Abstract
HPV vaccination coverage is suboptimal. Previous research largely focused on vaccinating girls. This study aimed to identify factors associated with HPV vaccination among male and female adolescents. We conducted secondary analyses using the National Immunization Survey-Teen. We specified parallel logistic models to examine associations of adolescent, caregiver, and provider characteristics with vaccination status among boys and girls. The primary outcome was HPV vaccination status defined as unvaccinated, initiated, or completed. Additionally, we analyzed caregivers’ intent to initiate or complete the three-dose series. The vaccination completion rate was 26%. Among teens aged 13–17 years, 19% initiated, but did not complete the vaccine. Additionally, 14% of males completed the 3-dose series as compared to 38 % of females. Vaccination rates were higher among teens receiving a provider recommendation [girls: adjusted odds ratio (AOR) = 3.33, 95% confidence interval (CI) (2.44, 4.55); boys: AOR=10.0, 95% CI (7.69, 12.5)]. Moreover, provider recommendation was associated with caregivers’ intent to initiate vaccination [girls: AOR = 2.32, 95% CI (1.77, 3.02); boys: AOR = 2.76, 95% CI (2.22, 3.43)]. Other associations differed by gender. Higher vaccine initiation rates were associated with younger age and residing in the mid-west for girls and racial/ethnic minority and eligibility for the “Vaccine for Children” program for boys. Provider recommendation for vaccination was the strongest predictor for both genders; however, it is insufficient to achieve high coverage rates, especially among boys. Factors associated with HPV vaccination were different for males and females. These findings suggest providers should consider gender bias with regard to HPV vaccination.
Keywords: HPV vaccination, Gender, Adolescents, Secondary data
Introduction
HPV is responsible for 70% of all cervical cancers and 90 % of all genital warts in the United States [1,2]. Despite recommendations from major medical societies [1, 3–6], the rate of HPV vaccination is lower than many other adolescent vaccines [7], and rates are lower for boys as compared to girls [8]. The Center for Disease Control and Prevention (CDC) reported that in 2013, 57.3 % of girls 13–17 years of age received one or more doses and 37.6% completed the series [9]. For boys of the same age, the rates are 34.6 and 13.9% respectively [9, 10]. In comparison, other 3-step vaccines have achieved much higher coverage rates, including the hepatitis B vaccine, with a 92.8 % coverage rate among adolescents aged 13 to 17 years and the tetanus, diphtheria and pertussis (Tdap) vaccine, with 84.6 % coverage [11]. Poor coverage and adherence to the HPV vaccine puts adolescents at risk for HPV-related sequelae [12–14]. To advance evidence-based HPV vaccine promotion strategies, we sought to identify facilitators and barriers to HPV vaccination among adolescents of both genders across the continuum of vaccine adherence. While previous research examining HPV acceptability largely focuses on girls, we chose to compare factors associated with teen boys and girls in order to elucidate reasons behind variations in vaccine acceptance across genders.
In recent years the number of nonmedical vaccine exemptions has increased [15–17] resulting in vulnerability to outbreaks of vaccine preventable diseases [6, 18–20]. The reasons for vaccine refusal are numerous and complex; including parental concern about safety, mistrust of vaccines, and religious or philosophical beliefs [21–25]. HPV vaccination introduces an additional challenge, the contentious issues of adolescent sexuality and sexual debut. Specific to HPV, a lack of perceived need for the vaccine [14, 26] has been frequently reported as a reason for refusing HPV vaccination, including the belief that a child is not sexually active [14, 27, 28]. In addition, caregivers have expressed a concern that vaccination can lead to an early sexual debut and promote risky sexual behavior [29], but there has been no evidence to support this [30].
Given the low levels of HPV vaccination, it is important to identify opportunities to develop evidence-based strategies to promote vaccination. There are relatively few studies of HPV vaccine acceptance among males and even fewer that include both sexes. This study sought to investigate facilitators and barriers related to the full spectrum of HPV vaccine adherence, including vaccine intention, initiation and completion, for males and females. We postulated that HPV vaccine uptake and adherence were different among boys and girls due to differences in caretakers’ knowledge and perceptions regarding the need for the vaccine for male and female adolescents.
Methods
Data Source
We analyzed data from the 2013 administration of the National Immunization Survey-Teen (NIS-Teen), a nationally representative survey conducted by the CDC to monitor vaccination trends among adolescents 13 to 17 years of age. In the first phase of the survey, data are collected using a random-digit-dialed telephone survey with parents/guardians, whom we term “caregivers”. In the second phase, provider confirmation of vaccination status is obtained. The 2013 NIS-Teen used a dual-frame sampling approach with independent random digit dial samples of landline and cellular telephones [8]. The response rates were 55.1 and 23.3 % for landline and cellular samples respectively [31].
Study Sample
The 2013 NIS-Teen dataset has a total sample of 33,949 adolescents, of which 18,264 (54%) had adequate provider data to confirm immunization status. Following previous work [8], we included participants with provider confirmed vaccination status to limit the effect of recall error and social desirability bias [32, 33]. We compared demographic characteristics of participants with and without provider-verified vaccination status and confirmed that there is no systematic difference between the two groups. Residents of the US Virgin Islands and Guam were excluded because the cell-phone sample was not fielded in this region.
