Abstract
The advent of human pluripotent stem cell biology has opened unprecedented opportunities for the use of tissue engineering to generate human cardiac tissue for in vitro study. Engineering cardiac constructs, that recapitulate human development and disease, require faithfully recreating the cardiac niche in vitro. Here, we discuss recent progress in translating the in vivo cardiac microenvironment into PSC models of the human heart. We review three key physiologic features required for recreating the cardiac niche and facilitating normal cardiac differentiation and maturation: the biochemical, biophysical and bioelectrical signaling cues. Finally, we discuss key barriers that must be overcome to fulfill the promise of stem cell biology in preclinical applications and ultimately in clinical practice.
Keywords: pluripotent stem cells, cardiac myocytes, tissue engineering, cellular microenvironment, cardiac niche, disease modeling
The Challenge to Engineer Cardiac Tissue In Vitro
The apparent simplicity of cardiac function belies the intricacy of cardiac development, the structural complexity of the adult heart and the difficulty in treating heart disease. Indeed, cardiovascular disease, including advanced heart failure, represents the leading cause of mortality and morbidity in the developed world [1]. The development of novel human systems for cardiac drug discovery and toxicology testing therefore represents a major public health priority. In addition, the adult heart has limited regenerative potential with an approximately 0.5% to 1% annual cardiac myocyte (CM) turnover rate [2, 3]. As a result, lost or damaged myocardium is not effectively replaced in adults with cardiac disease. Cardiac transplantation therefore remains the only proven long-term clinical therapy for end-stage heart failure. Nonetheless, the morbidities associated with heart transplantation and the limited organ supply necessitate the development of new stem cell-based approaches for regenerative medicine.
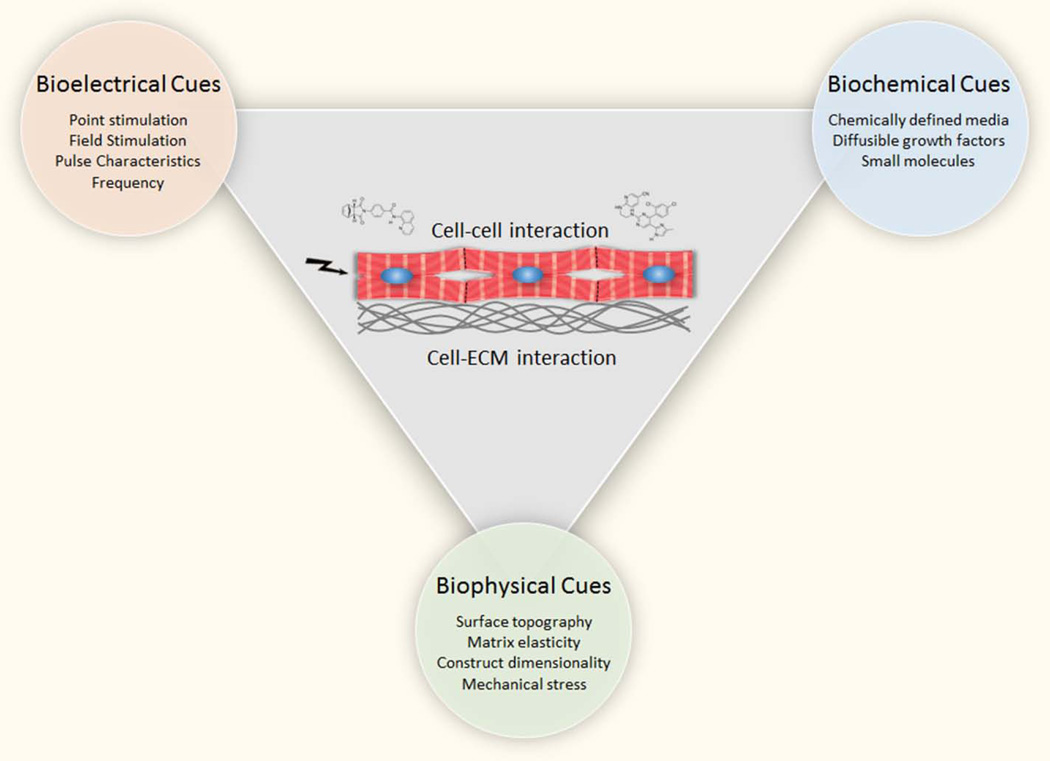
Engineering human patient and disease-specific myocardial tissue for in vitro applications and for in vivo regeneration requires the recapitulation of the native cardiac microenvironment [4–6]. The cardiac microenvironment represents a niche that harbors the biochemical, biophysical and bioelectrical cues required for normal cardiac function (Figure 1, Key Figure). In this review, we discuss current knowledge regarding how the cardiac microenvironment is recreated in vitro and examine key roadblocks that need to be overcome to effectively provide in vitro models of human heart biology and lay the foundation for cardiac regeneration.
Figure 1, Key Figure. Recreating the Cardiac Niche In Vitro.
The cardiac niche constitutes a cardiogenic microenvironment that controls cardiac development, function and disease. It harbors extrinsic cues that exhibit an interdependence between bioelectrical, biochemical and biophysical signals. These microenvironmental cues control cardiac myocyte biology and are interconnected by cell-cell and cell-ECM interactions. Examples of approaches to recreate this complex network of signals in vitro in for recapitulating the cardiac niche into cellular models of human heart development and disease.are shown within colored circles.
Biochemical Signaling during Cardiogenesis
The successful generation of human myocardial cells from renewable pluripotent stem cells (PSCs) ushered a new era for studying human cardiovascular biology and disease [7]. Replicating the biochemical cues driving in vivo cardiogenesis in vitro has long been hypothesized to enable the efficient generation of hPSC-derived CMs (hPSC-CMs). Due to the inherent challenges in studying human cardiac development, much of what we know about mammalian heart development is based on murine studies. Both human and murine PSCs differentiate into diverse sets of CMs in a stage-wise manner from mesodermal progenitors to cardiac progenitor cells and ultimately myocardial cells [8–10]. In vivo, signaling cues that promote the sequential development of heart cells originate from adjacent cell populations. Endocardial cells, for example, directly control normal CM cellular differentiation and cardiac morphogenesis [11]. Similarly, the spatiotemporally regulated expression of multiple families of secreted growth factors critically controls cardiogenesis, including diverse members of the transforming growth factor beta (TGF-β) superfamily, Wnt proteins, and fibroblast growth factors (FGFs) [12, 13].
