SUMMARY
Granulocyte transfusions have a long history of being used in patients with neutropenia or neutrophil dysfunction to prevent and treat invasive fungal infections. However, there are limited and conflicting data concerning its clinical effectiveness, considerable variations in current granulocyte transfusion practices, and uncertainties about its benefit as an adjunct to modern antifungal therapy. In this review, we provide an overview on granulocyte transfusions and summarize the evidence on their role in the prevention and treatment of invasive fungal infections.
Keywords: granulocytes, transfusions, neutropenia, fungal infection, leukapheresis
RATIONALE FOR GRANULOCYTE TRANSFUSION IN INVASIVE FUNGAL INFECTION
The association between absolute or qualitative deficiency of circulating granulocytes and propensity for bacterial and invasive fungal infections (IFI) has been known for 50 years (Bodey et al, 1966). As prompt and effective antibiotic therapy has continued to improve the outcome of bacterial infections, fungal infections have become an increasingly important cause of morbidity and mortality in high risk patients, such as those with leukaemia or undergoing haematopoietic stem cell transplant (HSCT) (Sahin et al, 2016). Although late post-transplant fungal infections may occur in non-neutropenic patients on immunosuppressive therapy (Grow et al, 2002), prolonged neutropenia is a major risk factor for IFI.
Mortality rates secondary to invasive Candida albicans have decreased in HSCT recipients because of the widespread use of fluconazole prophylaxis (Marr et al, 2000). However, the spectrum of infections in neutropenic patients has shifted, with multidrug-resistant bacteria and mould infections, such as Aspergillus, Fusarium, Scedosporium and Mucorales, emerging as major determinants of morbidity and mortality (Marr et al, 2002). Without correction of neutropenia, either by recovery of endogenous or graft-related granulocytopoiesis, antimicrobials alone may not resolve infections against which neutrophils form the primary line of defence. Therefore, in cases of delayed neutrophil reconstitution or drug-resistant fungal infection, granulocyte transfusion (GTX) remains a logically attractive solution.
HISTORY OF GRANULOCYTE TRANSFUSION
The introduction of the plastic bag for blood collection (Walter & Murphy, 1952) and refrigerated centrifuge in 1953 allowed for safe and easy preparation of multiple blood components from a single unit of whole blood. This afforded the opportunity to address specific cytopenias by transfusing only the cells of interest. The theoretical potential for leucocyte transfusion was established by early animal studies. Brecher et al (1953) showed that granulocytes transfused to neutropenic dogs migrated to areas of infection. Later, animal models of bacterial and fungal infection were supportive of the efficacy of transfused donor granulocytes (Dale et al, 1976; Ruthe et al, 1978).
Buffy coats prepared from whole blood as a source of granulocytes were limited by the low numbers of neutrophils obtainable from a single healthy donor, about 5 ×108 to 1 × 109 (Reiss et al, 1982). Thus granulocytes for transfusion were collected from donors with chronic myeloid leukaemia (CML). This practice, while understandably controversial to modern readers due to the transfusion of malignant cells, was considered a viable option at the time. Neutrophil yields ranged from 2.6 × 109 to 1.8 × 1011 (Freireich et al, 1964); transfused cells disappeared from the recipient’s circulation with a half-time of one day. Patients were noted to have neutrophil increments (median 1.0 × 109/l) and clinical responses to doses exceeding 1 × 1010 granulocytes.
The development of the automated blood cell separator enabled increased collection efficiency via apheresis, the process of separation of blood components in an extracorporeal circuit. Apheresis allowed selective collection of a larger dose of granulocytes than would be retrieved from a unit of whole blood, with the added advantage of minimal donor red cell loss (Graw et al, 1971), eventually obviating the need for donors with CML. Cell kinetics studies showed that transfused granulocytes were of normal appearance and viability (De Fliedner et al, 1974) and migrated to sites of inflammation (Dutcher et al, 1981). Filtration leukapheresis, due to reduced intravascular recovery and abnormal kinetics of collected granulocytes, as well as adverse reactions in both donors and recipients, was supplanted by centrifugation apheresis (McCullough, 1979; Eckermann and Strauss, 1984).
Granulocytapheresis was further enhanced by the intravenous administration of macromolecule starch solutions (Bearden et al, 1977; Iacone et al, 1981) to the donor before the procedure, which sediments red cells, separating them from the granulocyte layer and hence increases the granulocyte yield (Mishler et al, 1983). Corticosteroids were administered to donors to increase the circulating white cell count, by both increasing marrow release of granulocytes and decreasing efflux from peripheral blood. However, steroid-stimulated donors yielded granulocyte doses of 2–3 × 1010, about half of what is produced daily by normal bone marrow. Functional tests of granulocytes from both steroid-stimulated and unstimulated donors (Glasser & Huestis, 1979) showed statistically significant decreases in chemotaxis, candidacidal activity, and phagocytosis at 24 h of ex vivo storage. Randomized controlled trials (RCTs) of both prophylactic and therapeutic GTX were conducted, but the reported benefit ranged from clear to marginal to none in some studies, and some authors reported significant adverse effects. For these reasons, GTX therapy fell out of favour.
In the early 1990s, the availability of recombinant granulocyte colony-stimulating factor (G-CSF), allowing even higher white cell counts to be achieved by marrow stimulation of healthy donors (Inaba et al, 1992), led to renewed interest in GTX. A single injection of G-CSF alone or combined with an oral dose of steroids enabled the collection of up to 6 – 8 × 1010 granulocytes (Stroncek et al, 2001). Co-administration with systemic steroids enabled reduction in G-CSF dose, ameliorating associated side effects, including bone pain, headache and fever (Heuft et al, 2002). Donor granulocyte count elevations were sustained longer when G-CSF was administered subcutaneously rather than intravenously (Stroncek et al, 2002). The therapeutic efficacy of G-CSF given directly to patients for prevention or as adjunctive treatment of severe refractory infections is not well-defined. Therefore, transfusion of granulocyte concentrates still holds clinical and research interest.
ANTIFUNGAL THERAPY IN IFI: EVOLUTION AND CURRENT PRACTICE
The management of IFI has changed over the past 15 years due to the availability of two new classes of drugs (echinocandins and mould-active azoles), the increased use of computed tomography and the development of new biomarkers (galactomannan antigen in serum and bronchoalveolar lavage for aspergillosis and serum β-D-glucan for several fungal infections) to aid with diagnosis (Lehrnbecher et al, 2016). Systemic antifungal agents have three applications in clinical practice: prophylaxis (i.e., administration of antifungal agents to prevent infection), treatment of a documented specific fungal infection, and treatment of a suspected fungal infection (triggered by a particular constellation of signs and symptoms, but in the absence of definite proof of fungal infection).
Candida species occur as part of normal human flora of the gastrointestinal tract and, often, the skin. Consequently, invasive candidiasis and candidaemia occur due to endogenous organisms in the setting of neutropenia and/or mucosal damage caused by chemotherapy, radiation and/or instrumentation in critically ill patients in concert with disruption of bacterial flora by broad-spectrum antibiotics. Moulds, on the other hand, are not part of the normal flora of the human respiratory tract; mould infections usually follow systemic corticosteroid use or periods of prolonged neutropenia. Fluconazole (Goodman et al, 1992) or micafungin (van Burik et al, 2004) are recommended for targeted prophylaxis against invasive candidiasis. Meta-analyses of prophylaxis trials conclude that second-generation azoles [posaconazole (Ullmann et al, 2007; Cornely et al, 2007a) and voriconazole (Wingard et al, 2010)] are more effective than fluconazole to prevent invasive aspergillosis (IA) (Bow et al, 2015; Ping et al, 2013; Ethier et al, 2012).
Professional societies, such as the Infectious Diseases Society of America (IDSA), the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) and the European Confederation of Medical Mycology (ECCM), issue guidelines that provide evidence-based recommendations for the treatment of established infections with the most common fungal agents, i.e. candidiasis (Pappas et al, 2016) and aspergillosis (Patterson et al, 2016). Echinocandins, azoles and lipid formulations of amphotericin B are considered acceptable treatment for of invasive candidiasis in neutropenic patients. Randomized trials have provided evidence to optimize antifungal therapy for aspergillosis. Specifically, voriconazole is superior to amphotericin B (Herbrecht et al, 2002); 10 mg/kg/day of liposomal amphotericin B is not superior to 3 mg/kg/day (Cornely et al, 2007b); the combination of voriconazole with an echinocandin may be superior to voriconazole in a subgroup of higher-risk patients (Marr et al, 2015); and isavuconazole is non-inferior to voriconazole and has less hepatotoxicity (Maertens et al, 2016). The role of therapeutic drug monitoring and pharmacogenetics for voriconazole and posaconazole remain controversial (Lee et al, 2013; Hamada et al, 2013; Chau et al, 2014; Ashbee et al, 2014; Laverdiere et al, 2014; Moriyama et al, 2015), but most experts agree it should be considered in high-risk patients.
Due to low overall incidence, the evidence for management of established infection due to uncommon fungal organisms, such as mucormycosis (Cornely et al, 2014), dematiaceous fungi (Chowdary et al, 2014), rare yeasts (Arendrup et al, 2014) and hyalohyphomycoses (Tortorano et al, 2014), is sparse and limited to case series and expert opinion. Invasive mucormycosis, the third most frequent cause of IFI, is still associated with high mortality. Surgical resection is life saving in many cases. There are no biomarkers for the disease, and only liposomal amphotericin B and posaconazole are effective (Ruping et al, 2010). Recovery of neutrophil counts is shown to improve outcomes (Pagano et al, 2004); thus, GTX may play an important therapeutic role. Fusarium is a refractory mould in which granulocytes might be the primary driver of a clinical response (Kadri et al, 2015).
The approach to suspected IFI is evolving. The decades-old practice of adding amphotericin B after 4–7 days of persistent fever during neutropenia (Pizzo et al, 1982), which resulted in many patients without fungal infection being exposed to the toxic agent, is being superseded by approaches that attempt to identify those patients who truly have IFI by intensive use of computed tomography, biomarker tests and PCR (Cordonnier et al, 2009; Maertens et al, 2011; Maschmeyer et al, 2013; Morrissey et al, 2013).
The most common clinical presentation of a suspected or “possible” invasive fungal infection is of dense, well-circumscribed pulmonary nodules or infiltrates in patients with prolonged neutropenia in the absence of microbiological confirmation (De Pauw et al, 2008). Evidence-based recommendations that can guide the practitioner in this setting are currently lacking. However, prompt empiric therapy upon suspicion of IFI seems prudent, given that withholding treatment when infection truly exists can be catastrophic. The available evidence suggests that the most common breakthrough fungal infection is Aspergillus in those on fluconazole prophylaxis, and non-Aspergillus mould among those on micafungin prophylaxis (van Burik et al, 2004). Some case series have suggested increased frequency of mucormycosis in patients on voriconazole prophylaxis (Imhof et al, 2004; Chamilos et al, 2005). These data serve to guide empiric therapy choices especially in the absence of microbiological or biomarker data. A fundamental concept in the management of IFIs is that host factors, such as neutropenia, that render the patient susceptible to the infection should be corrected whenever possible, as antifungal agents alone have a significant failure rate (Maertens et al, 2016).
