Abstract
Introduction
Chromosomal rearrangements involving the ROS proto-oncogene 1 receptor tyrosine kinase gene (ROS1) define a distinct molecular subset of non-small cell lung cancer (NSCLC) with sensitivity to ROS1 inhibitors. Recent reports have suggested a significant overlap between ROS1 fusions and other oncogenic driver alterations, including mutations in epidermal growth factor receptor (EGFR) and KRAS proto-oncogene (KRAS).
Methods
We identified patients at our institution with ROS1-rearranged NSCLC who had undergone testing for genetic alterations in additional oncogenes, including EGFR, KRAS, and anaplastic lymphoma kinase (ALK). Clinicopathologic features and genetic testing results were reviewed. We also examined a separate database of ROS1-rearranged NSCLCs identified through a commercial FoundationOne assay.
Results
Among 62 patients with ROS1-rearranged NSCLC evaluated at our institution, none harbored concurrent ALK fusions (0%) or EGFR activating mutations (0%). KRAS mutations were detected in two cases (3.2%), one of which harbored a concurrent non-canonical KRAS I24N mutation of unknown biological significance. In a separate ROS1 FISH-positive case, targeted sequencing failed to confirm a ROS1 fusion, but instead identified a KRAS G13D mutation. No concurrent mutations in BRAF, ERBB2, PIK3CA, AKT1, or MAP2K1 were detected. Analysis of an independent dataset of 166 ROS1-rearranged NSCLCs identified by FoundationOne demonstrated rare cases with co-occurring driver mutations in EGFR (1/166) and KRAS (3/166), and no cases with co-occurring ROS1 and ALK rearrangements.
Conclusions
ROS1 rearrangements rarely overlap with alterations in EGFR, KRAS, ALK, or other targetable oncogenes in NSCLC.
Keywords: ROS1, non-small cell lung cancer, NSCLC
INTRODUCTION
ROS proto-oncogene 1 receptor tyrosine kinase (ROS1) is a validated therapeutic target in non-small cell lung cancer (NSCLC). Chromosomal rearrangements involving the ROS1 gene occur in 1–2% of NSCLCs,1–4 and are clinically associated with never smoking history, younger age, and adenocarcinoma histology.2 NSCLC cells harboring oncogenic ROS1 fusions are dependent on ROS1 signaling for their viability.1,5
In the clinic, identification of patients with NSCLC harboring ROS1 fusions is crucial, as these patients can have marked responses to ROS1-targeted tyrosine kinase inhibitors (TKIs). In an early-phase study of crizotinib in advanced ROS1-rearranged NSCLC, the objective response rate was 72% and median progression-free survival was 19.2 months.6 Two additional studies since then have demonstrated similarly high response rates (ranging 71–80%) to crizotinib in ROS1-rearranged NSCLC, although the median progression-free survival in these studies was shorter at 9–10 months.7,8 Based on its safety and efficacy, crizotinib was granted approval by the United States Food and Drug Administration and the European Medicines Agency for treatment of advanced ROS1-positive NSCLC. Several additional inhibitors with ROS1 activity are now being developed, including lorlatinib (NCT01970865), cabozantinib (NCT01639508), entrectinib (NCT02568267), ceritinib (NCT02186821), and DS-6051b (NCT02279433).
Genetic alterations in oncogenic drivers in NSCLC, including KRAS proto-oncogene (KRAS), epidermal growth factor receptor (EGFR), and anaplastic lymphoma kinase (ALK), are generally deemed mutually exclusive.9 Initial studies suggested that ROS1 rearrangements do not overlap with EGFR mutations or ALK rearrangements.2,4,6,10 However, conflicting findings have since been reported.11,12 For example, in a recent analysis of 25 NSCLCs positive for ROS1 rearrangement by immunohistochemistry (IHC), 36% were reported to harbor concomitant oncogenic driver mutations (including in EGFR, KRAS, PIK3CA, and BRAF).12 Five of the six patients with tumors harboring concurrent EGFR mutations in this cohort derived significant clinical benefit from an EGFR inhibitor and did not receive a ROS1-targeted therapy,12 raising the question of whether ROS1 rearrangements truly define a distinct molecular subset of NSCLC.
