resistant organisms [1]. Understanding the mechanisms by which bacteria evade killing by antimicrobials is therefore imperative to address the burden of disease caused by these organisms.
Although bacteria can acquire mutations or novel genes to gain resistance to antimicrobials, many bacteria are intrinsically resistant to some classes of antimicrobials [2–13]. Such intrinsic resistance can either be constitutive or inducible [14–18, 6, 19–21]. In the case of inducible resistance, there are many known mechanisms by which bacteria can sense and respond to the threat of antimicrobials. Two-component regulatory systems have long been a recognized mechanism by which bacteria respond to extracellular signals to alter gene expression, and many two-component systems are known to increase expression of antimicrobial resistance genes [22–29]. Alternative sigma factors are another common mechanism by which bacteria can alter their gene expression in response to a stimulus. The extracytoplasmic function (ECF) sigma factors are a unique class of alternative sigma factors that also regulate gene expression in response to extracellular signals. ECF sigma factors are present in a wide range of species [30, 31]. This review will provide an overview of the role of ECF sigma factors in regulating resistance to antimicrobials.
After a brief summary of how ECF sigma factors function, we will examine several examples of how ECF sigma factors can regulate resistance to antimicrobial compounds in both Gram-negative and Gram-positive species. Because antimicrobial resistance is primarily a concern for organisms that pose a threat to human health, this review will mostly focus on clinically relevant pathogens, with the exceptions of Streptomyces coelicolor, which is included for historical importance, and Bacillus subtilis, which is included because it is a well-studied model organism.
2. Overview of ECF sigma factors
This review does not seek to be a comprehensive review of all ECF sigma factors or a review of the pathways that regulate ECF sigma factor function. A number of excellent reviews cover these topics, and the reader is referred to them for further information [30, 32–37]. Nevertheless, a very brief introduction to ECF sigma factors will be necessary before delving into the specifics of how ECF sigma factors regulate antimicrobial resistance.
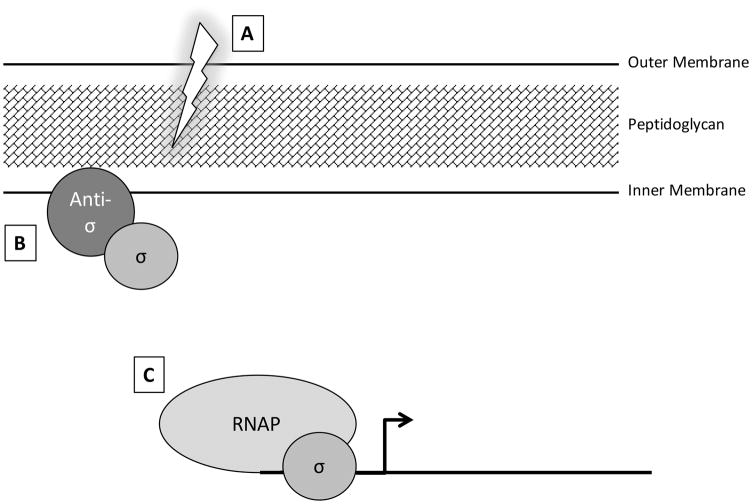
Core RNA polymerase (RNAP) requires a sigma factor to create the RNAP holoenzyme and initiate transcription. Sigma factors confer promoter specificity and can therefore direct RNAP to transcribe specific sets of genes (Fig. 1C). The ECF sigma factors constitute a subfamily within the larger σ70 family of sigma factors. Similar to other sigma factors, the ECF sigma factors contain the σ2 and σ4 domains, which are involved in binding the -10 and -35 promoter regions, respectively. However, ECF sigma factors lack the σ1.1 and σ3 domains, which are involved in RNAP binding and extended -10 binding, respectively [35, 38, 39, 31, 40]. The name extracytoplasmic function refers to the fact that the common function of these factors is to regulate processes that affect the cell envelope, yet the specific processes that they regulate can vary greatly between different ECF factors. For example, ECF sigma factors have been implicated in regulating processes as diverse as iron uptake, cell wall maintenance, and motility [30, 40–47]. Although a great deal of variation and nuance exists in how ECF sigma factor activity is regulated, the majority of ECF sigma factors are held inactive after translation by a membrane-bound anti-sigma factor [32, 31, 48–54] (Fig. 1A). An extracytoplasmic signal then initiates a proteolytic cascade that results in release of the sigma factor from the anti-sigma factor to initiate transcription [32, 55–59] (Fig. 1B, C).
Figure 1. Model of ECF sigma factor activation.
(A) ECF sigma factors (labeled σ) are typically kept inactive via sequestration at the cell membrane by an anti-sigma factor (labeled anti-σ). (B) An extracellular signal or perturbation initiates a proteolytic cascade that cleaves the anti-sigma factor to release the sigma factor. (C) Upon release, the sigma factor is free to bind to DNA and direct the RNA polymerase (RNAP) to transcribe specific genes.
Finally, a note on the nomenclature of the ECF sigma factors: most Gram- negative bacteria follow the convention established in E. coli of using an rpo designation for sigma factor genes, whereas most Gram-positive bacteria follow the convention of using a sig (σ) gene designation [30].
3. ECF sigma factor-mediated systems in Gram-negative bacteria
3.1 Escherichia coli
RpoE is one of two ECF sigma factors in Escherichia coli. Although specific RpoE-dependent antimicrobial-resistance genes have not been identified, RpoE bears mention here because RpoE is one of the most-studied ECF sigma factors. RpoE activity is induced in response to misfolded proteins in the outer membrane [60, 61]. It is therefore not surprising that the RpoE regulon contains genes for foldases, proteases, LPS biosynthesis proteins, and chaperone proteins [62]. Although these genes are not implicated directly in antimicrobial resistance, they are necessary for maintaining cell membrane integrity, which has consequences for how well E. coli copes with antimicrobial-induced damage. Deletion of rpoE is lethal in E. coli strain K-12 [63]. Lower levels of RpoE also has consequences in E. coli, as overexpressing RseA, the RpoE anti-sigma factor, leads to membrane blebbing and eventual cell death [64]. Unlike laboratory strains, some clinical isolates of E. coli can survive rpoE deletion. Upon rpoE deletion, these strains exhibit increased sensitivity to polymyxin B [65].
3.2 Salmonella enterica
The Salmonella RpoE is a homolog of E. coli RpoE and appears to play a role in regulating resistance to certain antimicrobials in serovar Typhimurium. An S. Typhimurium rpoE mutant is more sensitive to polymyxin B, P2 (a derivative of bactericidal and permeability increasing protein produced by neutrophils), and the murine defensin, cryptdin-4 [66, 67]. P2 alters the electron motive force. RpoE may be able to mediate resistance to P2 because it induces expression of fdhD, which encodes a formate dehydrogenase that allows S. Typhimurium to utilize alternate terminal electron acceptors [68, 67]. Another gene of the RpoE regulon, smpA, may also play a role in providing antimicrobial resistance. smpA encodes an outer-membrane lipoprotein that plays an essential role in an outer membrane protein assembly complex. An smpA mutant is more susceptible to rifampicin, SDS, and EDTA, indicating its important role in maintaining proper outer membrane protein composition [69]. Thus, RpoE is implicated in two different mechanisms that enable S. Typhimurium to survive the insults of antimicrobials.
In contrast to the increased sensitivity to antimicrobials seen in the S. Typhimurium rpoE mutant, an S. Typhi rpoE mutant has increased resistance to several antimicrobials, including penicillins, cephalosporins, aminoglycosides, and ciprofloxacin [70]. The S. Typhi rpoE mutant has reduced expression of ompC and ompF, which encode outer membrane proteins associated with antimicrobial passage into the periplasm [70]. Expression of ramA, a gene encoding a transcriptional regulator of the AcrAB-TolC multidrug efflux pump, was increased in the rpoE mutant [70]. The lower expression of ompC and ompF and the increased expression of ramA may, at least partially, explain the higher resistance to some antimicrobials observed in the S. Typhi rpoE mutant. However, it remains unclear why RpoE has opposite effects on antimicrobial resistance in S. Typhi and S. Typhimurium.
