Abstract
The promise of revolutionary insights into intraocular pressure (IOP) and aqueous humor outflow homeostasis, IOP pathogenesis, and novel therapy offered by engineered mouse models has been hindered by a lack of appropriate tools for studying the aqueous drainage tissues in their original 3-dimensional (3D) environment. Advances in 2-photon excitation fluorescence imaging (TPEF) combined with availability of modalities such as transgenic reporter mice and intravital dyes have placed us on the cusp of unlocking the potential of the mouse model for unearthing insights into aqueous drainage structure and function. Multimodality 2-photon imaging permits high-resolution visualization not only of tissue structural organization but also cells and cellular function. It is possible to dig deeper into understanding the cellular basis of aqueous outflow regulation as the technique integrates analysis of tissue structure, cell biology and physiology in a way that could also lead to fresh insights into human glaucoma. We outline recent novel applications of two-photon imaging to analyze the mouse conventional drainage system in vivo or in whole tissues: (1) collagen second harmonic generation (SHG) identifies the locations of episcleral vessels, intrascleral plexuses, collector channels, and Schlemm’s canal in the distal aqueous drainage tract; (2) the prospero homeobox protein 1-green fluorescent protein (GFP) reporter helps locate the inner wall of Schlemm’s canal; (3) Calcein AM, siGLO™, the fluorescent reporters m-Tomato and GFP, and coherent anti-Stokes scattering (CARS), are adjuncts to TPEF to identify live cells by their membrane or cytosolic locations; (4) autofluorescence and sulforhodamine-B to identify elastic fibers in the living eye. These tools greatly expand our options for analyzing physiological and pathological processes in the aqueous drainage tissues of live mice as a model of the analogous human system.
Keywords: Multiphoton microscopy, second harmonic generation, aqueous humor outflow, conventional outflow, Schlemm’s canal, collector channel, glaucoma
INTRODUCTION
We barely understand the cellular basis of intraocular pressure (IOP) hemostasis let alone triggers of glaucomatous dysregulated IOP. IOP is determined by the aqueous humor dynamic. The aqueous drainage system has an intricate 3-dimensional (3D) organization comprising a number of tissues cooperating in a complex interplay to maintain physiological IOP. The basic functional units of this drainage system are distinct cellular subsystems that typically have been studied in isolated form instead of within the more complex tissue or in vivo environment. It has been hard to achieve the latter mainly for lack of appropriate techniques and overcoming this difficulty could bridge an important scientific gap. It would provide capacity to translate cellular knowhow obtained in vitro to an in vivo context, helping us better understand tissue function and dysfunction. It could pave the way to apply myriad biological advances to better reveal and treat the pathophysiology of glaucoma.
2-photon (2P) imaging that permits direct tissue-based observation of cell and extracellular matrix (ECM) events offers a unique glimpse of cellular mechanisms in vivo (Masedunskas et al., 2011; Masedunskas and Weigert, 2008; Rothestein et al., 2016; Schenke-Layland, 2008) and in the live aqueous drainage tissues (Gonzalez Jr and Tan, 2013a). This expands our capacity to probe and better understand the cellular regulation of IOP. Apart from providing unique high-resolution structural views of the drainage tissues, 2P imaging permits direct observation of cells and their behavior within tissues in 2D and 3D so that cell interactions may be analyzed within their original tissue context. Whereas cellular analysis is classically performed in cell culture, we now have an opportunity to analyze the same cells within their native tissue environment without first disrupting the tissue or removing the cells for culture.
Among the benefits offered by 2P imaging are deep tissue penetration, precise optical sectioning, subcellular resolution within tissues, and reduced phototoxicity compared with traditional 1-photon (1P) imaging. We have adopted a 2P excitation fluorescence imaging (TPEF) approach combining multiple modalities including selective identification of naturally occurring endogenous fluorophores, transgenic fluorophores and exogenous labeling. Second harmonic generation (SHG) is a further label-free method to specifically isolate and visualize certain molecules such as structural collagen. Combining high-resolution deep tissue optical sectioning with computational image analysis provides versatile options for analyzing these signals in tissues without disrupting the mouse eye (Fig. 1). Thus biological events in drainage tissues of the intact mouse eye may be observed in vivo or ex vivo without conventional histological sectioning, combined with simple bench top techniques. Foregoing studies describe this in postmortem human eyes (Chu et al., 2014b; Gonzalez Jr et al., 2016a, 2016b, 2014, 2013, 2012; Gonzalez Jr and Tan, 2013b; Huang et al., 2013; Park et al., 2015; Tan et al., 2012; Tan et al, 2013), postmortem mouse eyes (Ammar et al., 2011, 2010; Gibson et al., 2011; Ko et al, 2016; Masihzadeh et al., 2011; Tan et al., 2013) and live mouse eyes (Gonzalez Jr and Tan, 2013a). The purpose of this article is to describe development and application of 2P multimodality imaging to the live mouse aqueous drainage system as a model of the analogous human drainage tract. We illustrate this with examples of mouse in vivo imaging and correlation with ex vivo studies.
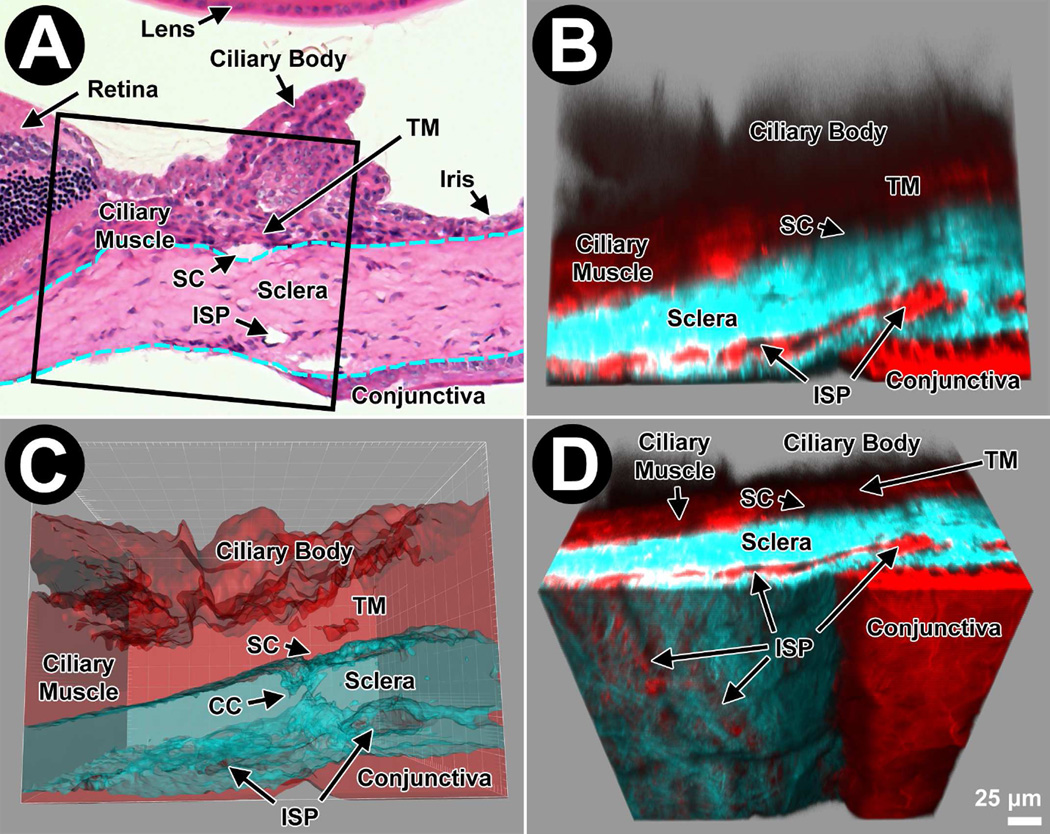
Figure 1.
This figure orientates the reader to the imaging perspective created by deep tissue 2-photon microscopy of the mouse aqueous drainage tissues. A: Light micrograph of a hematoxylin and eosin-stained cryosection from a Balb/c mouse reveals its anatomy. Lens is at the top (internal); cornea to the right (anterior; stroma); choroid to the left (posterior); and sclera and conjunctiva at the bottom (external). Schlemm’s canal (SC) is a space between the trabecular meshwork (TM) and sclera. Intrascleral plexus (ISP) channels are openings in the mid to external sclera. In this instance, the postmortem iris is flush with the corneal endothelium (right). Dashed cyan lines indicate inner and outer limits of the sclera (and corneal stroma to the right) featuring dense collagen. The box indicates the 2P imaging volume at 630X power as represented in panels B–D. B: Mouse TPEF of labeled F-actin (Alexa Fluor 568-conjugated phalloidin; red) and SHG of collagen (cyan) mirrors the organization shown in panel A. The dense collagen is collagen SHG-positive and intertwined collagen fiber bundles are seen in the rotated en face aspect of panel D (see later). F-actin lines ISPs. C: Isosurface volume reconstruction integrating F-actin TPEF (red) and SHG (cyan, flat) signals, and signal voids of channel lumen (darker cyan, contoured). The SHG signal is rendered transparent, revealing the 3D-mapped signal voids representing collector channels (CC) connecting to ISP within the sclera. D: The image volume is rotated obliquely and surface structures cut away in the software to reveal ISP just deep to the ocular surface.