Because of these findings, the in vitro replication of the cardiac biochemical milieu has largely focused on cell-cell interactions as well as secreted diffusible factors (Figure 2). Endodermal signaling, for example, has been mimicked in vitro by co-culture of PSCs with mouse visceral endoderm-like (END2) stromal cells. PSCs growing in the presence of END2 stromal cells or in END2-conditioned media differentiate towards the cardiac lineage However, the relatively low differentiation efficiency (ranging between 1% and 10%) and the poor mechanistic understanding of the differentiation technique has prevented the widespread adoption of this approach [14, 15]. Other efforts at directed stem cell differentiation to the cardiac lineage relies on the generation of three-dimensional (3D) constructs called embryoid bodies (EBs) and their treatment with a staged program of signaling molecules including BMP4, bFGF, Activin A, and VEGF among others [16]. While this method is still considered a robust way to generate CMs from murine PSCs, hPSCs do not seem to tolerate the dissociation into single cells for the production of EBs. This method has been limited by the low differentiation efficiency (ranging as low as 1%) and the inconsistency between experiments [7, 17, 18]. Cardiac differentiation has also been achieved with a two-dimensional (2D) monolayer technique that exposes a narrowly controlled stem cell monolayer to a combination of growth factors and/or small molecules to induce cardiogenesis (Figure 1). Importantly, intercellular communication between differentiating hPSC appears to be a critical factor in cardiac differentiation, as too little or too extensive physical interactions hinder cardiac differentiation [19]. The timing and concentration of the growth factors or small molecules to sequentially induce mesoderm formation, cardiac specification and cardiac differentiation are key for efficient production of hPSC-CMs. It is notable, however, that multiple different combinations of these growth factors can promote efficient cardiac differentiation, suggesting that a common downstream pathway ultimately controls cardiac differentiation [20–22]. Intriguingly, the stage-wise modulation of Wnt signaling alone is sufficient to drive efficient cardiogenesis in vitro [21]. Since activating this key downstream pathway is sufficient for cardiogenic induction, replicating the entire normal in vivo developmental program may not be necessary. An important advantage of the 2D monolayer technique is that it is highly scalable and highly efficient [19, 21], making this method the most efficient and cost-effective differentiation approach to date. Readers are referred to more comprehensive reviews on cardiac differentiation methods for detailed information [23].
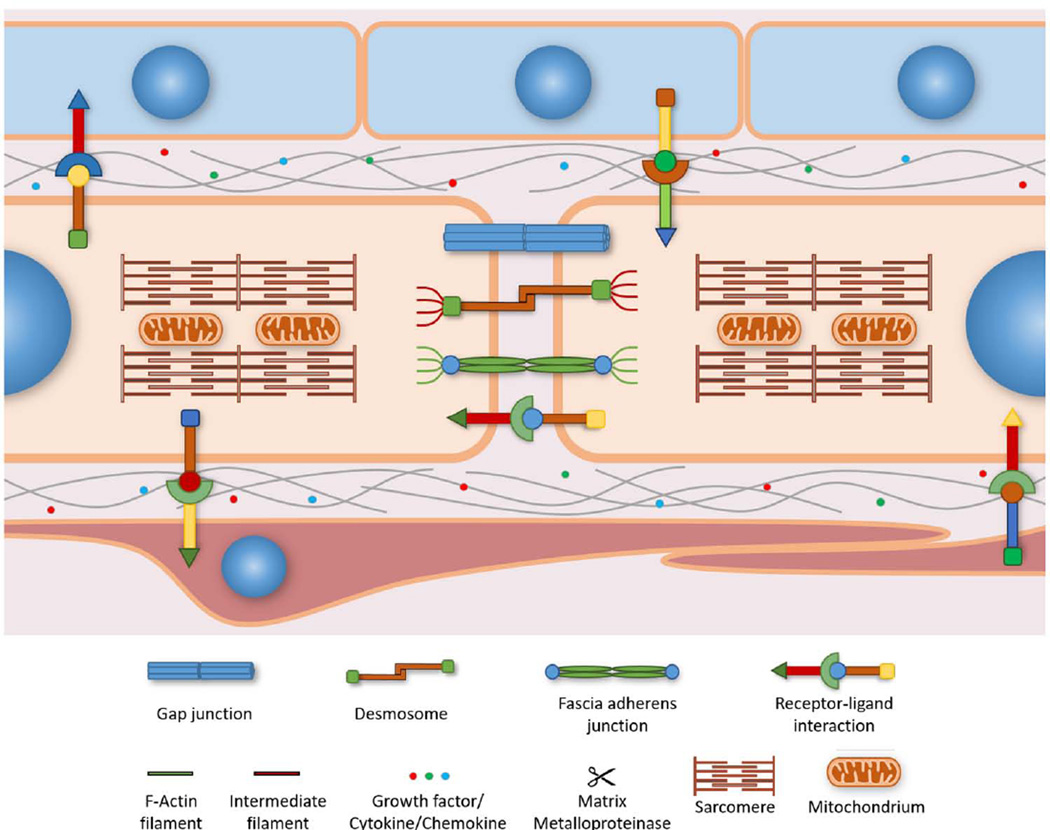
Figure 2. Cell-Cell Interactions in the Myocardium.
Cardiac myocytes directly interact with each other and with adjacent cell populations to receive signaling cues critical for normal heart development. Two important cell populations are epicardial (depicted in blue) and endocardial (depicted in red) cells. Fibroblasts and vascular cells have been omitted for visual clarity. Biochemical signaling between cells centers on transmembrane ligand-receptor interactions as well as diffusible growth factors that regulate intracellular signaling pathways. Intercalated discs at the longitudinal borders of cardiac myocytes connect the myocardium into a functional syncytium. Biophysical signaling relies on fascia adherens junctions and desmosomes to sense and transmit mechanical forces in a bi-directional and longitudinal way between neighboring cells. Fascia adherens junctions consist of cadherins that link intracellular actin filaments, while desmosomes link desmosomal cadherins to intracellular intermediate filaments. Both anchors form attachment sites between adjacent cells that allow cytoskeletal remodeling in response to intercellular mechanical stress. Bioelectrical signaling between cardiac myocytes is mediated by gap junctions that transmit electrical impulses between cells.
Directed Cardiac Differentiation towards Sub-lineages
An inherent limitation to PSC cardiac differentiation has been the heterogeneous nature of the resulting CMs. Current protocols routinely yield a mixture of nodal, atrial, ventricular and other types of CMs [19, 21, 24]. In addition, the CM yield is variable between different hPSC lines [20, 25]. Consequently, directed differentiation protocols result in significant cellular heterogeneity with respect to lineage commitment and degree of maturation. These difficulties are further compounded by batch-to-batch variability even within the same cell line. As a result, detailed analysis of the cell biology and physiology of specific cardiac subtypes has been difficult. While recent technical advances have facilitated the genetic and metabolic purification of hPSC-CMs [24, 26, 27], the generation of highly purified CM subtypes remains an important focus. Live-cell phenotyping using fluorescent reporters [28, 29] or comprehensive physiological assessment [30] have established a number of options to identify specific CM subtypes. An alternative approach has been to develop differentiation protocols that direct PSC differentiation toward specific cardiac sub-lineages. By modulating the combination, timing, and concentration of morphogen treatment, stem cell differentiation could be geared toward the atrial or ventricular sub-lineage (modulating noggin/retinoic acid pathways [31]), sinoatrial or atrioventricular sub-lineage (repressing the NRG-1β/ErbB signaling pathways [32]), or the epicardial sub-lineage (stage-specific activation of BMP/Wnt signaling pathways [33]). These methods have improved existing protocols and should prove useful for studies in lineage specification, disease pathogenesis, and regenerative medicine.