3.3 Pseudomonas aeruginosa
The ubiquitous bacterium, P. aeruginosa, encodes an ortholog to the E. coli RpoE: the sigma factor, AlgU (also known as AlgT) [71]. AlgU/T shares 78% similarity and 66% identity with RpoE and can initiate transcription from E. coli RpoE promoters [72, 71]. Although AlgU/T most prominently controls conversion to the mucoid phenotype by inducing production of the exopolysaccharide, alginate, it also regulates the expression of roughly 35 other genes that are important for virulence and generation of an inflammatory response in the host [73, 74].
There are several lines of evidence that suggest AlgU/T is important for intrinsic antibiotic resistance in P. aeruginosa due to its role in alginate production. The mucoid phenotype stimulated by AlgU/T is associated with increased resistance of the bacterium to a variety of antimicrobials [75, 76]. Mucoidy also contributes to biofilm formation, which itself is associated with increased antimicrobial resistance [77–79]. Additionally, alginate binds tobramycin and hinders its diffusion through liquids and agar [80]. This finding suggests that alginate could contribute to antibiotic resistance by impeding antibiotics from reaching their targets, although no differences in diffusion rates of tobramycin were observed in microcolonies or biofilms [80]. Alginate also confers protection against oxidative stress, as demonstrated by increased sensitivity to hydrogen peroxide and paraquat in non-mucoid strains [81]. Many antimicrobials induce oxidative stress, and alginate may prevent the damage caused by these antimicrobials [82]. However, an algU/T mutant is more sensitive to hypochlorite than an algD (alginate synthesis) mutant, suggesting that AlgU/T contributes to resistance through mechanisms beyond just regulating alginate production [83].
A more specific mechanism by which AlgU/T can regulate antimicrobial resistance is through control of ampR transcription [84]. AmpR regulates expression of the ampC and poxB β-lactamases [84]. Several studies found that alginate production was induced in the presence of a variety of β-lactam antibiotics, suggesting that induction of AlgU/T activity might play a role in responding to these compounds [85, 86]. Similarly, mutations in any of the regulatory components of the AlgU/T system, including algW, mucB, mucD, or algU/T, lead to increased sensitivity to the β-lactam antibiotic, imipenim [87]. Based on this evidence, Balasubramanian et al. were able to demonstrate that the β-lactamase regulator, ampR, is under control of AlgU/T [84]. Although AmpR is active even in an Alg− strain (in which AlgT is held inactive by the MucA anti-sigma factor), auto-induction of the ampR promoter in the presence of β-lactams is about three times higher in an Alg+ strain (in which AlgT is constitutively active due to a mucA mutation) [84]. Thus, AlgU/T plays a positive role in the induction of β-lactamase production.
In addition, AlgU/T impacts antimicrobial resistance by inducing expression of the mexCD-oprJ efflux pump. AlgU/T is necessary for induction of mexCD-oprJ in response to chlorhexidine exposure, and consequently, an algU/T mutant is more sensitive to chlorhexidine [88]. Moreover, AlgU/T is necessary for full induction of mexCD-oprJ expression in response to polymyxin B and several other cationic antimicrobial peptides (CAMPs), all of which are potential substrates of this efflux pump [88].
Whereas much progress has been made to elucidate the mechanisms by which AlgU/T confers resistance, much less is currently known about the functions of an additional pseudomonal ECF sigma factor, σX. The P. aeruginosa σX shares 49% similarity to Bacillus subtilis σW, which is induced by antibiotics that act on the cell wall [82, 58]. A P. aeruginosa sigX mutant is more sensitive to imipenem and polymyxin B [89]. Several genes of the σX regulon have been identified, including ion-gated channels involved in iron homeostasis, a lipid A deacylase, a glucose porin, and the outer membrane porin, oprF [90, 89]. oprF mutants are more sensitive to lysozyme, suggesting that a lack of this porin results in changes to the cell surface structure [91]. Because all of the genes thus far identified as part of the σX regulon are membrane-associated, σX is likely involved in maintaining the composition of the membrane and may therefore play a critical role in modulating the ability of antimicrobials to reach their targets in the cell wall.
Although P. aeruginosa is predicted to have 19 ECF sigma factors, so far only AlgU/T, σX, and the pyoverdin regulator, PvdS, have received much attention [82]. In the related organism, Pseudomonas putida, mutants in ECF-10 are more resistant to β-lactams, sulfonamides, and chloramphenicol due to upregulation of an efflux pump [92]. ECF-10 shares 67% similarity with σI of P. aeruginosa [93]. Whether σI plays a similar role in P. aeruginosa has not been determined. Nevertheless, it remains possible that some of the other ECF sigma factors also contribute to regulation of antimicrobial resistance mechanisms in P. aeruginosa.
4. ECF sigma factor-mediated systems in Gram-positive bacteria
4.1 Streptomyces coelicolor
Streptomyces coelicolor is predicted to encode roughly 50 ECF sigma factors, of which σE is the most extensively studied [30]. σE of S. coelicolor was the first ECF sigma factor to be described [94, 95, 40, 30]. Although its initial discovery focused on its role in transcription of the agarase gene, dagA [94], further studies tied σE to antimicrobial resistance. Paget et al. found that a sigE mutant is more sensitive to lysozyme and other muramidases [47]. Although their analysis found that mutation of sigE does not alter which components are present in the cell wall, a sigE mutant does have altered ratios of a number of cell wall components, which may impact the ability of S. coelicolor to survive insults from cell wall-active antimicrobials.
One other S. coelicolor ECF sigma factor, σR, has been tied to antibiotic resistance [96]. In the presence of translation-inhibiting antibiotics such as chloramphenicol and erythromycin, expression of a stable isoform of σR increases [96]. A sigR mutant is more sensitive than the parent strain to the antibiotics that induce σR expression, but is not more sensitive to unrelated antibiotics, such as ampicillin [96]. The σR response is therefore likely to induce expression of genes that are important for specifically responding to stalled translation, but antibiotic resistance genes within the σR regulon have yet to be identified. Aside from σE and σR, few of the many ECF sigma factors of S. coelicolor have been studied.
4.2 Bacillus subtilis
The model Gram-positive organism, Bacillus subtilis, has seven ECF sigma factors: σM, σW, σX, σV, σY, σZ, and σYlaC [30, 97, 98]. Of these, σW, σM, and σX are the best studied, and appear to be the primary ECF sigma factors in B. subtilis that are responsible for conferring antibiotic resistance, based on the phenotype of a σMWX triple mutant, which is as sensitive to a wide variety of antibiotic classes as a mutant lacking all seven ECF sigma factors [99]. Although their regulons are often overlapping, some of the ECF sigma factors have distinct effects on resistance to specific compounds. For example, σW appears to be the main ECF sigma factor that confers resistance to fosfomycin, whereas σM appears to be the main ECF sigma factor that confers resistance to moenomycin [97].
Some of the earliest studies on σW identified a role for this ECF sigma factor in mediating antimicrobial resistance during stationary phase [100]. By searching for consensus σW promoter sequences within the genome, Huang et al. identified several genes directly regulated by σW that function in cell wall structure and detoxification, including a penicillin binding protein and several ATP-binding cassette (ABC) transporters [101]. More recent studies have identified several genes in the σW regulon with direct antimicrobial resistance functions. For example, fosB, a metallothiol transferase that confers resistance to fosfomycin, is transcribed by σW [102]. Additionally, a σW promoter within the fabHa coding sequence, a gene necessary for initiation of fatty acid synthesis, reduces FabHa production and increases expression of FabF, a protein responsible for fatty acid elongation. The change in production of these two proteins results in decreased membrane fluidity and greater resistance to detergents [103].