DRAINAGE TRACT FUNCTIONAL ORGANIZATION
Primate aqueous humor outflow follows conventional and unconventional drainage routes and similar routes likely serve aqueous outflow in mice. It is not inconceivable that structural or cellular aberration within any part of these complex drainage routes contributes to elevated IOP and glaucoma.
The conventional pathway comprising trabecular meshwork (TM), Schlemm’s canal (SC) and the intrascleral aqueous drainage channels is located deep to the region of the corneoscleral limbus in primates and the same organization is seen in mice (Aihara et al., 2003a; Ko et al., 2014; Ko et al., 2016; Lei et al., 2011; Overby et al., 2014; Smith and John, 2001). The TM and inner wall endothelium of SC (IWSC) are of clinical and physiological importance because they are sites of high outflow resistance, and by implication play a pivotal role in determining IOP. The TM is comprised of contractile cells attached to an intricately organized extracellular matrix (ECM) rich in elastic fibrils (Lütjen-Drecoll, 1999; Tian et al., 2009). Aqueous humor percolates through several TM cell layers, then by paracellular and/or transcellular transit through the endothelial layer of IWSC before entering SC (Johnson, 2006; Johnson and Erickson, 2000; Overby, 2011). From SC, aqueous drains down collector channels (CC) into intrascleral plexuses (ISP), aqueous veins, and episcleral veins of the venous system. The intrascleral portion of the conventional pathway distal to the TM may account for up to half of total outflow resistance (Bahler et al., 2004; Battista et al., 2008; Grant, 1958, 1955; Ellingsen and Grant, 1972; Johnston and Grant, 1973; Moses, 1981; Rosenquist et al., 1989; Schuman et al., 1999; Van Buskirk and Grant, 1973).
The mouse ciliary muscle extends anteriorly from a region posterior to SC to the anterior chamber angle (Ko and Tan, 2013). Along this longitudinal course, the ciliary muscle is sandwiched between the ciliary body with its processes internally, and TM and SC externally. The ECM and interstitial spaces of the ciliary muscle provide an anterior access point for aqueous flowing posteriorly from the anterior chamber into the uveoscleral drainage route. From here aqueous enters the suprachoroidal space then crosses the sclera or perhaps enters the choroid. There is evidence of uveoscleral outflow in rodents based on tracer studies (Bernd et al., 2004; Lindsey and Weinreb, 2002).
As in primates, pressure-dependent outflow is seen in mice during in vivo and ex vivo anterior chamber perfusion. Pressure-dependent outflow, or outflow facility - the inverse of outflow resistance - is an important measure as it is a major determinant of IOP. In primates, pressure-dependent outflow is attributed largely to conventional outflow, although the pressure dependency of uveoscleral outflow may change under certain circumstances such as inflammation or exposure to prostaglandins (Becker and Neufeld, 2002; Bill, 2003; Gabelt and Kaufman, 1990; Takagi et al., 2004; Toris et al., 1987; Toris and Pederson, 1987). These observations made in primates have been applied to mice to model relative contributions of the conventional and unconventional routes to total aqueous outflow (Aihara et al., 2003a; Goel et al., 2010; Lei et al., 2011; Lindsey and Weinreb, 2002).
Benefits of studying the mouse model - chief of which are similarity of aqueous drainage structure and function to that of primates, and ready availability of many mouse genetic strains - ought to be weighed against a notable disadvantage of the mouse eye being so tiny. The mouse anterior chamber volume (about 6 microliters) is some 50 times smaller than that of humans and does not readily lend itself to analysis by methods conventionally used in larger mammals. This limits the extent to which the benefits of the mouse model may be realized. Realizing the potential of mice requires a rethinking of the way we analyze the mouse drainage tissues and aqueous hydrodynamic. To this end we have established novel techniques to stably and reproducibly measure (Ko et al., 2014; 2016) and biologically probe (Kim and Tan, 2014; Ko et al., 2015; 2016) aqueous outflow function in live mice. These techniques represent steps to overcome the mouse problem of size in vivo. Coupling our in vivo physiological and pharmacological techniques with in vivo 2P imaging are next logical steps to enhance biological analysis of the live mouse eye.
We have learned from our 2P studies in human TM and mice that tissue-based cell biological analysis may be achieved with the resolution and easy accessibility expected of in vitro methods (Chu et al., 2014b). Capacity to deliver pharmacological (Gonzalez Jr et al, 2016a; Ko et al., 2016) and biological probes (Gonzalez Jr and Tan, 2015, 2014; Ko and Tan, 2015) to the TM and stably and reproducibly measure outflow dynamics and IOP in live mice (Chu et al, 2014a; Ko et al., 2016) enhances further the potential of 2P studies. This has led us to turn to the mouse as an in vivo platform on which to unearth a better understanding of IOP and aqueous outflow regulation in health and disease. Establishing a system of 2P imaging in live mice that permits direct observation and analysis of the aqueous drainage tissues and fluid dynamics within their original 3D ocular environment could drive a new understanding of IOP hemostasis and pathogenic IOP elevation.
TRANS-SCLERAL DEEP TISSUE IMAGING
The mouse sclera is relatively thin, lending itself to trans-scleral deep tissue visualization by 2P techniques (i.e., outside-in imaging) in the live mouse (Fig. 2) or enucleated mouse eye (Figs. 1, 3–5). Trans-scleral imaging yields en face views of the drainage structures lying within sclera and deep to it. To achieve sufficient tissue penetration and preservation of subcellular-level resolution requires an optimal setup of laser excitation in a near-infrared range that minimizes tissue scatter, energy absorption and phototoxicity (Aptel et al., 2010; Schenke-Layland, 2008; Zipfel et al., 2003), coupled with sensitive detectors (see Method supplement). Our 2P studies show that the mouse SC and TM lie between 60–100 microns from the ocular surface (Fig. 3), but the maximal scleral penetration of 1P imaging is typically not more than 50 µm. Use of high numerical aperture objectives may increase the depth of 2P-imaging to 100–200 µm. Low power objectives may further increase this to 600 µm or more. Imaging at these depths is needed to visualize deeper structures such as ciliary muscle and ciliary processes lying internal to the TM (Tan et al., 2013). Lack of pigment in albino mice permits relatively unimpeded laser penetration and excellent views of the deeper aqueous drainage tissues (Fig. 3). Capacity to view the same structures is no less in pigmented mice as we have found good 2P signal integrity in the inner drainage structures of pigmented strains. Pigment is sporadically present in the TM and ciliary body, however, and care should be taken to calibrate and reduce laser power (eg., ≤15 mW at the objective; Masihzadeh et al., 2012) in these regions to avoid thermal damage.
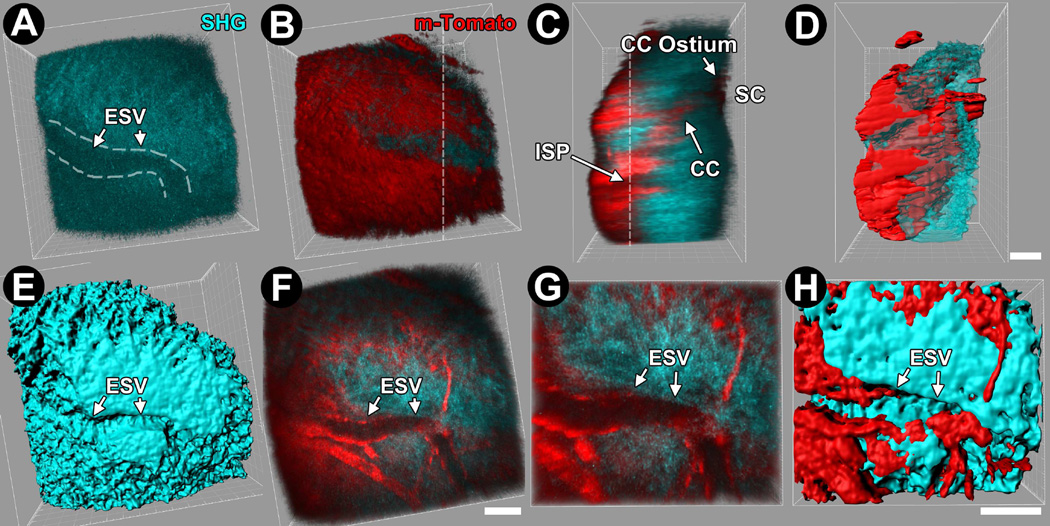
Figure 2.