Measures
We based our analyses on the Vaccine Perceptions, Acceptability, and Adherence conceptual model developed by Katz et al. [34]. The primary outcomes were vaccine adherence, based on the stages of the HPV vaccination continuum, including vaccine initiation and completion, and intent to initiate and complete the series. Vaccination status was determined by provider-verified records. Initiation was defined as receiving one or two shots of the three-dose HPV series. Intention was based on the caregiver’s response to the question “How likely is it the “TEEN” will receive HPV shots in next 12 months?” Guided by previous studies [34–37], we identified three types of correlates of vaccination status: (1) characteristics of the child, including: age (13 to 17 years of age in single year increments); race/ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, or non-Hispanic other); history of being uninsured (yes, no, or unknown based on the answer to the question: “Since age 11, was there any time when the teen was not covered by any health insurance?”); time since last medical check-up (less than one year, one year, or two years or more defined by the difference between current age and age at last check-up); region of residence (Northeast, Midwest, South or West); insurance type and eligibility for the federal cost-free Vaccines For Children (VFC) program (private insurance, VFC-eligible, other and unknown, all derived by a set of questions about insurance coverage) [38]; (2) characteristics of the caregiver including: relationship of the survey respondent and adolescent (mother or other family member/friend); maternal age (less than or equal to 34 years of age, 35 to 44 years of age, greater than or equal to 45 years of age); educational level (less than 12 years; 12 years; greater than 12 years, but no-college degree; or college graduate); and marital status (married or unmarried); household income (grouped into categories by the federal poverty level (FPL): below poverty, 100 to 200% of FPL, above 200% FPL); and language used to administer NIS-Teen (English or Spanish/other languages); and (3) characteristics of the healthcare system, including facility type (public facilities, hospital facilities, private facilities, STD/school/teen clinics or other facilities, or mixed) and provider recommendation for the vaccine based on caregivers’ self-report on the question: “Has a doctor or other health care professional ever recommended that the “TEEN” receive HPV shots?” (yes or no).
Statistical Analyses
Rao-Scott Chi-Square tests were used for bivariate analyses. Multinomial logistic regression models were specified to estimate adjusted odds ratios (AORs) of being unvaccinated vs initiating HPV vaccination and completing all three shots vs initiating HPV vaccination. Additionally, we used multinomial logistic regression to analyze intent to vaccinate in the next 12 months among unvaccinated sub-populations and those that initiated, but had not completed the series. We also examined the main reason for refusing HPV vaccine reported by respondents who expressed no intention to vaccinate their teens by gender of the adolescents. Statistical tests were two tailed with a critical a of 0.05. To obtain nationally representative estimates, we applied the weights in the data files to adjust for the multistage stratified sampling design [31]. Analysis was based on appropriate estimates of variance for subsample analyses. All analyses were conducted in SAS version 9.3.1. This study was approved by the Boston University Institutional Review Board (Protocol H-33168).
Results
Characteristics of Study Sample
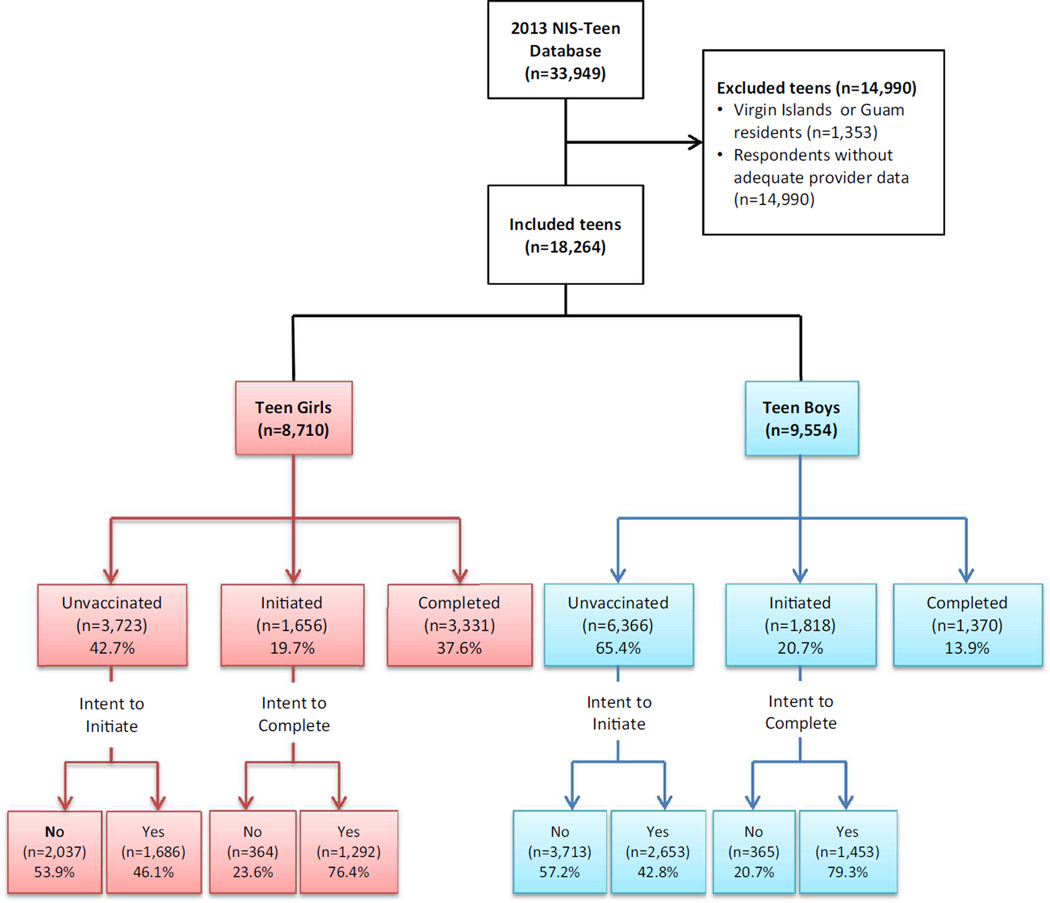
The 2013 NIS-Teen dataset has a total sample of 33,949 adolescents aged 13–17 years. We excluded 14,990 subjects who did not have provider-confirmed vaccination records or resided in Virgin Islands or Guam (Fig. 1). A demographic comparison of participants with and without provider-verified vaccination status confirmed that there were no systematic difference between two groups. The study sample consisted of 8,710 female and 9,554 male adolescents, representing about 10.1 and 10.6 million teen girls and boys nationally.
Fig. 1.
Flow chart of study sample selection
The overall vaccine completion rate was 26%. While girls were significantly more likely than boys to initiate the vaccine (57 vs 33% respectively, p < 0.0001), overall rates of completion remained low in both populations, particularly among boys (38 % for girls vs 14 % for boys, p < 0.0001). Vaccination intention of caregivers did not vary by gender of adolescents. About half of the caregivers declared intention to vaccinate their children against HPV; while more than three-quarters of parents intended to complete vaccine series.