Additionally, σW plays a key role in resistance to lantibiotics and other antimicrobials produced by competing bacteria [104, 17]. Genes within the σW regulon that confer resistance to the lantibiotic nisin include sppA, a signal peptide peptidase, yvlC and pspA, phage shock protein homologues, and the yceGHI operon. The mechanisms by which these genes contribute to nisin resistance remain unverified, yet together they fully account for the increased sensitivity of a sigW mutant to nisin [104]. σW also provides innate resistance to a variety of antimicrobials that are produced by other Bacillus species. For example, through regulation of fosB and ydbST (membrane proteins of unknown function), σW confers resistance to amylocyclicin produced by Bacillus amyloliquefaciens FZB42 [17, 105]. Via an unknown mechanism, the σW-dependent operon yqeZyqfAB provides resistance to sublancin when the SPβ resistance genes are absent [17]. Similarly, the σW-dependent operons yfhLM and yknWXYZ confer resistance to the toxic SdpC protein when the SdpI resistance protein is absent [17]. These findings establish σW as important for regulating expression of a number of genes that enable B. subtilis to resist killing by antimicrobials produced by competitor species in the environment.
Evidence suggests that σM also facilitates the ability of B. subtilis to respond to insults to the cell surface. Unlike σW, which is most important during stationary phase, σM has greatest activity during early and exponential growth [106]. σM is further induced in the presence of high extracellular salt concentrations, ethanol, heat shock, acidity, paraquat, and the antimicrobials vancomycin, rhamnolipids, bacitracin, fosfomycin, daptomycin, and friulimicin B [106–108]. Given these stimuli, it is not surprising that the σM regulon contains genes involved in cell division, cell membrane composition, DNA repair, and detoxification [109].
More specifically, Luo and Helmann identified several genes in the σM regulon that increase resistance to antimicrobials that target the cell wall. For example, in the presence of β-lactams, abh expression is increased in a σM-dependent manner [110]. Abh is a transcriptional regulator that activates SlrR, which in turn represses transcription of several autolysins [111, 110]. By transcribing abh in the presence of β-lactams, σM makes B. subtilis less susceptible to the autolytic effects of β-lactams and does so independently of the role of Abh in exopolysaccharide production [110, 112]. Additionally, Abh induces biofilm formation, and biofilms themselves contribute to greater resistance to antimicrobials [111, 113]. Another important resistance gene in the σM regulon is spx [109, 114]. Spx helps cells deal with oxidative stress and is induced in bacitracin and enduracidin [115]. Moreover, σM increases expression of disA, a diadenylate cyclase (DAC). A mutant lacking disA is moderately more sensitive to moenomycin, and c-di-AMP levels influence maintenance of the cell wall [110]. Therefore, σM may contribute to resistance to antimicrobials targeting the cell wall by controlling disA expression. σM also regulates ltaSa in response to nisin exposure [104]. LtaSa is a lipoteichoic acid (LTA) synthase that produces longer LTA than the primary synthase, which may impede access of nisin to lipid-II and the membrane, thereby conferring resistance [104].
The mechanism by which σM confers resistance to bacitracin has also been examined. The key member of the σM regulon that is induced in the presence of bacitracin is bcrC. This gene encodes an undecaprenyl pyrophosphate (UPP) phosphatase. BcrC helps cells to overcome the inhibition of cell wall synthesis that occurs due to the inhibition of UPP dephosphorylation caused by bacitracin [116]. Although several two-component systems, including the BceRS, YvcPQ, and LiaRS systems, respond to bacitracin at lower concentrations than are necessary for inducing σM, the σM-dependent induction of bcrC nevertheless remains an important mechanism by which B. subtilis can resist killing by bacitracin [116].
The third ECF sigma factor that plays a significant role in regulating antimicrobial resistance in B. subtilis is σX. There are several genes in the σX regulon with clear effects on resistance. For example, σX regulates the dlt operon, which is responsible for D-alanylating teichoic acids, a process that decreases the negative charge on the cell surface and thereby provides protection against CAMPs [117]. σX has an additional effect on cell surface charge by inducing the pss operon, which is responsible for synthesizing phosphatidylethanolamine, a zwitterionic lipid that can reduce the net negative charge of the bacterial surface by decreasing the proportion of negative lipids in the membrane [117]. Like σM, σX transcribes abh transcription, which enhances resistance to β-lactams [100, 118, 119, 112]. Even though σM appears to be far more important than σX for inducing abh, σX has an additional unique effect on autolysins by inducing lytR, which inhibits autolysin production [119].
Despite the clear importance of the three ECF sigma factors described above, the roles of the remaining four ECF sigma factors in B. subtilis remain largely obscure. It appears that the σV regulon overlaps considerably with that of σM, σW, and σX and includes bcrC and dltABCDE [120]. In addition, σV contributes to lysozyme resistance by inducing the expression of both dltABCDE and oatA, a peptidoglycan acetylating enzyme [121, 122]. σY, on the other hand, has a unique and limited regulon consisting only of itself and a gene of unknown function, ybgB [123]. Additional functions for these remaining ECF sigma factors are hinted at in the study by Luo et al. that identified several phenotypes that appear only in a mutant of all seven ECF sigma factors, but not in a σMWX triple mutant [99]. These phenotypes included decreased production of exopolysaccharide and increased sensitivity to cefuroxime, ciprofloxacin, and oflaxacin. Single σV, σY, σZ, or σYlaC mutants, however, did not display any of these phenotypes, making it unclear which factor(s) are responsible for these phenotypes in the heptad mutant [99]. Further investigations will be necessary to tease out the functions of these other ECF sigma factors.
4.3 Clostridium difficile
Research on ECF sigma factors in B. subtilis laid the groundwork for much of what is known about the ECF sigma factors in the intestinal pathogen, Clostridium difficile. Ho and Ellermeier identified three ECF sigma factors in C. difficile: σT, σU, and σV (also known as CsfT, CsfU, and CsfV), all of which appear to play a role in regulating antimicrobial resistance [124]. All three ECF sigma factors are induced in the presence of bacitracin and lysozyme [124]. In the presence of bacitracin or lysozyme, the protease, PrsW, releases σT and σU from their anti-sigma factors, RsiT and RsiU, respectively. A prsW mutant, which consequently has inactive σT and σU, is more sensitive to bacitracin and lysozyme [124]. A similar protease has not been found for regulation of σV, but lysozyme binds to the σV anti-sigma factor, RsiV, which may allow for direct induction of σV activity by lysozyme as it does in B. subtilis [125]. Induction of σV is necessary for lysozyme resistance, an effect that is partly mediated by one of the genes in the σV regulon, pdaV. This gene encodes a peptidoglycan deacetylase, which may help C. difficile avoid damage from lysozyme by making its peptidoglycans less susceptible to lysozyme cleavage [126]. Moreover, σV upregulates dlt expression in C. difficile in response to lysozyme, and a dlt mutant is significantly more sensitive to lysozyme and polymyxin B [127, 128]. Other genes in the regulons of these ECF sigma factors may also contribute to their ability to modulate antimicrobial resistance but have yet to be identified.
4.4 Enterococcus faecalis
Enterococcus faecalis is predicted to encode two ECF sigma factors, although to date only σV has been extensively studied [30]. Like σV in B. subtilis and C. difficile, σV in E. faecalis is critical for lysozyme resistance [129]. Le Jeune et al. demonstrated that sigV is the most important gene for lysozyme resistance, because a sigV oatA dltA triple mutant was significantly more sensitive to lysozyme than an oatA dltA double mutant, even though oatA and dltA are the key mediators of lysozyme resistance. Although oatA and dltA are regulated by the orthologous σV in B. subtilis, transcription of oatA and dltA is not controlled by σV in E. faecalis [129]. This finding suggests that there are additional σV-controlled mechanisms that mediate lysozyme resistance in E. faecalis.
The E. faecalis σV regulon has not been fully determined; however, Varahan et al. have identified a few mechanisms of σV-mediated lysozyme resistance in this bacterium. For one, they found that overexpression of σV (via mutation of its anti-sigma factor, rsiV) results in cell chaining, which implies that σV regulates autolysin activity [130]. Autolysins play a role in lysozyme-mediated damage, in part because autolysin activity is enhanced in lysozyme [131, 132]. Moreover, both rsiV and sigV mutants bind more lysozyme, suggesting that σV regulates components of the cell surface that can deter lysozyme binding [130]. Additional research is needed to determine additional genes in the σV regulon.
