Abstract
Atrial fibrillation (AF) is an age-related arrhythmia of enormous socioeconomic significance. In recent years, our understanding of the basic mechanisms that initiate and perpetuate AF has evolved rapidly, catheter ablation of AF has progressed from concept to reality, and recent studies suggest lifestyle modification may help prevent AF recurrence. Emerging developments in genetics, imaging, and informatics also present new opportunities for personalized care. However, considerable challenges remain. These include a paucity of studies examining AF prevention, modest efficacy of existing antiarrhythmic therapies, diverse ablation technologies and practice, and limited evidence to guide management of high-risk patients with multiple comorbidities. Studies examining the long-term effects of AF catheter ablation on morbidity and mortality outcomes are not yet completed. In many ways, further progress in the field is heavily contingent on the feasibility, capacity, and efficiency of clinical trials to incorporate the rapidly evolving knowledge base and to provide substantive evidence for novel AF therapeutic strategies. This review outlines the current state of AF prevention and treatment trials, including the foreseeable challenges, as discussed by a unique forum of clinical trialists, scientists, and regulatory representatives in a session endorsed by the Heart Rhythm Society at the 12th Global CardioVascular Clinical Trialists Forum in Washington, DC, December 3–5, 2015.
Keywords: Atrial fibrillation, Randomized controlled trial, Prevention, Ablation, Personalized medicine
Introduction
Atrial fibrillation (AF) is a public health concern of global and epidemic proportions, inextricably linked to an aging population, expanding burden of predisposing factors, and enhanced arrhythmia surveillance.1–3 Symptoms associated with AF may be severe and disabling, and AF represents an independent risk factor for stroke, heart failure (HF), dementia, and death.4,5 Patients with AF are hospitalized twice as often as those without AF, and the incremental costs attributable to AF-related care present important challenges for existing health care systems.4–6 Accordingly, the treatment and prevention of AF have become key priorities for clinical and translational research efforts.7–9
Significant advances in our understanding of the basic mechanisms underlying AF initiation and maintenance have been made in recent years.10,11 It has become clear that aging, genetics, environmental factors, and cardiac and noncardiac conditions further contribute to a favorable atrial substrate.12 Moreover, surgical and catheter ablation techniques for AF have been at the forefront of rapid technological innovation.4,5,13 Central to these endeavors, integration of basic science and observational findings into a defined therapeutic strategy and its uniform application and validation within a randomized controlled trial (RCT) has remained the benchmark for safety and efficacy required for any change in clinical practice.14
Currently, however, there are insufficient high-quality and generalizable RCT data to support the needs of “real-world” clinical practice.15 Broadly speaking, prevailing challenges with respect to AF management include (1) limited RCT evidence relating to lifestyle and risk factor modification, prediction, and prevention of AF; (2) diverse ablation practices, and underrepresentation of long-term and patient-reported outcomes within existing AF intervention trials; and (3) evolving demands for design and validation of personalized and mechanism-orientated AF therapies in order to improve patient adherence and outcomes. A comprehensive discussion of these issues and the current state of AF prevention and treatment RCTs took place within a unique forum composed of clinical trialists, scientists, and regulatory representatives at the 12th Global CardioVascular Clinical Trialists (CVCT) Forum in Washington, DC, December 3–5, 2015, the details of which are outlined in this review. Note that AF-related stroke prevention and anticoagulation, although essential to AF management, are beyond the scope of this article.
AF prevention trials
Primary and secondary AF prevention
Epidemiologic studies have described an array of potentially modifiable risk factors for AF, including hypertension, obesity, metabolic syndrome, diabetes mellitus, obstructive sleep apnea, cigarette smoking, and excessive alcohol intake. Many of these are also risk factors for atherosclerotic cardiovascular disease, myocardial infarction, and HF, which themselves predispose to AF. Recent consensus documents advocate targeting prevention efforts to individuals with the highest risk, typically those with multiple predisposing conditions.8,16 Putative risk scores have been developed with this in mind, although they are not yet widely in use.17
Unfortunately, the current framework for scientific investigation limits feasibility of dedicated AF primary prevention RCTs by the large population size and prolonged duration required to achieve an adequate number of recognized end-points. A first presentation of symptomatic AF may occur years after recruitment, and the expediency of prolonged ECG monitoring in asymptomatic individuals is low. Looking forward, smartphone-based ECG applications and implantable and convenient wearable recorders with single-lead ECG recording capabilities are likely to become more pervasive in RCTs. Emerging literature supports their utility and diagnostic performance in population-based settings.18–20 Furthermore, detection of frequent atrial ectopy may prove to be a precursor or surrogate marker of AF, allowing enrichment of study populations with individuals at sufficiently high risk.21 Presently, however, further delineation of its natural history is required.
Secondary prevention of AF (delaying recurrence of AF after an initial episode or delaying progression from paroxysmal to persistent AF) has received greater attention, albeit a clinical rather than pathologic classification. Basic research in animal models and humans has demonstrated progressive atrial electrical and structural remodeling occurring in the setting of cardiometabolic risk factors, which are thought to be responsible for AF initiation and perpetuation of AFmaintenance.11 Because this process develops insidiously, AF risk is realistically a continuum and in most individuals will result from a combined effect of several interacting factors, often without definite threshold values. This may explain why isolated treatment of hypertension, although arguably one of the strongest contributors to AF burden,22 has not been shown to reduce AF risk consistently, and no target blood pressure has been identified. Hence, contemporary RCTs, as in clinical practice, have recognized the need to incorporate a strategy of comprehensive risk factor modification with individual AF prevention and treatment interventions. The fact that AF induces further electrical remodeling in animal models23 (“AF begets AF”) also highlights the importance of early intervention.
Nonpharmacologic approaches to AF prevention
Among candidate nonpharmacologic interventions, inaugural studies of weight loss and exercise have shown efficacy for secondary, though not yet primary, AF prevention within a comprehensive risk factor modification program (Table 1).
Table 1. Clinical studies of lifestyle modification for atrial fibrillation prevention.
| Study | Design | Patient population | N | Intervention | Comparator | Endpoints | Follow-up (mean ± SD) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Abed et al24 | RCT Single-blind Single-center |
Obese (BMI >27 kg/m2) PAF (58%) or persistent AF Mean age 60 ± 10 years 67% male |
150 | Physician-led weight loss and RFM program | Self-directed general lifestyle measures |
Primary: Symptom burden and severity Secondary: AF burden and echocardiographic parameters |
Control 12.0 ± 4.0 months Intervention 12.9 ± 2.6 months |
RFM resulted in greater weight loss (from mean BMI 32.8 kg/m2 to 27.2 kg/m2) and improved cardiometabolic profile, reduced symptom burden and severity, decreased AF burden, LA area, and LV wall thickness |
| ARREST-AF Pathak et al25 |
Prospective cohort Single-center | Obese (BMI ≥27 kg/m2) ≥1 cardiometabolic risk factor Initial catheter ablation Symptomatic PAF (60%) or persistent AF despite antiarrhythmic therapy Pooled mean age 58 ± 10 years 64% male |
149 | Physician-led weight loss and RFM program | Self-directed general lifestyle measures |
Primary: Recurrent AF Secondary: AF frequency, duration, and symptom burden |
Control 42.1 ± 14.2 months Intervention 41.6 ± 12.5 months |
RFM resulted in greater weight loss and improved cardiometabolic profile, greater freedom from AF after a single or multiple ablation procedures, and greater reduction in AF frequency, duration, symptoms, and symptom severity |
| LEGACY Pathak et al26 |
Prospective cohort Single-center | Obese (BMI ≥ 27 kg/m2) PAF (53%) and persistent AF Weight loss categorized as ≥10% (group 1), 3%-9% (group 2), <3% (group 3) Pooled mean age 63 ± 11 years 66% male |
825 | Physician-led weight loss and RFM program | N/A | Primary: AF burden Secondary: Echocardiographic parameters | 34 ± 15 months | Greatest improvement in AF burden and symptom severity in group 1 Greatest benefit in patients with stable (vs fluctuating) weight after initial weight loss Groups 1 and 2 had reductions in LA area and LV wall thickness |
| CARDIO-FIT Pathak et al27 |
Prospective cohort | Obese (BMI ≥27 kg/m2) and able to undergo exercise training Symptomatic PAF (53%) or persistent AF Baseline CRF categorized as low (<85% predicted METs), adequate (86%–100% predicted METs), high (>100% predicted METs) Improvement in CRF categorized as ≥2 METs vs <2 METs Pooled mean age 60 ± 11 years 48% male |
308 | Physician-led weight loss, RFM, and tailored exercise program | N/A |
Primary: Freedom from AF and symptom burden Secondary: Echocardiographic parameters |
49 ± 19 months | Greatest ablation and drug-free AF-free survival and reduction in symptoms in patients with high CRF (vs low or adequate CRF) Greater reduction in AF burden and symptom severity in patients with ≥2 METs gain in CRF (vs <2 METs) More favorable reductions in LA area and LV wall thickness in patients with ≥2 METs gain in CRF (vs <2 METs) |
| Malmo et al28 | RCT Single-blind Single-center |
Paroxysmal (57%) or persistent AF, able to undergo exercise training Pooled mean age 59 ± 8 years 82% male |
51 | Aerobic interval training | Self-directed usual exercise regimen |
Primary: AF burden Secondary: VO2, echocardiographic parameters, lipid status, quality of life, AF-related hospital admissions |
4 weeks | Aerobic interval training was associated with reduced AF burden, improvement in AF symptoms, peak VO2, quality of life, and LA and LV function |
AF = atrial fibrillation; BMI = body mass index; CRF = cardiorespiratory fitness; LA = left atrium; LV = left ventricular; METs = metabolic equivalents; PAF = paroxysmal atrial fibrillation; RCT = randomized controlled trial; RFM = risk factor management; VO2 = oxygen uptake.