The distributions of demographic and other characteristics were similar between genders, with more than one-fourth of teens living below the 2011 FPL and around one-third being eligible for VFC. One notable exception related to messaging from healthcare providers, specifically, 64 % of girls received a recommendation for the HPV vaccine as compared to 42 % of boys (Table 1).
Table 1.
Characteristics of study sample by gender
| Girls |
Boys |
|||
|---|---|---|---|---|
| Sample | p-valuea | Sample | p-valueb | |
| N (weighted N) | 8710 (10,162,055) | 9554 (10,649,852) | ||
| Characteristic of teens (weighted %) | ||||
| Age | <0.0001 | 0.812 | ||
| 13 | 19.57 | 20.01 | ||
| 14 | 19.58 | 21.23 | ||
| 15 | 21.59 | 19.74 | ||
| 16 | 22.65 | 20.17 | ||
| 17 | 16.61 | 18.84 | ||
| Race/ethnicity | <0.0001 | <0.0001 | ||
| Hispanic | 21.34 | 22.80 | ||
| NH White | 55.78 | 54.34 | ||
| NH Black | 13.37 | 14.13 | ||
| NH Others | 9.52 | 8.72 | ||
| Insurance type | 0.0063 | <0.0001 | ||
| Private | 58.23 | 57.23 | ||
| VFC-eligible* | 30.09 | 31.22 | ||
| Others | 3.88 | 3.44 | ||
| Unknown | 7.79 | 8.10 | ||
| Had ever been uninsured since age 11 | 0.2663 | 0.703 | ||
| No | 84.26 | 84.97 | ||
| Yes | 8.22 | 7.60 | ||
| Unknown | 7.51 | 7.43 | ||
| Time from last check-up visit (year) | <0.0001 | <0.0001 | ||
| 0 | 43.89 | 41.73 | ||
| 1 | 41.00 | 41.20 | ||
| 2+ | 13.43 | 15.72 | ||
| Unknown | 1.68 | 1.35 | ||
| Census region based on true state of residence | 0.0017 | <0.0001 | ||
| Northeast | 16.86 | 16.87 | ||
| Midwest | 21.70 | 21.68 | ||
| South | 37.50 | 37.47 | ||
| West | 23.93 | 23.98 | ||
| Characteristic of caregiver (weighted %) | ||||
| Relationship of respondent to the teen | 0.005 | 0.045 | ||
| Mother | 72.86 | 72.54 | ||
| Other family member/friend | 27.12 | 27.45 | ||
| Mother’s age | 0.0231 | 0.0009 | ||
| ≤34 | 10.53 | 9.62 | ||
| 35–44 | 43.79 | 45.20 | ||
| ≥45 | 45.69 | 45.18 | ||
| Education level of mother | <0.0001 | <0.0001 | ||
| <12 years | 13.69 | 13.92 | ||
| 12 years | 24.01 | 24.28 | ||
| >12 years, no-college degree | 26.30 | 25.56 | ||
| College graduate | 36.00 | 36.24 | ||
| Marital status of mother | <0.0001 | <0.0001 | ||
| Unmarried | 35.02 | 34.10 | ||
| Married | 64.98 | 65.90 | ||
| Income level | 0.0014 | <0.0001 | ||
| Below poverty | 26.81 | 28.52 | ||
| 100–200% FPL | 20.64 | 20.78 | ||
| Above 200% FPL | 52.55 | 50.70 | ||
| Language in which survey was conducted | <0.0001 | <0.0001 | ||
| English | 88.82 | 87.46 | ||
| Spanish/other | 11.18 | 12.54 | ||
| Characteristic of provider (weighted %) | ||||
| Facility type | 0.0059 | 0.2267 | ||
| Public facilities | 15.90 | 14.47 | ||
| Hospital facilities | 8.30 | 8.92 | ||
| Private facilities | 47.50 | 50.99 | ||
| STD/school/teen clinics or other facilities | 3.35 | 2.78 | ||
| Mixed | 22.76 | 20.43 | ||
| Unknown | 2.19 | 2.41 | ||
| Provider recommended HVP vaccine | <0.0001 | <0.0001 | ||
| No | 28.31 | 49.42 | ||
| Yes | 64.42 | 41.64 | ||
| Unknown | 7.27 | 8.93 | ||
Vaccine for Children (VFC) program provides vaccines at no cost to children who might not otherwise be vaccinated because of inability to pay. Children who are eligible for VFC are entitled to receive those vaccines recommended by the Advisory Committee on Immunization Practices, including HPV for girls. Children who are eligible for the VFC program include enrollees of Medicaid, S-CHIP, or Indian health service, uninsured, and those covered by military health care, Tricare, CHAMPUS, or champ-VA
Rao-Scott Chi-Square Test was performed to measure the associations between individual covariates and HPV vaccination status
Factors Associated with Vaccination Initiation and Completion
Providers promoting HPV immunization significantly increased the odds of HPV vaccine initiation, regardless of the gender of the child (adjusted odds ratio (AOR) = 3.33, 95 % confidence interval (CI) [2.44, 4.55] for girls; AOR = 10.0, 95 % CI [7.69, 12.5] for boys) (Table 2). However, provider recommendation was not related to completion of HPV vaccine. Risk factors associated with higher unvaccinated rates among teens of both genders included lack of routine check-up visits, English proficiency of caregivers, and marital status of mothers.
Table 2.