3-dimensional (3D) reconstructions and isosurface maps derived from serial optical sections through the limbal scleral region of live transgenic m-Tomato (membrane-directed td-Tomato) mice. Episcleral veins (ESV), collector channels (CC) and collector channel ostia were easily identified in live mice in which m-Tomato fluorescent reporter expression and second harmonic generation (SHG) imaging were combined. A–D: Transgenic m-Tomato expression (red) was seen in cell membranes revealing the location of an ESV in a scleral groove (A) near the conjunctival epithelium surface (B; red cellular sheet), and intrasceral plexus (ISP), CC (C), and Schlemm’s canal (SC) deeper in the sclera. D: 3D isosurface map of m-Tomato with respect to scleral collagen SHG closely resembles direct 3D reconstruction from optical sections (C). E, F: Isosurface map of SHG (E) and reconstruction of m-Tomato and SHG (F) cropped near the external ocular surface reveals ESVs as channel-like SHG voids (arrows) flanked by m-Tomato-expressing endothelial cells. G, H: 2X magnified views of reconstructions (G) and isosurface maps (H) of m-Tomato and SHG reveal submicron-level detail. Bar=25 µm.
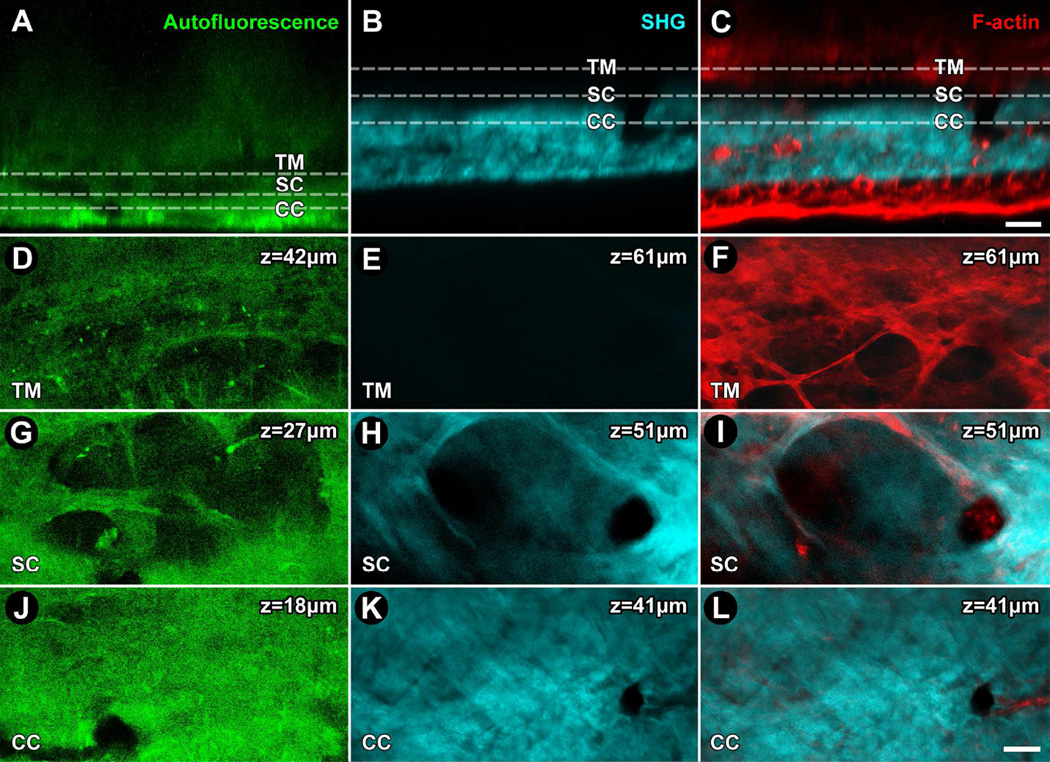
Figure 3.
2-photon (2P) navigation of the mouse conventional outflow pathway guided by autofluorescence, collagen second harmonic generation (SHG) and filamentous actin (F-actin) signals. Eyes were enucleated and not fixed (A, D, G, J) or fixed in paraformaldehyde and labeled with Alexa-568-conjugated phalloidin (B, C, E, F, H, I, K, L) and imaged with TPEF for autofluroescence (green; A, D, G, J), SHG (cyan; B, E, H, K), or SHG and F-actin (red; C, F, I, L show merged SHG and F-actin signals). A, B, C: Conjunctival epithelium on the corneoscleral limbal surfce is at the bottom of each panel. The 2P laser passes ‘outside-in’ through the ocular surface structures, then sclera, intrascleral plexuses (ISP), collector channels (CC), Schlemm's canal (SC), and the trabecular meshwork (TM) in succession. ISP, CC, SC, and TM are identifiable by the location and pattern of signal voids in autofluorescence and SHG images at the indicated depths. The signal voids of ISP, CC and SC were continuous through different scleral depths. At the level of the TM shown, a network of linear and branching elastin fibrils surrounding signal voids representing pores was seen by autofluorescence (D) but not SHG (E). The location of cells was indicated by F-actin. Cells associated with the branching fibrils surrounding pores with a diameter > 25um (F). In SC, a pattern of walls and septae as seen by autofluorescence (G) and SHG (H) produced an appearance of loculation. CC were seen in cross-section as oval or circular signal voids with diameters of 15–20 µm and associated with F-actin (L) in autofluorescence (J) and SHG (K, L) images. Bar=25 µm.
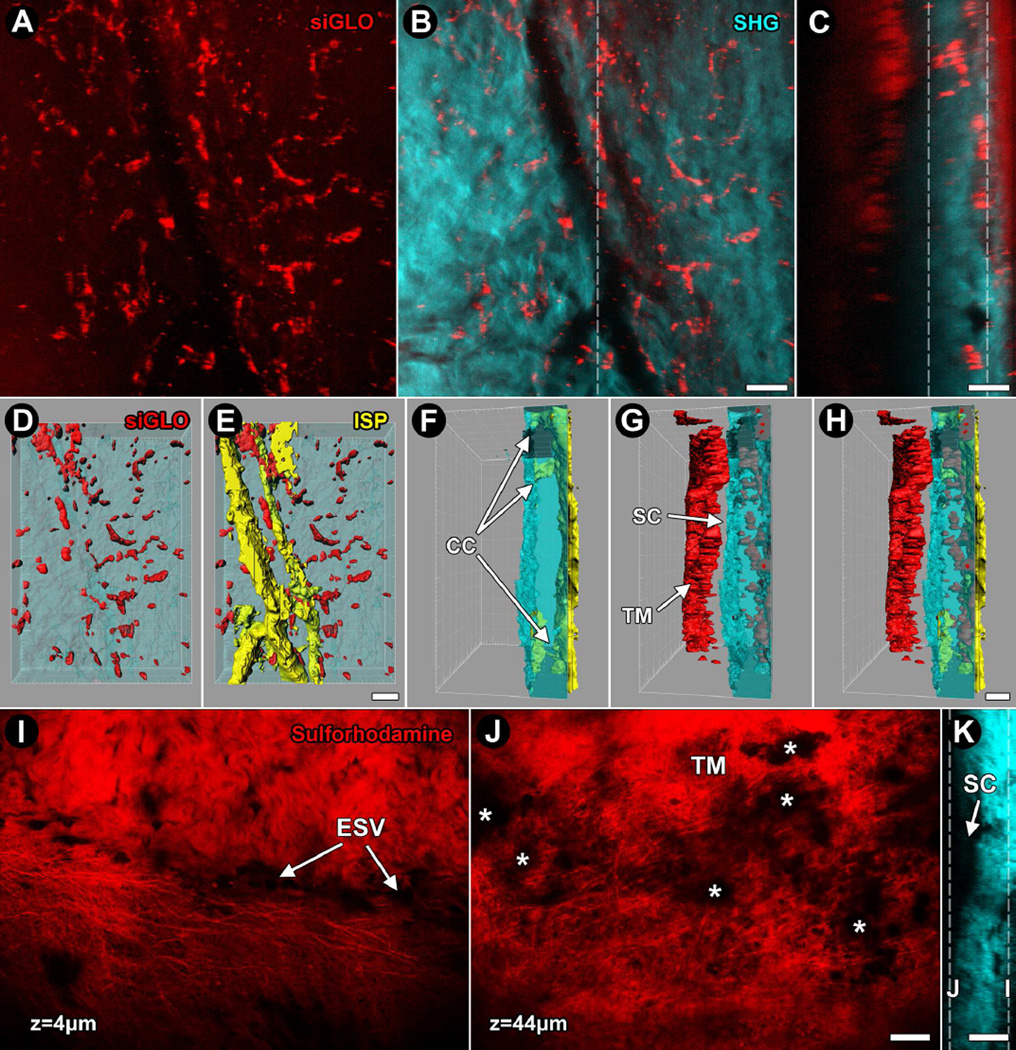
Figure 5.