Weight loss
Primary prevention RCT data regarding lifestyle intervention and weight loss in overweight and obese individuals with type II diabetes and no prior AF were available in the Look AHEAD trial.29 Secondary analyses did not demonstrate a reduction in AF incidence over 9 years of follow-up, although AF was not a prespecified endpoint, and the overall weight loss achieved was modest.29 Conversely, in overweight and obese patients with documented paroxysmal AF, randomized to a physician-instructed low-calorie diet and exercise routine, Abed et al24 reported a more significant reduction in body mass index (BMI) and improved blood pressure control at 15-month follow-up, associated with less frequent and shorter-duration AF episodes (Holter monitoring) and lower self-reported symptom severity, compared with patients receiving standard lifestyle and weight loss advice. Both groups received aggressive management of concomitant cardiometabolic risk factors. In ARREST-AF (Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation), overweight and obese patients undergoing first-time catheter ablation for either paroxysmal or persistent AF, who opted to undergo a focused cardiometabolic risk factor management program, showed consistently greater reductions in weight, systolic blood pressure, markers of AF burden, and freedom from AF recurrence (32.9% vs 9.7% in control subjects) at 42-month follow-up, compared with patients choosing standard postablation care.25 Finally, a longitudinal cohort study of overweight and obese patients with paroxysmal or persistent AF participating in a physician-led weight management clinic (Long Term Effect of Goal Directed Weight Management on Atrial Fibrillation Cohort: A 5 year Follow-Up Study [LEGACY-AF]) reported a dose–response relationship between weight loss and reduction in AF symptoms and arrhythmia burden (7-day Holter) at 5-year follow-up.26
Taken together, the CVCT believes that these seminal findings from a dedicated single-center team strongly support a strategy of purposeful weight loss to accompany risk factor modification for obese and overweight patients with existing AF. Replication of these findings across multiple centers is now a priority for the clinical community and will require appropriate support from funding agencies. Notably, only 1 of the aforementioned studies was randomized,24 and patients with significant valvular or ventricular dysfunction have so far been excluded.
Exercise and cardiovascular fitness
Regular physical activity and aerobic exercise training delay development of atherosclerotic cardiovascular disease.30 Exercise has been cautiously advocated in AF prevention and management because of an increased risk of AF among individuals engaging in high-intensity and endurance training. However, it is worth highlighting that proarrhythmic levels of exercise exceed those practiced by the majority of patients with AF, and recent data demonstrate an inverse relationship between physical activity and AF incidence in nonathlete cohorts,31,32 akin to a J-shaped phenomenon.
Indeed, short-term exercise training has been shown to benefit secondary prevention of AF.27,28 The CardioFIT (Impact of Cardiorespiratory Fitness on Arrhythmia Recurrence in Obese Individuals with Atrial Fibrillation) study of overweight and obese patients with symptomatic paroxysmal or persistent AF exhibited a dose–response relationship between baseline cardiorespiratory fitness and long-term freedom from AF without antiarrhythmic drugs or ablation.27 Cardiorespiratory fitness gain from a tailored program of aerobic and resistance training further prolonged AF freedom, over and above the effect of weight loss.27 Malmo et al28 corroborated these findings in a referral cohort with paroxysmal or persistent AF undergoing first-time ablation, in a different center, randomized to 12 weeks of adjuvant high-intensity aerobic interval training vs no exercise prescription. Despite fewer cardiometabolic risk factors in this cohort compared with prior prevention trials, exercise training yielded improvements in BMI, lipid profile, and exercise capacity with attendant reduction in mean AF time (implantable loop recording) and fewer and less severe self-reported AF symptoms, compared with the control group.28
These data demonstrate that short-term gains in cardiorespiratory fitness are attainable and safe, and confer reductions in arrhythmia burden, with and without rhythm control strategies, in ambulatory patients with symptomatic AF. Whether these benefits can be sustained long term now needs to be addressed. In the HF-ACTION trial, initial gains from supervised exercise training over 12 weeks were diluted by poor adherence when supervision ended, yielding modest overall improvement at final follow-up.33 Furthermore, in LEGACY-AF, >5% annual fluctuation in weight partially offset the benefits of weight loss and exercise on arrhythmia-free survival at 5 years.26 Thus, the CVCT Forum concluded that although public health-level arguments in favor of exercise and weight management programs are strong, funding and resources for long-term physician-led face-to-face counseling and exercise clinics will be needed to realize their potential for AF prevention in clinical practice.
Risk factor modification in patients with HF
Patients with AF and HF present a unique challenge to the weight loss hypothesis. There are currently no published RCT data investigating the effect of weight loss in patients with AF and HF, and whether severe atrial remodeling, as may occur in AF and HF, can be interrupted or reversed by weight loss, which is among its reported benefits, remains unclear. Furthermore, it is uncertain how weight loss interventions may impact the reported “obesity paradox,” in which overweight and mildly obese patients with HF appear to have better short-term outcomes compared with lean HF patients.34 Interestingly, a similar paradox has been reported in a large trial cohort of patients with AF (without HF) on oral anticoagulant therapy,35 despite compelling RCT data supporting weight loss for secondary AF prevention. Acknowledging that the nature of this paradox remains controversial, future AF trials may benefit from more refined characterization of an obesity phenotype, beyond BMI alone. At present, however, empirical evidence does not support the practice of asking HF patients to lose weight, and AF studies to date have not defined a target weight or weight range within which AF burden is minimized. Notably, cardiorespiratory fitness, itself associated with AF burden, reportedly alters the relationship between adiposity and prognosis in HF patients and attenuates the obesity paradox in younger patients with reduced ejection fraction.36 Thus, the combined impact of exercise and weight optimization in patients with AF and HF warrants prospective investigation.
Pharmacologic approaches to AF prevention
Upstream therapy refers to the use nonantiarrhythmic drugs to modify the atrial substrate or target specific mechanisms of AF.37,38 The concept is supported by compelling experimental evidence showing protective effects of renin–angiotensin–aldosterone system (RAAS) inhibitors, statins, and polyunsaturated fatty acids, on atrial structural and electrical remodeling.37,38 Unfortunately, however, the clinical performance of these agents in RCTs has been generally disappointing.
RAAS inhibitors have been associated with reduced incidence of AF in patients with left ventricular systolic dysfunction or hypertrophy in retrospective analyses of RCTs with ≥3 years of follow-up.38 The addition of a mineralocorticoid receptor antagonist, eplerenone, to RAAS inhibition and beta-blockade was associated with a lower incidence of AF in patients with systolic HF (left ventricular ejection fraction ≤35%) and mild symptoms (New York Heart Association functional class II) in the EMPHASIS trial.39 No convincing benefit of RAAS inhibitors or mineralocorticoid receptor antagonists has been observed in patients without underlying heart disease or for secondary AF prevention. In retrospective studies of patients undergoing cardiac surgery, statins appeared to protect against new-onset AF, although conflicting RCT data exist.40,41 There are insufficient clinical data to support the use of polyunsaturated fatty acids in primary or secondary prevention of AF.37,38 Only short-term colchicine use has been associated with lower rates of postoperative AF42 and reduced early AF recurrence after catheter ablation.43 Concomitant reductions in inflammatory markers, C-reactive protein and interleukin-6, support an antiinflammatory mechanism. Colchicine use appears to be safe and generally well tolerated, although the optimal dose and duration need to be confirmed.
Because cardiac surgery and catheter ablation initiate inflammation, it is likely that the anti-inflammatory action of colchicine represents a good example of matching pathology with treatment. The failure of other agents to prevent AF in RCTs, despite convincing experimental data, may relate to inappropriately heterogeneous patient cohorts or a failure of experimental models to account for potentially neutralizing effects of comorbidities accompanying AF. Upstream agents may be unable to reverse advanced atrial remodeling, and trials of generally ≤ 1 -year duration may have been too short to demonstrate their effects. Future RCTs may consider combining agents or refining AF ascertainment methods to assess the true impact on total AF burden. The Routine versus Aggressive upstream rhythm Control for prevention of Early AF in heart failure (RACE 3) RCT has taken this approach and will test the hypothesis that aggressive combination upstream therapy increases persistence of sinus rhythm in patients with early AF and mild-to-moderate early systolic or diastolic HF undergoing electrical cardioversion compared with conventional rhythm control alone (ClinicalTrials.gov Identifier: NCT00877643).44
AF treatment trials: Filling in the gaps
Rate vs rhythm control
The major goals of AF therapy are to reduce cardiovascular symptoms and AF-related morbidity and mortality. These aims should be pursued in parallel. Both rate and rhythm control strategies have demonstrated improvements in quality of life and exercise capacity; however, the first wave of large RCTs failed to show a mortality benefit with rhythm control.45–49 Reasons cited included a failure of rhythm control strategies to achieve and sustain sinus rhythm, a high rate of crossover between treatment arms, and harmful effects of antiarrhythmic drugs that may have offset the benefits of restoring sinus rhythm. Consequently, whether one strategy is superior to the other with respect to major cardiovascular endpoints remains uncertain. A prevailing view is that AF at least contributes to its associated adverse outcomes, as well as representing a marker of cardiovascular disease, and thus a more efficacious and less toxic strategy of restoring and maintaining sinus rhythm is expected to improve prognosis. On-treatment analysis of the AFFIRM trial suggested that sinus rhythm, when achieved, was associated with a reduction in mortality,47 thus providing qualification for ongoing efforts to optimize rhythm control.
Nonpharmacologic (invasive) approaches to AF treatment
State-of-the-art nonpharmacologic rhythm control therapy is based on radiofrequency endocardial catheter ablation, with pulmonary vein isolation (PVI) as the mainstay (Figure 1A). Prompted from a clinical observation,51 translational research has corroborated the importance of pulmonary vein (PV) triggers for AF initiation, and several RCTs have demonstrated the superiority of catheter ablation over antiarrhythmic drug therapy, with respect to freedom from arrhythmia recurrence, particularly in young patients with paroxysmal AF, and without major comorbidity (Table 2).53–55,57,58 Contemporary guidelines have endorsed catheter ablation for patients with symptomatic paroxysmal (class I) or persistent (class IIa) AF who are intolerant or resistant to 1 or more antiarrhythmic drugs.5,13 Importantly, however, this endorsement is not without caveats. The Forum emphasized that available RCT and registry data show that PVI in its current form does not represent a cure for AF, and several evidence gaps remain.
Figure 1.