5. Discussion
The ECF sigma factors that have demonstrated roles in the regulation of antimicrobial resistance genes are summarized in Table 1. Considering the diverse range of bacterial species described, it is clear that ECF sigma factors have proven useful throughout evolutionary history as a way for bacteria to modulate intrinsic antimicrobial resistance mechanisms. Constitutive expression of intrinsic resistance genes would be the most reliable way to ensure that a bacterium could survive the sudden introduction of antimicrobials into its environment; however, there is a long-established correlation between increased resistance and fitness cost [133]. If there is a fitness cost associated with a particular resistance mechanism, it would be advantageous for a bacterium to be able to exhibit the resistance phenotype only when a threat exists. Even if no fitness cost exists, the production of resistance-mediating proteins could impose an unnecessary metabolic burden on the bacterium if those proteins had no relevance in an antimicrobial-free environment. The regulation of antimicrobial resistance by ECF sigma factors allows a bacterium to quickly sense antimicrobial signals and respond by activating resistance genes and repair mechanisms that are specific to that antimicrobial, only when present.
Table 1.
Summary of ECF sigma factors with a demonstrated role in antimicrobial resistance regulation.
| Organism | ECF sigma factor(s) | Induced by antimicrobials? | Genes in regulon that affect antimicrobial resistance | Mechanism(s) of resistance | Confers resistance to |
|---|---|---|---|---|---|
| E. coli | RpoE | NDa | unknown | maintain cell membrane integrity | polymyxin B |
| S. enterica | RpoE (serovar Typhimurium) | Yes | fdhD, smpA | alternate terminal electron acceptor use, outer membrane protein assembly | polymyxin B, P2, cryptdin-4 |
| rpoE (serovar Typhi) | ND | ompC, ompF, ramA | antimicrobial access to periplasm, antimicrobial efflux | *sensitivity* to β-lactams, quinolones, aminoglycosides | |
| P. aeruginosa | AlgU/T | Yes | algD, ampR, mecCD-oprJ | alginate production, β-lactamase production, antimicrobial efflux | β-lactams, tobramycin, chlorhexidine |
| SigX | ND | oprF | porin | imipenem, polymyxin B | |
| S. coelicolor | σE | Yes | unknown | maintain ratios of cell wall components | lysozyme |
| σR | Yes | unknown | unknown | chloramphenicol, erythromycin, lincomycin, tetracycline | |
| B. subtilis | σW | Yes | fosB, fabHa, fabF, sppA, yvlC, pspA, yceGHI, ydbST, yqeZyqfAB, yfhLM, yknWXYZ | regulate membrane fluidity, other unknown mechanisms | fosfomycin, nisin, amylocyclicin, sublancin, bacteriocins |
| σM | Yes | abh, spx, disA, ltaSa, bcrC | decreased autolysin production, biofilm formation, response to oxidative stress, diadenylate cyclase regulation of cell wall, LTA synthesis, UPP phosphatase | moenomycin, vancomycin, β-lactams, nisin, bacitracin, enduracidin | |
| σX | Yes | dlt, pss, abh, lytR | cell surface charge, decreased autolysin production | β-lactams, nisin | |
| σV | Yes | dlt, bcrC, oatA | cell surface charge, UPP phosphatase, peptidoglycan acetylation | lysozyme | |
| C. difficileb | σT, σU | Yes | unknown | unknown | bacitracin, lysozyme |
| σV | Yes | pdaV, dlt | peptidoglycan deacetylation, cell surface charge | lysozyme | |
| E. faecalis | σV | Yes | unknown | cell wall turnover, prevention of lysozyme binding | lysozyme |
ND = Not determined
C. difficile ECF sigma factors are also annotated as CsfT, CsfU, and CsfV.
It is important to note, however, that not all of the ECF sigma factors that play a role in antimicrobial resistance do so through specific, antimicrobial-induced mechanisms. For example, RpoE in E. coli is induced by misfolded proteins in the outer membrane rather than by a specific antimicrobial, yet the downstream effects of RpoE activation provide protection against membrane-active antimicrobials such as polymyxin B. In this way, ECF sigma factors can play a role in both specific and broad mechanisms of resistance.
The importance of these regulatory systems is further highlighted by the overlap and redundancy evident in some species. The overlapping regulons of σM, σW, and σX in B. subtilis are perhaps the most striking example of ECF redundancy, but E. coli also has a measure of regulatory redundancy between RpoE and the CpxAR two-component system [134]. Such redundancy may serve several functions. First, it may simply provide a mechanism so that regulatory systems can respond differently based on antimicrobial concentration. Second, different regulators may be more important at certain times of growth, as seems to be the case in B. subtilis [106]. Finally, antimicrobial compounds that are very different structurally (and therefore may trigger different systems) may cause similar types of damage that require expression of a common set of defenses. Induction of bcrC expression in B. subtilis provides an example of this phenomenon; bacitracin induces expression of only σM, yet both σM and σX can activate bcrC transcription [135].
Functional redundancy could be one possible explanation for the absence of ECF sigma factors in some pathogens. For example, Campylobacter jejuni, Chlamydia trachomatis, Streptococcus pneumoniae, and Helicobacter pylori do not encode identifiable ECF sigma factors [30]. In these pathogens, other regulatory mechanisms, such as two-component systems may fulfill similar functions to compensate for the lack of ECF sigma factors [136–141]. Other pathogens, such as Haemophilus influenza, Neisseria gonnorhea, and Listeria monocytogenes have ECF sigma factors that are clearly involved in membrane stress responses, but their specific roles in antibiotic resistance have not been determined [142–145].
Finally, it is notable that ECF sigma factors are not only implicated in regulating resistance to antimicrobials, but they also often play an important role in regulating production of self-made antimicrobials [118, 146–149]. Although it is outside the scope of this review to discuss this function of ECF sigma factors in detail, this additional function emphasizes the close relationship between resistance and antimicrobial production.
6. Conclusion
ECF sigma factors provide a mechanism by which bacteria can respond to extracellular threats, such as antimicrobials, by inducing expression of resistance mechanisms. To date, they have not been as extensively characterized as two-component systems, but it is clear that ECF sigma factors are key factors in the response to antimicrobials for many species of bacteria. Even though sequence homology studies have identified hundreds of ECF sigma factors, few have been studied. It will be important to determine whether these unstudied ECF sigma factors also function in antimicrobial resistance regulation. By characterizing these pathways, we may uncover new therapeutic targets in the ongoing battle against antimicrobial-resistant pathogens.