IMPACT OF NEUTROPHILS ON FUNGAL PATHOGENESIS
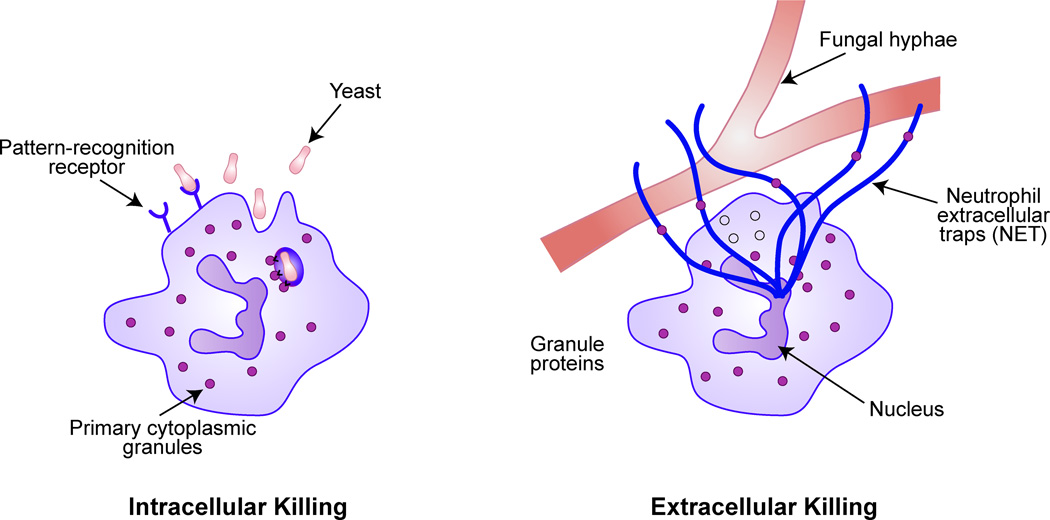
Neutrophils recognize and respond to fungal pathogens using pattern recognition receptors, including toll-like receptors and dectin-1 (Kennedy et al, 2007). After phagocytosis of the pathogen, the contents of the cytoplasmic granules are released into the vacuole and expressed onto the surface of the organism (Cohn & Hirsch, 1960). The azurophil (primary) granules contain myeloperoxidase (MPO) and three predominant neutral proteases; cathepsin G, elastase and proteinase 3. NADPH oxidase pumps electrons into the phagocytic vacuole, thereby inducing a charge across the membrane. The movement of compensating potassium ions produces conditions in the vacuole conducive to microbial killing and digestion by the enzymes released from the cytoplasmic granules (Reeves et al, 2002).
When fungal elements are too large to be phagocytosed, neutrophils release granule proteins and chromatin that together form extracellular fibres, dubbed neutrophil extracellular traps or NETs (see Fig. 1) that degrade conidia and hyphae (McCormick et al, 2010). Neutrophil-induced hyphal damage to A. fumigatus and resistant filamentous fungi, such as Scedosporium, is enhanced synergistically in the presence of newer triazole agents (voriconazole and posaconazole) (Walsh et al, 2002; Gil-Lamaignere et al, 2002).
Figure 1.
Neutrophil-mediated intracellular and extracellular killing of fungal pathogens. During phagocytosis, neutrophil azurophilic granules fuse with the phagosome and release contents (cathepsin G, elastase, proteinase 3, and myeloperoxidase) into the phagocytic vacuole. For larger structures like fungal hyphae, the neutrophil releases web-like extracellular traps (NETs) composed of decondensed chromatin in complex with antimicrobial proteins that trap and neutralize pathogens. Adapted with permission from Macmillan Publishers Ltd: (Wheeler, M.L. & Underhill, D. M. Time to cast a larger net. Nature Immunology, 11, 1000–1001), copyright (2014).
DONOR SELECTION AND COMPONENT PREPARATION
Identifying a suitable donor may take some time depending on the size of donor pool, and the requirements of the patient. Using community donors is more readily feasible than relying on related donors only (Hübel et al, 2002). Because granulocyte concentrates contain between 20 and 50 ml of red blood cells (RBCs) per product, ABO compatibility is ideal to prevent haemolytic transfusion reactions. Human leucocyte antigen (HLA) compatible donors may be indicated if the patient has a history of alloimmunization. For patients who are awaiting HSCT, prospectively avoiding transplant donor antigens is crucial to avoid the formation of donor-specific antibodies targeting the graft (O’ Donghaile et al, 2012). Cytomegalovirus (CMV) seronegative donors are generally recommended for seronegative recipients.
It usually takes at least a day to prepare a donor for granulocytapheresis, with subcutaneous G-CSF injection 12–18 h prior, and/or oral dexamethasone 8–12 h prior to collection. The apheresis procedure takes about 4 h. Alternatively, granulocytes derived from whole blood buffy coats may be used; a dose of ten buffy coats for adults and 10–20 ml/kg for children weighing less than 50 kg is recommended. A pooled granulocyte component is also available in the UK (Bashir et al, 2008); 10 buffy coats are pooled into a final volume of 200–250 ml, each pack containing approximately 1×1010 granulocytes. In the blood bank, a sample from the granulocyte donor undergoes a full RBC cross-match with the recipient. Red cell depletion by sedimentation may be required if there is major ABO incompatibility between donor and recipient (Bryant et al, 2010), adding a further 4 h to the process. Finally, the granulocyte concentrate must be irradiated before release to the patient to prevent graft-versus-host disease.
CLINICAL EFFICACY
We performed a structured narrative review of the existing literature on GTX to prevent or treat IFI. The details of the literature search are described in the supplement. Previous reviews have described the limited data on the use of GTX for the prevention or treatment of infections in general (Price, 2007; Strauss, 2012). The published literature largely comprises case reports and uncontrolled case series, with heterogeneous patient populations, intervention parameters and outcome measures. Obstacles to conducting RCTs include cost, logistical factors, and low enrolment partly due to the unwillingness of patients and physicians to potentially forfeit what they believe to be a life-saving intervention (Seidel et al, 2008; Price et al, 2015). Patients with IFI represent a fraction of cases in most studies; extracting a precise estimate of benefit from GTX in overall or species-specific fungal subgroups in mixed study populations remains challenging. Finally, advances in antifungal agents and supportive care may have diminished the role of GTX, making it difficult to show its benefit in recent randomized controlled studies. Notwithstanding, we provide a categorical summary of the existing evidence for GTX in IFIs from individual case reports, case series, matched cohort studies and clinical trials and, where possible, report on adults and paediatric populations separately.
Case Reports
We summarized the outcomes of 97 patients with fungal infection treated with GTX from individual case reports and small series (Table I). The most common underlying illnesses were acute leukaemia (45%), chronic granulomatous disease (26%) and aplastic anaemia (12%). Granulocyte dose and transfusion course varied considerably and were often not specified. In a third of the cases, patients also underwent surgical debridement or excision of locoregional disease. Overall, 77% reported clinical, radiological or microbiological improvement with GTX therapy; 2% reported stable disease. Adverse events, including febrile and pulmonary reactions, cytomegalovirus (CMV) transmission and HLA alloimmunization were described in 16%. These findings must be interpreted with caution, given the known propensity for positive publication bias.
Table I.
Summary of case reports and small series of therapeutic granulocyte transfusions in invasive fungal infections.
| Number of Cases |
Disseminated Infection |
Clinical Response |
Radiological Response |
Microbiologic al Response |
Overall response |
|
|---|---|---|---|---|---|---|
|
Pre-G-CSF Era (1973 – 1992) |
16 | 5 (31%) | 14 (87%) | 4 (25%) | 3 (19%) | 14/16 (87%) |
| Aspergillus | 11 | 2 (18%) | 9 (82%) | 3 (27%) | 2 (18 %) | 9/11 (82%) |
| Fusarium | 4 | 3 (75%) | 4 (100%) | 1 (25%) | 1 (25%) | 4/4 (100%) |
| Candida | 1 | 0 (0%) | 1 (100%) | NS | NS | 1/1 (100%) |
|
G-CSF Era (1993 – 2015) |
81 | 33 (41%) | 54 (67%) | 16 (20%) | 10 (12%) | 61/81 (75%) |
| Aspergillus | 46 | 10 (22%) | 27 (59%) | 11 (24%) | 3 (7%) | 32/46 (70%) |
| Mucorales | 12 | 3 (25%) | 8 (67%) | 1 (8%) | 1 (8%) | 10/12 (83%) |
| Fusarium | 10 | 9 (90%) | 9 (90%) | 1 (10%) | 4 (40%) | 9/10 (90%) |
| Candida | 9 | 9 (100%) | 8 (88%) | 1 (11%) | 2 (22%) | 8/9 (88%) |
| Other fungia | 4 | 2 (50%) | 2 (50%) | 2 (50%) | 0 (0%) | 2/4 (50%) |
| Total | 97 | 38 (39%) | 68 (70%) | 20 (20%) | 13 (13%) | 75/97 (77%) |
Invasive fungal infections were classified according to the Revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group definitions (De Pauw et al, 2008).
Outcomes of therapeutic granulocyte transfusion, where specified, were classified as: (1) clinical response (defervescence, reduction or disappearance of local symptoms of infection, or decrease in size or appearance of visible lesions), (2) radiological response (decreased size or disappearance of lesions on imaging) or (3) microbiological response (clearance of previously positive cultures or antigen tests).
Trichosporon, and Scedosporium spp.
G-CSF, granulocyte colony-stimulating factor; NS, not specified.
Case Series of IFIs in the G-CSF Era
Seven case series specifically reported on the treatment or prevention of fungal infections using G-CSF mobilized granulocytes (Table II). Hester et al (1995) and Dignani et al (1997) described a group of 15 adult patients with haematological malignancies and refractory fungal infections. Eleven patients were determined to have favourable responses (9 improved, 2 stable) and 4 had progression of infection; 8 patients remained free of infection 3 weeks after therapy.
Table II.
Case series of specific IFIs treated with G-CSF-stimulated granulocyte transfusions.
| Study | N (controls) |
Underlying Condition |
Study Design | Infections | Average GTX Dose |
Intervention | Outcomes | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Hester et al (1995) | 15 | HM | Uncontrolled | 11 moulds, 4 yeast |
4.1 × 1010 | Therapeutic | 73% stable or improved, 20% 3 month survival |
Pulmonary reactions (30%) |
| Hermann et al (2001) | 4 | HSCT, HM | Uncontrolled | IA, Mucor | 4.6 × 1010 | Pre-emptivea | 75% regression of fungal lesions, 100% survival > 1 year |
No serious side effects |
| Kerr et al (2003) | 9 (18) | HSCT, HM/AA |
Controls matched for conditioning regimen |
IA | 6.4 × 1010 | Pre-emptiveb | 57% showed radiological improvement, GTX group less likely to become febrile (p < 0.05), fewer days of fever (p < 0.05), no survival benefit |
Fever, bronchospasm (11%) |
| Safdar et al (2004) | 29 (462) | HSCT, HM | Controls, unmatched |
Candidaemia | 5.6 × 1010 | Therapeutic | Fewer CR in GTX group (p < 0.001), no difference in attributable mortality (p = 0.5) |
NS |
| Yenicesu et al (2011) | 5 | HSCT, HM/AA |
Uncontrolled | IPA, Invasive Candidiasis |
3.49 × 1010 | Therapeutic | 60% full clinical recovery | |
| Raad et al (2013) | 53 (75) | HM | Controls, unmatched |
Proven and probable IA |
5.5 × 1010 | Therapeutic | IPA less likely to respond to antifungal therapy (p = 0.03), more likely to die of IA (p = 0.009) in GTX group |
Pulmonary reactions (53%) |
| Kadri et al (2015) | 11 | HSCT, HM/AA |
Uncontrolled | Invasive Fusariosis |
6.84 × 1010 | Therapeutic | 91% 30-day survival, 73% 90-day survival |
Pulmonary reactions (18%), HLA alloimmunization (9%) |
AA, aplastic anaemia; CR, complete response; G-CSF, granulocyte colony-stimulating factor; GTX, granulocyte transfusion; HLA, human leucocyte antigen; HM, haematological malignancy; HSCT, haematopoietic stem cell transplant; IA, invasive aspergillosis; IFI, invasive fungal infection; IPA, invasive pulmonary aspergillosis; NS, not specified.
Patients had existing fungal infection at the time of HSCT.
Patients were considered at high risk due to history of IA or because of prolonged neutropenia. Many patients had evidence of disease at the time of the study.
Hermann et al (2001) reported 4 older patients (median age 62 years) with leukaemia who had fungal infections at the time of HSCT. A combination of reduced intensity conditioning, GTX and G-CSF was employed to reduce the period of neutropenia. Three of four patients had documented regression of fungal lesions, and all four patients survived without relapse of leukaemia over a year post-transplant. Kerr et al (2003) reported favourable clinical outcomes of GTX in 9 HSCT patients at high risk for IFI (due to existing or previous IA, or prolonged neutropenia) compared to a control group, although there was no survival difference. Four of seven patients with radiological abnormalities prior to transplant showed improvement on imaging. Yenicesu et al (2011) reported full clinical and radiological recovery in 3 of 5 patients with active IFI who had undergone HSCT with GTX support.