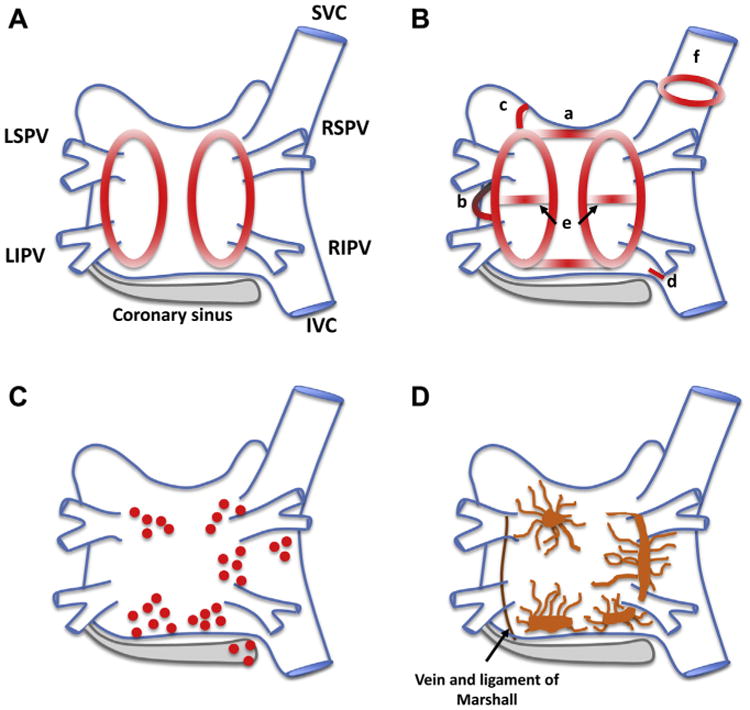
Atrial fibrillation ablation strategies. Schematic posterior view of the left and right atria with example ablation lesion sets. Pulmonary vein isolation with circumferential ablation lesions (A) and linear lesion sets (B), including (a) roof line, (b) mitral isthmus line, (c) anterior linear lesion, (d) cavo-tricuspid isthmus line, (e) additional linear lesions between the superior and inferior pulmonary veins, and (f) electrical isolation of the superior vena cava. C: Common sites of ablation when complex fractionated electrograms are targeted. D: Sites of the major left atrial autonomic ganglionated plexi (GP) and axons (superior left GP, inferior left GP, anterior right GP, inferior right GP, and ligament of Marshall). IVC = inferior vena cava; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein. (Figure adapted from Calkins et al.50)
Table 2. Randomized clinical trials of catheter ablation for atrial fibrillation.
| Study | Design | Patient population | N | Intervention | Comparator | Endpoints | Follow-up (mean ± SD) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| First-line therapy | ||||||||
| RAAFT-1 Wazni et al52 |
RCT Multicenter |
Symptomatic PAF (96%) and persistent AF Pooled mean age 53 ± 8 years % male not reported |
70 | PVI | AAD | Primary: Recurrent AF Secondary: Hospitalization, quality of life | 12 months | 87% PVI vs 37% AAD group freedom from AF recurrence 9% PVI vs 54% AAD hospitalization Greater improvement in quality of life with PVI |
| MANTRA-PAF Cosedis Nielson et al53 |
RCT Multicenter |
Symptomatic PAF Pooled mean age 55 ± 10 years 70% male |
294 | PVI + additional ablation at operator's discretion | AAD |
Primary: Burden of AF Secondary: Freedom from AF, symptoms, time to first recurrence, quality of life |
24 months | No significant difference in cumulative burden of AF 9% PVI vs 18% AAD AF burden at 24 months 85% PVI vs 71% AAD freedom from any AF recurrence Quality of life improved equally in both groups |
| RAAFT-2 Morillo et al54 |
RCT Multicenter |
Symptomatic PAF Pooled mean age 55 ± 10 years 76% male |
127 | PVI + additional ablation at operator's discretion | AAD | Primary: Recurrent AF Secondary: Symptomatic AF, quality of life | 24 months | 45% PVI vs 28% AAD freedom from AF recurrence 53% PVI vs 41% AAD freedom from recurrent symptomatic AF Quality of life improved equally in both groups |
| Second-line therapy | ||||||||
| A4 Study Jais et al55 |
RCT Multicenter Superiority |
Symptomatic drug-refractory PAF Pooled mean age 51 ± 11 years 84% male |
108 | PVI + additional ablation at operator's discretion | AAD |
Primary: Recurrent AF at 3-12 months, freedom from AF after ≤3 procedures, changes in AAD within 3 months Secondary: Time to recurrent AF, quality of life, exercise capacity, AF burden |
12 months | Greater freedom from AF in the ablation (89%) vs AAD (23%) group More favorable symptom scores, exercise capacity, and quality of life in ablation group |
| APAF study Pappone et al56 |
RCT Single-center |
Symptomatic drug-refractory PAF Pooled mean age 56 ± 10 years 67% male |
198 | PVI + additional ablation at operator's discretion | AAD | Primary: Freedom from AF at 12 months | 12 months | Greater freedom from AF in the ablation vs AAD group (93% vs 35% at 1 year) Fewer cardiovascular hospitalizations in ablation group |
| Wilber et al57 | RCT Multicenter |
Symptomatic drug-refractory PAF Pooled mean age 56 years (95% CI 54.1–57.4) 67% male |
167 | PVI + additional ablation at operator's discretion | AAD | Primary: Freedom from symptomatic AF, safety Secondary: Freedom from any atrial arrhythmia, quality of life | PVI group: median 12.5 (95% CI 11.9–13.1) months AAD group: median 14.3 (95% CI 9.4–15.5) months |
Greater freedom from AF in the ablation vs AAD group (66% vs 16% at 9 months). Greater freedom from any atrial arrhythmia in the ablation vs AAD group (70% vs 19% at 9 months) More favorable quality of life scores in the ablation vs AAD group |
AAD = antiarrhythmic drug therapy; CI = confidence interval; PVI = pulmonary vein isolation. Other abbreviations as in Table 1.
Pulmonary vein isolation
Only 50% to 66% of patients with paroxysmal AF achieve sustained sinus rhythm after a single PVI procedure and 70% to 80% after a second attempt.59 Success rates are compromised further when follow-up is extended beyond 1 year.59 These real-world outcomes are inferior to the initial results reported by expert centers, prompting efforts to improve completeness of acute PVI (Table 3) and the discovery of PV reconnection as an important mechanism for AF recurrence.69
Table 3. Seminal clinical trials of novel techniques for atrial fibrillation ablation.
| Study | Design | Patient population | N | Intervention | Comparator | Endpoints | Follow-up (mean ± SD) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Contact force | ||||||||
| TOCCASTAR Reddy et al60 |
RCT Multicenter Noninferiority |
Symptomatic drug-refractory PAF Pooled mean age 60 ± 10 years 65% male |
295 | Ablation with CF-sensing catheter | Ablation with non-CF catheter |
Primary: Efficacy (acute PVI, freedom from symptomatic AF off AAD); safety Secondary: Optimal vs nonoptimal CF, quality of life |
12 months | Efficacy: 67.8% CF vs 69.4% control (noninferiority endpoint met) Efficacy within stratified CF arm: 75.9% optimal CF vs 58.1% nonoptimal CF Serious adverse events in 2.0% CF vs 1.4% control (safety noninferior endpoint met) |
| Cryoablation | ||||||||
| FIRE and ICE Kuck et al61 |
RCT Multicenter Noninferiority |
Symptomatic drug-refractory PAF Pooled mean age 60 ± 10 years 61% male |
750 | Cryoablation | Standard ablation | Primary: Efficacy (time to first recurrence of arrhythmia, AAD use, or repeat ablation), safety Secondary: Quality of life | 18 months | Noninferiority efficacy and safety endpoints met No significant difference among the 4 types of ablation catheters |
| CFAE | ||||||||
| STAR AF Verma et al62 |
RCT Multicenter Superiority |
Symptomatic drug-refractory PAF (65%) or persistent AF Pooled mean age 57 ± 10 years 74% male |
100 | CFE alone CFE + PVI | PVI alone |
Primary: Freedom from AF Secondary: Freedom from any arrhythmia, complications, procedural characteristics |
12 months | PVI + CFE had the highest freedom from AF vs PVI alone or CFE alone CFE alone had lowest success rate after 1 or 2 procedures, and higher incidence of repeat procedures required |
| STAR AF II Verma et al63 |
RCT Multicenter |
Symptomatic drug-refractory persistent AF Pooled mean age 60 ± 9 years 78% male |
589 | PVI + CFAE PVI + linear ablation | PVI alone |
Primary: Freedom from atrial arrhythmia after index ablation off AAD or repeat ablation Secondary: Freedom from any AF after 2 procedures, freedom from any atrial arrhythmia, AAD use, complications, procedural characteristics |
18 months | No significant difference in outcomes between groups after a first or repeat procedure PVI alone tended to be associated with shorter procedure and radiation times |
| Rotor modulation | ||||||||
| CONFIRM Narayan et al64 |
RCT Single-center | Symptomatic PAF (28%) or persistent AF Pooled mean age 62 ± 8 years 95% male |
92 | FIRM-guided ablation + conventional ablation | Conventional ablation: WACA (+LA roof line for persistent AF cases) | Primary: Acute procedural termination of AF, long-term freedom from AF, safety Secondary: Freedom from AF after first ablation, freedom from all atrial arrhythmias | Median 22 months | Acute procedural endpoint achieved in 86% FIRM-guided vs 20% conventional ablation cases Total ablation time same for both groups Greater freedom from AF for FIRM-guided (82%) vs conventional (45%) after single procedure Safety: No significant difference in complication rates between groups |
| Dominant frequency ablation | ||||||||
| RADAR AF Atienza et al65 |
RCT Multicenter Single-blind Noninferiority |
Symptomatic PAF (50%) and persistent AF Pooled mean age 54 ± 10 years 80% male |
232 | PAF: HFSA Persistent AF: PVI |
PAF: PVI Persistent AF: PVI 1 HFSA |
Primary:Freedom from AF at 6 months after index ablation Secondary: Freedom from AF/AT at 6 and 12 months, periprocedural complications, adverse events, quality of life. | 12 months |
PAF: HFSA noninferior to PVI at 12 months (failed to achieve noninferiority at 6 months) Fewer serious adverse events in HFSA group Persistent AF: No significant difference between HFSA and PVI for primary or secondary endpoints, but a trend toward more serious adverse events with PVI + HFSA |
| Adenosine | ||||||||
| ADVICE Macle et al66 |
RCT Multicenter Superiority |
Symptomatic AF undergoing ablation Pooled mean age 60 ±10 years 71% male |
534 | PVI + adenosine-guided dormant conduction ablation | PVI alone |
Primary: Time to first recurrence of atrial tachyarrhythmia after index ablation or repeat ablation <1 year Secondary: Time to first recurrence of atrial tachyarrhythmia, AAD use, periprocedural complications |
12.2 ± 1.4 months | Adenosine unmasked dormant PV conduction in 53% of patients Adenosine-guided ablation associated with greater freedom from AF (69% vs 42%) Similar occurrence of serious adverse events in each group |
| UNDER-ATP Kobori et al67 |
RCT Multicenter Superiority |
Symptomatic PAF or persistent AF undergoing first-time ablation Pooled mean age 63.3 ± 10 years 74% male |
2113 | PVI + adenosine-guided dormant conduction ablation | PVI alone |
Primary: Recurrent atrial tachyarrhythmia <1 year Secondary: Repeat ablation for any atrial tachyarrhythmia, periprocedural complications |
Median 384 days (interquartile range 366–450 days) | No significant difference in incidence of recurrent atrial tachyarrhythmias at 1 year between the 2 groups |
| GP ablation | ||||||||
| Katritsis et al68 | RCT 2-center | Symptomatic PAF Pooled mean age 56 ± 8 years 66% male |
242 | PVI + GP ablation GP alone | PVI alone |
Primary: Freedom from AF/AT after index ablation Secondary: Radiofrequency delivery time, fluoroscopy time, adverse events |
2 years | Addition of GP ablation to PVI conferred significantly higher success rate compared with either PVI or GP alone in patients with PAF |
AT = atrial tachycardia; CF = contact force; CFAE = contact force atrial electrogram; CFE = contact force electrograms; FIRM = focal impulse and rotor modulation; GP = ganglion plexi; HFSA = high-frequency source ablation; PV = pulmonary vein; WACA = wide area circumferential ablation. Other abbreviations as in Tables 1 and 2.