The Extracellular Matrix Controls Cardiogenesis and Function
In addition to cell-cell interactions and soluble signaling cues, the extracellular matrix (ECM) contributes to the biochemical signaling cues of the cardiac niche (Figure 3). The ECM provides structural support to CMs, dynamically, modulates biochemical and biophysical signaling cues, and facilitates intercellular signaling within the heart [34–36]. ECM composition is tightly regulated during normal heart development and its dysregulation can result in structural and functional diseases [37–39]. Consequently, the choice of ECM for in vitro culture is recognized as a key consideration. In an effort to use defined ECM, recombinant laminin or vitronectin were used in early cardiac differentiation studies [19] and their simple composition (single ECM proteins) results in a less physiologic extracellular environment. A number of studies have shown that complex ECM, including ECM obtained from decellularized hearts, provides a superior microenvironment over single purified ECM with respect to CM function [40, 41] and structural organization [4, 42]. Because of this consideration, Matrigel, a poorly defined complex ECM isolated from the murine Engelbreth-Holm-Swarm tumor [43], has remained the principal ECM used in PSC maintenance and differentiation protocols. Matrigel consists primarily of laminin and collagen IV [43]. In contrast, the adult heart’s ECM is constituted primarily of collagen I [42]. With the elucidation of the ECM composition of the developing and adult heart [42], a new avenue for the tailored recombinant engineering of complex ECM hPSC differentiation and routine CM culture is now open.
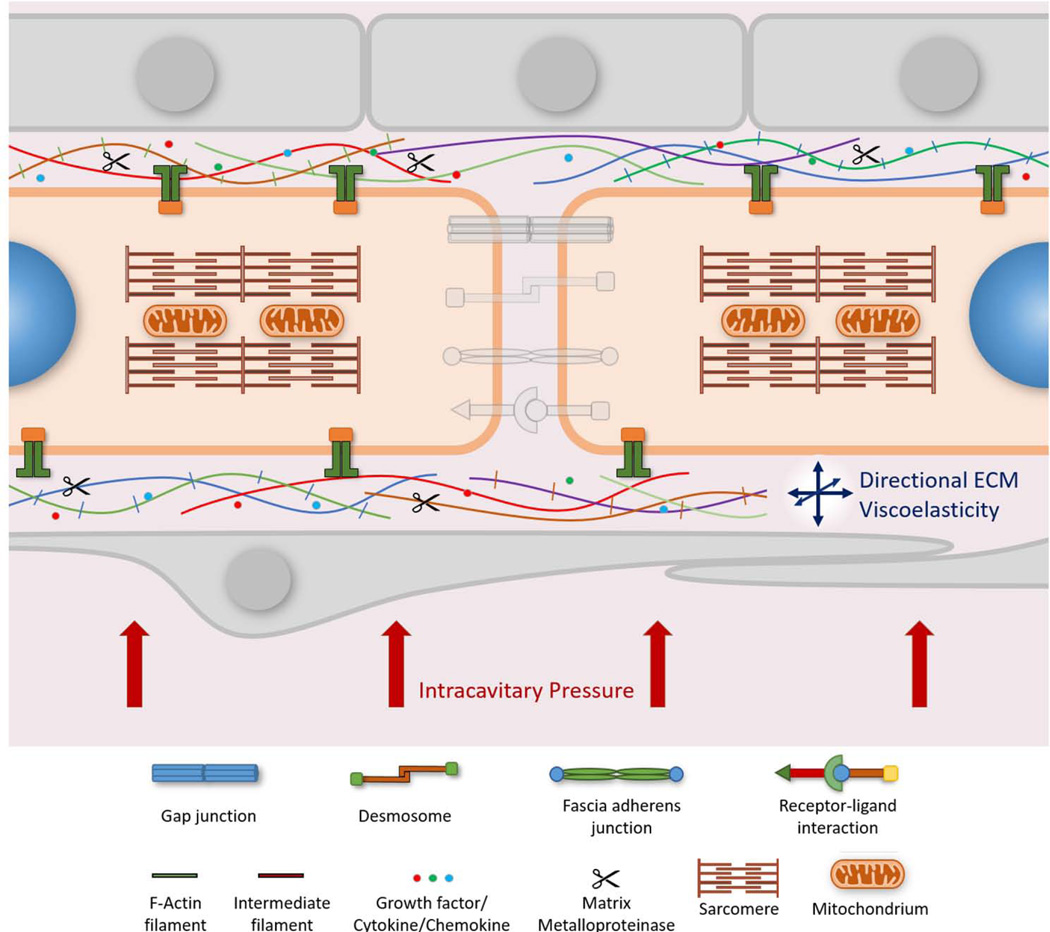
Figure 3. Cell-ECM Interactions in the Myocardium.
Cardiac cells are surrounded by the extracellular matrix (ECM). It is the anchoring source that provides structural as well as molecular support to cardiac cells. The ECM is composed of a wide variety of micro-and macromolecules including fibrous proteins, proteoglycans and incorporates growth factors, cytokines and chemokines. As such, the ECM provides important biochemical and biophysical signaling cues to cardiac myocytes. The balance between synthesis and degradation of the ECM is maintained by matrix metalloproteinases. Cardiac myocytes connect to the ECM at costamere complexes. Costameres are mechanotransducing complexes that mainly link Z-discs laterally to the ECM and consist of integrin and dystrophin-glycoprotein complexes. Integrins play a critical role in forming focal adhesions in vitro that couple actin filaments through linker proteins to the ECM. At costameres the incoming mechanical load regulates biochemical signaling pathways. Cytoskeletal organization and cell geometry are significantly influenced by how the ECM is presented to the cell. As a result dysregulation of the ECM may lead to cytoskeletal disarray and aberrant cell shape. The viscoelastic properties of the ECM also influence the contractile properties of the myocardium. In addition, the intracavitary pressure of the native heart is transmitted through the ECM and acts as a major determinant of the biomechanical forces exerted on myocardial cells. Aspects of cell-cell interactions have been deemphasized to reduce visual clutter.