Acknowledgments
The authors would like to thank William Shafer and members of the McBride lab for helpful criticism of this manuscript. This research was supported by the U.S. National Institutes of Health through research grants DK087763, DK101870, AI109526 and AI116933 to S.M.M. and T32 GM008169 to E.C.W. The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors do not have any conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.CDC. Antibiotic Resistance Threats in the United States, 2013. 2013. [Google Scholar]
- 2.El-Halfawy OM, Valvano MA. Non-genetic mechanisms communicating antibiotic resistance: rethinking strategies for antimicrobial drug design. Expert Opin Drug Discov. 2012;7:923–33. doi: 10.1517/17460441.2012.712512. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Ortega C, Wiegand I, Olivares J, Hancock RE, Martinez JL. The intrinsic resistome of Pseudomonas aeruginosa to beta-lactams. Virulence. 2011;2:144–6. doi: 10.4161/viru.2.2.15014. [DOI] [PubMed] [Google Scholar]
- 4.Bhullar K, Waglechner N, Pawlowski A, Koteva K, Banks ED, Johnston MD, et al. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One. 2012;7:e34953. doi: 10.1371/journal.pone.0034953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake KL, O'Neill AJ. Transposon library screening for identification of genetic loci participating in intrinsic susceptibility and acquired resistance to antistaphylococcal agents. J Antimicrob Chemother. 2013;68:12–6. doi: 10.1093/jac/dks373. [DOI] [PubMed] [Google Scholar]
- 6.Cox G, Wright GD. Intrinsic antibiotic resistance: mechanisms, origins, challenges and solutions. Int J Med Microbiol. 2013;303:287–92. doi: 10.1016/j.ijmm.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 7.D'Costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–61. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 8.Fajardo A, Martinez-Martin N, Mercadillo M, Galan JC, Ghysels B, Matthijs S, et al. The neglected intrinsic resistome of bacterial pathogens. PLoS One. 2008;3:e1619. doi: 10.1371/journal.pone.0001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez MJ, Neyfakh AA. Genes involved in intrinsic antibiotic resistance of Acinetobacter baylyi. Antimicrob Agents Chemother. 2006;50:3562–7. doi: 10.1128/AAC.00579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XZ, Livermore DM, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–41. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–8. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 12.Nawrocki KL, Crispell EK, McBride SM. Antimicrobial peptide resistance mechanisms of Gram-positive bacteria. Antibiotics (Basel) 2014;3:461–92. doi: 10.3390/antibiotics3040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBride SM, Sonenshein AL. Identification of a genetic locus responsible for antimicrobial peptide resistance in Clostridium difficile. Infect Immun. 2011;79:167–76. doi: 10.1128/IAI.00731-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pumbwe L, Ueda O, Yoshimura F, Chang A, Smith RL, Wexler HM. Bacteroides fragilis BmeABC efflux systems additively confer intrinsic antimicrobial resistance. J Antimicrob Chemother. 2006;58:37–46. doi: 10.1093/jac/dkl202. [DOI] [PubMed] [Google Scholar]
- 15.Poole K. Efflux-mediated multiresistance in Gram-negative bacteria. Clin Microbiol Infect. 2004;10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 16.Samuelsen O, Haukland HH, Jenssen H, Kramer M, Sandvik K, Ulvatne H, et al. Induced resistance to the antimicrobial peptide lactoferricin B in Staphylococcus aureus. FEBS Lett. 2005;579:3421–6. doi: 10.1016/j.febslet.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Butcher BG, Helmann JD. Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol Microbiol. 2006;60:765–82. doi: 10.1111/j.1365-2958.2006.05131.x. [DOI] [PubMed] [Google Scholar]
- 18.Barclay ML, Begg EJ, Chambers ST. Adaptive resistance following single doses of gentamicin in a dynamic in vitro model. Antimicrob Agents Chemother. 1992;36:1951–7. doi: 10.1128/aac.36.9.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daikos GL, Jackson GG, Lolans VT, Livermore DM. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis. 1990;162:414–20. doi: 10.1093/infdis/162.2.414. [DOI] [PubMed] [Google Scholar]
- 20.Dean AC. Adaptive drug resistance in Gram-negative bacteria. Proc R Soc Med. 1971;64:537. doi: 10.1177/003591577106400531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore RA, Chan L, Hancock RE. Evidence for two distinct mechanisms of resistance to polymyxin B in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;26:539–45. doi: 10.1128/aac.26.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez H, Rico S, Díaz M, Santamaría RI. Two-component systems in Streptomyces: key regulators of antibiotic complex pathways. Microbial Cell Factories. 2013;12:127. doi: 10.1186/1475-2859-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother. 2009;53:3628–34. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez L, Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock RE. Adaptive resistance to the "last hope" antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob Agents Chemother. 2010;54:3372–82. doi: 10.1128/AAC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunn JS, Miller SI. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178:6857–64. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother. 2004;48:3298–304. doi: 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meehl M, Herbert S, Gotz F, Cheung A. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:2679–89. doi: 10.1128/AAC.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otto M. Bacterial sensing of antimicrobial peptides. Contrib Microbiol. 2009;16:136–49. doi: 10.1159/000219377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suarez JM, Edwards AN, McBride SM. The Clostridium difficile cpr locus is regulated by a noncontiguous two-component system in response to type A and B lantibiotics. J Bacteriol. 2013;195:2621–31. doi: 10.1128/JB.00166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helmann JD. The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol. 2002;46:47–110. doi: 10.1016/s0065-2911(02)46002-x. [DOI] [PubMed] [Google Scholar]
- 31.Staro3 A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, Mascher T. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) s factor protein family. Mol Microbiol. 2009;74:557–81. doi: 10.1111/j.1365-2958.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 32.Ho TD, Ellermeier CD. Extra cytoplasmic function sigma factor activation. Curr Opin Microbiol. 2012;15:182–8. doi: 10.1016/j.mib.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–66. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 34.Bashyam MD, Hasnain SE. The extracytoplasmic function sigma factors: role in bacterial pathogenesis. Infect Genet Evol. 2004;4:301–8. doi: 10.1016/j.meegid.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Mascher T. Signaling diversity and evolution of extracytoplasmic function (ECF) sigma factors. Curr Opin Microbiol. 2013;16:148–55. doi: 10.1016/j.mib.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Staron A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, Mascher T. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol Microbiol. 2009;74:557–81. doi: 10.1111/j.1365-2958.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 37.Paget MS. Bacterial Sigma Factors and Anti-Sigma Factors: Structure, Function and Distribution. Biomolecules. 2015;5:1245–65. doi: 10.3390/biom5031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lonetto M, Gribskov M, Gross CA. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–9. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paget MS, Helmann JD. The sigma70 family of sigma factors. Genome Biol. 2003;4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lonetto MA, Brown KL, Rudd KE, Buttner MJ. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci U S A. 1994;91:7573–7. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun V. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch Microbiol. 1997;167:325–31. doi: 10.1007/s002030050451. [DOI] [PubMed] [Google Scholar]