Herein, we examined ROS1-rearranged NSCLC patients who underwent genotyping of other oncogenes including KRAS, EGFR, and ALK, in order to determine the frequency of concurrent driver alterations in ROS1-rearranged NSCLC.
METHODS
Study population
Seventy patients with ROS1-rearranged NSCLC seen at Massachusetts General Hospital (MGH) between 2007 and October 2016 were identified. Of these, 62 had known mutational status of KRAS (exon 2), EGFR (exons 18–21) and ALK, and these patients (the MGH cohort) were selected for an institutional review board-approved retrospective analysis. Records were reviewed to extract data on clinicopathologic characteristics and tumor genotyping. An independent group of 166 ROS1-rearranged NSCLC patients were identified using the FoundationOne next-generation sequencing (NGS) assay at Foundation Medicine (the FM cohort). A total of eight patients were included in both cohorts.
Molecular testing
In the MGH cohort, ROS1 testing was performed using fluorescence in situ hybridization (FISH), targeted RNA sequencing using anchored multiplex polymerase chain reaction as previously described,13 commercially available FoundationOne NGS (Foundation Medicine, Cambridge, MA), or a commercial real-time polymerase chain reaction (PCR) assay (Clarient/NeoGenomics Laboratories, Fort Myers, FL). FISH was performed on formalin-fixed paraffin-embedded (FFPE) tumor tissue using a break-apart assay as previously described,2 and determined to be positive if >15% of tumor cells demonstrated split signals.
More comprehensive genotyping data (defined as sequencing for hotspot mutations in >10 genes) was available for 44 patients in the MGH cohort. The sequencing assay used for each patient is listed in Supplemental Table 1. Genes analyzed in each sequencing platform are listed in Supplemental Table 2. The FoundationOne (Foundation Medicine, Cambridge, MA), Smart Genomics (PathGroup, Brentwood, TN), and LUNGSEQ (Medfusion, Lewisville, TX) panels are commercially available. The MGH SNaPshot13 and Dana-Farber Cancer Institute (DFCI) OncoPanel14 assays have been previously described.
RESULTS
Identification of ROS1 rearrangements
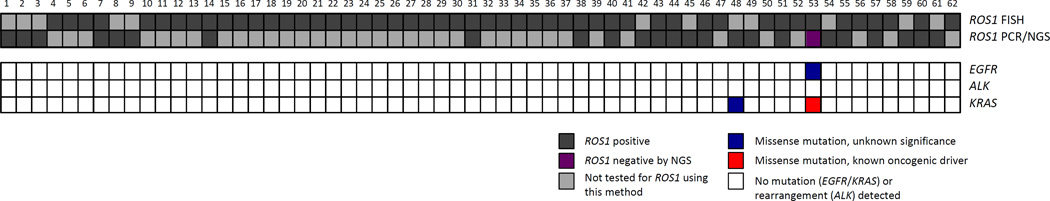
ROS1 fusions were identified in 62 patients in the MGH cohort using FISH (n = 38), targeted sequencing or PCR (n = 13), or both FISH and sequencing (n = 11). Clinicopathologic features of these 62 ROS1-rearranged NSCLC patients are summarized in Table 1. In the 24 cases of ROS1 fusions detected by NGS or PCR, four previously reported ROS1 fusion partners were identified: CD74 (n = 16), SDC4 (n = 4), EZR (n = 2), and SLC34A2 (n = 2).1–4 Twelve patients underwent ROS1 testing by both FISH and NGS, of whom 11 had concordant positive results (Figure 1). In the patient with discordant results (patient 53), FISH was positive with split signals in 44 of 50 tumor cell nuclei, but RNA sequencing on the same tumor did not detect a ROS1 fusion.
Table 1.