Recent efficacy studies of novel irrigated-tip catheters have suggested an optimal contact force (CF) between the catheter tip and atrial tissue (20g with a force–time integral of 400g per ablation lesion70), which may improve transmural lesion formation and eliminate conduction gaps by improving lesion continuity. The first prospective multicenter RCT comparing CF-guided PVI against conventional PVI (TaciCath Contact Force Ablation Catheter Study For Atrial Fibrillation; TOCCASTAR60) met its primary noninferiority safety and efficacy endpoints, and in secondary analyses reported that 76% of CF-PVI patients were free from arrhythmia at 12 months off antiarrhythmic drugs compared with 58% undergoing non-CF ablation. Nonradiofrequency approaches are also being considered. Cryoablation uses a balloon, positioned at the PV ostia, which is cooled with liquid nitrous oxide. Iterative modifications have led to changes in freeze duration, homogeneity, balloon pressure, and positioning, yielding greater efficacy and fewer serious complications (phrenic nerve paralysis, atrioesophageal ulceration, or fistula formation). The first prospective multicenter randomized trial of cryoablation vs conventional PVI recently met its noninferiority safety and efficacy end-points (Fire and Ice, Clinicaltrials.gov Identifier: NCT01490814) (Table 3).71 Additionally, periprocedural adenosine administration has been used to unmask “dormant” PVs ostensibly at risk of reconnecting, prompting further prophylactic lesion sets to be delivered during the index procedure. The multicenter ADVICE trial (Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicenter, randomized superiority trial66) demonstrated improvement in 12-month arrhythmia-free survival with this approach compared with standard ablation practice, without an increase in periprocedural complications.66 Still, the overall clinical impact of adenosine testing is likely to depend on the ablation technologies used, patient cohort, and resultant prevalence of dormant conduction. In the UNDER-ATP (UNmasking Dormant Electrical Reconduction by Adenosine TriPhosphate) trial, in which the prevalence of dormant conduction after ablation with combined anatomic and electrophysiologic guidance was 20%–30% among patients with paroxysmal, persistent, or longstanding persistent AF (vs 50% in the ADVICE trial), no significant reduction in the incidence of recurrent atrial arrhythmias was observed compared with conventional PVI (Table 3).67
It may be relevant that the positive aforementioned trials exclusively studied patients with paroxysmal AF. Even within this cohort, our understanding of the relevance of PV reconnection is derived from a biased subset of patients who have failed antiarrhythmic therapy and re-presented with symptomatic AF after a failed ablation. Purists may argue that PVI, at its conception, was never compared against a placebo control (i.e., sham procedure), and thus the true effect size may have been overestimated. Indeed, RCTs in drug-naïve patients comparing catheter ablation with antiarrhythmic drug therapy have shown either a lack of benefit or at best modest reduction in AF recurrence (Table 2).53,54 Salient data from electrophysiologic studies of patients without AF recurrence have highlighted that PV reconnection is also common in this setting.69,72,73 Hence, although PV sources are undoubtedly associated with AF initiation and maintenance in many patients, the current trend of performing multiple procedures in order to attain complete PVI is largely without corroborating evidence for long-term or universal clinical benefit.
Ablation of extra-PV targets
Ablation success rates for patients with persistent AF are lower than for paroxysmal AF, particularly in the setting of comorbidities,74 ranging between 30% and 40% after a single or multiple procedures.59,75 Current guidelines recommend consideration of more extensive ablation in this cohort in order to address a higher number of non-PV triggers.76 However RCT data for this approach have been inconsistent (Table 3).
Creation of linear lesions to compartmentalize the left atrium has been incorporated into clinical practice, adopted from the surgical Cox-maze procedure,77 and is thought to disrupt the critical mass of tissue needed to sustain AF (Figure 1B). Complex fractionated atrial electrograms (CFAEs) are electrograms of long duration, thought to highlight areas of slowed conduction and anchoring points for continuous reentry.78,79 Targeting CFAEs in patients with persistent AF has been associated with improved sinus rhythm maintenance in nonrandomized studies and subgroup analyses (Figure 1C).80,81 However, until recently, these empirical methods of substrate modification had few randomized or comparative data to support their use.
In the largest multicenter RCT to date (Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial Part II, STAR AF II63), 3 ablation strategies—PVI alone, PVI plus linear lesions (mitral isthmus and roof line), and PVI plus CFAE ablation—were compared in 589 patients undergoing first-time ablation for drug-refractory persistent AF. Contrary to expectations, no statistically significant difference in outcomes was observed after a first or repeat procedure (same strategy), with or without antiarrhythmic drugs, at 18-month follow-up. In fact, PVI alone tended to be superior at sinus rhythm maintenance and was associated with shorter procedure and radiation times, although the trial was not adequately powered for this assessment. These compelling data highlight the importance of scientifically rigorous investigation to guide standard of care. Thus, in patients with persistent AF, there is currently no clear advantage to adjuvant CFAE ablations or empiric linear lesions not targeting arrhythmogenic substrate.
That said, the discrepancy between index AF ablation success rates and long-term PVI has spurred interest in targeting other mechanisms of AF. When present, ablation of patient-specific focal triggers may allow less overall tissue ablation, upholding the recommendations of STAR AF II. Proposed targets include rapid drivers/dominant frequency, ganglion plexi ablation, and focal impulse or rotor modulation (FIRM).
Dominant frequency ablation predicates that AF is driven by areas of high-frequency activity not possessing the fractionation of CFAEs.82 The RADAR-AF trial demonstrated that high-frequency source ablation alone was not inferior to PVI for patients with paroxysmal AF at 12-month follow-up and displayed fewer complications, potentially because of less tissue ablation.65 Conversely, for patients with persistent AF in whom a superiority endpoint was sought, high-frequency source ablation failed to demonstrate incremental value when added to PVI.65 The autonomic nervous system may modulate AF triggers and substrate.83,84 In patients with paroxysmal AF, ablation of peri-PV ganglion plexi (Figure 1D) was inferior to PVI alone but when combined with PVI improved 2-year freedom from AF from 56% to 74%, without increasing the rate of iatrogenic atrial flutter.68 An incremental effect of ganglion plexi ablation in patients with persistent AF has not yet been studied. A further body of research has proposed that spiral reentrant waves, or “rotors,” perpetuate AF. FIRM mapping software may be used to identify and direct ablation of patient-specific rotors. The CONFIRM trial demonstrated that rotors were present in 97% of subjects with paroxysmal or persistent AF, and FIRM-guided cases of PVI exhibited higher rates of freedom from AF when compared with PVI alone.64 The same investigators presented data corroborating FIRM-guided ablation as a stand-alone procedure for patients with paroxysmal AF,85 although this was not published, and, controversially, others have been unable to replicate these findings.86,87
Summarizing these studies, the Forum stresses that there is currently insufficient randomized evidence to change the ablative approach for persistent AF beyond isolated PVI, at least for an index procedure. Moving forward, the field would benefit from standardization of the definitions used for extra-PV targets and the approach to ablation. It may be argued that discrepancies in the prevalence, electrophysiologic characteristics, and clinical outcomes between early studies largely result from methodologic differences. Furthermore, whether successful ablation of AF, regardless of technique, will result in reduced mortality is not known. This is under investigation in the Catheter Ablation versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA; ClinicalTrials.gov Identifier: NCT00911508) and Catheter Ablation versus Standard Conventional Treatment in Patients With LV Dysfunction and AF (CASTLE-AF; ClinicalTrials.gov Identifier: NCT0064318888) trials.
Additional considerations
The rapid evolution of catheter designs and ablation techniques presents a challenge for evidence-based medicine and the premium placed on RCTs. Stringent protocols, high costs, and cumbersome operational requirements have given rise to a number of short-duration trials, often with noninfer-iority endpoints, in which clinical decision-making and patient choice may be better guided by demonstrating superiority of one strategy over another and long-term outcomes. Equally disadvantageous is the prospect of a lengthy trial examining ablation strategies that vary markedly over time, in which the primary outcome may be biased by earlier used and less efficacious techniques. There are some concerns this may apply to the CABANA trial (ClinicalTrials.gov Identifier: NCT00911508).
Operator proficiency and center-related factors must also be considered. A worldwide survey in 2005 reported higher ablation success rates in high-volume compared with low-volume centers.89 Single-90 and multicenter91 studies have also demonstrated an independent association between individual operator volume and procedure efficacy, complication rates, and procedure times. Most importantly, the available evidence for AF catheter ablation has been derived from recognized regional, national, and international centers of expertise. As a result, a consensus statement from the Heart Rhythm Society and European Heart Rhythm Association specifying minimum institutional and operator criteria has been published.50
Patient-reported outcome measures
Symptoms are a major reason that patients with AF seek medical attention and are the primary justification for ablation. In recognition of this, contemporary AF RCTs are increasingly using quality of life and AF-specific symptom questionnaires when comparing treatment strategies. However, these data continue to serve secondary endpoints or ancillary analyses. Probable impediments to the adoption of patient-reported outcome measures as major endpoints in AF trials include the lack of an accepted gold standard assessment tool unique to AF, uncertainty regarding the fundamental mechanisms for symptoms or meaningful levels of change, and enormous heterogeneity in symptom profiles between and even within the same patients over time.