- 42.Leoni L, Orsi N, de Lorenzo V, Visca P. Functional analysis of PvdS, an iron starvation sigma factor of Pseudomonas aeruginosa. J Bacteriol. 2000;182:1481–91. doi: 10.1128/jb.182.6.1481-1491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sexton R, Gill PR, Jr, Callanan MJ, O'Sullivan DJ, Dowling DN, O'Gara F. Iron-responsive gene expression in Pseudomonas fluorescens M114: cloning and characterization of a transcription-activating factor, PbrA. Mol Microbiol. 1995;15:297–306. doi: 10.1111/j.1365-2958.1995.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 44.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. Fecl belongs to a new subfamily of sigma 70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–74. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 45.Pradel E, Locht C. Expression of the putative siderophore receptor gene bfrZ is controlled by the extracytoplasmic-function sigma factor BupI in Bordetella bronchiseptica. J Bacteriol. 2001;183:2910–7. doi: 10.1128/JB.183.9.2910-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward MJ, Lew H, Treuner-Lange A, Zusman DR. Regulation of motility behavior in Myxococcus xanthus may require an extracytoplasmic-function sigma factor. J Bacteriol. 1998;180:5668–75. doi: 10.1128/jb.180.21.5668-5675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paget MS, Chamberlin L, Atrih A, Foster SJ, Buttner MJ. Evidence that the extracytoplasmic function sigma factor sigmaE is required for normal cell wall structure in Streptomyces coelicolor A3(2) J Bacteriol. 1999;181:204–11. doi: 10.1128/jb.181.1.204-211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Las Penas A, Connolly L, Gross CA. The sigmaE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigmaE. Mol Microbiol. 1997;24:373–85. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 49.Gorham HC, McGowan SJ, Robson PR, Hodgson DA. Light-induced carotenogenesis in Myxococcus xanthus: light-dependent membrane sequestration of ECF sigma factor CarQ by anti-sigma factor CarR. Mol Microbiol. 1996;19:171–86. doi: 10.1046/j.1365-2958.1996.360888.x. [DOI] [PubMed] [Google Scholar]
- 50.Missiakas D, Mayer MP, Lemaire M, Georgopoulos C, Raina S. Modulation of the Escherichia coli sigmaE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol Microbiol. 1997;24:355–71. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 51.Xie ZD, Hershberger CD, Shankar S, Ye RW, Chakrabarty AM. Sigma factor-anti-sigma factor interaction in alginate synthesis: inhibition of AlgT by MucA. J Bacteriol. 1996;178:4990–6. doi: 10.1128/jb.178.16.4990-4996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anthony JR, Newman JD, Donohue TJ. Interactions between the Rhodobacter sphaeroides ECF sigma factor, sigma(E), and its anti-sigma factor, ChrR. J Mol Biol. 2004;341:345–60. doi: 10.1016/j.jmb.2004.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimura M, Asai K, Sadaie Y, Yoshikawa H. Interaction of Bacillus subtilis extracytoplasmic function (ECF) sigma factors with the N-terminal regions of their potential anti-sigma factors. Microbiology. 2004;150:591–9. doi: 10.1099/mic.0.26712-0. [DOI] [PubMed] [Google Scholar]
- 54.Campbell EA, Tupy JL, Gruber TM, Wang S, Sharp MM, Gross CA, et al. Crystal structure of Escherichia coli sigmaE with the cytoplasmic domain of its anti-sigma RseA. Mol Cell. 2003;11:1067–78. doi: 10.1016/s1097-2765(03)00148-5. [DOI] [PubMed] [Google Scholar]
- 55.Alba BM, Leeds JA, Onufryk C, Lu CZ, Gross CA. DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Genes Dev. 2002;16:2156–68. doi: 10.1101/gad.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellermeier CD, Losick R. Evidence for a novel protease governing regulated intramembrane proteolysis and resistance to antimicrobial peptides in Bacillus subtilis. Genes Dev. 2006;20:1911–22. doi: 10.1101/gad.1440606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hastie JL, Williams KB, Sepulveda C, Houtman JC, Forest KT, Ellermeier CD. Evidence of a bacterial receptor for lysozyme: binding of lysozyme to the anti-sigma factor RsiV controls activation of the ECF sigma factor sigmaV. PLoS Genet. 2014;10:e1004643. doi: 10.1371/journal.pgen.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schobel S, Zellmeier S, Schumann W, Wiegert T. The Bacillus subtilis sigmaW anti-sigma factor RsiW is degraded by intramembrane proteolysis through YluC. Mol Microbiol. 2004;52:1091–105. doi: 10.1111/j.1365-2958.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 59.Ades SE, Connolly LE, Alba BM, Gross CA. The Escherichia coli sigma(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor. Genes & Development. 1999;13:2449–61. doi: 10.1101/gad.13.18.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rouviere PE, De Las Penas A, Mecsas J, Lu CZ, Rudd KE, Gross CA. rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO J. 1995;14:1032–42. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mecsas J, Rouviere PE, Erikson JW, Donohue TJ, Gross CA. The activity of SigmaE, an Escheria coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–28. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 62.Dartigalongue C, Missiakas D, Raina S. Characterization of the Escherichia coli sigma E regulon. J Biol Chem. 2001;276:20866–75. doi: 10.1074/jbc.M100464200. [DOI] [PubMed] [Google Scholar]
- 63.De Las Penas A, Connolly L, Gross CA. SigmaE is an essential sigma factor in Escherichia coli. J Bacteriol. 1997;179:6862–4. doi: 10.1128/jb.179.21.6862-6864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayden JD, Ades SE. The extracytoplasmic stress factor, sigmaE, is required to maintain cell envelope integrity in Escherichia coli. PLoS One. 2008;3:e1573. doi: 10.1371/journal.pone.0001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulesus RR, Diaz-Perez K, Slechta ES, Eto DS, Mulvey MA. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect Immun. 2008;76:3019–26. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Humphreys S, Stevenson A, Bacon A, Weinhardt AB, Roberts M. The alternative sigma factor, sigmaE, is critically important for the virulence of Salmonella typhimurium. Infect Immun. 1999;67:1560–8. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crouch ML, Becker LA, Bang IS, Tanabe H, Ouellette AJ, Fang FC. The alternative sigma factor sigmaE is required for resistance of Salmonella enterica serovar Typhimurium to antimicrobial peptides. Mol Microbiol. 2005;56:789–99. doi: 10.1111/j.1365-2958.2005.04578.x. [DOI] [PubMed] [Google Scholar]
- 68.Barker HC, Kinsella N, Jaspe A, Friedrich T, O'Connor CD. Formate protects stationary-phase Escherichia coli and Salmonella cells from killing by a cationic antimicrobial peptide. Mol Microbiol. 2000;35:1518–29. doi: 10.1046/j.1365-2958.2000.01820.x. [DOI] [PubMed] [Google Scholar]
- 69.Lewis C, Skovierova H, Rowley G, Rezuchova B, Homerova D, Stevenson A, et al. Small outer-membrane lipoprotein, SmpA, is regulated by sigmaE and has a role in cell envelope integrity and virulence of Salmonella enterica serovar Typhimurium. Microbiology. 2008;154:979–88. doi: 10.1099/mic.0.2007/011999-0. [DOI] [PubMed] [Google Scholar]
- 70.Xie X, Zhang H, Zheng Y, Li A, Wang M, Zhou H, et al. RpoE is a putative antibiotic resistance regulator of Salmonella enteric Serovar Typhi. Curr Microbiol. 2016;72:457–64. doi: 10.1007/s00284-015-0983-7. [DOI] [PubMed] [Google Scholar]
- 71.DeVries CA, Ohman DE. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. J Bacteriol. 1994;176:6677–87. doi: 10.1128/jb.176.21.6677-6687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hershberger CD, Ye RW, Parsek MR, Xie ZD, Chakrabarty AM. The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor (sigma E) Proc Natl Acad Sci U S A. 1995;92:7941–5. doi: 10.1073/pnas.92.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Firoved AM, Boucher JC, Deretic V. Global genomic analysis of AlgU (sigma(E))-dependent promoters (sigmulon) in Pseudomonas aeruginosa and implications for inflammatory processes in cystic fibrosis. J Bacteriol. 2002;184:1057–64. doi: 10.1128/jb.184.4.1057-1064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Firoved AM, Deretic V. Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. J Bacteriol. 2003;185:1071–81. doi: 10.1128/JB.185.3.1071-1081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Govan JR. Antibiotic therapy and cystic fibrosis: increased resistance of mucoid Pseudomonas aeruginosa to carbenicillin. J Antimicrob Chemother. 1976;2:215–7. doi: 10.1093/jac/2.2.215. [DOI] [PubMed] [Google Scholar]
- 76.Govan JR, Fyfe JA. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978;4:233–40. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- 77.Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–24. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gristina AG, Jennings RA, Naylor PT, Myrvik QN, Webb LX. Comparative in vitro antibiotic resistance of surface-colonizing coagulase-negative staphylococci. Antimicrob Agents Chemother. 1989;33:813–6. doi: 10.1128/aac.33.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis. 2015;34:877–86. doi: 10.1007/s10096-015-2323-z. [DOI] [PubMed] [Google Scholar]