Baseline Characteristics.
| Characteristic | No (N = 62) | % |
|---|---|---|
| Age, years | ||
| Median | 52 | |
| Range | 22–84 | |
| Sex | ||
| Male | 23 | 37.1 |
| Female | 39 | 62.9 |
| Smoking history | ||
| Never smoker | 48 | 77.4 |
| Light smoker (<10 pack-years) | 5 | 8.1 |
| Smoker (≥10 pack-years) | 9 | 14.5 |
| Ethnicity | ||
| Asian | 9 | 14.5 |
| Caucasian | 45 | 72.6 |
| African-American | 5 | 8.1 |
| Hispanic | 2 | 3.2 |
| Unknown | 1 | 1.6 |
| Pathology | ||
| Adenocarcinoma | 62 | 100 |
| Squamous | 0 | 0 |
| Stage at diagnosis | ||
| IA | 3 | 4.8 |
| IB | 2 | 3.2 |
| IIA | 1 | 1.6 |
| IIB | 1 | 1.6 |
| IIIA | 7 | 11.3 |
| IIIB | 2 | 3.2 |
| IV | 46 | 74.2 |
Figure 1.
ROS1 rearrangements are generally mutually exclusive with oncogenic driver alterations in EGFR, KRAS, and ALK. One case (patient 53) in the MGH cohort had ROS1 testing that was positive by FISH (dark grey) but negative by NGS (purple). This case was found to harbor a KRAS G13D mutation (red) and an EGFR C781F mutation of unknown significance (blue). Another case (patient 48) had a KRAS I24N mutation of unknown significance (blue). All other ROS1-rearranged NSCLC cases in the MGH cohort tested negative (white) for concurrent EGFR and KRAS mutations and ALK rearrangements.
Genetic alterations of ALK, EGFR, and KRAS
All 62 cases were tested for ALK rearrangements, EGFR mutations, and KRAS mutations. None had a concurrent ALK fusion. A concurrent EGFR activating mutation was also not detected (Figure 1). The discordant ROS1 case (patient 53, mentioned above) was found to harbor an EGFR C781F mutation. This variant, which lies within the kinase domain, has not been previously reported, and its biological consequence is unknown.15,16
Two cases (3.2%; patients 53 and 48) had a KRAS mutation (Figure 1). Patient 53, the patient with discordant ROS1 testing (FISH-positive/NGS-negative) and EGFR C781F, was also found to harbor a KRAS G13D activating mutation. This patient, a 25-pack-year former smoker, was treated with crizotinib with no documented response, but experienced a sustained response to nivolumab. Patient 48 had a KRAS I24N mutation, which does not lie within a functional KRAS domain and is not a known oncogenic driver mutation. This patient responded to crizotinib for over seven months. The remaining 60 ROS1-positive cases had wild-type KRAS.
Other co-occurring genetic alterations
Forty-four of the 62 patients underwent more comprehensive tumor genotyping (i.e., sequencing of >10 genes). Among these 44 cases, 24 did not have additional genetic changes other than a ROS1 fusion detected. Twenty cases were found to have additional alterations, summarized in Table 2 and Supplemental Table 3. Recurrent co-occurring genetic alterations included TP53 mutations (11 of 43 tested cases, or 25.6%), CTNNB1 mutations (3 of 43 tested cases, or 7.0%), and CDKN2A/B loss (3 of 22 tested cases, or 13.6%).
Table 2.