Visualization of live cells and elastin in mouse eyes using siGLO™ and sulforhodamine-B, exogenous fluorescence labels. A–B: projection images from serial optical sections; C: orthogonal reconstruction; D–H: isosurface mapping of the corneoscleral limbus region of C57BL/6 mouse eye after exposure to siGLO™ (red). A: Projection image of sclera combining optical sections located between the vertical dashed lines in panel C showing cell-associated siGLO™ signal. B: SHG signal voids at mid-depth of sclera reveal the location of an intrascleral plexus (ISP). siGLO™ associates with ISP vessels (more clearly seen in C) but also extra-ISP regions of the sclera. C: Orthogonal reconstruction reveals strong siGLO™ in Schlemm's canal (SC) and distal intrascleral channels. D–H: Isosurface maps of siGLO™ (D) and ISP (E) within the sclera, shown as collagen SHG rendered transparent from an outside-in perspective. In the orthogonal perspective, collector channels (CC) are easily appreciated, extending from the ISP (yellow) to the outer wall of SC (blue craggy undulating structure on left; F). The siGLO™ signal is much brighter in cells of the TM and ciliary body (G, H), indicating preferentially labeling of these more proximal drainage structures. In G and H, a slit-like space between the TM (red) and outer wall of SC (red) indicates SC. I–K: Sulforhodamine-B rapidly labels elastin in live mouse eyes, seen here as distinct elastic fibrils with associated stromal and cellular components as the dye also labels cell membranes (I). Near the ocular surface of the corneoscleral limbus (depth of 4 µm) path of an episcleral vessel (ESV) is revealed by lack of sulforhodamine fluorescence surrounded by elastic fibrils and more amorphous labeling. J: Deeper (depth of 44 µm; deep to SC), sulforhodamine-B labels elastic fibers and cells of the TM. Asterisks show fluorescence obstructions caused by pigment (melanin) in the TM. K: Collagen SHG orthogonal reconstruction showing locations (dashed lines) of suflorhodamine-B-labeled optical sections of I and J with respect to SC. Bar=25 µm.
Prior studies of aqueous drainage structures, lymphatics and blood vessels in the mouse limbus region adopted methods of cross-sectional histology (Chang et al., 2001; Smith et al., 2000), whole mount immunostaining (Chan et al., 2004) and vascular corrosion casts (Ninomiya and Inomata, 2006), but results were variable (Chang et al., 2001; Smith et al., 2000). The organization of these structures at the limbus is hard to capture because of their complex branching and intermingled configuration. Moreover the structures extend across the scleral depth from a convex-shaped ocular surface, making it best to define them 3-dimensionally but more conventional histological methods used in previous studies were better suited to 2D capture, with the exception of the corrosion casts of Ninomiya and Inomata (2006). Studies in humans have tried to capture 3D aspects of the aqueous drainage system (Ashton, 1951, 1952; Ashton and Smith, 1953). More recently, characterization of biological markers such as PECAM-1 and LYVE-1 in whole mount preparations has yielded novel descriptions of these discrete systems (Aspelund et al., 2014; Karpinich et al., 2014; Park et al., 2014; Thomson et al., 2014; van der Merwe and Kidson, 2014).
AUTOFLUORESCENCE
Trabecular beams - collagen (eg., type I and III collagen) structures with a core of elastic fibrils - provide scaffolding around tissue pores representing aqueous drainage conduits through the TM. Apart from providing structural support, the precise functional role of trabecular beams is unclear but understanding it better could provide important clues to the modulation of aqueous outflow resistance. TM structural collagens and elastic fibrils represent endogenous fluorophores emitting autofluorescence of different intensities that may be visualized and distinguished from each other by TPEF without exogenous labeling (Figs. 3A, D) (Chu et al., 2014b; Huang et al., 2013; Tan et al., 2012). Distal to the TM, scleral walls surrounding Schlemm’s canal (SC) and collector channels are also richly invested with elastic fibrils (Hann et al., 2011) that are autofluorescent and characterized by TPEF (Fig. 3G) (Tan et al, 2012). Pigment itself is autofluorescent but prone to thermal injury due to a propensity to absorb laser energy. But with laser energy calibrated carefully, the pigment autofluorescent signal may be used to visualize pigmented structures of the iridocorneal angle (Gibson et al., 2011; Johnson et al., 2011; Masihzadeh, et al., 2012).
Coherent Anti-Stokes Raman Scattering (CARS) (Ammar et al., 2013) may be used as an adjunct to visualize lipid-rich membranes in combination with autofluorescence and SHG (see next). A multiphoton configuration is used with two objectives placed opposite each other on the limbus of an enucleated eye, with autofluorescence and SHG collected as backscatter through one objective, and CARS collected through the other objective. This allowed non-labeled visualization of cells by autofluorescence (NAD[P]H) and CARS (lipid-rich membranes), and tissue structures by SHG (collagen).
SECOND HARMONIC GENERATION
SHG is a frequency-doubling phenomenon arising by non-linear 2P excitation of non-centrosymmetric (asymmetric) macromolecular structures (Ammar et al., 2010; Chu et al., 2014b; Huang et al., 2013; Gonzalez Jr et al., 2016a; Johnson et al., 2011). SHG is generated by macromolecules such as type I collagen that simultaneously scatter and recombine two lower-energy photons as a single photon of twice the energy. The SHG signal of a particular molecule has its own narrow wavelength (half the excitation wavelength) detection range that can be used to distinguish it from other SHG-generating molecules with different wavelength detection ranges. SHG may be distinguished from tissue autofluorescence by detecting the SHG signal outside (typically below) the autofluorescence emission range using an appropriate 2P excitation wavelength and narrowband emission filter combination (Ammar et al., 2010; Chu et al., 2014b; Gonzalez Jr and Tan, 2013a; Gonzalez Jr et al., 2016b; Huang et al., 2013; Johnson et al., 2011; Schenke-Layland, 2008; Tan et al., 2013).
The scleral collagen SHG signature reveals wavy bundles (Fig. 2A). Collagen SHG signals are not homogenous across the sclera, however, as regions of signal voids representing localized absences of structural collagen are seen (Figs. 2A, E). If SHG signal voids are cell-lined we take them to represent intrascleral channels draining aqueous humor (eg., collector channels), blood (blood vessels) or lymph (lymphatic vessels). Alternatively nerves or regions of cellularity may be present. To confirm that a scleral SHG signal void truly represents an aqueous drainage channel requires that the signal void be cell-lined and traceable to SC in serial optical sections (Fig. 2C). We have identified cell layers bordering channel-shaped scleral SHG signal voids by exogenous dye-labeling (eg., cellular intravital dyes or phalloidin-conjugated Alexa568) or in transgenic reporter mice with fluorescently-tagged endothelium. A further confirmatory step would be to follow transit of a perfused fluorescent tracer from the anterior chamber to its appearance in an intrascleral channel. A caveat of using tracers is that transit should not be restricted by the TM-SC inner wall endothelium and the channel itself should be draining at the time, as due to aqueous preferential outflow, not all channels will have established drainage at a particular time or for a particular physiological condition. Thus absence of the tracer may not necessarily mean a structure is not an aqueous drainage channel.
Intrinsic collagen abnormality may itself lead to IOP elevation, as seen in collagen type I alpha 1 mutant mice (Aihara et al., 2003b). Elevated IOP of these mutants may be attributable to abnormal conventional or unconventional drainage. These types of mouse models could yield further information on the drainage system when studied by SHG imaging of collagen to characterize aberrations in collagen organization in the eye tissues.
TRANSGENIC FLUORESCENCE
Mice transgenically expressing fluorescent tags that are specific for a particular cell type or subcellular compartment may be used to segregate and specifically visualize structures of interest by TPEF. For example, a prospero homeobox protein 1-GFP (Prox1-GFP) reporter mouse that expresses tagged Prox1 in structures bearing a lymphatic identity strongly expresses Prox1-GFP in the IWSC endothelium (Fig. 4). Furthermore, Prox1-GFP expression varies with IOP and outflow through the IWSC (Park et al., 2014), indicating the potential usefulness of TPEF imaging in these mice to learn more about IOP regulation. It also makes it possible to specifically identify conventional drainage structures in 3D imaging studies (Choi et al., 2010; Karpinich and Caron, 2014;) when analyzed with respect to other markers such as lymphatic vessel endothelial hyaluronan receptor (LYVE-1), a biomarker for lymphatic endothelium (Kizhatil et al., 2014; Park et al., 2014) that is expressed in true limbal lymphatics but not SC (Figs. 3G–L). (Park et al., 2014). Work with this Prox1-GFP reporter mouse has already revealed intriguing insights into SC development (Kizhatil et al., 2014), pathogenesis from FOXC transcription factor mutations (Thomson et al., 2014), and glaucoma therapy with vascular endothelial growth factor C (Aspelund et al., 2014). Many of these observations were made by 1P confocal microscopy of dissected whole mount tissues but we propose that 2P imaging combined with biological probing and fluid dynamics studies in live mice will further extend our understanding of aqueous drainage regulation.