- 80.Nichols WW, Dorrington SM, Slack MP, Walmsley HL. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother. 1988;32:518–23. doi: 10.1128/aac.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin DW, Schurr MJ, Yu H, Deretic V. Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: relationship to sigma E and stress response. J Bacteriol. 1994;176:6688–96. doi: 10.1128/jb.176.21.6688-6696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potvin E, Sanschagrin F, Levesque RC. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol Rev. 2008;32:38–55. doi: 10.1111/j.1574-6976.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 83.Yu H, Boucher JC, Hibler NS, Deretic V. Virulence properties of Pseudomonas aeruginosa lacking the extreme-stress sigma factor AlgU (sigmaE) Infect Immun. 1996;64:2774–81. doi: 10.1128/iai.64.7.2774-2781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balasubramanian D, Kong KF, Jayawardena SR, Leal SM, Sautter RT, Mathee K. Co-regulation of beta-lactam resistance, alginate production and quorum sensing in Pseudomonas aeruginosa. J Med Microbiol. 2011;60:147–56. doi: 10.1099/jmm.0.021600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bagge N, Schuster M, Hentzer M, Ciofu O, Givskov M, Greenberg EP, et al. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production. Antimicrob Agents Chemother. 2004;48:1175–87. doi: 10.1128/AAC.48.4.1175-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wood LF, Leech AJ, Ohman DE. Cell wall-inhibitory antibiotics activate the alginate biosynthesis operon in Pseudomonas aeruginosa: Roles of sigma (AlgT) and the AlgW and Prc proteases. Mol Microbiol. 2006;62:412–26. doi: 10.1111/j.1365-2958.2006.05390.x. [DOI] [PubMed] [Google Scholar]
- 87.Alvarez-Ortega C, Wiegand I, Olivares J, Hancock RE, Martinez JL. Genetic determinants involved in the susceptibility of Pseudomonas aeruginosa to beta-lactam antibiotics. Antimicrob Agents Chemother. 2010;54:4159–67. doi: 10.1128/AAC.00257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fraud S, Campigotto AJ, Chen Z, Poole K. MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa: involvement in chlorhexidine resistance and induction by membrane-damaging agents dependent upon the AlgU stress response sigma factor. Antimicrob Agents Chemother. 2008;52:4478–82. doi: 10.1128/AAC.01072-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brinkman FS, Schoofs G, Hancock RE, De Mot R. Influence of a putative ECF sigma factor on expression of the major outer membrane protein, OprF, in Pseudomonas aeruginosa and Pseudomonas fluorescens. J Bacteriol. 1999;181:4746–54. doi: 10.1128/jb.181.16.4746-4754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duchesne R, Bouffartigues E, Oxaran V, Maillot O, Benard M, Feuilloley MG, et al. A proteomic approach of SigX function in Pseudomonas aeruginosa outer membrane composition. J Proteomics. 2013;94:451–9. doi: 10.1016/j.jprot.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 91.Bouffartigues E, Gicquel G, Bazire A, Bains M, Maillot O, Vieillard J, et al. Transcription of the oprF gene of Pseudomonas aeruginosa is dependent mainly on the SigX sigma factor and is sucrose induced. J Bacteriol. 2012;194:4301–11. doi: 10.1128/JB.00509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tettmann B, Dotsch A, Armant O, Fjell CD, Overhage J. Knockout of extracytoplasmic function sigma factor ECF-10 affects stress resistance and biofilm formation in Pseudomonas putida KT2440. Appl Environ Microbiol. 2014;80:4911–9. doi: 10.1128/AEM.01291-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Bueno MA, Tobes R, Rey M, Ramos JL. Detection of multiple extracytoplasmic function (ECF) sigma factors in the genome of Pseudomonas putida KT2440 and their counterparts in Pseudomonas aeruginosa PA01. Environ Microbiol. 2002;4:842–55. doi: 10.1046/j.1462-2920.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- 94.Buttner MJ, Fearnley IM, Bibb MJ. The agarase gene (dagA) of Streptomyces coelicolor A3(2): nucleotide sequence and transcriptional analysis. Mol Gen Genet. 1987;209:101–9. doi: 10.1007/BF00329843. [DOI] [PubMed] [Google Scholar]
- 95.Buttner MJ, Smith AM, Bibb MJ. At least three different RNA polymerase holoenzymes direct transcription of the agarase gene (dagA) of Streptomyces coelicolor A3(2) Cell. 1988;52:599–607. doi: 10.1016/0092-8674(88)90472-2. [DOI] [PubMed] [Google Scholar]
- 96.Yoo JS, Oh GS, Ryoo S, Roe JH. Induction of a stable sigma factor SigR by translation-inhibiting antibiotics confers resistance to antibiotics. Sci Rep. 2016;6:28628. doi: 10.1038/srep28628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mascher T, Hachmann AB, Helmann JD. Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function sigma factors. J Bacteriol. 2007;189:6919–27. doi: 10.1128/JB.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Helmann JD. Bacillus subtilis extracytoplasmic function (ECF) sigma factors and defense of the cell envelope. Curr Opin Microbiol. 2016;30:122–32. doi: 10.1016/j.mib.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luo Y, Asai K, Sadaie Y, Helmann JD. Transcriptomic and phenotypic characterization of a Bacillus subtilis strain without extracytoplasmic function sigma factors. J Bacteriol. 2010;192:5736–45. doi: 10.1128/JB.00826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang X, Fredrick KL, Helmann JD. Promoter recognition by Bacillus subtilis sigmaW: autoregulation and partial overlap with the sigmaX regulon. J Bacteriol. 1998;180:3765–70. doi: 10.1128/jb.180.15.3765-3770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang X, Gaballa A, Cao M, Helmann JD. Identification of target promoters for the Bacillus subtilis extracytoplasmic function sigma factor, sigma W. Mol Microbiol. 1999;31:361–71. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 102.Cao M, Bernat BA, Wang Z, Armstrong RN, Helmann JD. FosB, a cysteine-dependent fosfomycin resistance protein under the control of sigma(W), an extracytoplasmic-function sigma factor in Bacillus subtilis. J Bacteriol. 2001;183:2380–3. doi: 10.1128/JB.183.7.2380-2383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kingston AW, Subramanian C, Rock CO, Helmann JD. A sigmaW-dependent stress response in Bacillus subtilis that reduces membrane fluidity. Mol Microbiol. 2011;81:69–79. doi: 10.1111/j.1365-2958.2011.07679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kingston AW, Liao X, Helmann JD. Contributions of the sigma(W) , sigma(M) and sigma(X) regulons to the lantibiotic resistome of Bacillus subtilis. Mol Microbiol. 2013;90:502–18. doi: 10.1111/mmi.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scholz R, Vater J, Budiharjo A, Wang Z, He Y, Dietel K, et al. Amylocyclicin, a Novel Circular Bacteriocin Produced by Bacillus amyloliquefaciens FZB42. J Bacteriol. 2014;196:1842–52. doi: 10.1128/JB.01474-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thackray PD, Moir A. SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. J Bacteriol. 2003;185:3491–8. doi: 10.1128/JB.185.12.3491-3498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wecke T, Zuhlke D, Mader U, Jordan S, Voigt B, Pelzer S, et al. Daptomycin versus friulimicin B: in-depth profiling of Bacillus subtilis cell envelope stress responses. Antimicrob Agents Chemother. 2009;53:1619–23. doi: 10.1128/AAC.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wecke T, Bauer T, Harth H, Mader U, Mascher T. The rhamnolipid stress response of Bacillus subtilis. FEMS Microbiol Lett. 2011;323:113–23. doi: 10.1111/j.1574-6968.2011.02367.x. [DOI] [PubMed] [Google Scholar]
- 109.Eiamphungporn W, Helmann JD. The Bacillus subtilis sigma(M) regulon and its contribution to cell envelope stress responses. Mol Microbiol. 2008;67:830–48. doi: 10.1111/j.1365-2958.2007.06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo Y, Helmann JD. Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol. 2012;83:623–39. doi: 10.1111/j.1365-2958.2011.07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murray EJ, Strauch MA, Stanley-Wall NR. SigmaX is involved in controlling Bacillus subtilis biofilm architecture through the AbrB homologue Abh. J Bacteriol. 2009;191:6822–32. doi: 10.1128/JB.00618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murray EJ, Stanley-Wall NR. The sensitivity of Bacillus subtilis to diverse antimicrobial compounds is influenced by Abh. Arch Microbiol. 2010;192:1059–67. doi: 10.1007/s00203-010-0630-4. [DOI] [PubMed] [Google Scholar]
- 113.Vega NM, Gore J. Collective antibiotic resistance: mechanisms and implications. Curr Opin Microbiol. 2014;21:28–34. doi: 10.1016/j.mib.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jervis AJ, Thackray PD, Houston CW, Horsburgh MJ, Moir A. SigM-responsive genes of Bacillus subtilis and their promoters. J Bacteriol. 2007;189:4534–8. doi: 10.1128/JB.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rukmana A, Morimoto T, Takahashi H, Giyanto, Ogasawara N. Assessment of transcriptional responses of Bacillus subtilis cells to the antibiotic enduracidin, which interferes with cell wall synthesis, using a high-density tiling chip. Genes Genet Syst. 2009;84:253–67. doi: 10.1266/ggs.84.253. [DOI] [PubMed] [Google Scholar]