Kadri et al (2015) published a series of 11 neutropenic patients with invasive Fusarium infections. Ten of 11 (91%) patients had objective clinical, radiological or microbiological responses within the first few days of GTX, and survived 30 days post-GTX. The authors compared their results to those of 23 prior published cases of Fusarium infection treated with GTX, with clinical response in only 30%. Higher clinical response rates in this recent series might reflect wider use of voriconazole, G-CSF and dexamethasone stimulated donors, improvements in primary disease management and supportive care. Locally invasive sinus infections, which may carry a lower risk of mortality than disseminated fungaemia, were more common in the case series. Notably, there was a five-fold greater use of surgical debridement (100% vs. 17%) in patients with invasive Fusarium sinusitis compared to cases in the systematic review.
Safdar et al (2004) performed a single institution retrospective analysis of 491 patients with candidaemia, 29 of whom received GTX. The criteria for treatment with GTX included a positive blood culture for Candida species for > 72 h after appropriate systemic antifungal therapy was initiated, or when neutrophil count recovery was expected to be delayed for > 3–4 weeks after diagnosis of infection, or both. There was no difference in overall attributable mortality (48% in the transfused group, 45% in the control group, p = 0.5) but because various risk factors for higher mortality were more common in the transfused group, the authors interpreted their results to suggest that GTX had been beneficial.
Raad et al (2013) performed a single institution retrospective review of 128 patients with haematological malignancies and prolonged neutropenia with proven or probable IA. Fifty-three patients received GTX and 75 did not. Multivariate logistic regression analyses showed no significant association between GTX and response. Patients with invasive pulmonary aspergillosis (IPA) who received GTX were less likely to respond to antifungal therapy (p = 0.03), and more likely to die of IA (p = 0.009) when compared with the non-GTX group. In retrospective comparative effectiveness studies, however, it is difficult to account for confounding by indication for GTX (which may have been administered to sicker patients) in the absence of randomization or matching of cases (e.g. using propensity scores), and as such outcomes are difficult to interpret.
Case Series and Matched-cohort Studies Collectively Reporting Bacterial and Fungal Infections in Adult Patients
In many case series of infections treated with GTX, patients with bacterial infections had better outcomes than those with IFI (Table III and IV). Grigg et al (1996) reported a series of 8 patients with refractory infections. All three patients with bacterial infection cleared the infection and survived; all five patients with fungal infection, four of whom had Aspergillus pneumonia, died. Rutella et al (2003) administered granulocytes from HLA-matched siblings to 18 patients with haematological malignancies and refractory infections. Responses were seen in 6 of 9 patients with bacterial isolates, all 4 patients with fungaemia, but none of 3 with focal fungal infections. In a prospective cohort study, Mousset et al (2005) reported a 30-day overall response rate of 82%, which included 93% response in bacterial and 78% for fungal infections; infection-related mortality was very low. Infection did not recrudesce in any of the 23 patients in the secondary prophylaxis arm.
Table III.
Case series reporting bacterial and fungal infections in adult patients treated with GTX in the G-CSF era.
| Study | N (fungal /total) |
Underlying Condition |
Average GTX Dose |
Intervention | Outcome (Fungal) | Outcome (Bacterial) | Adverse Events |
|---|---|---|---|---|---|---|---|
| Grigg et al (1996) | 9/11 | BMT, HM, AA |
5·9 × 1010 | Therapeutic (8) Prophylactic (3) |
0% resolved, 67% successful prophylaxis (recent infection) |
100% resolved | No pulmonary reactions |
| Lee et al (2001) | 11/25 | HM | 6.6 × 1010 | Therapeutic | 73% favourable response | 45% favourable response | Pulmonary oedema (8%), hypoxia (4%), SVT (4%) |
| Illerhaus et al (2002) | 10/18 | HM | 2.6 × 1010 3.2 × 1010 |
Therapeutic (18), Prophylactic (16) |
55% IPA responded, no benefit of prophylactic GTX |
78% septicaemia responded |
CMV transmission, alloimmunization |
| Hübel et al (2002) | 57/74 | HM | 4·6–8·1 × 1010 | Therapeutic (paired controls) |
Non-progression in 18% mould, 55% yeast, no difference vs controls |
Fewer progressive bacterial infections in control group (p = 0.04) |
Fever (17.5%), chills (30%), desaturation and pulmonary oedema (1%) |
| Rutella et al (2003) | 7/20 | HM | 1.7 × 1010 | Therapeutic | 57% response, 100% fungaemia, 0% IFI |
54% response rate | Fever, chills, bronchospasm |
| Mousset et al (2005) | 31/44, 20/23 |
HSCT, HM | 4.3 × 1010 | Therapeutic (44), Prophylactic (23) |
78% response at 30 days, 58% survival at 100 days |
92% response at 30 days, 77% survival at 100 days |
NS |
| Safdar et al (2006) | 19/20 | HSCT, HM, | 5.6 × 1010 | Therapeutic | 45% CR or PR, 15% stable | NS | Transient dyspnoea (5%) |
| Ofran et al (2007) | 28/47 | HM, AA | 3.6 × 1010 | Therapeutic | 64% infection-related survival | 53% infection-related survival |
Pulmonary reactions (13%) |
| Quillen et al (2009a) | 18/32 | SAA | 6.8 × 1010 | Therapeutic | 44% survival, 33% CR, 22% PR, 17% stable, 28% progress |
58% overall survival to discharge |
HLA alloimmunization (17%), pulmonary (15%) |
| Al-Tanbal et al (2010) | 16/22 | SAA, HM, CGD |
2.8 × 1010/L | Therapeutic | 75% survival | 68% clinical improvement | TRALI (4.5%) |
| Ang & Linn (2011) | 13/15 | HM | 5.0 × 1010 | Therapeutic | 31% cleared | 63% cleared | NS |
| Kim et al (2011) | 37/128 | HM, AA | 0.96 × 109/kg | Therapeutic | 47% control of proven or probable IFI |
Overall control of infection 53% |
Fever (19%), hypotension (6.5%), respiratory (9%) |
| Safdar et al (2014) | 33/74 | HSCT, HM, other |
5.6 × 1010 | Therapeutic | 45% patients had IFI | 46% overall response | Fever (3%), respiratory (8%) |
| Wang et al (2014) | 31/56 | SAA | 0.92 × 1010 | Therapeutic | 87% 30-day, 58% 90-day, 52% 180-day survival |
92% 30-day, 84% 90-day, 84% 180-day survival |
Fever, chills, allergy, dyspnoea |
AA, aplastic anaemia; BMT, bone marrow transplantation; CGD, chronic granulomatous disease; CMV, cytomegalovirus; G-CSF, granulocyte colony-stimulating factor; GTX, granulocyte transfusion; HM, haematological malignancy; HSCT, haematopoietic stem cell transplant; IFI, invasive fungal infection; IPA, invasive pulmonary aspergillosis; NS, not specified; PR, partial response; SAA, severe aplastic anaemia; TRALI, transfusion associated acute lung injury.
Table IV.
Case series reporting bacterial and fungal infections in paediatric patients treated with GTX in the G-CSF era.
| Study | N (fungal /total) |
Underlying Condition |
Average GTX Dose |
Intervention | Outcome (Fungal) | Outcome (Bacterial) | Adverse Events |
|---|---|---|---|---|---|---|---|
| Grigull et al (2006) | 6/32 | HSCT, HM | 6.35 × 1010 | Therapeutic | 67% survival | 81% survival | Fever (9.6%), respiratory distress (5.3%) |
| Kikuta et al (2006) | 2/13 | HO | 6.4 × 108/kg | Therapeutic | 50% response | 73% response | Hypoxia (8%) |
| Sachs et al (2006) | 7 /27 | HO, AA | 2.0 × 1010 | Therapeutic | 100% response | 93% overall response | Mild fever, chills (7%), no pulmonary reactions |
| Drewniak et al (2008) | 13/16, 3/4 |
HO, HSCT | 2 × 109/kg | Therapeutic (16), Pre-emptivea (4) |
73% response (IA), no progression with pre- emptive GTX |
Overall 68% response | NS |
| Seidel et al (2009) | 31/69 | HO, HSCT | 6 × 109/kg in first 5 days |
Therapeutic | 28-day, 100-day survival probability 0.51 ± 0.12 and 0.40 ± 0.11 |
28-day, 100-day survival probability 0.89 ± 0.06 and 0.65 ± 0.09 |
Fever (14%), chills (3%), airway obstruction (3%) |
| Graham et al (2009) | 8/13 | HO | NS | Therapeutic | 50% survived to discharge |
100% survived to discharge |
Fever (15%) |
| Atay et al (2011) | 18/35 | HO, CGD | 2.7 × 1010 | Therapeutic | 55% clinical response, 78% infection-related survival |
65% clinical response, 88% infection-related survival |
No serious reactions |
| Oztürkmen et al (2013) | 4/10 | HM | 2.9 × 1010 0.6 × 109/kg |
Therapeutic | 50% response | 80% response | Oliguria and/or mild respiratory distress (23%) |
| Diaz et al (2014) | 5/18 | HM, CGD | 6.7 × 1010 | Therapeutic (13), Pre-emptivea (5) |
80% complete or partial response |
100% in non-fungal infection |
Respiratory (46%) |
| Nikolajeva et al (2015) | 14/28 | HM, AA, CGD |
3.6 × 1010 | Therapeutic | 50% radiological improvement, 43% stable disease, 79% 100-day survival |
50% 100-day survival | Fever (18%), mild respiratory symptoms (11%) |
GTX was used pre-emptively to prevent exacerbation of existing infection during HSCT.
AA, aplastic anaemia; CGD, chronic granulomatous disease; G-CSF, granulocyte colony-stimulating factor; GTX, granulocyte transfusion; HM, haematological malignancy; HO, haematology/oncology; HSCT, haematopoietic stem cell transplant; IA, invasive aspergillosis; NS, not specified.
Conversely, in a series of 25 patients with malignancies and severe refractory neutropenia-related infections, Lee et al (2001) reported that patients with fungal or gram-negative organisms isolated showed a more favourable response to GTX than those infected with gram-positive organisms (73%, 60%, and 31% respectively). In a single-centre retrospective study, Kim et al (2011) similarly reported better outcomes in fungal infections (60%) and gram-negative bacterial infections than in gram-positive infections (30%) in 128 patients with haematological disease. For gram-positive infections, antibiotics are usually highly efficacious and extensive drug resistance limiting antibiotic options is less common compared to gram-negative counterparts, such that appropriate initial therapy, and in turn, outcomes are likely to be better even in neutropenia, making GTX relatively more relevant in IFI and gram-negative compared to gram-positive bacterial infections.
Illerhaus et al (2002) reviewed 18 patients who received GTX to treat severe infection with an overall response rate of 67%, including a 55% response rate in patients with Aspergillus pneumonia. GTX was also administered to 8 high-risk patients with a history of serious infection, all of whom had a stable clinical course without severe infections until neutrophil recovery.
Safdar et al (2006) retrospectively evaluated 20 recipients of high-dose donor GTX (≈5.5 × 1010 neutrophils per transfusion) who had received concurrent rIFN-γ1b. Four weeks after therapy started, 9 patients (45%) had complete or partial resolution of infection; and, in another 3 patients (15%), progression of infection was halted.
Ofran et al (2007) reported a single centre retrospective analysis of 47 neutropenic patients treated with GTX for life-threatening infections. Patients with fungal infections (n=28) received more GTX than those with bacterial infections (median 8 vs. 4, p < 0.001), and 18 (64%) of GTX recipients with fungal infections survived. This study found no association between fungal infection and infection-related survival among recipients of GTX; the authors acknowledge the sample was probably underpowered to show this effect.
