Abstract
Objectives/Hypothesis
High‐mobility group box 1 (HMGB1) is a chromatin‐binding protein located in the cell nucleus. Following injury, immunocompetent cells secrete HMGB1 to the extracellular milieu under the stimulation of proinflammatory cytokines. Extracellular HMGB1 acts a danger signal that instigates the innate immunity and tissue repair. We previously reported HMGB1 in the vocal fold extracellular compartment between day 3 and day 7 following surgical injury. In this study, we further investigated the cell source of HMGB1 and the relationship of proinflammatory cytokine expression and HMGB1 translocation in wounded vocal folds.
Study Design
Prospective animal study.
Methods
Bilateral vocal fold injury was performed on 122 Sprague‐Dawley rats. An additional 18 rats served as uninjured controls. Animals were sacrificed at multiple time points up to 4 weeks after surgery. Immunohistochemical costaining was performed to identify the cell source of HMGB1. Cell markers ED1, fibroblast‐specific protein 1 (FSP1), and alpha smooth muscle actin (α‐SMA) were used to identify macrophages, fibroblasts, and myofibroblasts, respectively. Enzyme‐linked immunosorbent assays were performed to measure cytokine levels of interleukin‐1beta (IL‐1β) and tumor necrosis factor‐alpha (TNF‐α) in vocal fold tissue.
Results
Costaining of HMGB1 was strong with ED1 and FSP1 but was minimal with α‐SMA in injured vocal folds. Compared to uninjured controls, IL‐1β and TNF‐α expression increased significantly the first 2 days after injury.
Conclusions
Macrophages and fibroblasts were a major cell source of vocal fold HMGB1. Translocation of HMGB1 may be an active response to the early accumulation of IL‐1β and TNF‐α in the wounded vocal folds.
Level of Evidence
NA Laryngoscope, 127:E193–E200, 2017
Keywords: HMGB1, cytokines, vocal folds, inflammation, wound healing
INTRODUCTION
High‐mobility group box 1 (HMGB1) is a 30‐kDa chromatin‐binding protein that organizes the DNA structure in the cell nucleus. When the body is under stress such as cellular damage, HMGB1 is secreted to the extracellular compartment and functions as a damage‐associated molecular pattern molecule that signals the innate immune system to trigger a cascade of cell‐mediated inflammation and healing response.1, 2, 3, 4, 5, 6 HMGB1 can be passively diffused out of necrotic cells from early tissue damage. Immunocompetent cells, such as macrophages, can also actively secrete HMGB1 in response to the stimulation of proinflammatory cytokines.7 Extracellular HMGB1 interacts with multiple cell surface receptors such as the receptor for advanced glycation end products, Toll‐like receptor (TLR) 2, and TLR 4, which in turn activates the nuclear factor‐κB (NF‐κB) signal pathway. NF‐κB is a transcription factor found in almost all mammalian cell types,8 including human vocal fold fibroblasts.9, 10 The NF‐κB family of transcription factors plays a key role in the immune response by regulating a wide range of gene expression, including interleukins (ILs), tumor necrosis factors (TNFs), chemokines, growth factors, adhesion molecules, and inhibitors of apoptosis. In addition to its role in inflammation, extracellular HMGB1 plays an important role in soft tissue repair. HMGB1 promotes angiogenesis, neuronal differentiation,11 and fibroblast and stem cell migration and proliferation.12 Topical application of HMGB1 has been found to mitigate impaired wound healing in diabetic skin.13 Stem cell therapy, for instance, has been proposed to harness the property of extracellular HMGB1 in creating a favorable environment for stem cells to repair or regenerate the wounded tissue.12
We previously reported the temporal and spatial expression of HMGB1 in acute surgical vocal fold injury.14 Vocal fold HMGB1 was observed to translocate from nuclear to cytoplasmic and from cytoplasmic to extracellular within the first 3 days and between day 3 and day 7 postsurgery, respectively. In the present study, we investigated the cellular origin of HMGB1 in surgically injured rat vocal folds using immunohistochemistry (IHC) in addition to the synergy of HMGB1 and two other cytokines in the induction and propagation of vocal fold inflammation and repair. Protein levels of proinflammatory cytokines IL‐1β and TNF‐α were measured up to 4 weeks after vocal fold injury using enzyme‐linked immunosorbent protein assays (ELISAs).