- 116.Rietkotter E, Hoyer D, Mascher T. Bacitracin sensing in Bacillus subtilis. Mol Microbiol. 2008;68:768–85. doi: 10.1111/j.1365-2958.2008.06194.x. [DOI] [PubMed] [Google Scholar]
- 117.Cao M, Helmann JD. The Bacillus subtilis extracytoplasmic-function sigmaX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J Bacteriol. 2004;186:1136–46. doi: 10.1128/JB.186.4.1136-1146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luo Y, Helmann JD. Extracytoplasmic function sigma factors with overlapping promoter specificity regulate sublancin production in Bacillus subtilis. J Bacteriol. 2009;191:4951–8. doi: 10.1128/JB.00549-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang X, Helmann JD. Identification of target promoters for the Bacillus subtilis sigma X factor using a consensus-directed search. J Mol Biol. 1998;279:165–73. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 120.Zellmeier S, Hofmann C, Thomas S, Wiegert T, Schumann W. Identification of sigma(V)-dependent genes of Bacillus subtilis. FEMS Microbiol Lett. 2005;253:221–9. doi: 10.1016/j.femsle.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 121.Guariglia-Oropeza V, Helmann JD. Bacillus subtilis sigma(V) confers lysozyme resistance by activation of two cell wall modification pathways, peptidoglycan O-acetylation and D-alanylation of teichoic acids. J Bacteriol. 2011;193:6223–32. doi: 10.1128/JB.06023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ho TD, Hastie JL, Intile PJ, Ellermeier CD. The Bacillus subtilis extracytoplasmic function sigma factor sigma(V) is induced by lysozyme and provides resistance to lysozyme. J Bacteriol. 2011;193:6215–22. doi: 10.1128/JB.05467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao M, Salzberg L, Tsai CS, Mascher T, Bonilla C, Wang T, et al. Regulation of the Bacillus subtilis extracytoplasmic function protein sigmaY and its target promoters. J Bacteriol. 2003;185:4883–90. doi: 10.1128/JB.185.16.4883-4890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ho TD, Ellermeier CD. PrsW is required for colonization, resistance to antimicrobial peptides, and expression of extracytoplasmic function sigma factors in Clostridium difficile. Infect Immun. 2011;79:3229–38. doi: 10.1128/IAI.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hastie JL, Williams KB, Sepulveda C, Houtman JC, Forest KT, Ellermeier CD. Evidence of a bacterial receptor for lysozyme: binding of lysozyme to the anti-sigma factor RsiV controls activation of the ECF sigma factor sigmaV. PLoS Genetics. 2014;10:e1004643. doi: 10.1371/journal.pgen.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ho TD, Williams KB, Chen Y, Helm RF, Popham DL, Ellermeier CD. Clostridium difficile extracytoplasmic function sigma factor sigmaV regulates lysozyme resistance and is necessary for pathogenesis in the hamster model of infection. Infect Immun. 2014;82:2345–55. doi: 10.1128/IAI.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woods EC, Nawrocki KL, Suárez JM, McBride SM. The Clostridium difficile Dlt pathway is controlled by the ECF sigma factor, sV, in response to lysozyme. Infect Immun. 2016:IAI.00207–16. doi: 10.1128/IAI.00207-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McBride SM, Sonenshein AL. The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology. 2011;157:1457–65. doi: 10.1099/mic.0.045997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le Jeune A, Torelli R, Sanguinetti M, Giard J-C, Hartke A, Auffray Y, et al. The extracytoplasmic function sigma factor SigV plays a key role in the original model of lysozyme resistance and virulence of Enterococcus faecalis. PLoS One. 2010;5:e9658. doi: 10.1371/journal.pone.0009658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Varahan S, Iyer VS, Moore WT, Hancock LE. Eep confers lysozyme resistance to Enterococcus faecalis via the activation of the extracytoplasmic function sigma factor SigV. J Bacteriol. 2013;195:3125–34. doi: 10.1128/JB.00291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coonrod JD, Varble R, Yoneda K. Mechanism of killing of pneumococci by lysozyme. J Infect Dis. 1991;164:527–32. doi: 10.1093/infdis/164.3.527. [DOI] [PubMed] [Google Scholar]
- 132.Westmacott D, Perkins HR. Effects of lysozyme on Bacillus cereus 569: rupture of chains of bacteria and enhancement of sensitivity to autolysins. J Gen Microbiol. 1979;115:1–11. doi: 10.1099/00221287-115-1-1. [DOI] [PubMed] [Google Scholar]
- 133.Andersson DI, Levin BR. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2:489–93. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 134.Ruiz N, Silhavy TJ. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr Opin Microbiol. 2005;8:122–6. doi: 10.1016/j.mib.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 135.Cao M, Helmann JD. Regulation of the Bacillus subtilis bcrC bacitracin resistance gene by two extracytoplasmic function sigma factors. J Bacteriol. 2002;184:6123–9. doi: 10.1128/JB.184.22.6123-6129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raphael BH, Pereira S, Flom GA, Zhang Q, Ketley JM, Konkel ME. The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J Bacteriol. 2005;187:3662–70. doi: 10.1128/JB.187.11.3662-3670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mascher T, Heintz M, Zahner D, Merai M, Hakenbeck R. The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in beta-lactam resistance. J Bacteriol. 2006;188:1959–68. doi: 10.1128/JB.188.5.1959-1968.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–3. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 139.Loh JT, Gupta SS, Friedman DB, Krezel AM, Cover TL. Analysis of protein expression regulated by the Helicobacter pylori ArsRS two-component signal transduction system. J Bacteriol. 2010;192:2034–43. doi: 10.1128/JB.01703-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Loh JT, Cover TL. Requirement of histidine kinases HP0165 and HP1364 for acid resistance in Helicobacter pylori. Infect Immun. 2006;74:3052–9. doi: 10.1128/IAI.74.5.3052-3059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waidner B, Melchers K, Stahler FN, Kist M, Bereswill S. The Helicobacter pylori CrdRS two-component regulation system (HP1364/HP1365) is required for copper-mediated induction of the copper resistance determinant CrdA. J Bacteriol. 2005;187:4683–8. doi: 10.1128/JB.187.13.4683-4688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miller HK, Carroll RK, Burda WN, Krute CN, Davenport JE, Shaw LN. The extracytoplasmic function sigma factor SigmaS protects against both intracellular and extracytoplasmic stresses in Staphylococcus aureus. J Bacteriol. 2012;194:4342–54. doi: 10.1128/JB.00484-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodrigue S, Provvedi R, Jacques PE, Gaudreau L, Manganelli R. The sigma factors of Mycobacterium tuberculosis. FEMS Microbiol Rev. 2006;30:926–41. doi: 10.1111/j.1574-6976.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 144.Craig JE, Nobbs A, High NJ. The extracytoplasmic sigma factor, final sigma(E), is required for intracellular survival of nontypeable Haemophilus influenzae in J774 macrophages. Infect Immun. 2002;70:708–15. doi: 10.1128/IAI.70.2.708-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chaturongakul S, Raengpradub S, Wiedmann M, Boor KJ. Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol. 16:388–96. doi: 10.1016/j.tim.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sherwood EJ, Bibb MJ. The antibiotic planosporicin coordinates its own production in the actinomycete Planomonospora alba. Proc Natl Acad Sci U S A. 2013;110:E2500–9. doi: 10.1073/pnas.1305392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feng WH, Mao XM, Liu ZH, Li YQ. The ECF sigma factor SigT regulates actinorhodin production in response to nitrogen stress in Streptomyces coelicolor. Appl Microbiol Biotechnol. 2011;92:1009–21. doi: 10.1007/s00253-011-3619-2. [DOI] [PubMed] [Google Scholar]
- 148.Foulston LC, Bibb MJ. Microbisporicin gene cluster reveals unusual features of lantibiotic biosynthesis in actinomycetes. Proc Natl Acad Sci U S A. 2010;107:13461–6. doi: 10.1073/pnas.1008285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luo S, Sun D, Zhu J, Chen Z, Wen Y, Li J. An extracytoplasmic function sigma factor, sigma(25), differentially regulates avermectin and oligomycin biosynthesis in Streptomyces avermitilis. Appl Microbiol Biotechnol. 2014;98:7097–112. doi: 10.1007/s00253-014-5759-7. [DOI] [PubMed] [Google Scholar]