Chemically Defined PSC Differentiation
Since most growth factors present in media supplements or used to promote cardiac differentiation are polypeptides, they are expensive, have limited diffusion capacity, and are susceptible to batch-to-batch variability. Lineage specification during cardiogenesis cannot be rigorously studied without a defined biochemical microenvironment, therefore, a major priority for the field has been to transition to fully defined hPSC culture and differentiation media while maintaining efficient cardiac differentiation [44, 45]. Fully defined growth media consisting of RPMI 1640, recombinant albumin, ascorbic acid, and small molecule Wnt modulators are sufficient to robustly induce in vitro cardiac differentiation [19]. However, a recent report has shown that albumin is not required for efficient cardiac differentiation [46]. Importantly, eleven hiPSC lines were successfully differentiated into CMs using fully defined media, providing a reproducible system that should render in vitro studies more comparable in the future. This is particularly significant for the development of a standardized system that diminishes the variability in cardiac differentiation efficiency across different stem cell lines [20, 25].
Chemically defined media is preferable for cellular assays geared at factors that direct cardiac sub-lineage specification. Such systems should also ease the translation of basic research to clinical practice as animal proteins are eliminated. At the same time, however, one has to remain cautious about over-simplifying an extremely complex organ system by employing only a few compounds during in vitro differentiation. Given that numerous factors are at play during heart development, homeostasis and disease, a major challenge of recreating the cardiac biochemical microenvironment in vitro is the development of a cost-effective platform that incorporates the key complexities of known in vivo molecular signaling pathways.
Biophysical features of the cardiac niche
In vivo, myocardial cells and cardiac tissue are exposed to a myriad of biophysical stimuli that control normal development and physiology as well as pathophysiology. The biophysical properties of the heart are simulated in vitro by manipulation of ECM composition and growth substrate stiffness, tissue dimensionality and mechanical stress (Figure 1). While these factors impact myocardial function at vastly different scales (ranging from the nanometer scale of sarcomeres to the centimeter scale of the cardiac chamber), they are all critical for normal cardiac function. Biophysical cues are sensed by CMs through mechanosensors that convert the physical stimulus into a biochemical signal. These sensors typically consist of membrane-associated protein complexes that interact with the ECM and link it to the cytoskeletal scaffold. The mechanosensors thereby allow for the detection of the strength and orientation of the biophysical signal. Costameres [47] and intercalated discs [48] represent two families of mechanosensors extensively studied in CMs (Figures 2 and 3). In recent years, the intracellular protein titin [49] and the cytoskeletal microtubule network [50] have also been described as intracellular mechanosensors, adding another layer of complexity to how cells sense their microenvironment and adapt to it. Elucidating the molecular composition of CMs, and employing more sophisticated engineering techniques, is important for fully defining the biophysical cues from the cardiac microenvironment and delineating how they impact cardiac function.
Structural Control of 2D and 3D Myocardial Tissue
An important challenge in CM biology is the in vitro recapitulation of two and three dimensional myocardial microstructure (Figure 2). In 2D tissue engineering applications, customized growth surfaces pose an attractive opportunity to study structure-function relationships. The challenge to generate anisotropic myocardial tissue resembling tissue organization in vivo has driven tissue engineers to construct growth surfaces that enable the cellular alignment of hPSC-CMs. Multiple strategies have been developed to provide cells with topographical cues at the nano- and microscale. Aligned collagen fibrils in the native heart matrix are 100nm in diameter [51], suggesting a nanoscale interaction between CMs and the ECM. Indeed, it has been shown that the optimum width of the nanotopographic cue for aspects of CM function and maturation, such as cell area and sarcomere formation, is approximately 800nm in width [52], considerably smaller than the average width of a CM of 20–25µm. This suggests that anisotropic orientation is co-directed by cues at the nanoscale and although nanotopographical cues cannot physically constrain cell growth, CMs still align along the direction of the topographical cue [52, 53]. On the microscale, ECM proteins have been deposited onto growth surfaces to force CMs to adopt a specific shape [10, 40, 54–56]. Similar to nanopatterning approaches, microgrooved surfaces were fabricated to study the role of topographical cues on CM biology at the microscale [57]. Taken together, uniaxial alignment facilitates the study of CM biology in vitro and anisotropically grown CMs have more physiological calcium-handling properties and improved contractile force during systole [57–59].
In vivo, a typical left ventricular CM has cell-cell contacts with approximately eleven other CMs in a three dimensional oriented configuration [60]. Cells cultured in a two-dimensional monolayer lack this extensive network of cell-cell and cell ECM interactions. Notably, 3D cardiac tissue constructs exhibit more physiological electrical and contractile function when compared to equivalent 2D constructs [6, 61]. 3D myocardial tissue has been constructed by culturing CMs in hydrogels [62, 63] or prefabricated matrices from decellularized hearts [64, 65]. Culturing cells in pre-fabricated matrices enables the precise control of nano- and microfeatures that are present in the native heart, such as formation and orientation of ECM macromolecules. Tissue constructs can be further modified with conductive materials to improve electrical communication between cells and to overcome the electrical resistance synthetic materials typically possess [66]. Further, hydrogel and engineered matrix techniques allow the miniaturization of constructs to increase throughput [67, 68]. In an alternative approach, 3D cardiac tissue was generated by stacking multiple 2D cell sheets harvested using the thermo-responsive polymer poly-(N-isopropylacrylamide) (PIPAAm) [69, 70] or by using a paper-based scaffold that allows oxygen and nutrient transport between the individual sheets [71]. Recently, 3D bioprinting has emerged as an exciting technological advancement to construct 3D myocardial tissue [72–74]. With the use of bioinks, it is now possible to directly print cardiac tissues with native characteristics and spatial distribution of different cell types including myocardial and vascular cells.
Molecular diffusion is effective up to a thickness of approximately 100–200µm depending on the density of the tissue [75]. Although thin myocardial tissue constructs are sufficient for in vitro studies, clinically useful constructs for regenerative medicine must be several millimeters thick to meet the mechanical demand during each contraction cycle [76]. As 3D myocardial tissue is engineered into these thicker force-generating constructs, the diffusion limit is reached, necessitating perfusion or vascularization for oxygen and nutrient delivery. Cell type and cell density determine the metabolic activity of the tissue and influence whether vascularization is required. hPSC-CMs mainly rely on anaerobic glycolysis [77] and thus may be less affected by hypoxia. Neonatal rat CMs also exhibit some degree of hypoxia resistance [78]. As both of these cell types mature however in culture, their metabolic profile changes over time. A shift toward oxidative phosphorylation may therefore increase the requirement for oxygen to preserve construct viability. 3D tissue engineering may provide an avenue to overcome the metabolic limitations to myocardial tissue constructs of clinically relevant sizes by efficiently delivering oxygen and nutrients to all cells within the construct.