Although there is no simple solution to these challenges, it is no longer acceptable to omit qualitative endpoints in an era of patient-centered medicine. This is particularly important when tradeoffs may exist, for example, between modestly efficacious antiarrhythmic drugs and potential associated adverse effects. Furthermore, although restoration of sinus rhythm remains the desired clinical goal, for some patients with symptomatic AF, a reduction in frequency or duration of AF episodes (AF burden) may be an acceptable outcome. Alternatively, improvement in functional capacity was demonstrated to be a feasible and clinically relevant AF intervention endpoint in the ARC-HF trial.92 For other patients, a holistic approach that includes detection and treatment of comorbidities may offer greater benefit. The impact of spironolactone on exercise capacity, health-related quality of life, and left ventricular diastolic function in patients with AF and HF with preserved ejection fraction (HFpEF) is under investigation in the IMPRESS-AF trial (ClinicalTrials.gov Identifier: NCT02673463). Wokhlu et al93 observed that long-term improvements in quality of life were unrelated to rhythm status in patients undergoing index AF ablation, although the exact reasons for this finding were unclear.
Because the majority of tools used to assess quality of life, symptoms, and functional status were not specifically developed for patients with AF, future systematic research is warranted to define the pathophysiologic mechanisms underlying AF-related symptoms, their relationship to functional status, and their distinction, if any, from symptoms caused by other cardiac diseases such as HF and valvular disease.
AF ablation in patients with HF
The vacillation between rate and rhythm control strategies for the management of AF extends to patients with HF and reduced ejection fraction (HFrEF). Persistent AF is more common in this group, and adverse hemodynamic and neurohumoral factors present additional complexity. Nevertheless, among the few RCTs examining ablation as second-line therapy in dedicated HFrEF cohorts, overall efficacy rates are similar to those for patients without HF, although repeat procedures are more often required (Table 4). Improvements in quality of life, functional class, and exercise tolerance have also been documented after AF ablation compared with AV nodal ablation94 and pharmacologic rate control,92,96 although ablation was not superior to rate control in an early trial of digoxin therapy alone.95 Notably, definitions of adequate ventricular rate control in HF remain arbitrary. Whether AF ablation can reduce the risk of death or major adverse cardiovascular events is currently being investigated in the CABANA, RAFT AF (A Randomised Ablation-based Atrial Fibrillation Rhythm Control Trial in Patients with Heart Failure and High Burden Atrial Fibrillation), and CASTLE AF (Catheter Ablation Versus Standard Conventional Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) trials (Table 4). Encouraging results were recently obtained in the AATAC trial (Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device), in which AF ablation in HFrEF patients achieved greater freedom from AF, reduced mortality, and fewer unplanned hospital admissions compared with amiodarone therapy.97
Table 4. Randomized controlled trials of atrial fibrillation ablation in patients with heart failure.
| Study | Design | Patient population | N | Intervention | Comparator | Endpoints | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| PABA-CHF Khan et al94 |
RCT Multicenter |
Symptomatic HF (EF ≤40%) and PAF (49%) or persistent AF LVEF 27%–35% NYHA class II–III Pooled mean age 60 ± 8 years 91% male |
81 | PVI + additional ablation at operator's discretion | AV node ablation with biventricular pacing |
Primary: Composite of improvement in EF, 6MWD, QOL score vs baseline Secondary: Freedom from AF, LA dimensions |
6 months | Significance criteria for primary composite endpoint met Improved QOL and 6MWD in ablation group |
| MacDonald et al95 | RCT Multicenter |
Symptomatic HFrEF (EF<35%), persistent AF LVEF 36%–41% NYHA class II–IV, mean 2.9 Pooled mean age 63 ± 7 years 78% male |
41 | PVI + linear ablation + CFAE ablation + medical therapy | Medical therapy: HF therapy (+digoxin if HR >80 bpm) |
Primary: EF by CMR Secondary: EF by radionuclide imaging, 6MWD, NT-proBNP, quality of life, other CMR indices |
6 months | No significant difference in EF improvement between groups No difference in QOL and 6MWD between groups |
| ARC-HF Jones et al92 |
RCT Single center |
Symptomatic HFrEF (EF≤35%) and persistent AF LVEF 21%–32% NYHA class II–IV, mean 2.4 Pooled mean age 63 ± 9 years 87% male |
52 | PVI + linear ablation + CFAE ablation | Rate control |
Primary: Change in peak VO2 at 12 months Secondary: Quality of life, BNP, 6MWD, EF |
12 months | Improved QOL and peak VO2 (+3 mL/kg/min difference) and reduced BNP in ablation group Nonsignificant improvements in 6MWD and EF |
| CAMTAF Hunter et al96 |
RCT Single center |
Symptomatic HFrEF (EF<50%) and persistent AF LVEF 32%–40% NYHA class II–IV, mean 2.7 Pooled mean age 57 ± 11 years 87% male |
50 | PVI + additional ablation at operator's discretion | Rate control |
Primary: EF at 6 months Secondary: % reduction in LVESV at 6 months, peak VO2, BNP, NYHA class, quality of life |
6 months | Improved QOL, NYHA class, peak VO2, and reduced BNP in ablation group |
| AATAC Di Biase et al97 |
RCT Multicenter |
HFrEF (EF ≤40%), persistent AF, implanted device (CRT-D/ICD) LVEF 29% ± 5% NYHA class II–III Pooled mean age 61 ± 10 years 74% male |
203 | PVI + additional ablation at operator's discretion | Amiodarone |
Primary: Freedom from AF Secondary: All-cause mortality, unplanned AF- and HF-related hospital admissions, change in LVEF, 6MWD, quality of life |
2 years | Greater freedom from AF in ablation group (70%) vs amiodarone group (34%) after 2 years Improved QOL, exercise capacity, reduced unplanned hospital admissions, reduced mortality in ablation group |
| Trials in progress CASTLE AF Marrouche et al88 |
RCT Multicenter |
Symptomatic HFrEF and AF https://clinicaltrials.gov/ct2/show/NCT00643188 | 420 | PVI + additional ablation at operator's discretion | Conventional therapy | Primary:All-cause mortality or worsening HF requiring unplanned hospitalization | Minimum 3 years | |
| RAFT AF Tang, Wells et al |
RCT Multicenter |
Symptomatic HFrEF or HFpEF (NYHA class II–III) PAF or persistent or long-term persistent AF https://clinicaltrials.gov/ct2/show/NCT01420393 |
PVI + additional ablation at operator's discretion | Rate control | Primary:Composite of all-cause mortality and hospitalization for HF | 5 years | ||
| EAST Kirchhof et al |
RCT Multicenter |
Symptomatic PAF or persistent AF Includes patients with HFrEF (EF, 50%) https://clinicaltrials.gov/ct2/show/NCT01288352 |
Early rhythm control: AAD PVI Combined AAD + PVI |
Usual care: Rate control as first line, rhythm control for patients with refractory symptoms | Primary: Composite of cardiovascular death, stroke, or hospitalization due to worsening of HF or acute coronary syndrome | 8 years | ||
| CABANA Packer et al |
RCT Multicenter |
Symptomatic PAF, persistent or longstanding persistent AF. Includes patients with HFrEF and HFpEF https://clinicaltrials.gov/ct2/show/NCT00911508 |
PVI + additional ablation at operator's discretion | AAD or rate control | Primary: Composite of total mortality, disabling stroke, serious bleeding or cardiac arrest |
6MWD = 6-minute walk distance; AV = atrioventricular; BNP = brain natriuretic peptide; CMR = cardiac magnetic resonance; CRT-D = cardiac resynchronization therapy–defibrillator; EF = ejection fraction; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = heart rate; ICD = implantable cardioverter–defibrillator; LVEF = left ventricular ejection fraction; LVESV = left ventricular end systolic volume; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association class; QOL = quality of life. Other abbreviations as in Tables 1, 2, and 3.
Outstanding areas in need of RCT level evidence include the optimal AF management strategy for patients with HFpEF and the role of catheter ablation as first-line therapy for AF in patients with HF, although early rhythm control approaches are being considered in the CABANA and EAST AF trials (Table 4).
Pharmacologic approaches to AF treatment
Ventricular rate control is recommended for all patients with AF, and a lenient strategy (resting heart rate <110 bpm) is supported by the RACE II trial.98 Beyond this, there are few RCT data to inform optimal heart rate targets or the choice of negatively chronotropic agent with respect to morbidity, mortality, and symptoms. Contemporary strategies are under evaluation in the pilot RATE AF (Rate Control Therapy Evaluation in Permanent Atrial Fibrillation; ClinicalTrials.gov Identifier: NCT02391337) study.
Antiarrhythmic drugs are recommended as first-line therapy for patients with AF-related symptoms after adequate rate control.4,5 Unfortunately, however, existing agents are of modest and unpredictable efficacy and confer significant risks of proarrhythmia and off-target adverse effects. Flecainide (Vaughan Williams class Ic) is frequently used in patients without structural heart disease, although amiodarone has the widest repertoire of antiarrhythmic actions and approximately twice the efficacy of class Ic and class III agents.99 Even so, in the landmark Canadian Trial of Atrial Fibrillation (CTAF), 35% of patients receiving amiodarone experienced AF recurrence at long-term follow up (mean 16 months), and 18% experienced adverse events requiring discontinuation of the drug.99 The sheer size of the AF population and current restricted indications for catheter ablation indicate a large unmet need for more effective antiarrhythmic drugs to manage AF.
Dronedarone, the first new anti-AF agent to appear in several years, was engineered to have structural similarities to amiodarone without the iodine moiety, thus eliminating thyroid toxicity. Dronedarone successfully reduced cardiovascular hospitalization and death in patients with paroxysmal or persistent AF and 1 additional high-risk factor (ATHENA trial100). However, subsequent trials demonstrated increased mortality associated with its use in patients with structural heart disease and left ventricular dysfunction (PALLAS, ANDROMEDA101,102). Dronedarone is now contraindicated in this setting.
As with amiodarone, dronedarone has a nonspecific mode of action that increases the risk of off-target effects. This has prompted the development of atrial-specific agents with potentially superior efficacy and safety profiles. Although the majority are still in preclinical studies, vernakalant, a novel Na+- and K+-channel blocker, has demonstrated efficacy and an acceptable safety profile for acute termination of new-onset AF (<48 hours) in medium-sized placebocontrolled RCTs103–105 compared with amiodarone (Active-controlled, superiority study of Vernakalant versus amiodarone in Recent Onset atrial fibrillation; AVRO trial106). Vernakalant has been approved by the European Medicines Agency for patients without structural heart disease, although there has been limited uptake; postapproval studies are currently underway. In the United States and Canada it remains an investigational drug.