In a series of patients with severe aplastic anaemia treated with GTX (Quillen et al, 2009a), of 18 patients with IFI, 44% survived to hospital discharge, compared to 58% overall. Al Tanbal et al (2010) described 22 patients receiving at least three continuous days of GTX, most of whom had disseminated fungal infection (73%). Fifteen (68·2%) patients showed clinical improvement. Safdar et al (2014) reported 74 patients, 45 of whom had IFI with a 46% overall response with use of GTX. Wang et al (2014) treated 56 patients with SAA and severe infections with GTX combined with G-CSF. Among 31 patients who had IFI, survival at 30 days, 90 days and 180 days was 87%, 58% and 52% respectively.
Although still susceptible to the effect of unmeasured confounders, matching of cases in non-randomized studies offers a fairer assessment of clinical effectiveness of an intervention compared to case series. Hübel et al (2002) prospectively examined the effect of GTX therapy on survival and microbial response in 74 patients undergoing marrow transplantation with active infection compared to 74 matched concurrent or historic controls receiving antibiotics alone. The number of fatal or progressive fungal infections and survival was comparable in both groups.
Case Series Collectively Reporting Bacterial and Fungal Infections in Paediatric Patients
A number of series of paediatric patients in diverse countries have emerged in recent years (Table IV). In a retrospective review of 32 children transfused for proven or suspected infection, Grigull et al (2006) reported 73% survival for bacterial infection and 57% for fungal infection.
Kikuta et al (2006) conducted a pilot study of GTX collected from G-CSF-stimulated blood relatives without apheresis. Only 2 of 13 patients had fungal infections (one with disseminated C. albicans, who died on day 3, and one with oral A. flavus, who survived to day 30). Eight of 11 children with bacterial infections survived to day 30.
Sachs et al (2006) conducted an open, single-centre, prospective Phase II clinical trial to assess the feasibility, safety and efficacy of early-onset G-CSF mobilized GTX in neutropenic children with severe infections. Overall, 25 of 27 (93%) were able to clear the infection being treated with GTX. All six patients with invasive aspergillosis showed clinical and radiological improvement, one patient with disseminated C. krusei cleared blood cultures. This study boasts a remarkable response rate, but this may have been due to the lower proportion (7/27) of cases with fungal infection, and/or as claimed by the authors, attributed to the early initiation of GTX, i.e., after a median infection period of 6 days, compared with 8 days (Peters et al, 1999) and 12 days (Hester et al, 1995; Cesaro et al, 2003) in other studies.
Drewniak et al (2008) reported the outcomes of 16 severely ill children treated with GTX. Eight of 11 patients (73%) with proven Aspergillus infection showed clinical recovery and negative galactomannan levels within 10 days of starting GTX. Four additional children received pre-emptive GTX during HSCT due to chronic infections. All four survived transplantation without evidence of disseminated infection; three of these had chronic mould infections.
Seidel et al (2009) conducted a prospective study of 49 children and 10 young adults suffering from bacterial (n=55) and/or fungal (n=31) infections during neutropenia. The first 30 patients were reported in a prior publication (Peters et al, 1999). The 28-day and 100-day survival probability for patients with fungal infections was 0.51 ± 0.12 and 0.40 ± 0.11 respectively, compared to 0.89 ± 0.06 and 0.65 ± 0.09 for bacterial infections (p = 0.039).
In a retrospective analysis, Graham et al (2009) reported the outcomes of 13 paediatric oncology patients with proven or suspected serious infection. Eight of the 13 patients had fungal infections, four of who died prior to discharge; however the dose of granulocytes per transfusion was not specified. Atay et al (2011) reported 35 paediatric patients with high-risk febrile neutropenia or defective granulocyte functions who received GTX for 3 consecutive days during refractory infections. Ten of 18 (56%) patients with fungal infections responded favourably. Oztürkmen et al (2013) retrospectively reported 10 children with haematological disorders who developed 13 episodes of febrile neutropenia with or without microbiologically documented infection treated with GTX. During 7 of 13 of episodes (53.8%), patients received G-CSF as well as GTX. The overall clinical response and infection-related mortality rates were 69% and 31%, respectively. Two of three children with IFI responded, and one patient with candidaemia did not.
Diaz et al (2014) retrospectively reviewed 18 children with granulocyte dysfunction or severe neutropenia who received GTX. Four of five (80%) cases that received GTX for IFI demonstrated response and one case of invasive fusariosis progressed. Nikolajeva et al (2015) performed a retrospective analysis on 28 consecutive paediatric HSCT recipients treated with GTX. Seven of 14 patients with IFI showed radiological improvement, with 79% 100-day survival.
Randomized Controlled Trials of Prophylactic Granulocyte Transfusion
We identified 8 prospective controlled trials of prophylactic GTX, in which at least one patient in either the control group or the GTX group developed a fungal infection (Table V). Two of these studies (Clift et al, 1978; Gomez-Villagran et al, 1984) concluded that prophylactic GTX was protective; there were no breakthrough fungal infections in the prophylactic group. The other six (Schiffer et al, 1979; Winston et al, 1980; Winston et al, 1981; Strauss et al, 1981; Buckner et al, 1983; Petersen et al, 1986) reported little to no benefit, but an increased risk of complications, such as CMV infection and pulmonary complications. Notably, all of these studies employed unstimulated, low-dose granulocyte transfusions; none of the studies included patients with neutrophil dysfunction. No RCTs of prophylactic GTX have been conducted since the start of the G-CSF era.
Table V.
Randomized controlled trials of prophylactic granulocyte transfusion in non-infected patients.
| Study | N | Indication for GTX |
Stimulation average GTX Dose |
GTX Course | Overall Outcomes | Fungal Outcomes | Adverse Events |
|---|---|---|---|---|---|---|---|
| Clift et al (1978)a | 69 | BMT (HM/AA), ANC < 0.2 × 109/l |
US, 2.22 × 1010 (FL), 1.57 × 1010 (CFC) |
Daily, mean 12.4 (6–25) |
Fewer infections (2/29) in GTX group vs control group (17/40) 21-days post-HSCT |
Fewer deaths from fungal infection in GTX group (0/29) vs controls (2/40) |
NS |
| Schiffer et al (1979)a | 19 | AML ANC < 0.5 × 109/l |
D, 1.15 × 1010 | Alternate days, mean 11 (3- 19) |
No severe infection in GTX group, 3/9 in control group |
3/9 fungal infections in control group |
Fevers, pulmonary reactions alloimmunization |
| Winston et al (1980)a | 38 | BMT (HM/AA), ANC < 0.5 × 109/l |
US, 1.2 × 1010 | Daily, mean 23.4 (13–34) |
No significant difference | 1/19 fungal pneumonia each in GTX and control group |
NS |
| Strauss et al (1981) | 102 | AML ANC < 0.5 × 109/l |
US, 3.4.x 109/m2 | Daily, mean 18.5 (3–28) |
No significant difference | 5/54 IFI (GTX) vs 3/48 (controls) |
Transfusion reactions (72%), pulmonary infiltrates (57%) |
| Winston et al (1981)a | 46 | AML ANC < 0.5 × 109/l |
US, 0.56 × 1010 | Daily, median 24 (7–28) |
No significant difference (p = 0.48) |
1/21 IFI (controls) | 68% had reactions, CMV, pneumonitis |
| Buckner et al (1983)b | 182 | BMT (HM/AA), ANC < 0.2 × 109/l |
NS | Daily, median 13 (2–31) |
No significant difference in infection or mortality at 100 days |
3 IFI (GTX) vs 8 (controls) | Severe pulmonary reaction, CMV, pneumonitis |
| Gomez-Villagran et al (1984) | 35 | AML ANC < 0.5 × 109/l |
D, 1.24 × 1010 | Daily, mean 6.16 (5–11) |
Fewer life-threatening infections (p < 0.01), fewer infectious deaths (p < 0.05) in GTX group vs controls |
No fungal infections in GTX group, 2 oral candidiasis in control group |
Pulmonary (2.3%), febrile (57.1%) and allergic (1.5%) reactions, passive haemolysis |
| Petersen et al (1986)a | 112 | BMT (HM), ANC < 0.2 × 109/l |
NS | Daily, median 12 (6–27) |
No significant difference in 100- day mortality post-HSCT, death from infection |
1/67 IFI (GTX) vs 5/45 (controls) |
7% transfusion reactions, mostly pulmonary |
AA, aplastic anaemia; AML, acute myeloid leukaemia; ANC, absolute neutrophil count; BMT, bone marrow transplantation; CFC, continuous flow centrifugation; CMV, cytomegalovirus; D, dexamethasone; FL, filtration leukapheresis; GTX, granulocyte transfusion; HM, haematological malignancy; HSCT, haematopoietic stem cell transplant; IFI, invasive fungal infection; NS, not specified; US, unstimulated.
Patients were eligible to receive therapeutic GTX during the study if they developed infection.
Partially randomized: if only one intervention option was available, patients were allocated to that modality.
Data on prophylactic GTX in patients with neutropenia or neutrophil dysfunction has been reviewed extensively in a recent Cochrane database systematic review (Estcourt et al, 2015). The authors concluded that this intervention did not improve overall or infection-related mortality or incidence of localized breakthrough fungal infection; but in a subgroup analysis, there were fewer people with infections in the group receiving prophylactic transfusions at a dose of 1.0 to 4.0 × 1010 granulocytes per day. The suggestion of lower incidence of fungaemia among cases that received prophylactic GTX was supported by low quality evidence.
Randomized Controlled Trials of Therapeutic Granulocyte Transfusion
We identified 5 RCTs of therapeutic GTX including patients with proven or probable fungal infection (Table VI). Three early studies (Higby et al, 1975; Alavi et al, 1977; Vogler & Winton, 1977) reported some benefit of GTX; however, fungal infections represented a minority of cases in these studies. Two recent controlled trials failed to confirm or refute the benefit of therapeutic GTX. Seidel et al (2008) found that the probability of 28-day survival after randomization was > 80% in both groups, and no effect of GTX on survival until day 100 could be detected in patients with fungal, bacterial or unknown infection, but this study was underpowered due to low enrolment. Price et al (2015) conducted a multicentre RCT, the RING (Resolving Infection in Neutropenia with Granulocytes) study. Initially, only patients with chemotherapy-related neutropenia and documented infection were enrolled. Due to poor recruitment, the eligibility criteria were changed to include patients with presumed infection and patients with underlying marrow disease. The primary end-point of this study was a composite of survival plus microbial response at 42 days. For invasive infections, response was defined as resolution or evidence demonstrating clinical improvement; stable infection was considered to be a failure. Invasive fungal infections and fungaemia comprised 36% and 11% respectively. Differences in primary end-point success rates for granulocyte and control arms were not statistically significantly different for any infection type whether analysed by intention-to-treat or per protocol. However, this study was underpowered due to low accrual rates and may have missed a clinically positive effect. The granulocyte dose was also lower than anticipated; the target of ≥4.0 × 1010 granulocytes per transfusion was only achieved in 70% of subjects. In a post-hoc analysis, subjects who received an average dose per transfusion of ≥ 0.6×109 granulocytes/kg tended to have better outcomes than those receiving a lower dose.
Table VI.