Matrix Elasticity
For the heart to efficiently contract during systole and relax during diastole, the ECM must be compliant. In vitro, CMs seeded into 3D hydrogels are capable of contracting against the ECM [79]. In 2D cultures, the layer thickness of adsorbed ECM protein is typically in the nanometer range [80, 81]. Cells on 2D substrates sense the stiffness of the underlying surface less than 5µm beneath them [82], suggesting that CMs cultured on 2D growth substrates contract against the stiffness of the underlying growth substrate rather than the ECM. It is therefore possible to independently control substrate elasticity and ECM composition for in vitro studies. The elasticity of decellularized myocardial ECM is in the range of 10–15kPa during cardiogenesis, 15–20kPa during healthy adulthood and 35–70kPa in infarcted myocardium [83]. Interestingly, hPSC-CM functional maturation (including sarcomere pattern, cell spread area, cell viability and contractility) is most efficient at physiologically relevant range of substrate stiffness, suggesting that biomechanical cues control aspects of cell fate [55, 83–85]. On substrates that are much softer than the healthy myocardium (~1kPa), low levels of substrate resistance lead to low levels focal adhesion points and little tension. In addition, sarcomerogenesis is inhibited on substrates that are too soft. In contrast, rigid substrates such as glass or tissue culture polystyrene facilitate spreading of CMs and thus the generation of higher levels of tension. However, cells are less viable on stiff substrates and cardiac gene expression is downregulated [83, 86]. Taken together, it is noteworthy that efficient PSC differentiation toward the cardiac lineage does not necessitate a physiologic matrix elasticity while functional maturation of CMs does. One might therefore speculate that during early embryogenesis biochemical signaling provides most of the necessary developmental cues while later during cardiogenesis biophysical cues gain more importance. Optimal cell shape is also linked to matrix elasticity. In the healthy heart, longer CMs show a greater absolute shortening as more sarcomeres are added in series. In conditions with stiff ECM (seen for example in infiltrative heart disease), CMs with a lower aspect ratio have a functional advantage over longer cells, because the higher matrix elasticity prevents longer CMs from shortening to their full potential [87]. These studies underscore the importance of controlling matrix rigidity when creating model systems of normal cardiac physiology and disease.
Replication of Hemodynamics In Vitro
The heart is a biomechanical organ in which the mechanical stress on myocardial cells mainly arises from the hemodynamic load of the heart. During normal diastolic filling, the heart tissue is stretched by the filling pressure or preload. During systole, the heart must overcome the afterload of aortic pressure to eject blood. Dysregulation of either preload or afterload can contribute to the pathogenesis of congenital or adult heart disease [88, 89]. During systole, ventricular contraction results in chamber emptying. In the absence of valvular heart disease, the ventricular chamber pressure is equal to aortic pressure. The stress of contraction against chamber pressure is denoted as the wall stress. This wall stress is estimated at a peak of 15–18 kPa in early systole [90, 91] and a mean of ~11–12 kPa across all of systole. More recent estimates of the wall stress made by magnetic resonance imaging place left ventricular wall stresses almost twice as high at 22–24 kPa [92]. Thus during systole, chamber pressure, in addition to cardiac tissue stiffness, is a major determinant of the biomechanical forces exerted on myocardial cells.
Mechanical preload has been mostly recapitulated in vitro by applying static or cycling stress [93, 94]. Often a 5–15% strain is applied to replicate the in vivo preload. The use of cyclic stress improves upon existing techniques by applying mechanical load at a specific frequency to mimic isotonic contraction. Cyclic stress must be synchronized with the beating frequency or electric stimulation of the cardiac tissue to avoid contraction while the tissue is stretched. Currently the most physiologic and biomimetic approach is the application of auxotonic loading [95]. Here, the engineered tissue contracts against elastic boundaries with a defined stiffness and consequently relaxes with a defined force during diastole. Auxotonic loading resulted in improved anisotropy of myocardial cells and greater tissue contraction compared to static or phasic loading [95, 96]. A limitation of this type of approach, however, is the tendency of premature rupture of the tissue after seven to ten days. Nevertheless, tissue failure can be controlled by modulating the amount of ECM and stiffness of the anchoring pillars [97]. Under mechanical load cardiac tissues engineered with hPSC-CMs have improved cellular alignment and more mature levels of gene expression, calcium handling and contractile force [6, 98, 99]. However, contractile stress of these constructs reaches only 11.8 mN/mm2 [6], lower than that of the human heart (~16mN/mm2) [100]. Going forward, the construction of engineered 3D cardiac tissues could be greatly facilitated by employing ECM, cellular composition and density in accordance with the native human heart [101].
Bioelectrical features of the cardiac niche
The electrical impulse controlling cardiac contractions originate from a specialized population of cardiac conduction cells in the sinoatrial node. From there, the impulse propagates along the conduction pathway to excite the contractile myocardium in a synchronous manner. Since these rhythmic electrical stimuli ensure the coordinated ejection of blood, they should be replicated in the in vitro hPSC system. Cells grown in vitro, however, may differ in their assembly from cells growing in situ in the heart. Cells in the native myocardium are oriented longitudinally with gap junctions preferentially located along the long axis of the cell for efficient conduction of electric signals. In contrast, cells grown in a monolayer may grow isotropically and unevenly along the growth surface with individual cells or independent cell clusters forming poor electrical connections with other cell clusters. Myocardial cells in the native heart are excited by an electric point stimulation originating in the sinoatrial node and propagating by cell-cell contract. In most cell culture systems, cells are excited by electric field stimulation whereby membranes of all cells are simultaneously depolarized. This approach ensures even electric stimulation of CMs in the field but does not accurately reflect the native heart mechanism of excitation via cell-cell interactions. Cellular excitability is more efficient in cells aligned parallel to the electrical field compared to cells aligned perpendicular to the electric field [102]. Interestingly, hPSC-CMs have poorly aligned sarcomeres and thus lack clearly defined long and short axes. Furthermore, since the expression of ion channels changes with the developmental stage of a CM [103], the intensity of electric field stimulation has to be adjusted to account for the degree of cellular maturation. In addition, the hPSC-CMs membrane capacitance is four times smaller than membrane capacitance of adult atrial CMs [104] and ten times smaller than that of adult ventricular CMs [105].
These considerations have led to a diverse number of attempts to replicate the bioelectrical features of the cardiac niche in vitro. The parameters that determine the type and strength of the electric field can be defined by the following considerations: (i) current versus voltage-based system, (ii) monophasic vs biphasic waveforms, (iii) the field versus point stimulations, and (iv) the frequency, duration and strength of the electric field (Figure 1). To date, electric field strengths of 2–5V/cm with a frequency of 1Hz for a duration of 1–2ms have been used to pace CMs cultured in vitro [106–109]. Biphasic stimulation compared to monophasic stimulation decreases the excitation threshold and increases cell viability [107]. When hPSC-CMs resemble immature fetal CMs, electric stimulation at 1Hz is non-physiologic since the fetal heart has a beating rate between 2 and 3Hz [110]. In a recent study, stimulation at 6 Hz promoted cellular and functional maturation of hPSC-CMs [108]. Thus, electric stimulation results in improved conductive and contractile properties of CMs [109, 111, 112] as well as morphologic maturation including cellular elongation, striation and improved calcium cycling [109, 113, 114]. It is thus critical to implement bioelectrical stimulation in studies employing hPSC-CMs to maximize their potential as an adult human cardiac cell source.