Overall, the sparse and slow progress in drug therapy for AF reflects existing barriers to pharmacologic innovation. Even when limitations in AF experimental models and techniques have been navigated and initial validation in human studies attained, regulatory requirements for new drug development are strict. Understandable caution is exercised with regard to patient safety; however, it is widely appreciated that escalating operational complexity of drug RCTs and prohibitive costs have somewhat diminished interest from industry. Demands for large RCTs with mortality endpoints and the desire for profitable broad indications may also impede clinical translation of promising compounds. In view of these challenges, across the spectrum of cardiovascular disease, efforts are underway to improve clinical trial and regulatory enterprises.107
Surgical and hybrid therapy for AF
The suboptimal efficacy of either pharmacologic therapy or catheter ablation for AF has given rise to the concept of hybrid therapy. Antiarrhythmic drugs are frequently used in patients undergoing catheter ablation, although there is wide variation in clinical practice and no consensus or prospective randomized data confirming the optimal duration of adjuvant drug therapy. Open-chest surgical AF ablation is reserved for selected symptomatic patients who have failed catheter ablation and are typically undergoing cardiac surgery for another indication.4,5 In this setting, epicardial ablation adds little additional operative risk. A novel minimally invasive approach, performed through video-assisted thoracoscopic surgery, has also demonstrated superiority to catheter ablation in achieving 1-year freedom from AF among patients with paroxysmal or persistent AF, with a dilated left atrium and hypertension, or previous failed catheter ablation.108 However, periprocedural adverse event rates were notably higher in the surgical group, including pneumothorax, major bleeding, and conversion to sternotomy.108 The ongoing multicenter CASA-AF trial will provide a contemporary appraisal of its safety and efficacy exclusively among patients with longstanding persistent AF (ClinicalTrials.gov Identifier: NCT02755688).
A hybrid approach, combining epicardial and endocardial ablation, has also been developed, providing freedom from AF rates approaching 90% at 1 year. Two small prospective studies reported superior 1- and 2-year outcomes compared with conventional percutaneous ablation in patients with persistent AF.109,110 Importantly, however, this strategy is still in its infancy and restricted to specialist centers with surgical expertise. Nevertheless, for patients without prohibitive surgical risk and symptomatic AF resistant to other therapies, it broadens future therapeutic options. Further data will be available from the ongoing Hybrid versus Catheter Ablation in Persistent AF trial (HARTCAP-AF; ClinicalTrials.gov Identifier: NCT02441738).
Future directions: Toward personalized AF therapy
To some extent, the clinical management of AF is already personalized.4,5, The decision to undergo cardioversion or ablation or to receive antiarrhythmic drug therapy is based on patient symptoms and preference. Additional consideration is given to comorbidities, duration of AF, left atrial size, drug efficacy, pharmacologic interactions, and adverse effects. Likewise, thromboembolism prophylaxis is prescribed according to validated risk prediction scores based on clinical characteristics. However, despite nuanced clinical judgments, the complexity of AF remains a challenge, and AF recurrence, progression to persistent AF, and response to treatment are largely unpredictable. This has prompted calls to improve our taxonomy of AF in order to facilitate a personalized or “precision medicine” approach in which therapy is tailored to an individual's unique clinical, genetic, and molecular determinants of disease.
Substrate-guided therapy
The concept of electrical AF substrate characterization is widely acknowledged and continues to inform the development of ablation techniques. However, recent trials, including STAR AF II, have demonstrated that nonselective electrical substrate modification is not clearly associated with clinical benefit. To better tailor the interventional treatment of AF, more refined substrate characterization along with prospective delineation of AF triggers and drivers will be important.
The 4th AFNET/EHRA consensus conference proposed a new clinical classification of AF incorporating distinct (monogenic, focally induced, and postoperative AF) and complex subtypes (polygenic, valvular, and AF in the elderly).111 Patients would be assigned to the most relevant group, ostensibly reflecting the dominant pathology of AF, but AF not fulfilling these definitions would be “unclassified.” However, many patients will have overlapping mechanisms, and “unclassified AF” may be a common outcome. An alternative substrate-based approach has been proposed by an EHRA/HRS/APHRS/SOLAEC consensus group, describing the atrial cardiomyopathy associated with AF. EHRA classes I to IV identify primarily cardiomyocyte-dependent, fibroblast-dependent (fibrotic), mixed dependency, and primarily noncollagen atrial infiltration, respectively. 112 Although this classification is not intended to describe disease progression or severity and it may vary over time and atrial sites, the framework proposed may enable the design of therapeutic strategies tailored to an individual's histopathophysiology in which atrial tissue is available for examination. In the majority of patients with AF, for whom atrial tissue is unlikely to be available or the risks of biopsy are not justified, advanced imaging techniques may provide an alternative means of substrate characterization.
Left atrial enlargement, identified on echocardiography or cardiac magnetic resonance imaging (MRI), has traditionally been associated with failure of rhythm control in AF. More recently, left atrial fibrosis quantified by MRI has emerged as a potentially superseding structural marker of disease severity. Three-dimensional assessment of left atrial myocardial delayed enhancement (DE-MRI) as a marker of interstitial fibrosis has been correlated with areas of low voltage by electroanatomic mapping113 and more frequent AF recurrence after index catheter ablation. In the DECAAF multicenter prospective observational study of patients with paroxysmal and persistent AF (n = 272), the risk of recurrent AF increased from 15% for stage I fibrosis (<10% of the left atrial wall) to 69% for stage IV fibrosis (>30% left atrial wall fibrosis).114 This association persisted after covariate adjustment, including left atrial volume. Additional proof-of-concept studies from specialist centers have demonstrated DE-MRI visualization of catheter ablation lines115 and lesion gaps.116 These data may assist patient selection for ablation, enabling priority to be given to patients with lower left atrial fibrotic burden or providing anatomic guidance for patients requiring a repeat procedure. This is currently being investigated in the DECAAF II trial (ClinicalTrials.gov Identifier: NCT02529319). Importantly, however, left atrial imaging is technically difficult, and 17% of the initial DECAAF study cohort (n = 57 subjects) was excluded because of poor-quality DE-MRI images.114 Furthermore, whether cardiac MRI has sufficient resolution to identity focal regions with incomplete scar remains uncertain. Additional development of cardiac MRI techniques, expertise, and correlation with electroanatomic mapping will be an essential prerequisite to its widespread application. Similarly, the use of circulating biomarkers of extracellular matrix remodeling and novel molecular imaging using radiotracers specific for cardiac fibrosis-related targets, although promising, are still early in development.
Genotype-directed therapy
In the last decade, positional cloning and candidate-gene approaches have identified rare forms of AF with mendelian inheritance. Probands with AF, largely in the absence of structural heart disease, exhibit mutations within genes encoding cardiac ion channels,117–119 gap junction proteins,120 atrial natriuretic peptide,121 and nucleoporins (NUP155).122 For affected individuals, these mutations confer large effect sizes and might therefore permit gene-directed pharmacotherapy, although this approach has not yet been tested.
From a population perspective, the goal is to elucidate common genetic variants identifying AF-susceptible individuals or AF subtypes who may benefit from preventive interventions or differentially respond to therapy. Thus far, genome-wide association studies have identified 14 independent genetic loci with genome-wide significance.123 While recognizing the small effect size of individual single nucleotide polymorphisms, genetic risk may well be clinically meaningful in the presence of additional risk factors (“two hit hypothesis”).124, 125 For example, an AF susceptibility allele at chromosome 4q25 (near the PITX2 gene) is present in approximately one-third of the general population, and associated polymorphisms have been independently linked to AF recurrence after antiarrhythmic drug therapy,126 cardioversion, 127 and catheter ablation128 in European cohorts, but not replicated in an Asian population.129 Patients with a common beta1-adrenergic receptor polymorphism (G389R) appear more likely to respond to a rate-control strategy and require lower doses of rate-lowering medication (beta-blockers or calcium channel antagonists) compared with non-G389R carriers.130 Other common single nucleotide polymorphisms have also been directly or inversely associated with greater prevalence of non-PV triggers and enhanced left atrial scarring after catheter ablation.131
Notably, a recent report from the AF-Gen consortium highlighted minimal incremental discriminatory value of polygenic AF risk scores compared with recognized clinical risk factors for incident AF.132 Thus, there are currently insufficient data to recommend routine genetic testing in patients with AF,133 and caution must be attributed to case control studies and data mining of large registries in which genotype–phenotype associations may be skewed. RCT data are currently being sought after including a randomized genotype-directed sequential crossover study of flecainide and sotalol (ClinicalTrials.gov Identifier: NCT02347111) and a prespecified genotype association substudy of patients enrolled in the large CABANA trial (ClinicalTrials.gov Identifier: NCT00911508).
Pragmatic trial design for precision AF therapy
Our current classification of AF, based on duration of episodes, belies the diverse mechanisms underlying AF initiation and maintenance. Furthermore, classic RCTs informing AF management guidelines quantify an average treatment response for a uniformly applied therapeutic strategy compared with placebo or standard of care, typically within unselected populations. These approaches conceal individual phenotypic characteristics and treatment effects, including potentially informative data from outlying super-or nonresponders, which could inform a precision medicine approach. Prespecified subgroup analyses are limited to high-incidence subtypes that are frequently underpowered to demonstrate statistical benefit. Hence, although advances in genetics, informatics, and imaging technologies will enable more refined classification and selection of patients with AF, concomitant changes in clinical trial design and accepted metrics of efficacy will likely also be needed to advance a precision medicine approach.