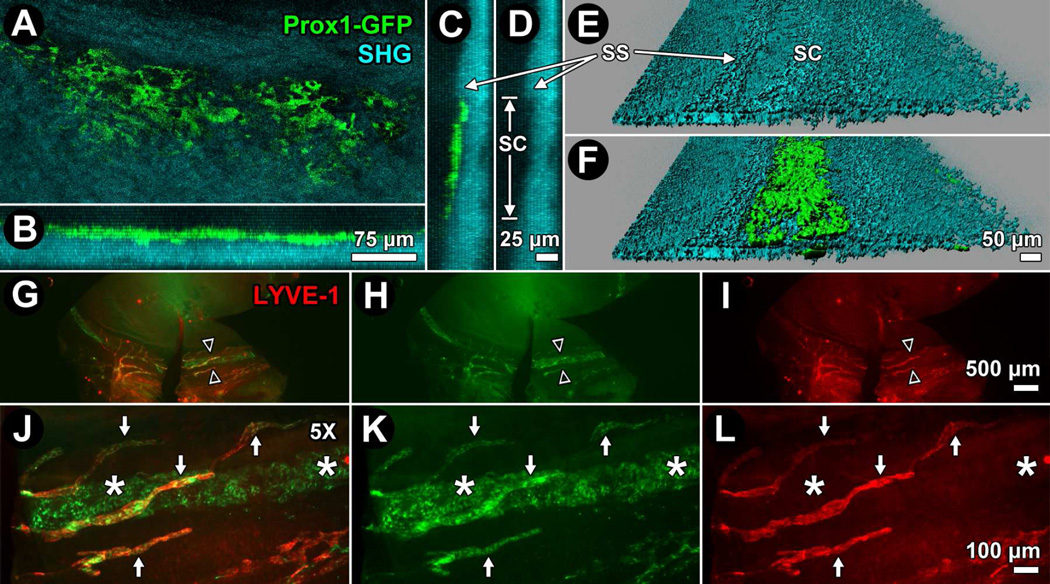
Figure 4.
Prox1-GFP reporter identifies the inner wall of Schlemm's canal (IWSC) in the mouse. A–F: 3D reconstructions and isosurface mapping (E, F) of Prox1-GFP (green) reporter mouse limbus after TPEF imaging. Prox1-GFP signal manifests as interconnected cells in a monolayer (A) aligned in a circumferential ring (B) just anterior to a scleral spur (SS; C), seen as a condensed ridge of structural collagen by second harmonic generation (SHG; D) and 3D reconstruction. D: Anterior-posterior distance of SC from SS to the anterior limit of Schlemm's canal (SC) was approximately 140 µm. E: Isosurface mapping of SHG (cyan) reveals the SS and a slight trench to the anterior (to the right) indicating the outer wall of SC. F: Isosurface map of Prox1-GFP is merged with SHG (from E), showing Prox1-GFP localization to the region of the IWSC. G–L: Whole mount staining of Prox1-GFP anterior segment with antibodies against lymphatic vessel endothelial hyaluronan receptor (LYVE-1; red) reveals location of the Prox1-GFP positive, LYVE-1 negative IWSC (asterisks) and the Prox1-GFP positive, LYVE-1 positive limbal lymphatic vessels (arrows). G, J: Merge of Prox1-GFP and LYVE-1. H, K: Prox1-GFP only. I, L: LYVE-1 only. J–L: 5X magnifications of regions indicated by opposing arrowheads in panels G–I.
EXOGENOUS LABELING
Intravital dyes are exogenous non-toxic water permeable fluorescent dyes labeling subcellular compartments for fluorescence imaging in live tissues. An example is Calcein AM, a non-fluorescent cell membrane-permeable dye that is converted to cytosolic fluorescent calcein by esterases in live cells but not dead cells, allowing selective identification of living cells within tissues. We have used it to identify live cells in the TM and quantitatively analyze tissue viability by 2P imaging (Gonzalez Jr et al., 2013; Gonzalez Jr and Tan, 2013b). The dye may also be perfusion-delivered to the mouse anterior chamber to selectively label live cells in the TM for 2P visualization with reference to the tissue’s structural ECM.
DY-547-labeled siGLO™ is taken up by cells in the TM, yielding a particularly bright signal (Figs. 5A–H). siGLO™ is a 22-nucleotide double-stranded RNA lacking known mRNA targets and serves as a RISC-independent positive control for siRNA transfection, but we have adapted its use for 2P visualization of cells within the TM (Fig. 5). A further technique is to transduce live cells in the TM with LifeAct (Hwang et al, 2013), a 17 amino acid peptide fluorescent tag that stains F-actin structures (not shown). This allowed us to analyze the contractile state of live cells in the human TM by TPEF. A transgenic LifeAct expressing mouse is also available that would be useful for 2P live mouse TM contractility studies without exogenous transduction.
Sulforhodamine-B that is typically used as a fluorescent dye to assess cell viability also labels elastic fibrils in vivo (Gaertner et al., 2012; Ricard et al., 2007). Brief mouse anterior chamber exposure to the dye not only labels live cells but also brightly labels elastic fibrils in the sclera and TM (Figs. 5I–K). Combined SHG and TPEF of sulforhodamine-B-labeled ECM of the TM provide powerful options for studying mechanoelastic properties of the drainage tissues.
3D TISSUE RECONSTRUCTION AND ISOSURFACE MAPPING
Using Imaris 7.3.0 from Bitplane Inc., we routinely perform software-assisted 3D reconstructions of the TM from 2P serial optical sections to analyze structural, cellular, and subcellular features of the tissue based on separately reconstructing and merging data from different 2P imaging channels (Chu et al., 2014b; Gonzalez Jr et al, 2016a, 2013; Gonzalez Jr and Tan, 2015, 2014, 2013a, 2013b; Huang et al, 2013; Tan et al., 2013; Figs. 1C, 2E, 2H, 4E, 4F, 5D–H). By this approach, tissue-based TPEF (autofluorescence, transgenic fluorescence and fluorescence from exogenous labeling) and SHG signals can be recombined for analysis. These techniques provide novel ways to explore aqueous drainage tissue cell biology and pathology. Specific features may be isolated, merged and mapped in relation to the fine architecture of the TM to qualitatively and quantitatively analyze associations in 3D tissue space. Cell-ECM interactions in situ may be studied by quantitative 3D co-localization analysis. Cellular entities, ECM and epitopes may be localized across the tissue as isosurface maps that represent not just stunning views of the tissue but large datasets displayed as coded 3D tissue plots. This output permits visualization of cellular, ECM and protein distribution combined in the 3D tissue in a way that would not otherwise be easily forthcoming by standard observation alone. For example, our isosurface mapping techniques qualitatively and quantitatively reveal TM porous expansion following pharmacologically-induced actomyosin relaxation (Gonzalez Jr et al., 2016a).
CONCLUSIONS: IN VIVO STRUCTURE-FUNCTION ANALYSIS AND FUTURE APPLICATIONS
Relative contributions of the mouse conventional and unconventional drainage routes to aqueous outflow dynamics and modulating outflow resistance remain unclear. Conventionally, these questions have been addressed using a mixture of direct and indirect techniques requiring prior assumptions of how the drainage system behaves in certain physiological and pharmacological contexts. Developing the capacity to directly resolve and measure cellular and ECM behavior with reference to fluid transit through different parts of the mouse aqueous drainage tract in vivo will provide unique capacity to better define and understand these important pathways and their cross-talk as they go about their business of regulating IOP. We have established mouse in vivo methods to predictably deliver pharmacological or cellular probes to the anterior chamber, stably and reproducibly measure aqueous drainage tissue fluid conductivity, and pharmacologically induce and resolve graded outflow responses (Kim and Tan, 2014; Ko and Tan, 2013; Ko et al., 2016). We have combined these in vivo drug delivery and physiological methods with ex vivo TPEF (Ko et al., 2016) and are now establishing more complete systems solely for in vivo analysis.
METHOD SUPPLEMENT
Animal husbandry
Mouse experiments were performed in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research and the National Institutes of Health guide for the care and use of Laboratory animals. Approval had been obtained from the University of Southern California and University of California, Los Angeles Institutional Animal Care and Use Committees (IACUC). The mice were raised and housed in air-filtered clear cages with a bedding of pine shavings, subject to a 12-hour light/dark cycle, and fed ad libitum. Mice were anesthetized with a mixture of ketamine (60–85 mg/Kg, Ketaject, Phoenix Pharmaceutical, Inc., St. Joseph, MO, USA), xylazine (6–8.5 mg/Kg, AnaSed; Lloyd Laboraties, Shenandoah, IA, USA) and acepromazine (1.5–2.5 mg/Kg, Boehringer Ingelheim, St. Joseph, MO, USA), injected intraperitoneally. Anesthesia was titrated to achieve a depth facilitating stable ocular imaging. One drop of topical proparacaine hydrochloride ophthalmic solution (0.5%, Akorn, Inc, Buffalo Grove, IL, USA) was applied to the cornea prior to imaging. Mice were rested on a warming platform or under a heating blanket (Homeothermic Blanket Systems, Harvard Bioscience, Inc., Holliston, MA, USA) to maintain body temperature during experiments.