Randomized controlled trials of therapeutic GTX for documented or suspected infection.
| Study | N | Underlying condition |
Stimulation average GTX Dose |
GTX Course | Outcome Measures |
Outcome of GTX | Fungal outcomes | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Pre-G-CSF Era | ||||||||
| Higby et al (1975) | 36 | HM, ANC < 0.5 × 109/l |
D, 2–5 × 1010 | Daily, 4 days | 20-day survival | 20-day survival 88% 15/17 (GTX), 5/19, 26% (controls) |
2 candida infections in GTX group, 1 in control groupa |
NS |
| Alavi et al (1977) | 31 | AML/ALL, ANC < 0.25 × 109/l |
HC, 5 × 1010 (3.3 × 1010 in children, 5.9 × 1010 in adults) |
Daily, 7 per episode (3–18) |
21-day survival | No significant difference overall; with persistent marrow failure, 21-day survival 75 % in GTX, 20 % controls,(p = 0.03) |
1 candida infection each in GTX and control groups |
Fever (16%) chills (7%), laryngospasm (1), urticaria (2) |
| Vogler & Winton (1977) | 30 | HM, AA, DIG ANC < 0.3 × 109/l |
US, HC or D, 2.68 ×1010 |
NS | Microbiological or clinical resolution of infection, survival |
Response in 10/17 GTX group vs 2/13 controls (p < 0.05) Median survival 22.5 days (GTX), vs 7.7 days (controls) p < 0.01 |
1 candida infection in control group |
No reactions |
| G-CSF Era | ||||||||
| Seidel et al (2008) | 79 | HM/AA/ST | G-CSF, 6.6 × 108/kg |
> 3 per week, median 3 (1–13) |
28-day survival after randomization |
No statistically significant difference in survival |
Fungal infections in 55 patients |
Pulmonary (4), other reactions (7) |
| Price et al (2015) | 97 | HM/AA, ANC < 0.5 × 109/l |
G-CSF+D, 5.49×1010 |
Median 5 GTX | Microbiological or clinical resolution of infection, survival at 42 days |
No difference in overall success rates for GTX (42%) vs controls (43%) by MITT (p> 0.99) or per protocol (49% and 41%, p=0.64) |
36% IFI, 11% fungaemia No difference in outcomes by infection type |
Mild fever, chills (41%), hypoxia, tachycardia, hypotension, allergic reactions (20%) |
AA, aplastic anaemia; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; ANC, absolute neutrophil count; D, dexamethasone; DIG, drug-induced granulocytopenia; G-CSF, granulocyte colony-stimulating factor; GTX, granulocyte transfusion; HC, hydrocortisone; HM, haematological malignancy; IFI, invasive fungal infection; MITT, modified intent-to-treat analysis; NS, not specified; ST, solid tumour; US, unstimulated.
Organisms only specified for patients < 45 years old.
A recent update of a Cochrane review on therapeutic granulocyte transfusion (Estcourt et al, 2016) concluded that in patients who are neutropenic due to myelosuppressive chemotherapy or HSCT, there is insufficient evidence to determine whether granulocyte transfusions affect all-cause mortality. There were no differences between the granulocyte dose subgroups (< 1 × 1010 per day versus ≥ 1 × 1010 per day); however there may be a reduction in all-cause mortality in participants receiving granulocyte transfusions compared to those that did not in studies published before the year 2000. There is low-grade evidence that therapeutic granulocyte transfusions may not increase the number of patients with clinical resolution of an infection. Notably, the Cochrane review did not offer specific recommendations on the subgroup with IFI.
ADVERSE EFFECTS
The collection process entails minimal risk to donors. The short-term side effects of G-CSF, such as bone pain, headache and myalgia are generally mild and treatable. Axdorph Nygell et al (2015) reported no serious short-term adverse events in 18 years of granulocytapheresis; long-term follow-up of granulocyte donors stimulated with G-CSF and dexamethasone after 10 years (Quillen et al, 2009b), suggests that granulocyte donation is safe.
Febrile transfusion reactions and pulmonary complications, including transfusion-related acute lung injury (TRALI) (Sachs & Bux, 2003), are well-recognized complications of GTX, and are more likely to occur in patients with pre-existing granulocyte-reactive (HLA or human neutrophil antigen, HNA) antibodies (Dutcher et al, 1990; Heim et al, 2011). Neutrophil antibodies decrease the localization of transfused granulocytes to sites of inflammation (Stroncek et al, 1996). Lee et al (2004) demonstrated pulmonary localization of technetium-99m–labelled granulocytes in patients with pneumonia; cells accumulated at the area of infection in responders but not in the non-responders, suggesting that efficacy depends on the cells’ ability to migrate to the site of infection. By selecting HLA-compatible granulocyte donors, appropriate increments can be obtained and adverse reactions minimized (Quillen et al, 2009a). GTX can also cause alloimmunization to HLA and HNA (Stroncek et al, 1996), resulting in subsequent platelet and leucocyte transfusion refractoriness (Heim et al, 2011). One group described massive haemoptysis (3.5%) and respiratory failure (5.9%) in GTX recipients (Kim et al, 2011). Pulmonary toxicity associated with co-infusion with Amphotericin B was reported in one publication (Wright et al, 1981). However, other authors (Bow et al, 1984; Dutcher et al, 1989) failed to prove any specific detrimental interaction, concluding that the relationship was a function of tropism of the transfused neutrophils for pulmonary sites of IFI.
Granulocyte transfusion poses many of the same infectious risks as other blood products, and increased risk of infection with intracellular pathogens, such as CMV (Hersman et al, 1982); transmission of West Nile Virus (Meny et al, 2011) has also been reported. Due to the presence of viable lymphocytes in the granulocyte product, patients are at risk of transfusion-associated graft-versus-host disease (TA-GVHD) (Rosen et al, 1978; Nikoskelainen et al, 1983), a rare but highly fatal complication that results when transfused T-lymphocytes engraft, proliferate and attack host tissue antigens in a recipient who is unable to reject the allogeneic cells, either due to immune compromise or HLA-similarity to the donor. To prevent this complication, granulocyte concentrates must be irradiated before transfusion.
Perhaps in the future, functionally mature neutrophils generated from induced pluripotent stem cells (iPSCs) (Morishima et al, 2011; Sweeney et al, 2014) may resolve the problems of supply, adverse reactions and burdens on donors.
EVIDENCE SUMMARY AND CONCLUSIONS
Morbidity and mortality from IFIs remains substantial. Transfusion of granulocytes from stimulated healthy donors is often accompanied by a significant increase in the patient’s neutrophil count; the cells are capable of localization to areas of infection and appear to function normally. The process of granulocyte collection is relatively safe for donors.
While there is low-grade evidence that GTX may reduce the incidence of fungaemia, non-selective prophylaxis for all neutropenic patients does not prevent mortality due to localized fungal infection, and is accompanied by significant risks to recipients; as such, we do not recommend this practice. GTX may have a role in preventing progression of existing fungal infection during HSCT-induced neutropenia (Borge et al, 2010; Hermann et al, 2001; Drewniak et al, 2008, Diaz et al, 2014).
Recipients of GTX with IFI tend to be quite ill with several competing risk factors for mortality; therefore clinical response might be a more realistic marker of benefit than survival. Studies of GTX in the post-G-CSF era generally reported higher response rates, even for some refractory mould species. Unfortunately, the quality of the data suggesting response to therapeutic GTX in IFI is low, and predominantly limited to individual cases and uncontrolled case series. It remains unclear whether the RING trial was truly a negative study or whether it was unable to demonstrate the benefit of GTX due to low sample size. No RCTs to date have reported specifically on comparative effectiveness of GTX by fungal species. Nevertheless, in light of the evidence and its limitations, the authors would still recommend use of GTX in IFI if rapidly available at sufficient cell doses (at least 1.0 × 1010 or ≥ 0.6 × 109 granulocytes/kg) in select circumstances, such as salvageable patients with anticipated recovery of neutropenia. The risks and benefits must be weighed on a case-by-case basis. Treatment schedules may vary, but responses have been reported using intervals of up to 3 days between transfusions, with criteria for discontinuation including neutrophil recovery or clinical resolution of infection. The GRANITE (Transfusion of granulocytes for patients with febrile neutropenia) study, an ongoing multicentre RCT based in Germany (German Clinical Trials Register number DRKS00000218), may yet offer helpful results. Future studies should evaluate high-dose GTX and aim to compare homogenous groups of patients to controls, evaluating clearly defined parameters of response to GTX.
Supplementary Material
Acknowledgments
The authors thank Judith Welsh, NIH Library Informationist, for developing the database search strategies and conducting the literature searches in support of this systematic review, Kelly Byrne for assistance in preparing the illustration and Dr. Anthony F. Suffredini for reviewing the manuscript draft.
Footnotes
The opinions expressed are the authors’ own and do not reflect those of the National Institutes of Health, Department of Health and Human Services or the US Federal Government.
Author Contributions
KW, JGB, DS and SK wrote and critically reviewed the manuscript.
Conflicts of Interest
The authors have no relevant conflicts of interest to disclose.
References
- Alavi JB, Root RK, Djerassi I, Evans AE, Gluckman SJ, MacGregor RR, Guerry D, Schreiber AD, Shaw JM, Koch P, Cooper RA. A randomized clinical trial of granulocyte transfusions for infection in acute leukemia. New England Journal of Medicine. 1977;296:706–711. doi: 10.1056/NEJM197703312961302. [DOI] [PubMed] [Google Scholar]
- Al Tanbal H, Al Humaidan H, Al-Nounou R, Roberts G, Tesfamichael K, Owaidah T. The value and practicality of granulocyte transfusion: a single oncology centre experience. Transfusion Medicine. 2010;20:160–168. doi: 10.1111/j.1365-3148.2009.00988.x. [DOI] [PubMed] [Google Scholar]
- Ang AL, Linn YC. Treatment of severe neutropenic sepsis with granulocyte transfusion in the current era--experience from an adult haematology unit in Singapore. Transfusion Medicine. 2011;21:13–24. doi: 10.1111/j.1365-3148.2010.01035.x. [DOI] [PubMed] [Google Scholar]
- Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clinical Microbiology and Infection. 2014;20(Suppl. 3):76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. The Journal of Antimicrobial Chemotherapy. 2014;69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atay D, Ozturk G, Akcay A, Yanasik M, Anak S, Devecioglu O. Effect and safety of granulocyte transfusions in pediatric patients with febrile neutropenia or defective granulocyte functions. Journal of Pediatric Hematology/Oncology. 2011;33:e220–e225. doi: 10.1097/MPH.0b013e31821ffdf1. [DOI] [PubMed] [Google Scholar]
- Axdorph Nygell U, Sollén-Nilsson A, Lundahl J. Eighteen years experience of granulocyte donations-acceptable donor safety? Journal of Clinical Apheresis. 2015;5:265–272. doi: 10.1002/jca.21373. [DOI] [PubMed] [Google Scholar]
- Bearden JD, 3rd, Coltman CA, Jr, Ratkin GA. Hydroxyethyl starch and prednisone as adjuncts to granulocyte collection. Transfusion. 1977;17:141–146. doi: 10.1046/j.1537-2995.1977.17277151918.x. [DOI] [PubMed] [Google Scholar]
- Bashir S, Stanworth S, Massey E, Goddard F, Cardigan R. Neutrophil function is preserved in a pooled granulocyte component prepared from whole blood donations. British Journal of Haematology. 2008;140:701–711. doi: 10.1111/j.1365-2141.2008.06996.x. [DOI] [PubMed] [Google Scholar]