Concluding Remarks
The past decade has been among the most striking and progressive periods for studying human heart biology and disease. With the advent of iPSC technology, human CMs can now be easily produced in vitro in large quantities from a renewable cell source and used for the study of human cardiac physiology and pathophysiology (Box 1). Current differentiation methods, however, yield CMs with a fetal phenotype that may not be well suited to model adult onset cardiac diseases. Cellular differentiation and maturation of hPSC-CMs require a microenvironment that more closely resembles the mature heart. New insights regarding cell-cell signaling need to be incorporated in these cellular models. Exosomes carrying non-coding RNA are increasingly recognized as important for intercellular communication in the heart [131]. Further, the emergence of micro RNA (miRNA) [13, 132] and long non-coding RNA (lncRNA) [133, 134] as critical regulators of cardiac development and disease may necessitate their incorporation into future efforts at recreating the cardiac microenvironment in vitro. Similarly, physiological energy sources and cellular metabolism characteristics must be incorporated into the cardiogenic niche. hPSC-CMs resembling fetal CMs mostly rely on glycolysis whereas adult CMs mostly utilize fatty acids to generate cellular energy. Consequently, exposing hPSC-CMs to a more lipogenic milieu promoting fatty acid β-oxidation improves their maturity and allows for the study of adult onset heart diseases, like diabetic cardiomyopathy [120] and arrhythmogenic right ventricular dysplasia [135].
Box 1. Applications of the cardiac Microenvironment in vitro.
The hPSCs offers an unprecedented opportunity to generate human myocardial cells from a renewable cell source for in vitro and in vivo study of human cardiac physiology and pathophysiology. To date, a diverse set of clinically relevant cardiac diseases have been modeled in vitro using stem cell derived CMs. These diseases include channelopathies [115, 116], cardiomyopathies [117, 118] and cardiac metabolic diseases [119, 120]. While current studies have mostly focused on recapitulating monogenic cardiac disorders, infectious [121] and metabolic diseases with a cardiac phenotype [120] have also been successfully modeled in vitro. Pluripotent stem cell biology also provides novel platforms for cardiac drug discovery and testing [122, 123]. These efforts may prove powerful not only in the development of new medical therapies but also in the understanding of drug-induced arrhythmogenicity and cardiotoxicity [124].
In developing these platforms an important question remains regarding how single CMs and myocardial constructs will be functionally evaluated. While gene expression is commonly used to assess myocardial differentiation and maturation, the extensive structure-function relationship in myocardial cells requires new approaches to delineate the functional characteristics of hPSC-CMs [125, 126]. The hallmarks of myocardial function are the generation of an action potential upon change of membrane potential, the influx of calcium ions, and the consequent cellular contraction and force generation. For a comprehensive assessment of myocardial function all of these processes have to be simultaneously evaluated. While the membrane potential and variations of intracellular calcium ions are commonly imaged optically with small molecule fluorescent indicators [127], a major and exciting step toward reducing adverse effects of these chemicals has been the development of hPSC lines stably expressing optogenetic sensors either individually [28, 128] or combined [128]. Novel systems to precisely measure multiple physiological features of myocardial function at the single-cell [30, 129] or tissue level [56, 130] have also been developed. The cardiovascular field is rapidly moving from a descriptive to a more mechanistic characterization of cell biology of the myocardium. It will be exciting to see how these techniques will aid in mechanistically elucidating functional alterations in hPSC disease models and drug development platforms.
In conclusion, in vitro models using hPSC-CMs have the potential to reflect many complex features of the adult human heart. To accomplish this goal it is necessary to engineer tissue that incorporates the key aspects of the three dimensional biology of the human heart including the biochemical signaling, the biomechanical milieu of the native heart, and the electrical signaling within the heart (see Outstanding Questions). Integrating these key features requires the control of multiple interrelated aspects of cardiac biology including cell-cell signaling, cell-ECM interaction, electrical coupling, and energy utilization.
Outstanding Questions.
Can live-cell phenotyping efficiently distinguish myocardial subtypes and reduce biases introduced by the cellular heterogeneity in human stem cell-derived cardiac myocytes?
How can tailored physiologic cardiac ECM be best used in cellular models of human cardiac physiology and disease?
How does the ECM modulate bioelectrical signaling in the native heart? Could the ECM of the native heart play an important role in controlling electrical propagation in vivo or in vitro?
What is the role of exosomes in cardiac differentiation and maturation and how can exosomes be best exploited in cell culture?
How can we recreate the physiologic cardiac myocyte metabolic milieu in vitro and how can we make the bioenergetics of human stem cell-derived cardiac myocytes more efficient?
Trends.
Biochemical, biophysical and bioelectrical signaling cues control cardiac myocyte differentiation, maturation and physiology. These signals may be mediated via cell-cell and cell-ECM interactions.
The development of cellular models of human myocardial physiology and pathophysiology requires the adaptation of these cues for in vitro applications.
A cardiogenic biochemical milieu can be enforced in vitro via small molecules modulating Wnt signaling.
Cardiogenic biophysical cues can be adapted in vitro by controlling growth substrate topography and elasticity, generating 3D myocardial tissue and applying mechanical stress to cells and tissues.
Electric point stimulation provides physiologic bioelectrical cues and is dependent on cell–cell interactions for signal propagation. Electrical field stimulation is suitable for excitation of cell islands or clusters vitro and ensures synchronous contraction of myocardial cells.