Multinomial logit models investigating predictors of HPV vaccination status (comparison group is teens who initiated HPV series), OR (95% CI)
| Unvaccinated vs. initiated (Girls: 3723 unvac- cinated; 1656 initiated Boys: 6366 unvacci- nated; 1818 initiated) |
Completed vs. initiated (Girls: 1656 initiated; 3331 completed Boys: 1818 initiated; 1370 completed) |
|||
|---|---|---|---|---|
| Characteristics | Girls | Boys | Girls | Boys |
| N | 7375 | 7856 | 7375 | 7856 |
| Age of teens (Ref = 17) | ||||
| 13 | 0.68 (0.46, 0.99) | 1.24(0.85, 1.80) | 0.26 (0.17, 0.38) | 0.63 (0.39, 1.03) |
| 14 | 0.65 (0.44, 0.96) | 0.96 (0.67, 1.39) | 0.35 (0.24, 0.52) | 0.65 (0.40, 1.05) |
| 15 | 0.86 (0.58, 1.28) | 1.07 (0.72, 1.57) | 0.61 (0.40, 0.92) | 0.95 (0.57, 1.56) |
| 16 | 0.80 (0.53, 1.22) | 0.93 (0.65, 1.33) | 0.66 (0.44, 1.00) | 0.64 (0.42, 0.98) |
| Race/ethnicity of teens (Ref=NH white) | ||||
| Hispanic | 1.10(0.69, 1.75) | 0.67 (0.45, 0.99) | 1.30(0.88, 1.92) | 1.16 (0.67, 2.00) |
| NH black | 0.90 (0.59, 1.35) | 0.46 (0.31, 0.68) | 0.91 (0.61, 1.35) | 0.96 (0.61, 1.50) |
| NH others | 0.75(0.51, 1.12) | 0.91 (0.62, 1.33) | 1.16(0.76, 1.77) | 0.88 (0.56, 1.39) |
| VFC-eligible(Ref=Yes) | 0.87 (0.62, 1.23) | 0.67 (0.48, 0.96) | 0.88 (0.62, 1.25) | 0.84 (0.54, 1.29) |
| Ever uninsured since age 11 (Ref=Yes) | 0.69 (0.45, 1.05) | 0.84(0.54, 1.32) | 0.78 (0.49, 1.23) | 0.74 (0.36, 1.53) |
| Time from last check-up visit (year) | ||||
| 1 vs. 0 | 1.14(0.88, 1.46) | 1.21 (0.94, 1.55) | 0.79 (0.61, 1.01) | 1.02 (0.75, 1.40) |
| 2+ vs. 0 | 1.87 (1.26, 2.77) | 2.20 (1.33, 3.65) | 0.93 (0.58, 1.49) | 0.86 (0.40, 1.84) |
| State of residence (Ref=Northeast) | ||||
| Midwest | 0.69 (0.50, 0.94) | 1.50 (1.11, 2.03) | 0.68 (0.50, 0.93) | 1.03 (0.72, 1.49) |
| South | 0.90(0.66, 1.22) | 1.60 (1.17, 2.18) | 0.83 (0.61, 1.14) | 1.21 (0.85, 1.74) |
| West | 0.59 (0.39, 0.90) | 0.75 (0.53, 1.07) | 0.70 (0.46, 1.06) | 0.64 (0.41, 1.02) |
| Mother was the survey respondent (Ref=Yes) | 1.70 (1.25, 2.30) | 1.08 (0.81, 1.44) | 1.24 (0.91, 1.67) | 1.10(0.78,1.58) |
| Mother’s age (Ref=45+ years) | ||||
| ≤34 | 0.93 (0.58, 1.47) | 0.77(0.51, 1.16) | 0.96 (0.61, 1.49) | 0.73 (0.42, 1.24) |
| 35–44 | 1.18 (0.90, 1.54) | 0.84(0.65,1.10) | 0.98 (0.74, 1.29) | 0.72 (0.51, 1.03) |
| Education level of mother (Ref ≤ 12 years) | ||||
| 12 years | 1.95 (1.18, 3.22) | 0.89 (0.53, 1.49) | 1.47(0.88,2.44) | 0.62(0.34, 1.15) |
| >12 years, non-college grad | 1.82 (1.10, 3.00) | 1.04 (0.63, 1.70) | 0.98 (0.58, 1.66) | 0.61 (0.33, 1.13) |
| college graduate | 1.77 (1.07, 2.92) | 0.65(0.38,1.10) | 1.10(0.63,1.91) | 0.50 (0.27, 0.93) |
| Mother is married (Ref=Yes) | 1.35 (1.00, 1.82) | 1.62 (1.21,2.18) | 0.96 (0.72, 1.28) | 1.33 (0.92, 1.93) |
| Income level (Ref ≥ 200% FPL) | ||||
| Below poverty | 0.74(0.50,1.11) | 0.76(0.51,1.12) | 0.96 (0.64, 1.43) | 0.90(0.51, 1.57) |
| 100–200% FPL | 0.93 (0.66, 1.30) | 0.91 (0.59, 1.40) | 0.98 (0.70, 1.39) | 1.06 (0.65, 1.75) |
| English proficiency (Ref=Yes) | 2.42 (1.15,5.09) | 3.94 (2.16,7.22) | 0.69 (0.36, 1.32) | 1.05(0.51,2.14) |
| Facility type (Ref=private facilities) | ||||
| Hospital | 0.61 (0.38, 1.00) | 0.87 (0.59, 1.28) | 0.87 (0.54, 1.41) | 1.02(0.64, 1.62) |
| Public facilities | 0.85 (0.56, 1.28) | 1.10(0.71, 1.72) | 0.63 (0.42, 0.94) | 1.03 (0.58, 1.83) |
| STD/school/teen clinics/other | 1.30(0.66,2.56) | 2.34 (0.92, 5.95) | 0.58(0.29, 1.17) | 0.88 (0.34, 2.28) |
| Mixed facilities | 0.93 (0.70, 1.24) | 0.77 (0.58, 1.03) | 0.96 (0.72, 1.27) | 0.91 (0.61, 1.34) |
| Provider recommended vaccine (Ref=Yes) | 0.30 (0.22, 0.41) | 0.10 (0.08, 0.13) | 1.27 (0.89, 1.80) | 1.31 (0.88, 1.93) |
Bold font indicates statistical significance at p<0.05
The impact of other factors on vaccine initiation differed by gender. Notably, unvaccinated rates were higher among girls of mothers with higher levels of educational attainment [AOR = 1.95, 95 % CI (1.18, 3.22) for mothers with 12-year education; AOR = 1.82, 95 % CI (1.10, 3.00) for mothers attending college; AOR = 1.77, 95 % CI (1.07, 2.92) for mothers who completed college]. Among boys, racial/ethnic minorities and those eligible for VFC program had higher rates of initiation.