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- Borge PD, Theobald N, DeCastro R, Malech HL, Leitman S, Kang EM. Successful control of preexistent active infection by granulocyte transfusions during conditioning induced cytopenia in patients with chronic granulomatous disease undergoing hematopoietic stem cell transplant. Blood. 2010;21:1329. [Google Scholar]
- Bow EJ, Schroeder ML, Louie TJ. Pulmonary complications in patients receiving granulocyte transfusions and amphotericin B. Canadian Medical Association Journal. 1984;130:593–597. [PMC free article] [PubMed] [Google Scholar]
- Bow EJ, Vanness DJ, Slavin M, Cordonnier C, Cornely OA, Marks DI, Pagliuca A, Solano C, Cragin L, Shaul AJ, Sorensen S, Chambers R, Kantecki M, Weinstein D, Schlamm H. Systematic review and mixed treatment comparison meta-analysis of randomized clinical trials of primary oral antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients. BMC Infectious Diseases. 2015;15:128. doi: 10.1186/s12879-015-0855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher G, Wilbur KM, Cronkite EP. Transfusion of separated leukocytes into irradiated dogs with aplastic marrows. Proceedings of the Society for Experimental Biology and Medicine. 1953;84:54–56. doi: 10.3181/00379727-84-20539. [DOI] [PubMed] [Google Scholar]
- Bryant BJ, Yau YY, Byrne PJ, Stroncek DF, Leitman SF. Gravity sedimentation of granulocytapheresis concentrates with hydroxyethyl starch efficiently removes red blood cells and retains neutrophils. Transfusion. 2010;50:1203–1209. doi: 10.1111/j.1537-2995.2009.02576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CD, Clift RA, Thomas ED, Hersman J, Sanders JE, Stewart PS, Wade JC, Murphy M, Counts G, Meyers JD. Early infectious complications in allogeneic marrow transplant recipients with acute leukemia: effects of prophylactic measures. Infection. 1983;11:243–250. doi: 10.1007/BF01641254. [DOI] [PubMed] [Google Scholar]
- Cesaro S, Chinello P, De Silvestro G, Marson P, Picco G, Varotto S, Pittalis S, Zanesco L. Granulocyte transfusions from G-CSF-stimulated donors for the treatment of severe infections in neutropenic pediatric patients with onco-hematological diseases. Supportive Care in Cancer. 2003;11:101–106. doi: 10.1007/s00520-002-0394-8. [DOI] [PubMed] [Google Scholar]
- Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clinical Infectious Diseases. 2005;41:60–66. doi: 10.1086/430710. [DOI] [PubMed] [Google Scholar]
- Chau MM, Kong DC, van Hal SJ, Urbancic K, Trubiano JA, Cassumbhoy M, Wilkes J, Cooper CM, Roberts JA, Marriott DJ, Worth LJ. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy. Internal Medicine Journal. 2014;44:1364–1388. doi: 10.1111/imj.12600. [DOI] [PubMed] [Google Scholar]
- Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, Arikan-Akdagli S, Akova M, Boekhout T, Caira M, Guinea J, Chakrabarti A, Dannaoui E, van Diepeningen A, Freiberger T, Groll AH, Hope WW, Johnson E, Lackner M, Lagrou K, Lanternier F, Lass-Flörl C, Lortholary O, Meletiadis J, Muñoz P, Pagano L, Petrikkos G, Richardson MD, Roilides E, Skiada A, Tortorano AM, Ullmann AJ, Verweij PE, Cornely OA, Cuenca-Estrella M European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clinical Microbiology and Infection. 2014;20(Suppl 3):47–75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- Clift RA, Sanders JE, Thomas ED, Williams B, Buckner CD. Granulocyte transfusions for the prevention of infection in patients receiving bone-marrow transplants. New England Journal of Medicine. 1978;298:1052–1057. doi: 10.1056/NEJM197805112981904. [DOI] [PubMed] [Google Scholar]
- Cohn ZA, Hirsch JG. The influence of phagocytosis on the intracellular distribution of granule-associated components of polymorphonuclear leucocytes. The Journal of Experimental Medicine. 1960;112:1015–1022. doi: 10.1084/jem.112.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, Dhédin N, Isnard F, Ades L, Kuhnowski F, Foulet F, Kuentz M, Maison P, Bretagne S, Schwarzinger M. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: A randomized, controlled trial. Clinical Infectious Diseases. 2009;48:1042–1051. doi: 10.1086/597395. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. New England Journal of Medicine. 2007a;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, Heussel CP, Lortholary O, Rieger C, Boehme A, Aoun M, Horst HA, Thiebaut A, Ruhnke M, Reichert D, Vianelli N, Krause SW, Olavarria E, Herbrecht R AmBiLoad Trial Study Group. Liposomal amphotericin B as initial therapy for invasive mold infection: A randomized trial comparing a high-loading dose regimen with standard dosing (AmBiload trial) Clinical Infectious Diseases. 2007b;44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Flörl C, Lortholary O, Meis JF, Meletiadis J, Muñoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, van Diepeningen A, Verweij P, Petrikkos G European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis. Clinical Microbiology and Infection. 2014;20(Suppl 3):5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- Dale DC, Reynolds HY, Pennington JE, Elin RJ, Herzig GP. Experimental Pseudomonas pneumonia in leukopenic dogs: comparison of therapy with antibiotics and granulocyte transfusions. Blood. 1976;47:869–876. [PubMed] [Google Scholar]
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of Invasive Fungal Disease From the European Organization For Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute Of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clinical Infectious Diseases. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fliedner V, Meuret G, Senn H. Normal granulocyte collection with a modified repetitive cycle filtration leukapheresis. Blut. 1974;29:265–276. doi: 10.1007/BF01635548. [DOI] [PubMed] [Google Scholar]
- Díaz R, Soundar E, Hartman SK, Dreyer Z, Teruya J, Hui SK. Granulocyte transfusions for children with infection and neutropenia or granulocyte dysfunction. Pediatric Hematology and Oncology. 2014;31:425–434. doi: 10.3109/08880018.2013.868562. [DOI] [PubMed] [Google Scholar]
- Dignani MC, Anaissie EJ, Hester JP, O’Brien S, Vartivarian SE, Rex JH, Kantarjian H, Jendiroba DB, Lichtiger B, Andersson BS, Freireich EJ. Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia. 1997;11:1621–1630. doi: 10.1038/sj.leu.2400811. [DOI] [PubMed] [Google Scholar]
- Drewniak A, Boelens JJ, Vrielink H, Tool AT, Bruin MC, van den Heuvel-Eibrink M, Ball L, van de Wetering MD, Roos D, Kuijpers TW. Granulocyte concentrates: Prolonged functional capacity during storage in the presence of phenotypic changes. Haematologica. 2008;93:1058–1067. doi: 10.3324/haematol.12489. [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Schiffer CA, Johnston GS. Rapid migration of 111indium-labeled granulocytes to sites of infection. New England Journal of Medicine. 1981;304:586–589. doi: 10.1056/NEJM198103053041007. [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Kendall J, Norris D, Schiffer C, Aisner J, Wiernik PH. Granulocyte transfusion therapy and amphotericin B: adverse reactions? American Journal of Hematology. 1989;31:102–108. doi: 10.1002/ajh.2830310206. [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Riggs C, Jr, Fox JJ, Johnston GS, Norris D, Wiernik PH, Schiffer CA. Effect of histocompatibility factors on pulmonary retention of Indium-111-labeled granulocytes. American Journal of Hematology. 1990;33:238–243. doi: 10.1002/ajh.2830330405. [DOI] [PubMed] [Google Scholar]
- Eckermann I, Strauss RG. Granulocyte collection: a comparison of Fenwal CS 3000, IBM 2997, and haemonetics cell separators. Journal of Clinical Apheresis. 1984;2:26–31. doi: 10.1002/jca.2920020107. [DOI] [PubMed] [Google Scholar]
- Estcourt LJ, Stanworth S, Doree C, Blanco P, Hopewell S, Trivella M, Massey E. Granulocyte transfusions for preventing infections in people with neutropenia or neutrophil dysfunction. The Cochrane Database of Systemic Reviews. 2015;6:CD005341. doi: 10.1002/14651858.CD005341.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estcourt LJ, Stanworth S, Hopewell S, Doree C, Trivella M, Massey E. Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction. The Cochrane Database of Systemic Reviews. 2016;4:CD005339. doi: 10.1002/14651858.CD005339.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier MC, Science M, Beyene J, Briel M, Lehrnbecher T, Sung L. Mould-active compared with fluconazole prophylaxis to prevent invasive fungal diseases in cancer patients receiving chemotherapy or haematopoietic stem-cell transplantation: A systematic review and meta-analysis of randomised controlled trials. British Journal of Cancer. 2012;106:1626–1637. doi: 10.1038/bjc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freireich EJ, Levin RH, Whang J, Carbone PP, Bronson W, Morse EE. The function and fate of transfused leukocytes from donors with chronic myelocytic leukemia in leukopenic recipients. Annals of the. New York. Academy of Sciences. 1964;113:1081–1089. doi: 10.1111/j.1749-6632.1964.tb40726.x. [DOI] [PubMed] [Google Scholar]
- Gil-Lamaignere C, Roilides E, Mosquera J, Maloukou A, Walsh TJ. Antifungal Triazoles and Polymorphonuclear Leukocytes Synergize to Cause Increased Hyphal Damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrobial Agents and Chemotherapy. 2002;46:2234–2237. doi: 10.1128/AAC.46.7.2234-2237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser L, Huestis DW. Characteristics of stored granulocytes collected from donors stimulated with dexamethasone. Transfusion. 1979;19:53–56. doi: 10.1046/j.1537-2995.1979.19179160266.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Villagran JL, Torres-Gómez A, Gomez-Garcia P, Martinez-Guibelalde F, Velasco-Jimena F. A controlled trial of prophylactic granulocyte transfusions during induction chemotherapy for acute nonlymphoblastic leukemia. Cancer. 1984;54:734–738. doi: 10.1002/1097-0142(1984)54:4<734::aid-cncr2820540424>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, Shadduck RK, Shea TC, Stiff P, Friedman DJ, Powderly WG, Silber JL, Horowitz H, Lichtin A, Wolff SN, Mangan KF, Silver SM, Weisdorf D, Ho WG, Gilbert G, Buell D. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. New England Journal of Medicine. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- Graham AS, Price TH, Brogan TV. Revisiting the use of granulocyte transfusions in pediatric oncology patients. Journal of Pediatric Hematology/Oncology. 2009;31:161–165. doi: 10.1097/MPH.0b013e318196a6e6. [DOI] [PubMed] [Google Scholar]
- Graw RG, Jr, Herzig GP, Eisel RJ, Perry S. Leukocyte and platelet collection from normal donors with the continuous flow blood cell separator. Transfusion. 1971;11:94–101. doi: 10.1111/j.1537-2995.1971.tb04383.x. [DOI] [PubMed] [Google Scholar]
- Grigg A, Vecchi L, Bardy P, Szer J. G-CSF stimulated donor granulocyte collections for prophylaxis and therapy of neutropenic sepsis. Australian and New Zealand Journal of Medicine. 1996;26:813–818. doi: 10.1111/j.1445-5994.1996.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Grigull L, Pulver N, Goudeva L, Sykora KW, Linderkamp C, Beilken A, Seidemann K, Schmid H, Welte K, Heuft HG. G-CSF mobilised granulocyte transfusions in 32 paediatric patients with neutropenic sepsis. Supportive Care in Cancer. 2006;14:910–916. doi: 10.1007/s00520-006-0041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grow WB, Moreb JS, Roque D, Manion K, Leather H, Reddy V, Khan SA, Finiewicz KJ, Nguyen H, Clancy CJ, Mehta PS, Wingard JR. Late onset invasive Aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 2002;29:15–19. doi: 10.1038/sj.bmt.1703332. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Tokimatsu I, Mikamo H, Kimura M, Seki M, Takakura S, Ohmagari N, Takahashi Y, Kasahara K, Matsumoto K, Okada K, Igarashi M, Kobayashi M, Mochizuki T, Nishi Y, Tanigawara Y, Kimura T, Takesue Y. Practice guidelines for therapeutic drug monitoring of voriconazole: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Journal of Infection and Chemotherapy. 2013;19:381–392. doi: 10.1007/s10156-013-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim KF, Fleisher TA, Stroncek DF, Holland SM, Gallin JI, Malech HL, Leitman SF. The relationship between alloimmunization and post-transfusion granulocyte survival: experience in a chronic granulomatous disease cohort. Transfusion. 2011;51:1154–1162. doi: 10.1111/j.1537-2995.2010.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. New England Journal of Medicine. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- Hermann S, Klein SA, Jacobi V, Thalhammer A, Bialleck H, Duchscherer M, Wassmann B, Hoelzer D, Martin H. Older patients with high-risk fungal infections can be successfully allografted using non-myeloablative conditioning in combination with intensified supportive care regimens. British Journal of Haematology. 2001;113:446–454. doi: 10.1046/j.1365-2141.2001.02747.x. [DOI] [PubMed] [Google Scholar]