Activated macrophages and fibroblasts have been documented as the primary cell types to release HMGB1 in response to injury in other parts of the body.6 Given that both cells were populated in native and injured vocal fold lamina propria,15, 16, 17 we hypothesized that activated macrophages and fibroblasts would be the primary cell source of extracellular HMGB1. We also hypothesized that the secretion of HMGB1 would be associated with a response of immunocompetent cells to the early accumulation of IL‐1β and TNF‐α in the injured lamina propria. Secreted HMGB1 is also known to induce an autocrine and paracrine stimulation of proinflammatory cytokines via the NF‐κB signaling pathway. As such, we hypothesized that the protein levels of HMGB1, IL‐1β, and TNF‐α would be sustained in the wounded lamina propria beyond the acute phase of injury.
MATERIALS AND METHODS
Rat Vocal Fold Surgery
The animal study was approved by the institutional animal care and use committees of the University of Wisconsin–Madison (protocol MO2358) and of the University of Maryland–College Park (protocol R‐12‐85). A total of 140 Sprague‐Dawley male rats, 4 to 6 months old (450–500 g), were used in the study. Vocal fold injuries were created by following an established protocol.18 In brief, animals were anesthetized and their vocal folds were injured bilaterally using a 25‐gauge needle to strip the vocal folds until the thyroarytenoid muscle was exposed. At the endpoint of the study, animals were euthanized via CO2 asphyxiation and larynges were removed immediately following euthanasia.
For histological analysis, a total of 12 rats were used. Two rats with injured vocal folds were euthanized at each of five postsurgery time points: 1 day, 3 days, 5 days, 1 week, and 4 weeks. An additional two animals were utilized as uninjured controls. For protein analysis of IL‐1β and TNF‐α, two additional postsurgery time points (2 days and 2 weeks) were included in the design to provide a finer time scale to understand the relationship of HMGB1 and other proinflammatory cytokines. Sixteen rats were euthanized as uninjured controls and at each of seven postsurgery time points (1 day, 2 days, 3 days, 5 days, 1 week, 2 weeks, and 4 weeks), respectively. A total of 128 rats were thus used for the protein analysis.
IHC of HMGB1 and Cell Markers
Colocalization of HMGB1, ED1, fibroblast‐specific protein 1 (FSP1), and alpha smooth muscle actin (α‐SMA) was assessed using immunofluorescent staining. ED1, also known as CD68, is a classical cell surface marker of tissue macrophages; FSP1, also known as S100A4, is a cytoplasmic calcium‐binding protein and highly expressed in fibroblasts19; α‐SMA is known to be mostly expressed in vascular smooth muscle cells and activated myofibroblasts.20, 21 Larynges were fixed in formalin and embedded in paraffin followed by 5‐μm coronal sectioning. Only sections from the midmembranous portion of the vocal folds underwent staining. First, antigen retrieval using sodium citrate and heat was performed and primary antibodies were applied overnight at 4°C (Table 1). The next day, slides were washed with phosphate‐buffered saline (PBS) and corresponding fluorescence‐conjugated secondary antibodies were applied for 60 minutes at room temperature. Slides were then washed with PBS again and mounted with VECTASHIELD Mounting Medium with 4,6‐diamidino‐2‐phenylindole (DAPI; Vector Laboratories, Burlingame, CA) to preserve fluorescence and to label cell nuclei. Negative controls were processed identically without application of primary antibodies. All stained sections were viewed and photographed with an ECLPSE NI‐U microscope and DS‐QI1 microscope digital camera, respectively (Nikon, Tokyo, Japan).
Table 1.