Among adolescents who had initiated HPV vaccination, we did not identify any significant predictors of completing the series that were the same for males and females. Factors associated with series completion among females were age, region of residence, and healthcare facility type. Among males, those with more highly educated mothers were less likely to be fully immunized.
Factors Associated with Intent to Initiate and Complete Vaccination
Approximately 50 % of caregivers of unvaccinated teens were “somewhat” or “very” likely to vaccinate their children in the next 12 months, while nearly 80 % intended to complete the series. This suggested uptake and adherence behaviors may be driven by different factors. Therefore, we further explored determinants of intent to initiate and complete HPV vaccine using multivariable logistic regression models.
Provider recommendation for HPV vaccine was a significant predictor of greater willingness to initiate and complete the HPV vaccine, regardless of the gender of teens (Table 3). Among unvaccinated adolescents, the intent to initiate was considerably higher among those receiving provider recommendations [AOR = 2.32, 95% CI (1.77, 3.02) for girls; AOR = 2.76, 95% CI (2.22, 3.43) for boys]. Among initiated teens, caregivers receiving vaccine recommendations from providers were also more likely to intend to complete the series [AOR = 1.97, 95 % CI (1.10, 3.52) for girls; AOR = 2.24, 95 % CI (1.27, 3.97) for boys].
Table 3.
Multinomial logit models investigating predictors of intent to initiate and intent to complete the HPV vaccination, OR (95 % CI)
| Among unvaccinated teens |
Among initiated teens |
|||
|---|---|---|---|---|
| Characteristics | Girls (3723) | Boys (6366) | Girls (1656) | Boys (1818) |
| N | 3085 | 5135 | 1384 | 1512 |
| Age of teens (Ref = 17) | ||||
| 13 | 1.54 (0.99, 2.40) | 1.09 (0.78, 1.52) | 2.20 (1.03, 4.70) | 1.87 (0.78, 4.47) |
| 14 | 1.50(0.96,2.33) | 0.98 (0.70, 1.36) | 1.80(0.81,4.02) | 0.95 (0.41, 2.21) |
| 15 | 1.34(0.85,2.12) | 1.05 (0.76, 1.46) | 1.35 (0.66, 2.77) | 1.30 (0.57, 2.97) |
| 16 | 1.09 (0.69, 1.73) | 1.15(0.83, 1.61) | 1.09 (0.52, 2.28) | 1.04 (0.49, 2.20) |
| Race/ethnicity of Teens (Ref=NH white) | ||||
| Hispanic | 2.10 (1.32, 3.33) | 1.71(1.17,2.49) | 0.90(0.42, 1.94) | 1.12 (0.51, 2.44) |
| NH black | 1.31(0.85,2.03) | 1.26 (0.90, 1.75) | 1.34 (0.64, 2.78) | 1.71 (0.86, 3.40) |
| NH others | 0.98 (0.63, 1.51) | 1.28 (0.89, 1.85) | 0.56 (0.25, 1.23) | 1.57 (0.73, 3.36) |
| VFC-eligible(Ref=Yes) | 0.77(0.53, 1.12) | 1.12(0.83, 1.51) | 0.76 (0.40, 1.47) | 1.18(0.58,2.42) |
| Ever uninsured since age 11 (Ref=Yes) | 0.72(0.41, 1.25) | 0.90(0.61, 1.34) | 0.86 (0.43, 1.69) | 1.32(0.56,3.10) |
| Time from last check-up visit (year) | ||||
| 1 vs. 0 | 1.29 (0.99, 1.70) | 1.04 (0.85, 1.29) | 0.54 (0.34, 0.85) | 1.24(0.73,2.11) |
| 2+ vs. 0 | 0.79 (0.55, 1.15) | 1.10(0.81, 1.51) | 0.67 (0.28, 1.64) | 0.71 (0.23,2.19) |
| State of residence (Ref=Northeast) | ||||
| Midwest | 0.86(0.61, 1.21) | 0.97 (0.73, 1.29) | 0.37 (0.19, 0.75) | 1.13(0.58,2.20) |
| South | 1.02(0.73, 1.43) | 1.00 (0.75, 1.33) | 0.43 (0.21,0.86) | 1.01 (0.57, 1.77) |
| West | 1.04 (0.67, 1.62) | 1.03 (0.72, 1.48) | 0.61 (0.27, 1.40) | 1.08(0.50,2.31) |
| Mother was the survey respondent (Ref=Yes) | 0.72 (0.53, 0.96) | 1.27 (1.00, 1.63) | 2.49 (1.46,4.22) | 1.99(1.19,3.33) |
| Mother’s age (Ref=45+ years) | ||||
| ≤34 | 1.13 (0.67, 1.89) | 0.91 (0.63, 1.32) | 0.70 (0.32, 1.52) | 0.69(0.31,1.51) |
| 35–44 | 0.82 (0.63, 1.07) | 1.06 (0.85, 1.33) | 0.96 (0.57, 1.64) | 0.73 (0.40, 1.32) |
| Education level of mother (Ref ≤ 12 years) | ||||
| 12 years | 0.71 (0.40, 1.27) | 0.84 (0.52, 1.35) | 0.41 (0.17, 1.00) | 0.93(0.41,2.12) |
| >12 years, non-college grad | 0.53 (0.29, 0.95) | 0.72(0.45,1.14) | 0.56 (0.23, 1.38) | 0.67 (0.28, 1.58) |
| College graduate | 0.45 (0.25, 0.81) | 0.68(0.42,1.11) | 0.60 (0.23, 1.59) | 0.67 (0.24, 1.86) |
| Mother is married (Ref=Yes) | 0.66 (0.48,0.92) | 0.67 (0.53,0.85) | 1.12(0.68, 1.82) | 1.12(0.66, 1.92) |
| Income level (Ref > 200% FPL) | ||||
| Below poverty | 1.06 (0.71, 1.57) | 1.00 (0.72, 1.40) | 1.55 (0.70, 3.43) | 0.34 (0.17, 0.68) |
| 100–200% FPL | 1.44(1.00,2.09) | 0.78 (0.58, 1.06) | 1.30(0.67,2.53) | 0.47 (0.24, 0.94) |
| English proficiency (Ref=Yes) | 0.75 (0.33, 1.74) | 0.65 (0.32, 1.30) | 0.37(0.11, 1.30) | 1.01 (0.38, 2.73) |
| Facility type (Ref=private facilities) | ||||
| Hospital | 1.17(0.75,1.84) | 0.97 (0.65, 1.43) | 0.70 (0.29, 1.67) | 1.38 (0.57, 3.38) |
| Public facilities | 1.20 (0.80, 1.79) | 0.83 (0.60, 1.15) | 0.42 (0.20, 0.85) | 0.84(0.36, 1.97) |
| STD/school/teen clinics/other | 0.76 (0.36, 1.61) | 1.08 (0.60, 1.98) | 0.68(0.18,2.48) | 0.28(0.06, 1.31) |
| Mixed facilities | 1.02 (0.73, 1.42) | 1.14(0.90, 1.46) | 0.76 (0.43, 1.32) | 1.15(0.63,2.12) |
| Provider recommended vaccine (Ref=Yes) | 2.32 (1.77,3.02) | 2.76 (2.22, 3.43) | 1.97 (1.10, 3.52) | 2.24 (1.27, 3.97) |
Bold font indicates statistical significance at p<0.05
Another factor related to uptake intention was educational attainment. Highly educated mothers were less willing to vaccinate their daughters against HPV [AOR = 0.53, 95% CI (0.29, 0.95) for mothers who attended college, AOR = 0.45, 95 % CI (0.25, 0.81) for mothers with a college degree], however, this was not the case for male adolescents.