C57BL/6 and Balb/c mice aged 3–4 months were purchased from Charles River Laboratories (Wilmington, MA, USA). In vivo and ex vivo studies were performed in hybrid GFP/membrane-targeted td-Tomato (m-Tomato) mice that were a generous gift from Dr. Roberto Weigert, NIDCR. The m-Tomato mouse was a product of crossing Friend Virus B-Type transgenic mice expressing EGFP (De Paola et al., 2003) and C57BL/6 mice expressing the membrane-targeted td-Tomato protein (m-Tomato) (Shaner et al., 2004). Both mice were originally purchased from the Jackson Laboratory. Crossed lines were bred to generate homozygotes. The expression of both transgenes was driven by the chicken β-actin promoter and the cytomegalovirus enhancer (Masedunskas et al., 2011). The anesthetized mice were placed with their eyes facing upward toward an objective inverter (LSM Technologies) attached to a 2P imaging platform, with intervening optical coupling gel (0.5% Carbomer 940, 300mM D-sorbitol, adjusted to pH 7.3 with triethanolamine [Snowdrift farm, Tucson, AZ, USA]); or downward in contact with glass and the objective of an inverted microscope, as described below. Ex vivo studies were performed in the following mice: Prox1-GFP mice (Choi et al., 2011) and Prox1-tdTomato (Tg(Prox1-tdTomato)TA76Gsat/Mmucd) were a generous gift from Dr. Young Hong, University of Southern California.
Imaging setup
We have used the following 2P microscopy configurations: (a) Olympus IX81 inverted confocal microscope (Olympus, Melville, NY, USA) modified for 2P imaging. A tunable Ti:Sapphire femtosecond laser, Chameleon Ultra II (Coherent, Santa Clara, CA, USA) provided the laser source with power modulated by neutral density filters (Chroma Technologies, Rockingham, VT, USA). A beam expander modulated beam size (LSM Technology Inc., Shrewsbury, PA, USA). A Fluoview 1000 scanning head (Olympus, Melville, NY) was used to direct the beam. The emitted signal was directed into a custom-made array of three non-descanned detectors (LSM Technology Inc.). A 680 nm barrier filter (Chroma Technologies, Rockingham, VT, USA) prevented scattered IR light from reaching the detectors. Three cooled PMTs (R6060-12; Hamamatsu Corporation, Bridgewater, NJ, USA) and the dichroic mirrors and barrier filters (Chroma Technologies) were used for signal detection: (i) PMT dichroic mirror 510 nm with barrier filter 400 nm-480 nm (e.g., Hoechst fluorescence, endogenous fluorescence, collagen SHG); (ii) PMT dichroic mirror 570 nm with barrier filter 505 nm-560 nm (e.g., FITC, Alexa 488, GFP/EGFP); (iii) PMT barrier filter 590 nm-650 nm (e.g. Texas red, td-Tomato). To enhance detection of endogenous fluorescence, the barrier filter was removed. Imaging on the live animal was performed in the upright configuration using an objective inverter (LSM Technology Inc.; Rothstein et al., 2016). For time lapse imaging the acquisition speed was set to 0.3 frames/sec. A UPLSAPO 60X NA 1.2 water immersion objective was used (Masedunskas and Weigert, 2008).
(b) Leica TCS SP5 AOBS MP system (Leica Microsystems, Heidelberg, Germany). Incident light was focused, and emitted signals were collected, with an inverted HCX PL APO CS 63X/1.3NA glycerol objective (Leica). The laser was centered at 850 nm. TPEF signals were collected in epifluorescence configuration, split with a dichroic mirror, passed through multiphoton bandpass filters (TPEF = 525/50 or 500–550 nm [Leica]; and red epifluorescence = 635/90 or 590–680 nm [Chroma]) and guided onto a non-descanned photomultiplier tube detector (Hamamatsu). TPEF signals were collected through multiphoton bandpass filters (eg., 500–550 nm for autofluorescence; 415–435 nm for collagen SHG; and 590–680 nm for Alexa568 to reveal filamentous actin (F-actin)). Ocular images were collected as series of optical sections (X, Y, Z; 600Hz, bidirectional) using 1024×1024 pixel frames, 16-bit resolution and 16X line averaging. Images were captured using LAS AF (Leica) and analyzed with Volocity 5.4.1 (PerkinElmer; Waltham, MA, USA), LAS AF Lite 2.2.1 (Leica), Imaris 7.3.0 (Bitplane; Zurich, Switzerland), and Image J (NIH; Bethesda, MD, USA). Images were cropped, resized and fit into figures using Photoshop CS5 (Adobe).
(c) Zeiss 710NLO [Carl Zeiss AG, Oberkochen, Germany] confocal microscope system coupled to Chameleon Ultra-II multiphoton lasers (Coherent). Eyes were imaged in the inverted configuration or upright using a objective inverter coupled to an optical gel. The Zeiss 710NLO system was supplemented with a BiG non-descanned detector [Zeiss] to increase sensitivity and fidelity for capturing deeper tissue fluorescence and SHG. SHG of mouse iridocorneal angle (not shown) was used to identify the region of the TM just deep to Schlemm’s canal by a trans-scleral approach.
Reagents, antibodies and fluorescent dyes
Alexa Fluor 568-conjugated phalloidin was purchased from Life Technologies (Grand Island, NY, USA). Paraformaldehyde (PFA), Triton X-100, and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO, USA). Dharmacon™ siGLO™ Red transfection indicator (100 µM; GE Healthcare, Wauwatosa, WI, USA) was incubated with ex vivo enucleated mouse eyes for 24 h in Accell™ siRNA delivery media (GE Healthcare) at 37°C and 8% CO2. siGLO™ labeled-eyes were imaged by trans-scleral 2P microscopy without fixation.
Light Microscopy
Enucleated Balb/c mouse eyes were quickly embedded in Tissue-Tek® Optimum Cutting Temperature compound (Sakura Finetek Inc., Torrance, CA, USA). Cryosections (7 µm thickness) were fixed with 4% PFA and further permeablized/blocked in the blocking solution (5% BSA and 0.3% Triton X-100) for 1 hour at room temperature (RT). These sections were stained with hematoxylin and eosin and the mouse limbus was imaged by light microscopy using a Keyence BZ-X700 microscope (Keyence Corporation of America, Itasca, IL, USA).
Volumetric 3-dimensional (3D) reconstructions
Isosurface maps were generated with Imaris to recreate volumetric renderings of tissue SHG, autofluorescence, F-actin, Prox1-GFP, siGLO™, and signal voids representing intrascleral channels, as we have previously described (Chu et al., 2014b; Gonzalez Jr et al., 2016a, 2012; Huang et al, 2013). Volumes were rendered only above a predetermined threshold of size and fluorescence intensity. Optimum threshold parameters were determined in separate testing as: (a) voxels of 0.24 × 0.24 × 1 µm in X, Y, Z axes, respectively. This captured features with (a) dimensions ≥ 0.24 µm; (b) fluorescence intensity cutoff greater than 10,025 but less than 65,540 per voxel, based on 16-bit color depth. For focused isosurface modeling of deeper structures, reconstructions were cropped in the z-axis to segment blocks of optical slices 25 µm in depth before rendering. This preserved detail at depths where fluorescence dynamic range was narrow relative to the dynamic range of shallower regions.
Volumetric 3D reconstructions from signal voids
The SHG signal was reconstructed as isosurface volumes using the following parameters: surface grain size of 2 µm, largest sphere diameter or 1.803 µm, fluorescence intensity minimum cutoff of 3,600, and minimum voxel size of 10 µm. A solid block of fluorescence was then generated in software to serve as a mask (all voxels within the z-stack set to a value of 20,000) from which to carve out a negative imprint of the SHG. The SHG isosurface volume was then subtracted from the solid mask to generate a 3D representation of the collector channels. Isosurface volumes of the voids were reconstructed using the following parameters: surface grain size of 0.481 µm, largest sphere diameter of 1.803 µm, automatic (default) fluorescence intensity minimum cutoff value, and a minimum voxel size of 10 µm.
Table 1.