- Hersman J, Meyers JD, Thomas ED, Buckner CD, Clift R. The effect of granulocyte transfusions on the incidence of cytomegalovirus infection after allogeneic marrow transplantation. Annals of Internal Medicine. 1982;96:149–152. doi: 10.7326/0003-4819-96-2-149. [DOI] [PubMed] [Google Scholar]
- Hester JP, Dignani MC, Anaissie EJ, Kantarjian HM, O’Brien S, Freireich EJ. Collection and transfusion of granulocyte concentrates from donors primed with granulocyte stimulating factor and response of myelosuppressed patients with established infection. Journal of Clinical Apheresis. 1995;10:188–193. doi: 10.1002/jca.2920100406. [DOI] [PubMed] [Google Scholar]
- Heuft HG, Goudeva L, Sel S&Blasczyk R. Equivalent mobilization and collection of granulocytes for transfusion after administration of glycosylated G-CSF (3 microg/kg) plus dexamethasone versus glycosylated G-CSF (12 microg/kg) alone. Transfusion. 2002;42:928–934. doi: 10.1046/j.1537-2995.2002.00133.x. [DOI] [PubMed] [Google Scholar]
- Higby DJ, Yates JW, Henderson ES, Holland JF. Filtration leukapheresis for granulocyte transfusion therapy. Clinical and laboratory studies. New England Journal of Medicine. 1975;292:761–766. doi: 10.1056/NEJM197504102921501. [DOI] [PubMed] [Google Scholar]
- Hübel K, Carter RA, Liles WC, Dale DC, Price TH, Bowden RA, Rowley SD, Chauncey TR, Bensinger WI, Boeckh M. Granulocyte transfusion therapy for infections in candidates and recipients of HPC transplantation: a comparative analysis of feasibility and outcome for community donors versus related donors. Transfusion. 2002;42:1414–1421. doi: 10.1046/j.1537-2995.2002.00249.x. [DOI] [PubMed] [Google Scholar]
- Iacone A, Di Bartolomeo P, Di Girolamo G, Torlontano G. Hydroxyethyl starch and steroid improved collection of normal granulocytes with continuous flow centrifugation gravity leukapheresis. Haematologica. 1981;66:645–655. [PubMed] [Google Scholar]
- Illerhaus G, Wirth K, Dwenger A, Waller CF, Garbe A, Brass V, Lang H, Lange W. Treatment and prophylaxis of severe infections in neutropenic patients by granulocyte transfusions. Annals of Hematology. 2002;81:273–281. doi: 10.1007/s00277-002-0439-6. [DOI] [PubMed] [Google Scholar]
- Imhof A, Balajee SA, Fredricks DN, Englund JA, Marr KA. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clinical Infectious Diseases. 2004;39:743–746. doi: 10.1086/423274. [DOI] [PubMed] [Google Scholar]
- Inaba S, Takamatsu Y, Yamamoto A, Shimoda K, Fukuda T, Ohga S, Hamaguchi K, Ueda K. The use of recombinant granulocyte-colony-stimulating factor for granulocyte harvest. Transfusion. 1992;32:690–691. doi: 10.1046/j.1537-2995.1992.32792391049.x. [DOI] [PubMed] [Google Scholar]
- Kadri SS, Remy KE, Strich JR, Gea-Banacloche J, Leitman SF. Role of granulocyte transfusions in invasive fusariosis: a systemic review and a single-centre experience. Transfusion. 2015;55:2076–2084. doi: 10.1111/trf.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AD, Willment JA, Dorward DW, Williams DL, Brown GD, DeLeo FR. Dectin-1 promotes fungicidal activity of human neutrophils. European Journal of Immunology. 2007;37:467–478. doi: 10.1002/eji.200636653. [DOI] [PubMed] [Google Scholar]
- Kerr JP, Liakopolou E, Brown J, Cornish JM, Fleming D, Massey E, Oakhill A, Pamphilon DH, Robinson SP, Totem A, Valencia AM, Marks DI. The use of stimulated granulocyte transfusions to prevent recurrence of past severe infections after allogeneic stem cell transplantation. British Journal of Haematology. 2003;123:114–118. doi: 10.1046/j.1365-2141.2003.04583.x. [DOI] [PubMed] [Google Scholar]
- Kikuta A, Ohto H, Nemoto K, Mochizuki K, Sano H, Ito M, Suzuki H. Therapeutic transfusions of granulocytes collected by simple bag method for children with cancer and neutropenic infections: results of a single-centre pilot study. Vox Sanguinis. 2006;91:70–76. doi: 10.1111/j.1423-0410.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- Kim KH, Lim HJ, Kim JS, Kim BS, Bang SM, Kim I, Han KS, Kim BK, Lee SM, Yoon SS. Therapeutic granulocyte transfusions for the treatment of febrile neutropenia in patients with hematologic diseases: a 10-year experience at a single institute. Cytotherapy. 2011;13:490–498. doi: 10.3109/14653249.2010.529889. [DOI] [PubMed] [Google Scholar]
- Laverdiere M, Bow EJ, Rotstein C, Autmizguine J, Broady R, Garber G, Haider S, Hussaini T, Husain S, Ovetchkine P, Seki JT, Théorêt Y. Therapeutic drug monitoring for triazoles: A needs assessment review and recommendations from a Canadian perspective. The Canadian Journal of Infectious Disease and Medical Microbiology. 2014;25:327–343. doi: 10.1155/2014/340586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Chung IJ, Park MR, Kook H, Hwang TJ, Ryang DW, Kim HJ. Clinical efficacy of granulocyte transfusion therapy in patients with neutropenia-related infections. Leukemia. 2001;15:203–207. doi: 10.1038/sj.leu.2402007. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Song HC, Chung IJ, Bom HS, Cho D, Kim HJ. Clinical efficacy and prediction of response to granulocyte transfusion therapy for patients with neutropenia-related infections. Haematologica. 2004;89:632–633. [PubMed] [Google Scholar]
- Lee YJ, Lee SO, Choi SH, Kim YS, Woo JH, Chun S, Kim DY, Lee JH, Lee JH, Lee KH, Kim SH. Initial voriconazole trough blood levels and clinical outcomes of invasive aspergillosis in patients with hematologic malignancies. Medical Mycology. 2013;51:324–330. doi: 10.3109/13693786.2012.694082. [DOI] [PubMed] [Google Scholar]
- Lehrnbecher T, Robinson PD, Fisher BT, Castagnola E, Groll AH, Steinbach WJ, Zaoutis TE, Negeri ZF, Beyene J, Phillips B, Sung L. Galactomannan, Beta-D-Glucan and PCR-Based Assays for the Diagnosis of Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016 Nov 15;63(10):1340–1348. doi: 10.1093/cid/ciw592. [DOI] [PubMed] [Google Scholar]
- Marr KA, Seidel K, Slavin MA, Bowden RA, Schoch HG, Flowers ME, Corey L, Boeckh M. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96:55–61. [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clinical Infectious Diseases. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee DG, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR, Maertens JA. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Annals of Internal Medicine. 2015;162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- Maertens J, Marchetti O, Herbrecht R, Cornely OA, Flückiger U, Frêre P, Gachot B, Heinz WJ, Lass-Flörl C, Ribaud P, Thiebaut A, Cordonnier C Third European Conference on Infections in Leukemia. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: Summary of the ECIL 3--2009 update. Bone Marrow Transplantation. 2011;46:709–718. doi: 10.1038/bmt.2010.175. [DOI] [PubMed] [Google Scholar]
- Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee DG, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, 3rd, Lee M, Maher RM, Schmitt-Hoffmann AH, Zeiher B, Ullmann AJ. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. The Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- Maschmeyer G, Heinz WJ, Hertenstein B, Horst HA, Requadt C, Wagner T, Cornely OA, Löffler J, Ruhnke M IDEA study investigators. Immediate versus deferred empirical antifungal (IDEA) therapy in high-risk patients with febrile neutropenia: A randomized, double-blind, placebo-controlled, multicentre study. European Journal of Clinical Microbiology and Infectious Diseases. 2013;32:679–689. doi: 10.1007/s10096-012-1794-4. [DOI] [PubMed] [Google Scholar]
- McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, Heesemann J, Ebel F. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes and Infection. 2010;12:928–936. doi: 10.1016/j.micinf.2010.06.009. [DOI] [PubMed] [Google Scholar]
- McCullough J. Leukapheresis and granulocyte transfusion. CRC Critical Reviews in Clinical Laboratory Sciences. 1979;10:275–327. doi: 10.3109/10408367909147137. [DOI] [PubMed] [Google Scholar]
- Meny GM, Santos-Zabala L, Szallasi A, Stramer SL. West Nile virus infection transmitted by granulocyte transfusion. Blood. 2011;117:5778–5779. doi: 10.1182/blood-2011-02-335901. [DOI] [PubMed] [Google Scholar]
- Mishler JM, Hester JP, Huestis DW, Rock GA, Strauss RG. Dosage and scheduling regimens for erythrocyte-sedimenting macromolecules. Journal of Clinical Apheresis. 1983;1:130–143. doi: 10.1002/jca.2920010304. [DOI] [PubMed] [Google Scholar]
- Morishima T, Watanabe K, Niwa A, Fujino H, Matsubara H, Adachi S, Suemori H, Nakahata T, Heike T. Neutrophil differentiation from human-induced pluripotent stem cells. Journal of Cellular Physiology. 2011;226:1283–1291. doi: 10.1002/jcp.22456. [DOI] [PubMed] [Google Scholar]
- Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, Szer J, Halliday CL, Gilroy NM, Moore J, Schwarer AP, Guy S, Bajel A, Tramontana AR, Spelman T, Slavin MA Australasian Leukaemia Lymphoma Group and the Australia and New Zealand Mycology Interest Group. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: A randomised controlled trial. The Lancet Infectious Diseases. 2013;13:519–528. doi: 10.1016/S1473-3099(13)70076-8. [DOI] [PubMed] [Google Scholar]
- Mousset S, Hermann S, Klein S, Bialleck H, Duchscherer M, Bomke B, Wassmann B, Böhme A, Hoelzer D, Martin H. Prophylactic and interventional granulocyte transfusions in patients with haematological malignancies and life-threatening infections during neutropenia. Annals of Hematology. 2005;84:734–741. doi: 10.1007/s00277-005-1055-z. [DOI] [PubMed] [Google Scholar]
- Moriyama B, Kadri S, Henning SA, Danner RL, Walsh TJ, Penzak SR. Therapeutic Drug Monitoring and Genotypic Screening in the Clinical Use of Voriconazole. Current Fungal Infection Reports. 2015;9:74–87. doi: 10.1007/s12281-015-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajeva O, Mijovic A, Hess D, Tatam E, Amrolia P, Chiesa R, Rao K, Silva J, Veys P. Single-donor granulocyte transfusions for improving the outcome of high-risk pediatric patients with known bacterial and fungal infections undergoing stem cell transplantation: a 10-year single-centre experience. Bone Marrow Transplantation. 2015;50:846–849. doi: 10.1038/bmt.2015.53. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen J, Söderström KO, Rajamäki A, Meurman L, Korvenranta H, Kalliomäki JL, Toivanen A. Graft-versus-host reaction in 3 adult leukaemia patients after transfusion of blood cell products. Scandinavian Journal of Haematology. 1983;31:403–409. doi: 10.1111/j.1600-0609.1983.tb01533.x. [DOI] [PubMed] [Google Scholar]