Other factors associated with completion intention included age of teens, facilities where teens sought routine care, and family income. However, impact of these factors was gender specific and no consistent trend was observed.
Reasons for Refusing the HPV Vaccine
Table 4 summarizes the leading reasons for refusing the HPV vaccine overall, and by gender of the teens. Overall, the most common reason for refusal were that the vaccine was not recommended (21.3 %), belief that the vaccine was unneeded (16.8 %), lack of knowledge (16.3 %), safety concerns (9.7 %), and teen is not sexually active (8.7 %). Gender comparisons suggested that caregivers of male teens were more likely to report that the vaccine was not recommended by a provider or lack of perceived need for the vaccine; while guardians of females were more likely to decline vaccination based on safety concerns.
Table 4.
Rao-Scott Chi-Square tests investigating gender-based differences for reasons for HPV vaccine refusal
| Reason for refusal | All (n = 6166) |
Girls (n = 2297) |
Boys (n = 3869) |
p-value |
|---|---|---|---|---|
| Not recommended | 21.3% | 16.1% | 24.6% | <0.0001 |
| Not needed or necessary |
16.8% | 14.6% | 18.3% | 0.018 |
| Lack of knowledge | 16.3% | 16.4% | 16.2% | 0.877 |
| Safety concern/side effects |
9.7% | 14.1% | 6.8% | <0.0001 |
| Not sexually active | 8.7% | 10.1% | 7.8% | 0.077 |
Discussion
Results strongly suggest that factors influencing initiation of the HPV vaccination are different for male and female adolescents. The most common reasons for refusing the HPV vaccine were: lack of vaccine endorsement by the healthcare provider, lack of perceived need for the vaccine, including the belief that the child is not sexually active, lack of knowledge and safety concerns, and caregivers’ concerns about safety or potential side effects. These findings are consistent with the prior literature [10, 14, 25–29, 39]. Reasons for vaccine refusal by gender shows marked differences in providers’ and caregivers’ attitudes toward vaccination for boys and girls. We found that caregivers of male children were significantly more likely to report that the vaccine was not recommended by the provider (24.6 % for boys, 16.1 % for girls; p-value <0.0001), and that it was not necessary (18.3 % for boys, 14.6 % for girls; p-value = 0 0.018). In contrast, caregivers of girls were significantly more likely to report concerns for safety (14.1 % for girls, 6.8 % for boys; p-value <0.0001). In addition, caregivers more frequently reported that their daughters were not sexually active, although this was not statistically significant (10.1 % for girls, 7.8 % for boys; p-value 0.077). These findings suggest that healthcare providers need to be attentive to potential gender bias with regard to HPV vaccination.
Provider recommendation was the most robust and consistent predictor of receipt of HPV vaccine and intention to receive an HPV vaccination among caregivers, regardless of the gender of the teen. These findings are consistent with prior research [40–42]. The impact on vaccination rates due to provider recommendation was a 70 and 90 % reduction in the adjusted unvaccinated rates among girls and boys, respectfully.
Provider recommendation was also associated with a two-fold increase in the odds of intention to initiate and complete the HPV vaccine series. Provider recommendation, however, was not associated with completion of the three-shot series among those who started, suggesting that other unmeasured factors may be driving series completion. A critical factor that was not measured in this data set was whether providers issued reminders to complete the vaccine series beyond the first dose. Research indicates that immunization recall and reminder systems increase vaccination rates against HPV and other vaccine preventable diseases [43, 44].
Prior studies indicate that the likelihood of a provider recommending the vaccine is related to the gender of the adolescent and other characteristics of the adolescent-caregiver dyad [39, 45, 46]. A survey fielded nationally among pediatricians and family physicians found providers less frequently recommended the vaccine on time for boys (60 %) as compared to girls (25 %) [39]. Another qualitative study showed providers were hesitant to offer HPV vaccines to males because they felt that parents were more reluctant to vaccinate their sons than their daughters [45]. Furthermore, a large fraction of providers make vaccine recommendations based on perceived risk for HPV infection or an abnormal result of Papanicolaou or HPV testing [39, 46]. This goes against national guidelines recommending universal vaccination of adolescents within eligible age groups [MMWR, 2014]. Furthermore, earlier research has shown that physicians express a belief that educating parents about the HPV vaccine requires more time than it might for other vaccines and that providers are less likely to endorse the vaccine if they perceived the caregiver or parents did not value it [39].