Concomitant Genetic Alterations in ROS1-Rearranged NSCLC.
| Patient | Genes with Alterations | Prior Systemic Therapya |
|---|---|---|
| 1 | ATM, CTNNB1, TP53, DNMT3A | Yes |
| 2 | TP53 | No |
| 6 | TP53 | No |
| 10 | TP53 | No |
| 14 | TP53, SMAD4, APC | No |
| 24 | TP53, ROS1 (p.G2032R) | Yesb |
| 42 | MAP2K4, SF3B1 | No |
| 43 | TP53 | Yes |
| 45 | TSC2 | No |
| 46 | TP53 | No |
| 48 | KRAS (p.I24N), CTNNB1, TP53 | No |
| 49 | BAP1, CHEK2 | No |
| 50 | CTNNB1 | Yes |
| 53c | KRAS (p.G13D), EGFR (p.C781F), KIT, IDH1 | No |
| 54 | CDKN2A, TP53 | No |
| 55 | FLT1, PRKDC, RUNX1 | No |
| 57 | TP53 | No |
| 59 | CCND1, ARID1A, CDKN2A/B, FGF19, FGF4, FGF3 | No |
| 60 | MSH6, CDKN2A | No |
| 61 | CDKN2A/B | No |
Prior systemic therapy includes chemotherapy and/or crizotinib.
This tumor sample was derived post-crizotinib. The pre-crizotinib tumor sample did not harbor the ROS1 G2032R mutation, which is a known crizotinib-resistant mutation.23
This tumor sample tested positive for ROS1 rearrangement by FISH, but targeted RNA sequencing did not detect a ROS1 fusion transcript.
Notably, all 44 cases with additional genotyping were found to be wild-type for BRAF V600. Thirty-seven of the 44 cases were tested for BRAF non-V600 mutations, and all were wild-type. Among 39 cases tested for ERBB2 exon 20 insertions, none harbored these mutations. Similarly, oncogenic mutations in PIK3CA, MAP2K1, AKT1, and NRAS were not detected in the tested cases (n = 44, 37, 39, and 44, respectively), indicating that ROS1 fusions are generally mutually exclusive with other driver mutations in NSCLC.
In the recently published report by Wiesweg and colleagues,12 36% (9 of 25) of the ROS1-positive cases were found to harbor overlapping oncogenic mutations in EGFR, KRAS, PIK3CA, or BRAF. If this were the true frequency of overlap, then we would expect approximately 22 cases in the MGH cohort to have a concurrent driver mutation. However, only 3.2% (2 of 62)—a statistically significantly lower proportion (P < 0.001)—of the ROS1-rearranged cases in this cohort had a mutation detected in these oncogenes.
Independent analysis of 166 ROS-rearranged NSCLCs
In order to validate our findings regarding the frequency of driver mutations that co-occur with ROS1 rearrangements, a separate dataset of NSCLCs sequenced at Foundation Medicine was queried. Among a total of 17,538 NSCLC cases that underwent sequencing, 166 cases (0.95%) harbored a ROS1 fusion. Of note, eight of these cases were included in the MGH cohort described above.
Among the 166 ROS1-rearranged NSCLCs in the FM cohort, no concomitant ALK fusions (0%) were detected. One case (0.6%) had a concurrent EGFR activating mutation (L858R), and three (1.8%) had a concurrent KRAS driver mutation (Q61R, G12R, G12C). In addition, five cases (3.0%) had a concurrent PIK3CA mutation (E453Q, E453K, E545K, E726K, and E970K), while none (0%) had a BRAF V600E or a mutation in ERBB2, MAP2K1, or AKT1, again highlighting the significantly low prevalence of concurrent driver mutations. Of note, clinical information was not available for patients in this dataset; therefore, whether the co-occurring mutations arose de novo or post-treatment is unknown.
DISCUSSION
Current guidelines recommend upfront molecular testing for all patients with advanced lung adenocarcinoma. Identification of an actionable driver mutation directs patients to first- and often second-line targeted therapy, which typically results in durable clinical responses.3 Importantly, at this time, detection of EGFR, ALK, or ROS1 also directs patients away from first-line treatment with the PD-1 inhibitor pembrolizumab.17 Thus, establishing the correct tumor genotype is critical for patient management.