Summary of modalities available for 2-photon imaging of the mouse eye limbus.
| Modality | Ex vivo | In vivo | Tissue/Target labelled | Comment |
|---|---|---|---|---|
| Autofluorescence | Yes | Yes | Elastin and structural collagen | Signal is dim in living mouse eyes |
| Phalloidin-Alexa-568 | Yes | No | Filamentous actin, cells | Requires paraformaldehyde fixation |
| Coherent Anti-Stokes Raman Scattering | Yes | Yes | Lipid; Plasma membranes, cells | Requires a specialized platform |
| Second Harmonic Generation (SHG) | Yes | Yes | Structural collagen; Sclera | Primary modality for finding landmarks |
| Fluorescent Transgenes: | ||||
| Prox1-GFP | Yes | Yes | Inner wall of Schlemm's canal | SHG identifies the outer wall of Schlemm's |
| LifeAct | Yes | Yes | Filamentous actin, live cells | Delivery by viral transduction |
| Intravital Dyes: | ||||
| Calcein AM | Yes | Yes | Live cells | Thirty minute incubation at 37°C required |
| DY-547-siGLO™ | Yes | Yes | Live cells | Incubated with Accell™ siRNA delivery media |
| Sulforhodamine-B | Yes | Yes | Elastin and cells | Very bright signal. At first preferentially labels elastin, but gradually labels cytoplasm |
| Isosurface Mapping | Yes | Yes | All fluorescently labeled tissues | Allows new perspectives and morphometric and volumetric analysis |
Acknowledgments
National Institutes of Health, Bethesda, MD, Grants EY020863 (JCHT), EY03040 (Doheny Vision Research Institute Imaging Core), 1S10RR024754 (USC Multiphoton Core), and a Kirchgessner Foundation Research Grant (JCHT), American Glaucoma Society Mentoring for Physician Scientists Award and Young Clinician Scientist Award (JCHT), Career Development Award from Research to Prevent Blindness (JCHT), and an unrestricted grant from the Research to Prevent Blindness, Inc., New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors do not have conflicting relationships to report.
Author Contributions
JCHT and JMG devised the project and wrote the manuscript. MKK carried out the IHC. JMG, AM and RW carried out the live mouse 2P imaging. YK provided the Prox1-GFP and Prox1-tdTomato mice. RW provided the m-Tomato mice. JMG performed the ex vivo 2P microscopy, image reconstruction analysis, and prepared all figures.
References
- Aihara M, Lindsey JD, Weinreb RN. Aqueous humor dynamics in mice. Invest. Ophthalmol. Vis. Sci. 2003a;44:5168–5173. doi: 10.1167/iovs.03-0504. [DOI] [PubMed] [Google Scholar]
- Aihara M, Lindsey JD, Weinreb RN. Ocular hypertension in mice with a targeted type I collagen mutation. Invest. Ophthalmol. Vis. Sci. 2003b;44:1581–1585. doi: 10.1167/iovs.02-0759. [DOI] [PubMed] [Google Scholar]
- Ammar DA, Lei TC, Gibson EA, Kahook MY. Two-photon imaging of the trabecular meshwork. Mol. Vis. 2010;16:935–944. [PMC free article] [PubMed] [Google Scholar]
- Ammar DA, Lei TC, Masihzadeh O, Gibson EA, Kahook MY. Trans-scleral imaging of the human trabecular meshwork by two-photon microscopy. Mol. Vis. 2011;17:583–590. [PMC free article] [PubMed] [Google Scholar]
- Ammar DA, Lei TC, Kahook MY, Masihzadeh O. Imaging the intact mouse cornea using coherent anti-Stokes Raman scattering (CARS) Invest. Ophthalmol. Vis. Sci. 2013;54:5258–5265. doi: 10.1167/iovs.12-11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. Part I. Aqueous veins. Br. J. Opthalmol. 1951;35:291–303. doi: 10.1136/bjo.35.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. II. Aqueous veins. Br. J. Opthalmol. 1952;36:265–267. doi: 10.1136/bjo.36.5.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N, Smith R. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. III. Arterial relations of Schlemm's canal. Br. J. Opthalmol. 1953;37:577–586. doi: 10.1136/bjo.37.10.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Tammela T, Antila S, Nurmi H, Leppänen V-M, Zarkada G, Stanczuk L, Francois M, Mäkinen T, Saharinen P, Immonen I, Alitalo K. The Schlemm’s canal is a VEGF-C/VEGFR-3– responsive lymphatic-like vessel. J. Clin. Invest. 2014;124:3975–3986. doi: 10.1172/JCI75395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler CK, Smedley GT, Zhou J, Johnson DH. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am. J. Ophthalmol. 2004;138:988–994. doi: 10.1016/j.ajo.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest. Ophthalmol. Vis. Sci. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Neufeld AH. Pressure dependence of uveoscleral outflow. J. Glaucoma. 2002;11:464. doi: 10.1097/00061198-200210000-00017. [DOI] [PubMed] [Google Scholar]
- Bernd AS, Aihara M, Lindsey JD, Weinreb RN. Influence of molecular weight on intracameral dextran movement to the posterior segment of the mouse eye. Invest. Ophthalmol. Vis. Sci. 2004;45:480–484. doi: 10.1167/iovs.03-0462. [DOI] [PubMed] [Google Scholar]
- Bill A. Some thoughts on the pressure dependence of uveoscleral flow. J. Glaucoma. 2003;12:88–94. doi: 10.1097/00061198-200302000-00017. [DOI] [PubMed] [Google Scholar]
- Chan CK, Pham LN, Chinn C, Spee C, Ryan SJ, Akhurst RJ, Hinton DR. Mouse strain-dependent heterogeneity of resting limbal vasculature. Invest. Ophthalmol. Vis. Sci. 2004;45:441–447. doi: 10.1167/iovs.03-0869. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Peters M, Savinova OV, Hawes NL, Zabaleta A, Nusinowitz S, Martin JE, Davisson ML, Cepko CL, Hogan BL, Johjn SW. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001;2:18. doi: 10.1186/1471-2156-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Chung HK, Ramu S, Lee HN, Kim KE, Lee S, Yoo J, Choi D, Lee YS, Aguilar B, Hong YK. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 2011;117:362–365. doi: 10.1182/blood-2010-07-298562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ER, Kim EK, Gonzalez JM, Jr, Ko MH, Liew EC, Tan JC. Intraocular pressure measurement in acepromazine-sedated mice. Clin. Experiment Ophthalmol. 2014a;42:395–397. doi: 10.1111/ceo.12157. [DOI] [PubMed] [Google Scholar]
- Chu ER, Gonzalez JM, Jr, Tan JCH. Tissue-based imaging model of human trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014b;30:191–201. doi: 10.1089/jop.2013.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Arber S, Caroni P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat. Neurosci. 2003;6:491–500. doi: 10.1038/nn1046. [DOI] [PubMed] [Google Scholar]
- Ellingsen BA, Grant WM. Trabeculotomy and sinusotomy in enucleated human eyes. Invest. Ophthalmol. Vis. Sci. 1972;11:21–28. [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest. Ophthalmol. Vis. Sci. 1990;31:2384–2388. [PubMed] [Google Scholar]
- Gaertner M, Cimalla P, Meissner S, Kuebler WM, Koch E. Three-dimensional simultaneous optical coherence tomography and confocal fluorescence microscopy for investigation of lung tissue. J. Biomed. Opt. 2012;17:071310. doi: 10.1117/1.JBO.17.7.071310. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. The effect of prostaglandin F2α on trabecular outflow facility in cynomolgus monkeys. Exp. Eye Res. 1990;51:87–91. doi: 10.1016/0014-4835(90)90174-s. [DOI] [PubMed] [Google Scholar]
- Gibson EA, Masihzadeh O, Lei TC, Ammar DA, Kahook MY. Multiphoton microscopy for ophthalmic imaging. J. Ophthalmology. 2011 doi: 10.1155/2011/870879. ID: 870879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol. J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Jr, Heur M, Tan JCH. Two-photon immunofluorescence characterization of the trabecular meshwork in situ. Invest. Ophthalmol. Vis. Sci. 2012;53:3395–3404. doi: 10.1167/iovs.11-8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Jr, Hamm-Alvarez S, Tan JCH. Analyzing live cellularity in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2013;54:1039–1047. doi: 10.1167/iovs.12-10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Jr, Tan JCH. Multimodal microscopy of aqeuous drainage channels in live mice. Invest. Ophthalmol. Vis. Sci. 2013a;54:3350. [ARVO abstract] [Google Scholar]
- Gonzalez JM, Jr, Tan JCH. Semi-automated vitality analysis of human trabecular meshwork. IntraVital. 2013b;2(3):e27390. [Google Scholar]
- Gonzalez JM, Jr, Hsu HY, Tan JCH. Observing live actin in the human trabecular meshwork. Clin. Experiment. Ophthalmol. 2014;42:502–504. doi: 10.1111/ceo.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Jr, Tan JCH. Tissue-based caldesmon silencing by naked siRNA increases F- actin in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2014;55:5653. [ARVO abstract] [Google Scholar]
- Gonzalez JM, Jr, Tan JC. Mapping abnormal elastin maintenance in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2015;56:3299. [Google Scholar]
- Gonzalez JM, Jr, Ko MK, Pouw A, Tan JCH. Tissue-based multiphoton analysis of actomyosin and structural responses in human trabecular meshwork. Sci. Rep. 2016a;6:e21315. doi: 10.1038/srep21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Jr, Ammar M, Ko MK, Tan JCH. Optimizing 2-photon multiple fluorophore imaging of human trabecular meshwork. Mol. Vis. 2016b in press. [PMC free article] [PubMed] [Google Scholar]