Acknowledgments
This work was supported by grants from the NIH/National Heart, Lung, and Blood Institute (U01HL100408-01). A. Atmanli is supported by an American Heart Association predoctoral fellowship grant (15PRE22220009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moran AE, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129(14):1483–1492. doi: 10.1161/CIRCULATIONAHA.113.004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senyo SE, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedada FB, et al. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports. 2014;3(4):594–605. doi: 10.1016/j.stemcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun YW, et al. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials. 2015;67:52–64. doi: 10.1016/j.biomaterials.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehat I, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bu L, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460(7251):113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 9.Wu SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Domian IJ, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326(5951):426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luxan G, et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med. 2013;19(2):193–201. doi: 10.1038/nm.3046. [DOI] [PubMed] [Google Scholar]
- 12.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Developmental Biology. 2003;258(1):1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 13.Rao J, et al. Stepwise Clearance of Repressive Roadblocks Drives Cardiac Induction in Human ESCs. Cell Stem Cell. 2016;18(3):341–353. doi: 10.1016/j.stem.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Mummery C, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107(21):2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 15.Passier R, et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 2005;23(6):772–780. doi: 10.1634/stemcells.2004-0184. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 17.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotech. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104(4):e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burridge PW, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11(8):855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burridge PW, et al. Improved Human Embryonic Stem Cell Embryoid Body Homogeneity and Cardiomyocyte Differentiation from a Novel V-96 Plate Aggregation System Highlights Interline Variability. STEM CELLS. 2007;25(4):929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 21.Lian X, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109(27):E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Mummery CL, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111(3):344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott DA, et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8(12):1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 25.Moore JC, et al. Distinct cardiogenic preferences of two human embryonic stem cell (hESC) lines are imprinted in their proteomes in the pluripotent state. Biochem Biophys Res Commun. 2008;372(4):553–558. doi: 10.1016/j.bbrc.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tohyama S, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12(1):127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Hattori F, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7(1):61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 28.Leyton-Mange JS, et al. Rapid Cellular Phenotyping of Human Pluripotent Stem Cell-Derived Cardiomyocytes using a Genetically Encoded Fluorescent Voltage Sensor. Stem Cell Reports. 2014;2(2):163–170. doi: 10.1016/j.stemcr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dempsey GT, et al. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J Pharmacol Toxicol Methods. 2016 doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Kijlstra JD, et al. Integrated Analysis of Contractile Kinetics, Force Generation, and Electrical Activity in Single Human Stem Cell-Derived Cardiomyocytes. Stem Cell Reports. 2015;5(6):1226–1238. doi: 10.1016/j.stemcr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21(4):579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu WZ, et al. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107(6):776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witty AD, et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat Biotechnol. 2014;32(10):1026–1035. doi: 10.1038/nbt.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christalla P, et al. The cardiogenic niche as a fundamental building block of engineered myocardium. Cells Tissues Organs. 2012;195(1–2):82–93. doi: 10.1159/000331407. [DOI] [PubMed] [Google Scholar]
- 35.Frantz C, et al. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li A-H, et al. Dynamic Changes in Myocardial Matrix and Relevance to Disease: Translational Perspectives. Circulation Research. 2014;114(5):916–927. doi: 10.1161/CIRCRESAHA.114.302819. [DOI] [PubMed] [Google Scholar]
- 38.Buikema JW, et al. Wnt/beta-catenin signaling directs the regional expansion of first and second heart field-derived ventricular cardiomyocytes. Development. 2013;140(20):4165–4176. doi: 10.1242/dev.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Passer D, et al. Atypical Protein Kinase C-Dependent Polarized Cell Division Is Required for Myocardial Trabeculation. Cell Rep. 2016;14(7):1662–1672. doi: 10.1016/j.celrep.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atmanli A, et al. Molecular etching: a novel methodology for the generation of complex micropatterned growth surfaces for human cellular assays. Adv Healthc Mater. 2014;3(11):1759–1764. doi: 10.1002/adhm.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philp D, et al. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23(2):288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 42.Williams C, et al. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 2014;10(1):194–204. doi: 10.1016/j.actbio.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes CS, et al. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10(9):1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 44.Burridge PW, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6(4):e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Titmarsh DM, et al. Microbioreactor arrays for full factorial screening of exogenous and paracrine factors in human embryonic stem cell differentiation. PLoS One. 2012;7(12):e52405. doi: 10.1371/journal.pone.0052405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lian X, et al. Chemically defined, albumin-free human cardiomyocyte generation. Nat Methods. 2015;12(7):595–596. doi: 10.1038/nmeth.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danowski BA, et al. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118(6):1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutstein DE, et al. The organization of adherens junctions and desmosomes at the cardiac intercalated disc is independent of gap junctions. J Cell Sci. 2003;116(Pt 5):875–885. doi: 10.1242/jcs.00258. [DOI] [PubMed] [Google Scholar]
- 49.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77(4):637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 50.Kerr JP, et al. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun. 2015:6. doi: 10.1038/ncomms9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson TF, et al. Morphology, composition, and function of struts between cardiac myocytes of rat and hamster. Cell Tissue Res. 1987;249(2):247–255. doi: 10.1007/BF00215507. [DOI] [PubMed] [Google Scholar]
- 52.Carson D, et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl Mater Interfaces. 2016;8(34):21923–21932. doi: 10.1021/acsami.5b11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DH, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010;107(2):565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atmanli A, Domian IJ. Generation of aligned functional myocardial tissue through microcontact printing. J Vis Exp. 2013;(73):e50288. doi: 10.3791/50288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribeiro AJ, et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112(41):12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feinberg AW, et al. Muscular thin films for building actuators and powering devices. Science. 2007;317(5843):1366–1370. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- 57.Rao C, et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34(10):2399–2411. doi: 10.1016/j.biomaterials.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feinberg AW, et al. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials. 2012;33(23):5732–5741. doi: 10.1016/j.biomaterials.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ribeiro MC, et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro--correlation between contraction force and electrophysiology. Biomaterials. 2015;51:138–150. doi: 10.1016/j.biomaterials.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 60.Saffitz JE, et al. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ Res. 1994;74(6):1065–1070. doi: 10.1161/01.res.74.6.1065. [DOI] [PubMed] [Google Scholar]
- 61.Daily NJ, et al. Improving Cardiac Action Potential Measurements: 2D and 3D Cell. 2015;5(3):4. doi: 10.4172/2155-9538.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Black LD, 3rd, et al. Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Eng Part A. 2009;15(10):3099–3108. doi: 10.1089/ten.tea.2008.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dixon JE, et al. Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proc Natl Acad Sci U S A. 2014;111(15):5580–5585. doi: 10.1073/pnas.1319685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ott HC, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 65.Guyette JP, et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ Res. 2016;118(1):56–72. doi: 10.1161/CIRCRESAHA.115.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dvir T, et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol. 2011;6(11):720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCain ML, et al. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials. 2014;35(21):5462–5471. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boudou T, et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18(9–10):910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masumoto H, et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2014;4:6716. doi: 10.1038/srep06716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams C, et al. Stacking of aligned cell sheets for layer-by-layer control of complex tissue structure. Biomaterials. 2011;32(24):5625–5632. doi: 10.1016/j.biomaterials.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 71.Mosadegh B, et al. Three-dimensional paper-based model for cardiac ischemia. Adv Healthc Mater. 2014;3(7):1036–1043. doi: 10.1002/adhm.201300575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotech. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 73.Park SJ, et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science. 2016;353(6295):158–162. doi: 10.1126/science.aaf4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pati F, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radisic M, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286(2):H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 76.Vunjak-Novakovic G, et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16(2):169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rana P, et al. Characterization of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Bioenergetics and Utilization in Safety Screening. Toxicological Sciences. 2012;130(1):117–131. doi: 10.1093/toxsci/kfs233. [DOI] [PubMed] [Google Scholar]