In this study, we examined two separate cohorts of ROS1-rearranged NSCLC patients. Taking into account the eight patients included in both cohorts, the total number of ROS1-rearranged NSCLC patients in this study is 220. This represents the largest series of ROS1-positive NSCLC patients with additional molecular assessments published to date. Among the 220 patients, there were no cases of ROS1 fusions co-occurring with ALK fusions and only one case with co-occurring ROS1 fusion and EGFR activating mutation. Interestingly, a total of four cases of the 220 harbored a KRAS activating mutation. However, one of these cases had discordant ROS1 FISH and NGS testing, and was likely ROS1-negative. While the FM cohort demonstrated co-occurrence of ROS1 rearrangement and PIK3CA mutations in five cases, no overlap with other oncogenic drivers, including BRAF (V600E), ERBB2, NRAS, AKT1, and MAP2K1 were identified. Altogether, these findings indicate that ROS1 rearrangements rarely overlap with other driver mutations in NSCLC.
These findings are in line with early studies suggesting minimal overlap between ROS1 fusions and ALK fusions or EGFR mutations,2,4,6,10 but are in contrast to other recent reports.11,12,18–21 One explanation for the discrepancy may be the difference in ROS1 testing techniques. At present, options for ROS1 detection include IHC, FISH, RT-PCR, and DNA or RNA sequencing. Each detection method is associated with distinct advantages and challenges. ROS1 break-apart FISH was used as the diagnostic assay in the global crizotinib study6 and is widely regarded as the gold standard. However, FISH can be technically challenging, and interpretation can vary depending on the laboratory, leading to false positives and false negatives. ROS1 IHC is not a validated screening assay for ROS1 rearrangement and is more complicated than ALK IHC given background expression of ROS1.22 In one recent study reporting a high prevalence of concurrent driver mutations with ROS1, 25 ROS1 IHC-positive cases were examined.12 Of these, only roughly half (n = 13) were positive for ROS1 rearrangement by FISH. Several of the cases found to harbor concomitant mutations in EGFR, KRAS, BRAF, and PIK3CA were, in fact, ROS1 FISH-negative,12 suggesting that the IHC result for these cases may have represented false positives. Lastly, NGS offers an alternative diagnostic option with the advantage that it can identify the fusion partner, detect novel fusions, and allow for multiplex testing. On the other hand, NGS requires significantly more tissue and time for data analysis than FISH or IHC, and additionally carries the theoretical risk of identifying novel fusion variants that may not be functionally relevant. Given the distinct characteristics of each diagnostic modality, ROS1 testing using orthogonal methods may be informative in the face of inconclusive initial screening results or inconsistent clinical behavior (e.g., lack of response to a TKI despite a positive testing result), as illustrated by patient 53 in the MGH cohort.
While concomitant mutations in currently targetable oncogenes were rare, a number of additional genetic aberrations were detected by NGS in our ROS1-rearranged NSCLC cohort, including TP53 mutations (in 25.2%), CDKN2A/B loss (in 13.6%), and CTNNB1 mutations (in 7%). Future investigations in larger patient cohorts are needed to define the true frequencies of co-occurring genomic alterations, and to understand whether the genetic changes that co-occur with ROS1 fusions may be biologically and therapeutically relevant.
There are several potential limitations of this study. First, concomitant genetic alterations may have been missed if they were present at very low allelic frequencies below the analytic sensitivity threshold of the targeted NGS platforms (< 5%), and if they occurred outside the hotspot regions covered by the specific assays. Second, tumor biopsy specimens carry the inherent limitation that they do not capture inter-metastatic tumor heterogeneity. While driver mutations are generally thought to be truncal events present at all sites of disease, other co-occurring genetic alterations could have evolved later and have been present at sites other than the one biopsied. Liquid biopsies (i.e., circulating tumor DNA analysis) and deeper sequencing technologies could help overcome these limitations.
In summary, we have found that ROS1 rearrangements rarely co-occur with other oncogenic drivers. These findings establish ROS1-rearranged NSCLC as a distinct molecular subset of lung cancer. Advanced NSCLC patients found to harbor ROS1 fusions should be treated with a ROS1 inhibitor. If concurrent driver mutations are identified, an orthogonal testing methodology should be considered to confirm the molecular diagnosis before proceeding with targeted therapy.