- Grant WM. Facility of flow through the trabecular meshwork. AMA Arch. Ophthalmol. 1955;54:245–248. doi: 10.1001/archopht.1955.00930020251012. [DOI] [PubMed] [Google Scholar]
- Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch. Ophthalmol. 1958;60:523–533. doi: 10.1001/archopht.1958.00940080541001. [DOI] [PubMed] [Google Scholar]
- Hann CR, Fautsch MP. The Elastin Fiber System between and Adjacent to Collector Channels in the Human Juxtacanalicular Tissue. Invest. Ophthalmol. Vis. Sci. 2011;52:45–50. doi: 10.1167/iovs.10-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AS, Gonzalez JM, Jr, Le PV, Heur M, Tan JCH. Sources of structural autofluorescence in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2013;54:4813–4820. doi: 10.1167/iovs.12-11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Gonzalez JM, Tan JCH. Live Imaging of Actin Structure in Human Trabecular Meshwork. Invest. Ophthalmol. Vis. Sci. 2013;54:3528. doi: 10.1167/iovs.12-10479. [ARVO abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Ammar DA, Kahook MY. Two-photon imaging of the mouse eye. Invest. Ophthalmol. Vis. Sci. 2011;52:4098–4105. doi: 10.1167/iovs.10-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Erickson K. Mechanisms and Routes of Aqueous Humor Drainage. In: Albert DM, Jakobiec FA, editors. Principles and Practices of Ophthalmology. Philadelphia: WB Saunders Co.; 2000. pp. 2577–2595. [Google Scholar]
- Johnson M. What controls aqueous humor outflow resistance? Exp. Eye Res. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinich NO, Caron KM. Schlemm’s canal: more than meets the eye, lymphatics in disguise. J. Clin. Invest. 2014;124:3701–3703. doi: 10.1172/JCI77507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Tan JCH. Simulating anterior chamber aqueous exchange and drug delivery by single needle perfusion in mice. Invest. Ophthalmol. Vis. Sci. 2014;55:5275. [ARVO abstract] [Google Scholar]
- Kizhatil K, Ryan M, Marchant JK, Henrich S, John SWM. Schlemm’s Canal Is a Unique Vessel with a Combination of Blood Vascular and Lymphatic Phenotypes that Forms by a Novel Developmental Process. PLoS Biology. 2014;12:e1001912. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MK, Tan JCH. Contractile markers distinguish structures of the mouse aqueous drainage tract. Mol. Vis. 2013;19:2561–2570. [PMC free article] [PubMed] [Google Scholar]
- Ko MK, Yelenskiy A, Gonzalez JM, Jr, Tan JCH. Feedback-controlled constant pressure anterior chamber perfusion in live mice. Mol. Vis. 2014;20:163–170. [PMC free article] [PubMed] [Google Scholar]
- Ko MK, Tan JC. In vivo RhoA activation increases trabecular meshwork contraction and decreases outflow facility. Invest. Ophthalmol. Vis. Sci. 2015;56:3287. [ARVO abstract] [Google Scholar]
- Ko MK, Kim EK, Gonzalez JM, Jr, Tan JC. Dose- and time-dependent effects of actomyosin inhibition on live mouse outflow resistance and aqueous drainage tissues. Sci. Rep. 2016;6:e21492. doi: 10.1038/srep21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Overby DR, Boussommier-Calleja A, Stamer WD, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest. Ophthalmol. Vis. Sci. 2011;52:1865–1871. doi: 10.1167/iovs.10-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JD, Weinreb RN. Identification of the mouse uveoscleral outflow pathway using fluorescent dextran. Invest. Ophthalmol. Vis. Sci. 2002;43:2201–2205. [PubMed] [Google Scholar]
- Lütjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog. Retin. Eye. Res. 1989;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9:1801–1810. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13552–13557. doi: 10.1073/pnas.1016778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masihzadeh O, Ammar DA, Lei TC, Gibson EA, Kahook MY. Real-time measurements of nicotinamide adenine dinucleotide in live human trabecular meshwork cells: Effects of acute oxidative stress. Exp. Eye Res. 2011;93:316–320. doi: 10.1016/j.exer.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masihzadeh O, Lei TC, Ammar DA, Kahook MY, Gibson EA. A multiphoton microscope platform for imaging the mouse eye. Mol. Vis. 2012;18:1840–1848. [PMC free article] [PubMed] [Google Scholar]
- Moses RA. The conventional outflow resistances. Am. J. Ophthalmol. 1981;92:804–810. doi: 10.1016/s0002-9394(14)75634-x. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Inomata T. Microvasculature of the mouse eye: scanning electron microscopy of vascular corrosion casts. J. Exp. Anim. Sci. 2006;43:129–159. [Google Scholar]
- Overby DR. The Mechanobiology of Aqueous Humor Transport across Schlemm's Canal Endothelium. In: Nagatomi J, editor. Mechanobiology Handbook. Boca Raton, Florida: Taylor & Francis Group; 2011. pp. 367–390. [Google Scholar]
- Overby DR, Bertrand J, Schicht M, Paulsen F, Stamer WD, Lütjen-Drecoll E. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest. Ophthalmol. Vis. Sci. 2014;55:3727–3736. doi: 10.1167/iovs.13-13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Lee JK, Zhang C, Chuck RS. New details of the human corneal limbus revealed with second harmonic generation imaging. Invest. Ophthalmol. Vis. Sci. 2015;56:6058–6066. doi: 10.1167/iovs.15-16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D-Y, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong Y-K, Koh GY. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J. Clin. Invest. 2014;124:3960–3974. doi: 10.1172/JCI75392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard C, Vial JC, Douady J, van der Sanden B. In vivo imaging of elastic fibers using sulforhodamine B. J. Biomed. Opt. 2007;12:064017. doi: 10.1117/1.2821421. [DOI] [PubMed] [Google Scholar]
- Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr. Eye. Res. 1989;8:1233–1240. doi: 10.3109/02713688909013902. [DOI] [PubMed] [Google Scholar]
- Rothstein EC, Nauman M, Chesnick S, Balaban RS. Multi-photon excitation microscopy in intact animals. J. Microsc. 2016;222:58–64. doi: 10.1111/j.1365-2818.2006.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenke-Layland K. Non-invasive multiphoton imaging of extracellular matrix structures. J. Biophotonics. 2008;1:451–462. doi: 10.1002/jbio.200810045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman JS, Chang W, Wang N, de Kater AW, Allingham RR. Excimer laser effects on outflow facility and outflow pathway morphology. Invest. Ophthalmol. Vis. Sci. 1999;40:1676–1680. [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Smith RS, John SWM, Nishina PM, Sundberg JP. Systematic Evaluation of the Mouse Eye. Boca Raton, Florida: CRC Press; 2001. [Google Scholar]
- Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE, Nishimura DY, Alward WL, Hogan BL, John SW. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum. Mol. Genet. 2000;9:1021–1032. doi: 10.1093/hmg/9.7.1021. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nakajima T, Shimazaki A, Kageyama M, Matsugi T, Matsumura Y, Gabelt BT, Kaufman PL, Hara H. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp. Eye Res. 2004;78:767–777. doi: 10.1016/j.exer.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Tan JCH, Gonzalez JM, Jr, Hamm-Alvarez S, Song J. In situ autofluorescence visualization of human trabecular meshwork structure. Invest. Ophthalmol. Vis. Sci. 2012;53:2080–2088. doi: 10.1167/iovs.11-8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JCH, Gonzalez JM, Jr, Ko MK. In situ 3D Distribution of filamentous actin in mouse trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2013;54:3553. E-Abstract 3553. [Google Scholar]
- Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong Y-K, Fawzi A, Liu X, Kume T, Quaggin SE. A lymphatic defect causes ocular hypertension and glaucoma in mice. J. Clin. Invest. 2014;124:4320–4324. doi: 10.1172/JCI77162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88:713–717. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toris CB, Gregerson DS, Pederson JE. Uveoscleral outflow using different-sized fluorescent tracers in normal and inflamed eyes. Exp. Eye Res. 1987;45:525–532. doi: 10.1016/s0014-4835(87)80063-5. [DOI] [PubMed] [Google Scholar]
- Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Invest. Ophthalmol. Vis. Sci. 1987;28:477–481. [PubMed] [Google Scholar]
- Van Buskirk EM, Grant WM. Lens depression and aqueous outflow in enucleated primate eyes. Am. J. Ophthalmol. 1973;76:632–640. doi: 10.1016/0002-9394(73)90555-2. [DOI] [PubMed] [Google Scholar]
- van der Merwe EL, Kidson SH. The three-dimensional organization of the post-trabecular aqueous outflow pathway and limbal vasculature in the mouse. Exp. Eye Res. 2014;12:226–235. doi: 10.1016/j.exer.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]