- 78.Pritchett-Corning KR. Euthanasia of neonatal rats with carbon dioxide. J Am Assoc Lab Anim Sci. 2009;48(1):23–27. [PMC free article] [PubMed] [Google Scholar]
- 79.Shapira-Schweitzer K, Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 2007;3(1):33–41. doi: 10.1016/j.actbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Al-Jawad M, et al. Fibronectin adsorption studied using neutron reflectometry and complementary techniques. Eur Phys J E Soft Matter. 2009;30(2):175–179. doi: 10.1140/epje/i2009-10472-0. [DOI] [PubMed] [Google Scholar]
- 81.Kohen NT, et al. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases. 2009;4(4):69–79. doi: 10.1116/1.3274061. [DOI] [PubMed] [Google Scholar]
- 82.Buxboim A, et al. How deeply cells feel: methods for thin gels. Journal of physics. Condensed matter : an Institute of Physics journal. 2010;22(19):194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Engler AJ, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(Pt 22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 85.Hazeltine LB, et al. Temporal impact of substrate mechanics on differentiation of human embryonic stem cells to cardiomyocytes. Acta Biomater. 2014;10(2):604–612. doi: 10.1016/j.actbio.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhana B, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105(6):1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 87.McCain ML, et al. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am J Physiol Heart Circ Physiol. 2014;306(11):H1525–H1539. doi: 10.1152/ajpheart.00799.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kowalski WJ, et al. Investigating developmental cardiovascular biomechanics and the origins of congenital heart defects. Front Physiol. 2014;5:408. doi: 10.3389/fphys.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toischer K, et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122(10):993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grossman W, et al. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56(1):56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colan SD, et al. Developmental modulation of myocardial mechanics: age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19(3):619–629. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 92.Alter P, et al. B-type natriuretic peptide and wall stress in dilated human heart. Mol Cell Biochem. 2008;314(1–2):179–191. doi: 10.1007/s11010-008-9779-4. [DOI] [PubMed] [Google Scholar]
- 93.Eschenhagen T, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. Faseb j. 1997;11(8):683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 94.Mansour H, et al. Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res. 2004;94(5):642–649. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann WH, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12(4):452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 96.Legant WR, et al. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc Natl Acad Sci U S A. 2009;106(25):10097–10102. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H, et al. Necking and failure of constrained 3D microtissues induced by cellular tension. Proc Natl Acad Sci U S A. 2013;110(52):20923–20928. doi: 10.1073/pnas.1313662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruan JL, et al. Mechanical Stress Promotes Maturation of Human Myocardium From Pluripotent Stem Cell-Derived Progenitors. Stem Cells. 2015;33(7):2148–2157. doi: 10.1002/stem.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thavandiran N, et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci U S A. 2013;110(49):E4698–E4707. doi: 10.1073/pnas.1311120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Makarenko I, et al. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95(7):708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 101.Naito H, et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114(1 Suppl):I72–I78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 102.Tung L, et al. Influence of electrical axis of stimulation on excitation of cardiac muscle cells. Circ Res. 1991;69(3):722–730. doi: 10.1161/01.res.69.3.722. [DOI] [PubMed] [Google Scholar]
- 103.Sartiani L, et al. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25(5):1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 104.Cai B, et al. Difference of sodium currents between pediatric and adult human atrial myocytes: evidence for developmental changes of sodium channels. Int J Biol Sci. 2011;7(6):708–714. doi: 10.7150/ijbs.7.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sheng X, et al. Human pluripotent stem cell-derived cardiomyocytes: response to TTX and lidocain reveals strong cell to cell variability. PLoS One. 2012;7(9):e45963. doi: 10.1371/journal.pone.0045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Au HT, et al. Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials. 2007;28(29):4277–4293. doi: 10.1016/j.biomaterials.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiu LL, et al. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids. Tissue Eng Part A. 2011;17(11–12):1465–1477. doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nunes SS, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10(8):781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Radisic M, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101(52):18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pildner von Steinburg S, et al. What is the “normal” fetal heart rate? PeerJ. 2013;1:e82. doi: 10.7717/peerj.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martherus RS, et al. Electrical signals affect the cardiomyocyte transcriptome independently of contraction. Physiol Genomics. 2010;42a(4):283–289. doi: 10.1152/physiolgenomics.00182.2009. [DOI] [PubMed] [Google Scholar]
- 112.Lieu DK, et al. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol. 2013;6(1):191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Godier-Furnemont AF, et al. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 2015;60:82–91. doi: 10.1016/j.biomaterials.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eng G, et al. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun. 2016;7:10312. doi: 10.1038/ncomms10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davis RP, et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation. 2012;125(25):3079–3091. doi: 10.1161/CIRCULATIONAHA.111.066092. [DOI] [PubMed] [Google Scholar]
- 116.Yazawa M, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lan F, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12(1):101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang G, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20(6):616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ebert AD, et al. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci Transl Med. 2014;6(255):255ra130. doi: 10.1126/scitranslmed.3009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Drawnel FM, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9(3):810–821. doi: 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 121.Sharma A, et al. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ Res. 2014;115(6):556–566. doi: 10.1161/CIRCRESAHA.115.303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mercola M, et al. Induced pluripotent stem cells in cardiovascular drug discovery. Circ Res. 2013;112(3):534–548. doi: 10.1161/CIRCRESAHA.111.250266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liang P, et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127(16):1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eschenhagen T, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13(1):1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- 125.Drew NK, et al. Metrics for assessing cytoskeletal orientational correlations and consistency. PLoS Comput Biol. 2015;11(4):e1004190. doi: 10.1371/journal.pcbi.1004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pasqualini FS, et al. Structural phenotyping of stem cell-derived cardiomyocytes. Stem Cell Reports. 2015;4(3):340–347. doi: 10.1016/j.stemcr.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Herron TJ, et al. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res. 2012;110(4):609–623. doi: 10.1161/CIRCRESAHA.111.247494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shinnawi R, et al. Monitoring Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes with Genetically Encoded Calcium and Voltage Fluorescent Reporters. Stem Cell Reports. 2015;5(4):582–596. doi: 10.1016/j.stemcr.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodriguez ML, et al. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J Biomech Eng. 2014;136(5):051005. doi: 10.1115/1.4027145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alford PW, et al. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials. 2010;31(13):3613–3621. doi: 10.1016/j.biomaterials.2010.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ailawadi S, et al. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2015;1852(1):1–11. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fu JD, et al. Distinct roles of microRNA-1 and −499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2011;6(11):e27417. doi: 10.1371/journal.pone.0027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klattenhoff CA, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152(3):570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wamstad JA, et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell. 2012;151(1):206–220. doi: 10.1016/j.cell.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim C, et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494(7435):105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]