Finally, we found that receiving provider recommendations for HPV vaccine did not lead to a higher degree of acceptability among caregivers with certain characteristics. Although providers were more likely to recommend the vaccine to girls with highly educated mothers, highly educated mothers were immune to provider recommendations, suggesting that they may arrive at clinic visits with preconceived notions of what is appropriate for their child. This paradox suggests some caregivers may perceive messages conveyed by clinicians as an option rather than a direct recommendation. A previous study showed message framing plays an essential role in decision-making about HPV vaccination [47]. Failure to address the skepticism of caregivers about HPV vaccines may significantly weaken the effectiveness of provider recommendations. This trend was not observed in boys, suggesting that the mechanism of how educational attainment mediates caregivers’ decision making differs by the gender of the teen. Highly educated caregivers may be more hesitant about HPV vaccine when making decisions for their daughters. Most vaccine-related decision-making largely depends upon the caregivers’ perceptions of their child’s risk of exposure [48], normative beliefs about vaccines [49], and provider attitudes and reactions to vaccine programs [50]. These findings emphasize a need for enhanced efforts to educate caregivers about the safety and efficacy of the vaccine.
This study has several limitations. Vaccination and adolescent sexuality are controversial and sensitive topics and, therefore, social desirability bias may influence caregivers’ responses to survey questions in a perceived favorable direction. While we limited the study to respondents with provider confirmed vaccination status, this bias also may have influenced the responses to questions regarding intent to vaccinate. Another limitation is that NIS-Teen only collects information on attitudes about vaccination from the caregivers who refused HPV vaccination. This provides little input on beliefs about susceptibility to HPV-related diseases and perceptions of vaccine effectiveness among those who vaccinated their teens. Asking caregivers who vaccinated their children against HPV about their attitudes toward the vaccine has the potential to bring a more robust understanding of factors that contribute to vaccine acceptance which could further inform strategies for vaccine promotion. Finally, the NIS-Teen does not capture other factors that potentially impact initiation or completion of the HPV vaccine, such as differences in the nature of healthcare systems, the functioning of clinical practices and providers’ attitudes toward vaccines. Geographic region of the country, facility type, and insurance type were included in the analyses to control for unmeasured factors that might drive differential access and attitudes toward vaccines. Given the broad geographic representation of the data, we were unable to look at these factors at a more granular level.
In conclusion, the factors associated with HPV vaccination were different for males and female adolescents. Provider recommendation for the HPV vaccines is the strongest predictor of vaccination for teens of both genders; however, provider recommendation itself is insufficient to achieve high coverage rates, especially among males. Furthermore, provider recommendation did not lead to a higher rate of vaccine acceptance among girls with highly educated mothers. Further investigation is needed to identify the differences in caregivers’ perceptions of the vaccine for their male and female children. Tailoring and targeting the recommendations to address the unique concerns of caregivers of male and female teens may improve acceptability and uptake of the HPV vaccine.
Acknowledgments
This research was conducted as a doctoral student project and was not supported by any funding sources.
Footnotes
Compliance with Ethical Standards
Conflict of interest The Authors declare that there is no conflict of interest.
References
- 1.Saslow D, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. C CA: A Cancer Journal for Clinicians. 2007;57(1):7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz LE, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the advisory committee on immunization practices (ACIP) MMWR Recommendations and Reports. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 3.Committee on Infectious Diseases. HPV vaccine recommendations. Pediatrics. 2012;129(3):602–605. doi: 10.1542/peds.2011-3865. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morbidity and Mortality Weekly Report. 2010;59(20):626–629. [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. Committee opinion no. 467: Human papillomavirus vaccination. Obstetrics & Gynecology. 2010;116(3):800–803. doi: 10.1097/AOG.0b013e3181f680c8. [DOI] [PubMed] [Google Scholar]
- 6.Friedman L, et al. Human papillomavirus vaccine: An updated position statement of the Society for Adolescent Health and Medicine. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine. 2011;48(2):215–216. doi: 10.1016/j.jadohealth.2010.11.254. [DOI] [PubMed] [Google Scholar]
- 7.Statistics, N.C.f.H. Health, United States, 2013: With special feature on prescription drugs. 2013 [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years-United States, 2012. MMWR Morbidity and Mortality Weekly Report. 2013;62(34):685–693. [PMC free article] [PubMed] [Google Scholar]
- 9.Stokley S, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morbidity and Mortality Weekly Report. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 10.Lu PJ, et al. HPV vaccination coverage of male adolescents in the United States. Pediatrics. 2015;136(5):839–849. doi: 10.1542/peds.2015-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis RC, MacNeil J, Hariri S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2013. MMWR Morbidity andMortality Weekly Report. 2014;63(29):625–633. [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz N, et al. Impact of human papillomavirus (HPV)-6/l1/16/18 vaccine on all HPV-associated genital diseases in young women. Journal of the National Cancer Institute. 2010;102(5):325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 13.Cogliano V, et al. Carcinogenicity of human papillomaviruses. The Lancet Oncology. 2005;6(4):204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 14.Dorell CG, et al. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128(5):830–839. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 15.Wang E, et al. Nonmedical exemptions from school immunization requirements: A systematic review. American Journal of Public Health. 2014;101:e1–e23. doi: 10.2105/AJPH.2014.302190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constable C, Blank NR, Caplan AL. Rising rates of vaccine exemptions: Problems with current policy and more promising remedies. Vaccine. 2014;32(16):1793–1797. doi: 10.1016/j.vaccine.2014.01.085. [DOI] [PubMed] [Google Scholar]
- 17.CDC. Vaccination coverage among children in kindergarten—United States, 2011–12 school year. MMWR. Morbidity andMortality Weekly Report. 2012;61(33):647–652. [PubMed] [Google Scholar]