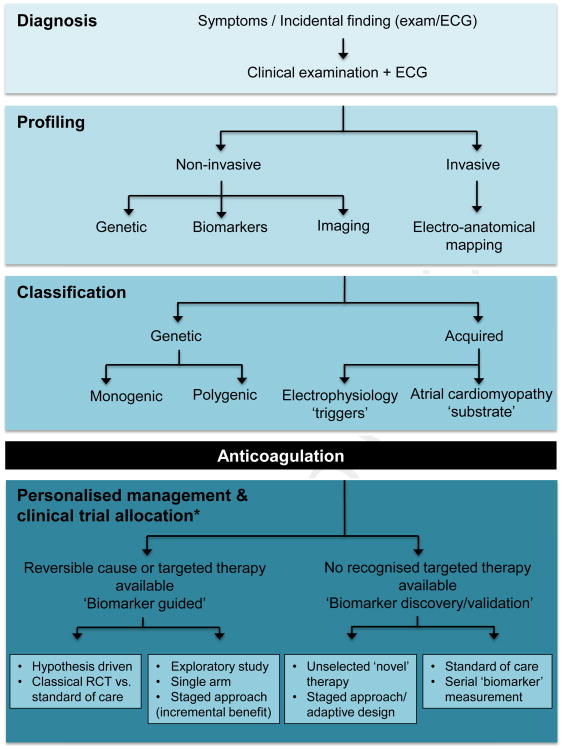
Envisioned future management algorithms for AF may involve comprehensive genetic, biochemical, and cardiac structural (imaging) profiling alongside routine clinical and ECG assessments (Figure 2). These data could be integrated into electronic health records and informatics platforms, with links to clinical registries and registry networks, preferably equipped with intelligent real-time decision support tools, to assist classification of patients into relevant, small-incidence subtypes based on their dominant or relative contributions of different AF mechanisms. Extrapolating from principles of patient-centric trials in oncology,134 all patients eligible for treatment may be presented with 1 of several rate or rhythm control treatment options within a single trial or network of trials (Figure 2). When a reversible cause or suitable biomarker is available, therapies may be cause-specific and biomarker-guided. Unselected new therapeutic strategies may still be investigated for incremental benefit in single-arm, staged designs. When no reversible cause is recognized, novel therapies may be compared with standard of care, in which patients are still monitored to provide opportunities for biomarker discovery and validation. A majority of AF patients screened would be assigned to a treatment option within a trial, thereby maximizing therapeutic development while minimizing the psychosocial, financial, and time costs of screening failures. Large registries may also be used as a cost-effective and convenient platform for large-scale, simple clinical trials.8, 135
Figure 2.
Proposed algorithm for personalized atrial fibrillation care. (*Management & clinical trial allocation adapted from Biankin et al.134)
With respect to data analyses, traditional interim analyses may be substituted by adaptive trial design features to allow modification of trial elements (e.g., sample size, randomization ratio, number of treatment arms) based on accumulated results, with full control of type I error.136 Greater use of repeated measures (e.g., serial imaging, assessment of patient-reported outcome measures) would allow detection of temporal changes in disease characteristics and treatment responses for individual patients. Consideration should be given to selecting endpoints most appropriate to the patients under study. Restoration and maintenance of sinus rhythm may remain the desired outcome for symptomatic patients with isolated paroxysmal AF, whereas in patients with AF and HF, delineating global determinants of impaired functional capacity may be of greater clinical value. Increasingly, remote or implantable monitoring technologies can be used to quantify AF burden as well as symptomatically “silent” AF recurrences after intervention. The association between device-detected AF and adverse outcomes, including stroke risk, is an active area of investigation, and the field awaits the definition of an acceptable threshold of AF burden, if any.137 Finally, recent literature has highlighted the shifting epidemiology of AF, with HF now representing the most common incident nonfatal event after AF diagnosis.138 Moving forward, it is incumbent on AF registries and long-term intervention trials to include incident HF as a legitimate endpoint alongside mortality and stroke rates.
Ultimately, successful and integrated development of AF precision medicine strategies will require wide collaboration between investigators, industry, and regulatory groups to ensure standardization of practice, broad access to therapeutics in development, and adequate scientific rigor to support clinical translation.
Conclusion
Several decades of concerted research has increased our understanding of the mechanisms underlying AF and given rise to novel therapeutic strategies. Systematic validation of these strategies in RCTs has reliably informed evidence-based practice and produced broad improvements in health outcomes. However, significant evidence gaps remain in our understanding of the AF substrate, and traditional RCTs designed for the “average” patient incompletely address the complexity of the AF population. In realizing the value of individual patient characteristics and treatment responses, the concept of precision medicine offers exciting opportunities to accelerate clinical translation of targeted interventions designed to treat and prevent AF.
Acknowledgments
Dr. Van Wagoner reports research grants from the National Institutes of Health and Amgen. Dr. Heist is a consultant for Biotronik, Boston Scientific, Janssen, Medtronic, Pfizer, St. Jude Medical; and receives research support from Boston Scientific and St. Jude Medical. Dr. Kowey is a consultant for Sanofi, Gilead, Medtronic, BI, Daiichi, BMS, and J&J. Dr. Mentz receives research support from Medtronic. Dr. Cleland is a consultant for Amgen, Bayer, BMS, CVRx, Novartis, Rietan, Sanofi, Servier, Stealth Biopharmaceuticals, Torrent, Vifor, and Zoll; and receives research support from Amgen, CVRx, Novartis, Rietan, Stealth Pharmaceuticals, and Torrent. Dr. Pitt is a consultant for Bayer, Merck, Astra Zeneca, Boehringer Ingelheim, Takeda, Stealth Peptides, Cytopherex, Sarfez, Relypsa*, scPharmaceuticals*, PharmaIN*, KBP Pharmaceuticals*, Tricida, *DaVinci Therapeutics*, and AuraSense* (*stock options); and has a patent pending for site-specific delivery of eplerenone to the myocardium. Dr. Zannad is chair of the steering committee for Janssen. Dr. Linde reports research grants, speaker honoraria, and consulting fees from Astra Zeneca, St. Jude Medical, Biotronik, Medtronic, Vifor, Cardio3, and Novartis.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camm AJ, Kirchhof P, Lip GY, et al. European Heart Rhythm Association and European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Comparative Effectiveness Research Prioritization. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 8.Van Wagoner DR, Piccini JP, Albert CM, et al. Progress toward the prevention and treatment of atrial fibrillation: a summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9-10, 2013. Heart Rhythm. 2015;12:e5–e29. doi: 10.1016/j.hrthm.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirchhof P, Breithardt G, Bax J, et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/ European Heart Rhythm Association consensus conference. Europace. 2016;18:37–50. doi: 10.1093/europace/euv304. [DOI] [PubMed] [Google Scholar]
- 10.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 11.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 12.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO) Eur Heart J. 2016;18:1609–1678. [Google Scholar]
- 14.Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357:373–380. doi: 10.1016/S0140-6736(00)03651-5. [DOI] [PubMed] [Google Scholar]
- 15.Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC., Jr Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301:831–841. doi: 10.1001/jama.2009.205. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 19.Chan PH, Wong CK, Poh YC, Pun L, Leung WW, Wong YF, Wong MM, Poh MZ, Chu DW, Siu CW. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc. 2016;5:e003428. doi: 10.1161/JAHA.116.003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinhubl SR, Mehta RR, Ebner GS, et al. Rationale and design of a home-based trial using wearable sensors to detect asymptomatic atrial fibrillation in a targeted population: the mHealth Screening To Prevent Strokes (mSToPS) trial. Am Heart J. 2016;175:77–85. doi: 10.1016/j.ahj.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. doi: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997;96:3710–3720. doi: 10.1161/01.cir.96.10.3710. [DOI] [PubMed] [Google Scholar]
- 24.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 25.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: the CARDIO-FIT Study. J Am Coll Cardiol. 2015;66:985–996. doi: 10.1016/j.jacc.2015.06.488. [DOI] [PubMed] [Google Scholar]
- 28.Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll O, Wisloff U, Loennechen JP. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133:466–473. doi: 10.1161/CIRCULATIONAHA.115.018220. [DOI] [PubMed] [Google Scholar]
- 29.Alonso A, Bahnson JL, Gaussoin SA, Bertoni AG, Johnson KC, Lewis CE, Vetter M, Mantzoros CS, Jeffery RW, Soliman EZ, Look ARG. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J. 2015;170:770–777.e5. doi: 10.1016/j.ahj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Physical activity is associated with a reduced risk of atrial fibrillation in middle-aged and elderly women. Heart. 2015;101:1627–1630. doi: 10.1136/heartjnl-2014-307145. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56:409–414. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD, Wallentin L. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J. 2016;37:2869–2878. doi: 10.1093/eurheartj/ehw124. [DOI] [PubMed] [Google Scholar]
- 36.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, Arena R. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–258. doi: 10.1016/j.mayocp.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace. 2011;13:308–328. doi: 10.1093/europace/eur002. [DOI] [PubMed] [Google Scholar]
- 38.Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace. 2011;13:610–625. doi: 10.1093/europace/eur023. [DOI] [PubMed] [Google Scholar]
- 39.Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H, Vincent J, Pitt B EMPHASIS-HF Investigators. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 40.Chen WT, Krishnan GM, Sood N, Kluger J, Coleman CI. Effect of statins on atrial fibrillation after cardiac surgery: a duration- and dose-response meta-analysis. J Thorac Cardiovasc Surg. 2010;140:364–372. doi: 10.1016/j.jtcvs.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Z, Jayaram R, Jiang L, Emberson J, Zhao Y, Li Q, Du J, Guarguagli S, Hill M, Chen Z, Collins R, Casadei B. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374:1744–1753. doi: 10.1056/NEJMoa1507750. [DOI] [PubMed] [Google Scholar]
- 42.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124:2290–2295. doi: 10.1161/CIRCULATIONAHA.111.026153. [DOI] [PubMed] [Google Scholar]
- 43.Deftereos S, Giannopoulos G, Kossyvakis C, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. 2012;60:1790–1796. doi: 10.1016/j.jacc.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 44.Alings M, Smit MD, Moes ML, et al. Routine versus aggressive upstream rhythm control for prevention of early atrial fibrillation in heart failure: background, aims and design of the RACE 3 study. Neth Heart J. 2013;21:354–363. doi: 10.1007/s12471-013-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U STAF Investigators. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 46.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD Atrial Fibrillation Follow-up Investigation of Rhythm Management Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 47.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 48.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 49.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 50.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 52.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 53.Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 54.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS Natale A; RAAFT-2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 55.Jais P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 56.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48:2340–2347. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 57.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 58.Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370–378. doi: 10.1093/europace/euu376. [DOI] [PubMed] [Google Scholar]
- 59.Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation. 2015;132:907–915. doi: 10.1161/CIRCULATIONAHA.114.014092. [DOI] [PubMed] [Google Scholar]
- 61.Kuck KH, Brugada J, Furnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 62.Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, Novak P, Calzolari V, Guerra PG, Nair G, Torrecilla EG, Khaykin Y. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010;31:1344–1356. doi: 10.1093/eurheartj/ehq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 64.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atienza F, Almendral J, Ormaetxe JM, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol. 2014;64:2455–2467. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 66.Macle L, Khairy P, Weerasooriya R, et al. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet. 2015;386:672–679. doi: 10.1016/S0140-6736(15)60026-5. [DOI] [PubMed] [Google Scholar]
- 67.Kobori A, Shizuta S, Inoue K, et al. Adenosine triphosphate-guided pulmonary vein isolation for atrial fibrillation: the UNmasking Dormant Electrical Reconduction by Adenosine TriPhosphate (UNDER-ATP) trial. Eur Heart J. 2015;36:3276–3287. doi: 10.1093/eurheartj/ehv457. [DOI] [PubMed] [Google Scholar]