- O’ Donghaile D, Childs RW, Leitman SF. Blood consult: granulocyte transfusions to treat invasive aspergillosis in a patient with severe aplastic anaemia awaiting mismatched hematopoietic progenitor cell transplantation. Blood. 2012;119:1353–1355. doi: 10.1182/blood-2011-10-345751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofran Y, Avivi I, Oliven A, Oren I, Zuckerman T, Bonstein L, Rowe JM, Dann EJ. Granulocyte transfusions for neutropenic patients with life-threatening infections: a single centre experience in 47 patients, who received 348 granulocyte transfusions. Vox Sanguinis. 2007;93:363–369. doi: 10.1111/j.1423-0410.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- Oztürkmen S, Altuntaş F, Olcay L. Granulocyte transfusion therapy in paediatric patients with severe neutropenic infection. Transfusion and Apheresis Science. 2013;48:381–385. doi: 10.1016/j.transci.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Picardi M, Corvatta L, D’Antonio D, Girmenia C, Martino P, Del Favero A. Mucormycosis in Hematologic Patients. Haematologica. 2004;89:207–214. [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TF, Thompson GR3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Minkov M, Matthes-Martin S, Potschger U, Witt V, Mann G, Hocker P, Worel N, Stary J, Klingebiel T, Gadner H. Leucocyte transfusions from rhG-CSF or prednisolone stimulated donors for treatment of severe infections in immunocompromised neutropenic patients. British Journal of Haematology. 1999;106:689–696. doi: 10.1046/j.1365-2141.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- Petersen FB, Buckner CD, Clift RA, Nelson N, Counts GW, Meyers JD, Thomas ED. Prevention of nosocomial infections in marrow transplant patients: a prospective randomized comparison of systemic antibiotics versus granulocyte transfusions. Infection Control. 1986;7:586–592. doi: 10.1017/s0195941700065437. [DOI] [PubMed] [Google Scholar]
- Ping B, Zhu Y, Gao Y, Yue C, Wu B. Second- versus first-generation azoles for antifungal prophylaxis in hematology patients: A systematic review and meta-analysis. Annals of Hematology. 2013;92:831–839. doi: 10.1007/s00277-013-1693-5. [DOI] [PubMed] [Google Scholar]
- Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. American Journal of Medicine. 1982;72:101–111. doi: 10.1016/0002-9343(82)90594-0. [DOI] [PubMed] [Google Scholar]
- Price TH. Granulocyte transfusion: current status. Seminars in Hematology. 2007;44:15–23. doi: 10.1053/j.seminhematol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Price TH, Boeckh M, Harrison RW, McCullough J, Ness PM, Strauss RG, Nichols WG, Hamza TH, Cushing MM, King KE, Young JA, Williams E, McFarland J, Holter Chakrabarty J, Sloan SR, Friedman D, Parekh S, Sachais BS, Kiss JE, Assmann SF. Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection. Blood. 2015;126:2153–2161. doi: 10.1182/blood-2015-05-645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen K, Wong E, Scheinberg P, Young NS, Walsh TJ, Wu CO, Leitman SF. Granulocyte transfusions in severe aplastic anaemia: an eleven-year experience. Haematologica. 2009a;94:1661–1668. doi: 10.3324/haematol.2009.010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen K, Byrne P, Yau YY, Leitman SF. Ten-year follow-up of unrelated volunteer granulocyte donors who have received multiple cycles of granulocyte-colony-stimulating factor and dexamethasone. Transfusion. 2009b;3:513–8. doi: 10.1111/j.1537-2995.2008.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad II, Chaftari AM, Al Shuaibi MM, Jiang Y, Shomali W, Cortes JE, Lichtiger B, Hachem RY. Granulocyte transfusions in hematologic malignancy patients with invasive pulmonary aspergillosis: Outcomes and complications. Annals of Oncology. 2013;24:1873–1879. doi: 10.1093/annonc/mdt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Reiss RF, Pindyck J, Waldman AA, Raju M, Kulpa J. Transfusion of granulocyte rich buffy coats to neutropenic patients. Medical and Pediatric Oncology. 1982;10:447–454. doi: 10.1002/mpo.2950100504. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Huestis DW, Corrigan JJ., Jr Acute leukemia and granulocyte transfusion: Fatal graft-versus-host reaction following transfusion of cells obtained from normal donors. Journal of Pediatrics. 1978;93:268–270. doi: 10.1016/s0022-3476(78)80517-4. [DOI] [PubMed] [Google Scholar]
- Rüping MJ1, Heinz WJ, Kindo AJ, Rickerts V, Lass-Flörl C, Beisel C, Herbrecht R, Roth Y, Silling G, Ullmann AJ, Borchert K, Egerer G, Maertens J, Maschmeyer G, Simon A, Wattad M, Fischer G, Vehreschild JJ, Cornely OA. Forty-one recent cases of invasive zygomycosis from a global clinical registry. Journal of Antimicrobial Chemotherapy. 2010;65:296–302. doi: 10.1093/jac/dkp430. [DOI] [PubMed] [Google Scholar]
- Rutella S, Pierelli L, Piccirillo N, Sica S, Serafini R, Chiusolo P, Paladini U, Leone F, Zini G, D’Onofrio G, Leone G, Piccirillo N. Efficacy of granulocyte transfusions for neutropenia-related infections: retrospective analysis of predictive factors. Cytotherapy. 2003;5:19–30. doi: 10.1080/14653240310000047. [DOI] [PubMed] [Google Scholar]
- Ruthe RC, Andersen BR, Cunningham BL, Epstein RB. Efficacy of granulocyte transfusions in the control of systemic candidiasis in the leukopenic host. Blood. 1978;52:493–498. [PubMed] [Google Scholar]
- Sachs UJ, Bux J. TRALI after the transfusion of cross-match-positive granulocytes. Transfusion. 2003;43:1683–1686. doi: 10.1111/j.0041-1132.2003.00568.x. [DOI] [PubMed] [Google Scholar]
- Sachs UJ, Reiter A, Walter T, Bein G, Woessmann W. Safety and efficacy of therapeutic early onset granulocyte transfusions in pediatric patients with neutropenia and severe infections. Transfusion. 2006;46:1909–1914. doi: 10.1111/j.1537-2995.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- Safdar A, Hanna HA, Boktour M, Kontoyiannis DP, Hachem R, Lichtiger B, Freireich EJ, Raad II. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia: retrospective case-control analysis of 491 episodes of Candida species bloodstream infections. Cancer. 2004;101:2859–2865. doi: 10.1002/cncr.20710. [DOI] [PubMed] [Google Scholar]
- Safdar A, Rodriguez GH, Lichtiger B, Dickey BF, Kontoyiannis DP, Freireich EJ, Shpall EJ, Raad II, Kantarjian HM, Champlin RE. Recombinant interferon gamma1b immune enhancement in 20 patients with hematologic malignancies and systemic opportunistic infections treated with donor granulocyte transfusions. Cancer. 2006;106:2664–2671. doi: 10.1002/cncr.21929. [DOI] [PubMed] [Google Scholar]
- Safdar A, Rodriguez G, Zuniga J, Al Akhrass F, Pande A. Use of healthy-donor granulocyte transfusions to treat infections in neutropenic patients with myeloid or lymphoid neoplasms: experience in 74 patients treated with 373 granulocyte transfusions. Acta Haematologica. 2014;131:50–58. doi: 10.1159/000351174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. Journal of Infection and Chemotherapy. 2016;22:505–514. doi: 10.1016/j.jiac.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Schiffer CA, Aisner J, Daly PA, Schimpff SC, Wiernik PH. Alloimmunization following prophylactic granulocyte transfusion. Blood. 1979;54:766–774. [PubMed] [Google Scholar]
- Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, Silling G, Grimminger W, Einsele H. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplantation. 2008;42:679–684. doi: 10.1038/bmt.2008.237. [DOI] [PubMed] [Google Scholar]
- Seidel MG, Minkov M, Witt V, Matthes-Martin S, Pötschger U, Worel N, Leitner G, Stary J, Gadner H, Peters C. Granulocyte transfusions in children and young adults: does the dose matter? Journal of Pediatric Hematology/Oncology. 2009;3:166–172. doi: 10.1097/MPH.0b013e318196a6f9. [DOI] [PubMed] [Google Scholar]
- Strauss RG. Role of granulocyte/neutrophil transfusions for haematology/oncology patients in the modern era. British Journal of Haematology. 2012;158:299–306. doi: 10.1111/j.1365-2141.2012.09190.x. [DOI] [PubMed] [Google Scholar]
- Strauss RG, Connett JE, Gale RP, Bloomfield CD, Herzig GP, McCullough J, Maguire LC, Winston DJ, Ho W, Stump DC, Miller WV, Koepke JA. A controlled trial of prophylactic granulocyte transfusions during initial induction chemotherapy for acute myelogenous leukemia. New England Journal of Medicine. 1981;305:597–603. doi: 10.1056/NEJM198109103051101. [DOI] [PubMed] [Google Scholar]
- Stroncek DF, Leonard K, Eiber G, Malech HL, Gallin JI, Leitman SF. Alloimmunization after granulocyte transfusions. Transfusion. 1996;36:1009–1015. doi: 10.1046/j.1537-2995.1996.36111297091747.x. [DOI] [PubMed] [Google Scholar]
- Stroncek DF, Yau YY, Oblitas J, Leitman SF. Administration of G--CSF plus dexamethasone produces greater granulocyte concentrate yields while causing no more donor toxicity than G--CSF alone. Transfusion. 2001;41:1037–1044. doi: 10.1046/j.1537-2995.2001.41081037.x. [DOI] [PubMed] [Google Scholar]
- Stroncek DF, Matthews CL, Follmann D, Leitman SF. Kinetics of G-CSF-induced granulocyte mobilization in healthy subjects: effects of route of administration and addition of dexamethasone. Transfusion. 2002;42:597- 602. doi: 10.1046/j.1537-2995.2002.00091.x. [DOI] [PubMed] [Google Scholar]
- Sweeney CL, Merling RK, Choi U, Priel DL, Kuhns DB, Wang H, Malech HL. Generation of functionally mature neutrophils from induced pluripotent stem cells. Methods in Molecular Biology. 2014;1124:189–206. doi: 10.1007/978-1-62703-845-4_12. [DOI] [PubMed] [Google Scholar]
- Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Munoz P, Johnson E, Meletiadis J, Pana ZD, Lackner M, Verweij P, Freiberger T, Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Guinea J, Guarro J, de Hoog S, Hope W, Kathuria S, Lortholary O, Meis JF, Ullmann AJ, Petrikkos G, Lass-Flörl C European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clinical Microbiology and Infection. 2014;20(Suppl 3):27–46. doi: 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]
- Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. New England Journal of Medicine. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clinical Infectious Diseases. 2004;39:1407–16. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- Vogler WR, Winton EF. A controlled study of the efficacy of granulocyte transfusions in patients with neutropenia. The American Journal of Medicine. 1977;63:548–555. doi: 10.1016/0002-9343(77)90200-5. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Lutsar I, Driscoll T, DuPont B, Roden M, Ghahramani P, Hodges M, Groll AH, Perfect JR. Voriconazole in the treatment of aspergillosis, scedosporiosis, and other invasive fungal infections in children. The Pediatric Infectious Disease Journal. 2002;21:240–248. doi: 10.1097/00006454-200203000-00015. [DOI] [PubMed] [Google Scholar]
- Walter CW, Murphy WP., Jr A closed gravity technique for the preservation of whole blood in ACD solution utilizing plastic equipment. Surgery, Gynecology & Obstetrics. 1952;94:687–692. [PubMed] [Google Scholar]
- Wang H, Wu Y, Fu R, Qu W, Ruan E, Wang G, Liu H, Song J, Xing L, Guan J, Li L, Liu C, Shao Z. Granulocyte transfusion combined with granulocyte colony stimulating factor in severe infection patients with severe aplastic anemia: a single center experience from China. PLoS One. 2014;9:e88148. doi: 10.1371/journal.pone.0088148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ML, Underhill DM. Time to cast a larger net. Nature Immunology. 2014;11:1000–1001. doi: 10.1038/ni.3013. [DOI] [PubMed] [Google Scholar]
- Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, Gersten ID, Mendizabal AM, Leather HL, Confer DL, Maziarz RT, Stadtmauer EA, Bolaños-Meade J, Brown J, Dipersio JF, Boeckh M, Marr KA. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston DJ, Ho WG, Young LS, Gale RP. Prophylactic granulocyte transfusions during human bone marrow transplantation. American Journal of Medicine. 1980;68:893–897. doi: 10.1016/0002-9343(80)90223-5. [DOI] [PubMed] [Google Scholar]
- Winston DJ, Ho WG, Gale RP. Prophylactic granulocyte transfusions during chemotherapy of acute nonlymphocytic leukemia. Annals of Internal Medicine. 1981;94:616–622. doi: 10.7326/0003-4819-94-5-616. [DOI] [PubMed] [Google Scholar]
- Wright DG, Robichaud KJ, Pizzo PA, Deisseroth AB. Lethal pulmonary reactions associated with the combined use of amphotericin B and leukocyte transfusions. New England Journal of Medicine. 1981;304:1185–1189. doi: 10.1056/NEJM198105143042001. [DOI] [PubMed] [Google Scholar]
- Yenicesu I, Sucak G, Dilsiz G, Akı SZ, Yeğin ZA. Hematopoietic stem cell transplantation in a very high risk group of patients with the support of granulocyte transfusion. Indian Journal of Hematology and Blood Transfusion. 2011;27:146–151. doi: 10.1007/s12288-011-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.