Supplementary Material
Acknowledgments
JFG has served as a compensated consultant or received honoraria from Novartis, Bristol-Myers Squibb, Merck, Ariad, Loxo, Genentech, Boehringer-Ingelheim, Jounce Therapeutics, Incyte, Clovis, and Kyowa Hakko Kirin. MMK has served as a compensated consultant for Merrimack Pharmaceuticals, ACD, and H3 Biomedicine. AJI has received personal fees from ArcherDx, Roche, and Chugai, and has a patent issued to ArcherDx. ATS has served as a compensated consultant or received honoraria from Pfizer, Novartis, Genentech/ Roche, Ariad, Ignyta, Daiichi-Sankyo, Taiho, Blueprint Medicines, Loxo, EMD Serono, and Foundation Medicine. SMA, MB, ABS, and VAM are employees of, and have equity interest, in Foundation Medicine, Inc.
Funding source:
This work was supported by the National Institutes of Health (5R01CA164273, to ATS), Be a Piece of the Solution, and LungStrong.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The remaining authors (JJL, LLR, LAF, JKL) have no other disclosures to declare.
LIST OF SUPPLEMENTAL DIGITAL CONTENT
Supplemental Table 1. Genotyping platforms.
Supplemental Table 2. Genes analyzed in the genotyping platforms.
Supplemental Table 3. Concomitant genetic alterations in ROS1-rearranged NSCLC: List of mutations.
REFERENCES
- 1.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch FR, Suda K, Wiens J, et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388:1012–1024. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, et al. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol. 2012;7:1086–1090. doi: 10.1097/JTO.0b013e3182570919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 8.Moro-Sibilot D, Faivre L, Zalcman G, et al. Crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC). Preliminary results of the ACSé phase II trial; Poster presented at: American Society of Clinical Oncology Annual Meeting; 2015 May 29–Jun 2; Chicago, IL. [Google Scholar]
- 9.Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR and KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida A, Kohno T, Tsuta K, et al. ROS1-rearranged lung cancer: a clinicopathologic and molecular study of 15 surgical cases. Am J Surg Pathol. 2013;37:554–562. doi: 10.1097/PAS.0b013e3182758fe6. [DOI] [PubMed] [Google Scholar]
- 11.Warth A, Muley T, Dienemann H, et al. ROS1 expression and translocations in non-small-cell lung cancer: clinicopathological analysis of 1478 cases. Histopathology. 2014;65:187–194. doi: 10.1111/his.12379. [DOI] [PubMed] [Google Scholar]
- 12.Wiesweg M, Eberhardt WE, Reis H, et al. High prevalence of concomitant oncogene mutations in prospectively identified patients with ROS1-positive metastatic lung cancer. J Thorac Oncol. 2016 doi: 10.1016/j.jtho.2016.08.137. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 14.Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucl Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pemrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016 doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 18.Go H, Kim DW, Kim D, et al. Clinicopathologic analysis of ROS1-rearranged non-small-cell lung cancer and proposal of a diagnostic algorithm. J Thorac Oncol. 2013;8:1445–1450. doi: 10.1097/JTO.0b013e3182a4dd6e. [DOI] [PubMed] [Google Scholar]
- 19.Kim HR, Lim SM, Kim HJ, et al. The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann Oncol. 2013;24:2364–2370. doi: 10.1093/annonc/mdt220. [DOI] [PubMed] [Google Scholar]
- 20.Ju L, Han M, Zhao C, et al. EGFR, KRAS, and ROS1 variants coexist in a lung adenocarcinoma patient. Lung Cancer. 2016;95:94–97. doi: 10.1016/j.lungcan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Scheffler M, Schultheis A, Teixido C, et al. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget. 2015;6:10577–10585. doi: 10.18632/oncotarget.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubendorf L, Büttner R, Al-Dayel F, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch. 2016;469:489–503. doi: 10.1007/s00428-016-2000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368:2395–2401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.