- 18.Parker Fiebelkorn A, et al. Measles in the United States during the postelimination era. The Journal of Infectious Diseases. 2010;202(10):1520–1528. doi: 10.1086/656914. [DOI] [PubMed] [Google Scholar]
- 19.Gastanaduy PA, et al. Measles—United States, January 1-May 23, 2014. MMWR. Morbidity and Mortality Weekly Report. 2014;63(22):496–499. [PMC free article] [PubMed] [Google Scholar]
- 20.Atwell JE, et al. Nonmedical vaccine exemptions and pertussis in California, 2010. Pediatrics. 2013;132(4):624–630. doi: 10.1542/peds.2013-0878. [DOI] [PubMed] [Google Scholar]
- 21.Omer SB, et al. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. The New England Journal of Medicine. 2009;360(19):1981–1988. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 22.Salmon DA, Siegel AW. Religious and philosophical exemptions from vaccination requirements and lessons learned from conscientious objectors from conscription. Public Health Reports (Washington, DC: 1974) 2001;116(4):289–295. doi: 10.1093/phr/116.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith PJ, et al. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Reports. 2011;126(Suppl 2):135–146. doi: 10.1177/00333549111260S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudino JA, Robison S. Risk factors associated with parents claiming personal-belief exemptions to school immunization requirements: Community and other influences on more skeptical parents in Oregon, 2006. Vaccine. 2012;30(6):1132–1142. doi: 10.1016/j.vaccine.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics. 2008;122(1):149–153. doi: 10.1542/peds.2008-0987. [DOI] [PubMed] [Google Scholar]
- 26.Myers E, et al. The current and future role of screening in the era of HPV vaccination. Gynecologic Oncology. 2008;109(2 Suppl):S31–S39. doi: 10.1016/j.ygyno.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Oldach BR, Katz ML. Ohio Appalachia public health department personnel: Human papillomavirus (HPV) vaccine availability, and acceptance and concerns among parents of male and female adolescents. Journal of Community Health. 2012;37(6):1157–1163. doi: 10.1007/s10900-012-9613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: Findings from the 2006–2008 National Survey of Family Growth. Vaccine. 2012;30(16):2676–2682. doi: 10.1016/j.vaccine.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine. 2006;24(22):4812–4820. doi: 10.1016/j.vaccine.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Vamos CA, McDermott RJ, Daley EM. The HPV vaccine: Framing the arguments FOR and AGAINST mandatory vaccination of all middle school girls. The Journal of School Health. 2008;78(6):302–309. doi: 10.1111/j.1746-1561.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- 31.Chicago, N.a.t.U.o. National immunization survey-teen: A user’s guide for the 2012 public-use data file. 2012 C.f.D.C.a. Prevention, Editor. [Google Scholar]
- 32.Dorell C, Yankey D, Strasser S. Parent-reported reasons for nonreceipt of recommended adolescent vaccinations, national immunization survey: Teen, 2009. Clinical Pediatrics. 2011;50(12):1116–1124. doi: 10.1177/0009922811415104. [DOI] [PubMed] [Google Scholar]
- 33.Ojha RP, et al. The accuracy of human papillomavirus vaccination status based on adult proxy recall or household immunization records for adolescent females in the United States: Results from the National Immunization Survey-Teen. Annals of Epidemiology. 2013;23(5):281–285. doi: 10.1016/j.annepidem.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Katz IT, et al. Scaling up human papillomavirus vaccination: A conceptual framework of vaccine adherence. Sexual Health. 2010;7(3):279–286. doi: 10.1071/SH09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Preventive Medicine. 2007;45(2–3):107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Holman DM, et al. Barriers to human papillomavirus vaccination among US adolescents: A systematic review of the literature. JAMA Pediatrics. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessels SJ, et al. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30(24):3546–3556. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 38.CDC. Vaccines for children program. 2012 http://www.cdc.gov/vaccines/programs/vfc/index.html.
- 39.Gilkey MB, et al. Quality of physician communication about human papillomavirus vaccine: Findings from a national survey. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2015;24(11):1673–1679. doi: 10.1158/1055-9965.EPI-15-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiter PL, Katz ML, Paskett ED. Correlates of HPV vaccination among adolescent females from Appalachia and reasons why their parents do not intend to vaccinate. Vaccine. 2013;31(31):3121–3125. doi: 10.1016/j.vaccine.2013.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorell C, et al. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old: National Immunization Survey-Teen, 2010. Clinical Pediatrics. 2013;52(2):162–170. doi: 10.1177/0009922812468208. [DOI] [PubMed] [Google Scholar]
- 42.Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. American Journal of Public Health. 2013;103(1):164–169. doi: 10.2105/AJPH.2011.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson RM, et al. The most effective and promising population health strategies to advance human papillomavirus vaccination. Expert Review Vaccines. 2016;15(2):257–269. doi: 10.1586/14760584.2016.1116947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tierney CD, et al. Adoption of reminder and recall messages for immunizations by pediatricians and public health clinics. Pediatrics. 2003;112(5):1076–1082. doi: 10.1542/peds.112.5.1076. [DOI] [PubMed] [Google Scholar]
- 45.Perkins RB, Clark JA. Providers’ attitudes toward human papillomavirus vaccination in young men: Challenges for implementation of 2011 recommendations. American Journal of Men’s Health. 2012;6(4):320–323. doi: 10.1177/1557988312438911. [DOI] [PubMed] [Google Scholar]
- 46.Kepka D, et al. Human papillomavirus vaccine practices in the USA: Do primary care providers use sexual history and cervical cancer screening results to make HPV vaccine recommendations? Sexually Transmitted Infections. 2012;88(6):433–435. doi: 10.1136/sextrans-2011-050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerend MA, Lee SC, Shepherd JE. Predictors of human papillomavirus vaccination acceptability among under-served women. Sexually Transmitted Diseases. 2007;34(1):468–471. doi: 10.1097/01.olq.0000245915.38315.bd. [DOI] [PubMed] [Google Scholar]
- 48.Bingham A, Drake JK, LaMontagne DS. Soci-ocultural issues in the introduction of human papillomavirus vaccine in low-resource settings. Archives of Pediatrics and Adolescent Medicine. 2009;163(5):455–461. doi: 10.1001/archpediatrics.2009.50. [DOI] [PubMed] [Google Scholar]
- 49.Conroy K, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. Journal of Women’s Health (2002) 2009;18(10):1679–1686. doi: 10.1089/jwh.2008.1329. [DOI] [PubMed] [Google Scholar]
- 50.Zimet GD. Understanding and overcoming barriers to human papillomavirus vaccine acceptance. Current Opinion in Obstetrics and Gynecology. 2006;18(Suppl 1):s23–s28. doi: 10.1097/01.gco.0000216317.10690.8f. [DOI] [PubMed] [Google Scholar]