- 68.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–2325. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 69.Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 70.Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, Lambert H, Yulzari A, Wissner E, Kuck KH. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013;6:327–333. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- 71.Furnkranz A, Brugada J, Albenque JP, Tondo C, Bestehorn K, Wegscheider K, Ouyang F, Kuck KH. Rationale and design of FIRE AND ICE: a multicenter randomized trial comparing efficacy and safety of pulmonary vein isolation using a cryoballoon versus radiofrequency ablation with 3D-reconstruction. J Cardiovasc Electrophysiol. 2014;25:1314–1320. doi: 10.1111/jce.12529. [DOI] [PubMed] [Google Scholar]
- 72.Jiang RH, Po SS, Tung R, Liu Q, Sheng X, Zhang ZW, Sun YX, Yu L, Zhang P, Fu GS, Jiang CY. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm. 2014;11:969–976. doi: 10.1016/j.hrthm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 73.Cappato R, Negroni S, Pecora D, Bentivegna S, Lupo PP, Carolei A, Esposito C, Furlanello F, De Ambroggi L. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–1604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- 74.Jacobs V, May HT, Bair TL, Crandall BG, Cutler M, Day JD, Weiss JP, Osborn JS, Muhlestein JB, Anderson JL, Mallender C, Bunch TJ. The impact of risk score (CHADS2 versus CHA2DS2-VASc) on long-term outcomes after atrial fibrillation ablation. Heart Rhythm. 2015;12:681–686. doi: 10.1016/j.hrthm.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 75.Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, Hsu LF, Sanders P. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7:835–846. doi: 10.1016/j.hrthm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 76.Kurotobi T, Iwakura K, Inoue K, Kimura R, Okamura A, Koyama Y, Tosyoshima Y, Ito N, Fujii K. Multiple arrhythmogenic foci associated with the development of perpetuation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:39–45. doi: 10.1161/CIRCEP.109.885095. [DOI] [PubMed] [Google Scholar]
- 77.Cox JL, Schuessler RB, D'Agostino HJ, Jr, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–583. [PubMed] [Google Scholar]
- 78.Konings KT, Smeets JL, Penn OC, Wellens HJ, Allessie MA. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 79.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 80.Li WJ, Bai YY, Zhang HY, Tang RB, Miao CL, Sang CH, Yin XD, Dong JZ, Ma CS. Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: a meta-analysis. Circ Arrhythm Electrophysiol. 2011;4:143–148. doi: 10.1161/CIRCEP.110.958405. [DOI] [PubMed] [Google Scholar]
- 81.Verma A, Novak P, Macle L, Whaley B, Beardsall M, Wulffhart Z, Khaykin Y. A prospective, multicenter evaluation of ablating complex fractionated electro-grams (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008;5:198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 82.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 83.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 84.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Narayan SM, Krummen DE, Donsky A, Swarup V, Miller JM. Precise Rotor Elimination without Concomitant pulmonary vein Isolation for the Successful Elimination of Paroxysmal ATrial Fibrillation. PRECISE-PAF. Heart Rhythm. 2013;10 LBCT4. [Google Scholar]
- 86.Berntsen RF, Haland TF, Skardal R, Holm T. Focal impulse and rotor modulation as a stand-alone procedure for the treatment of paroxysmal atrial fibrillation: a within-patient controlled study with implanted cardiac monitoring. Heart Rhythm. 2016;13:1768–1774. doi: 10.1016/j.hrthm.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 87.Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar K, Mandapati R. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:554–561. doi: 10.1161/CIRCEP.115.002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marrouche NF, Brachmann J CASTLE-AF Steering Committee. Catheter ablation versus standard conventional treatment in patients with left ventricular dysfunction and atrial fibrillation (CASTLE-AF): study design. Pacing Clin Electrophysiol. 2009;32:987–994. doi: 10.1111/j.1540-8159.2009.02428.x. [DOI] [PubMed] [Google Scholar]
- 89.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 90.Sairaku A, Nakano Y, Oda N, Makita Y, Kajihara K, Tokuyama T, Kihara Y. Learning curve for ablation of atrial fibrillation in medium-volume centers. J Cardiol. 2011;57:263–268. doi: 10.1016/j.jjcc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Sairaku A, Yoshida Y, Nakano Y, Maeda M, Hirayama H, Hashimoto H, Kihara Y. Who is the operator, that is the question: a multicentre study of catheter ablation of atrial fibrillation. Europace. 2016;18:1352–1356. doi: 10.1093/europace/euv424. [DOI] [PubMed] [Google Scholar]
- 92.Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, McDonagh TA, Underwood SR, Markides V, Wong T. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 93.Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, Bradley DJ, Bluhm CM, Haroldson JM, Packer DL. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. doi: 10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 94.Khan MN, Jais P, Cummings J, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 95.MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, Denvir M, Bhagra S, Small S, Martin W, McMurray JJ, Petrie MC. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740–747. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 96.Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAM-TAF trial) Circ Arrhythm Electrophysiol. 2014;7:31–38. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 97.Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 98.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 99.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne P, Nattel S, Thibault B. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 100.Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–678. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 101.Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, Amlie J, Carlsen J Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 102.Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–2276. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 103.Roy D, Pratt CM, Torp-Pedersen C, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1518–1525. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 104.Pratt CM, Roy D, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S, Retyk E, Drenning DH Atrial Arrhythmia Conversion Trial Investigators. Usefulness of vernakalant hydrochloride injection for rapid conversion of atrial fibrillation. Am J Cardiol. 2010;106:1277–1283. doi: 10.1016/j.amjcard.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 105.Kowey PR, Dorian P, Mitchell LB, Pratt CM, Roy D, Schwartz PJ, Sadowski J, Sobczyk D, Bochenek A, Toft E Atrial Arrhythmia Conversion Trial Investigators. Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: a randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:652–659. doi: 10.1161/CIRCEP.109.870204. [DOI] [PubMed] [Google Scholar]
- 106.Camm AJ, Capucci A, Hohnloser SH, Torp-Pedersen C, Van Gelder IC, Mangal B, Beatch G AVRO Investigators. A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J Am Coll Cardiol. 2011;57:313–321. doi: 10.1016/j.jacc.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 107.Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J. 2016;37:747–754. doi: 10.1093/eurheartj/ehv213. [DOI] [PubMed] [Google Scholar]
- 108.Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M, Sandoval E, Calvo N, Brugada J, Kelder J, Wijffels M, Mont L. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 109.Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen J, Crijns HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012;60:54–61. doi: 10.1016/j.jacc.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 110.Muneretto C, Bisleri G, Bontempi L, Curnis A. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long-standing atrial fibrillation: a 30-month assessment with continuous monitoring. J Thorac Cardiovasc Surg. 2012;144:1460–1465. doi: 10.1016/j.jtcvs.2012.08.069. discussion 1465. [DOI] [PubMed] [Google Scholar]
- 111.Kirchhof P, Breithardt G, Aliot E, et al. Personalized management of atrial fibrillation: Proceedings from the fourth Atrial Fibrillation competence NETwork/ European Heart Rhythm Association consensus conference. Europace. 2013;15:1540–1556. doi: 10.1093/europace/eut232. [DOI] [PubMed] [Google Scholar]
- 112.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 115.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 116.Badger TJ, Daccarett M, Akoum NW, et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010;3:249–259. doi: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 118.Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 119.Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, George AL, Jr, Roden DM. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gollob MH, Jones DL, Krahn AD, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 121.Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett JC, Jr, Olson TM. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, Chen S, Yoo S, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 123.Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Darbar D, Roden DM. Genetic mechanisms of atrial fibrillation: impact on response to treatment. Nat Rev Cardiol. 2013;10:317–329. doi: 10.1038/nrcardio.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Otway R, Vandenberg JI, Guo G, et al. Stretch-sensitive KCNQ1 mutation A link between genetic and environmental factors in the pathogenesis of atrial fibrillation? J Am Coll Cardiol. 2007;49:578–586. doi: 10.1016/j.jacc.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 126.Parvez B, Vaglio J, Rowan S, Muhammad R, Kucera G, Stubblefield T, Carter S, Roden D, Darbar D. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol. 2012;60:539–545. doi: 10.1016/j.jacc.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parvez B, Shoemaker MB, Muhammad R, Richardson R, Jiang L, Blair MA, Roden DM, Darbar D. Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm. 2013;10:849–855. doi: 10.1016/j.hrthm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shoemaker MB, Bollmann A, Lubitz SA, et al. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2015;8:296–302. doi: 10.1161/CIRCEP.114.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi EK, Park JH, Lee JY, Nam CM, Hwang MK, Uhm JS, Joung B, Ko YG, Lee MH, Lubitz SA, Ellinor PT, Pak HN. Korean Atrial Fibrillation (AF) Network: genetic variants for af do not predict ablation success. J Am Heart Assoc. 2015;4:e002046. doi: 10.1161/JAHA.115.002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parvez B, Chopra N, Rowan S, Vaglio JC, Muhammad R, Roden DM, Darbar D. A common beta1-adrenergic receptor polymorphism predicts favorable response to rate-control therapy in atrial fibrillation. J Am Coll Cardiol. 2012;59:49–56. doi: 10.1016/j.jacc.2011.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mohanty S, Hall AW, Mohanty P, et al. Novel association of polymorphic genetic variants with predictors of outcome of catheter ablation in atrial fibrillation: new directions from a prospective study (DECAF) J Interv Card Electrophysiol. 2015;45:7–17. doi: 10.1007/s10840-015-0069-2. [DOI] [PubMed] [Google Scholar]
- 132.Lubitz SA, Yin X, Lin H, et al. Genetic risk prediction of atrial fibrillation. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.024143. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 134.Biankin AV, Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature. 2015;526:361–370. doi: 10.1038/nature15819. [DOI] [PubMed] [Google Scholar]
- 135.Hess CN, Rao SV, Kong DF, et al. Embedding a randomized clinical trial into an ongoing registry infrastructure: unique opportunities for efficiency in design of the Study of Access site For Enhancement of Percutaneous Coronary Intervention for Women (SAFE-PCI for Women) Am Heart J. 2013;166:421–428. doi: 10.1016/j.ahj.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Committee for Medicinal Products for Human Use (CHMP); European Medicine Agency. Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design. 2007 [Google Scholar]
- 137.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE. Device-detected atrial fibrillation and risk for stroke: an analysis of > 10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices) Eur Heart J. 2014;35:508–516. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]