Primary and Secondary Antibodies Used for Immunohistochemistry.
| Costaining Pair | Primary Antibody (dilution) | Catalogue | Secondary Antibody (dilution) | Catalogue |
|---|---|---|---|---|
| HMGB1–ED1 | Rabbit polyclonal HMGB1 (1:250) | LS‐C2691, LSBio, Seattle, WA | Antirabbit Alexa 488 (1:1,000) | A11008, Invitrogen, Carlsbad, CA |
| Mouse monoclonal ED1 (1:500) | LS‐C2691, LSBio, Seattle, WA | Antimouse Texas red (1:200) | A11031, Invitrogen, Carlsbad, CA | |
| HMGB1–FSP1 | Mouse monoclonal HMGB1 (1:100) | ab11354, Abcam, Cambridge, MA | Antimouse Alexa 488 (1:200) | A11029, Invitrogen, Carlsbad, CA |
| Rabbit polyclonal FSP1 (1:100) | ABF32, EMD Millipore, Billerica, MA | Antirabbit Texas red (1:200) | A11036, Invitrogen, Carlsbad, CA | |
| HMGB1–α‐SMA | Mouse monoclonal HMGB1 (1:100) | ab11354, Abcam, Cambridge, MA | Antimouse Alexa 488 (1:200) | A11029, Invitrogen, Carlsbad, CA |
| Rabbit polyclonal α‐SMA (1:100) | Ab5694, Abcam, Cambridge, MA | Antirabbit Texas red (1:200) | A11036, Invitrogen, Carlsbad, CA |
FSP1 = fibroblast‐specific protein 1; HMGB1 = high‐mobility group box 1; α‐SMA = alpha smooth muscle actin.
Quantification of IL‐1β and TNF‐α Proteins
Microdissection procedures of vocal fold mucosae were performed. For each larynx, both vocal fold mucosae were dissected from the thyroarytenoid muscle under a stereo dissection microscope (Fisher Scientific, Pittsburgh, PA). Based on previous work, four pairs of vocal folds were necessary to give one pooled sample for cytokine protein analysis. Therefore, per each time point, 16 pairs of vocal fold mucosae were dissected to give four workable testing samples for protein extractions. Pooled samples were homogenized on ice and lysed using T‐PER total protein extraction kit (Thermo Scientific, Rockford, IL) according to the manufacturer's instructions. Supernatant was collected and its protein concentration was measured using bicinchoninic acid (BCA) protein assay (Thermo Scientific) in duplicates. BCA data were used to normalize the downstream ELISA data for each corresponding sample.
ELISA kits (Abcam, Cambridge, MA) were used to quantify IL‐1β (ab100768) and TNF‐α (ab100785) proteins by following the manufacturer's instructions. Briefly, 100 μL of standards, positive control, and testing samples were added to each well and incubated for 2.5 hours at room temperature. Wells were washed four times with wash buffer and incubated for 1 hour at room temperature with 100 μL of biotinylated detection primary antibody. Subsequently, wells were washed four times in wash buffer and incubated for 45 minutes at room temperature with 100 μL of horseradish peroxidase–streptavidin solution. Wells were washed as previously described and incubated for 30 minutes at room temperature with 100 μL of color solution. One hundred fifty microliters of stop solution was added to each well. Wells were read at 450 nm on a KC4 microplate reader (BioTek, Winooski, VT). Experimental samples were run in duplicate.
Statistical Analysis
One‐way analysis of variance (ANOVA) was used to test differences in ELISA IL‐1β and TNFα expressions across time. If F tests revealed significant differences, post hoc pairwise comparisons using Bonferroni adjustments were carried out. An α level of .05 was employed for F tests. Adjusted α levels of .007 were used to protect type I (α) error for post hoc analyses. All data met the assumptions of ANOVA.
RESULTS
Colocalization of HMGB1 and Cell Markers in Injured Rat Vocal Folds
Accumulation of positively stained ED1 and FSP1 cells was evident from day 1 though day 7 postsurgery (Fig. 1F, J, N, and R for ED1; Fig. 2F, J, N, and R for FSP1), concomitantly with the increase in HMGB1 staining (Fig. 1E, I, M, and Q). Starting from day 1 postsurgery, injured vocal folds revealed double‐stained HMGB1‐ED1 cells (Fig. 1G) and HMGB1‐FSP1 cells (Fig. 2G) as compared to uninjured controls (Figs. 1C and 2C, respectively). Obvious cytoplasmic‐to‐extracellular deposition of HMGB1 was observed in the vicinity of ED1‐positive (Fig. 1M and Q) and FSP1‐positive cells (Fig. 2M and Q) on day 5 and day 7 postsurgery. On day 14, both ED1‐positive (Fig. 1V) and FSP1‐positive (Fig. 2V) cells returned to baseline as compared to uninjured controls.
Figure 1.
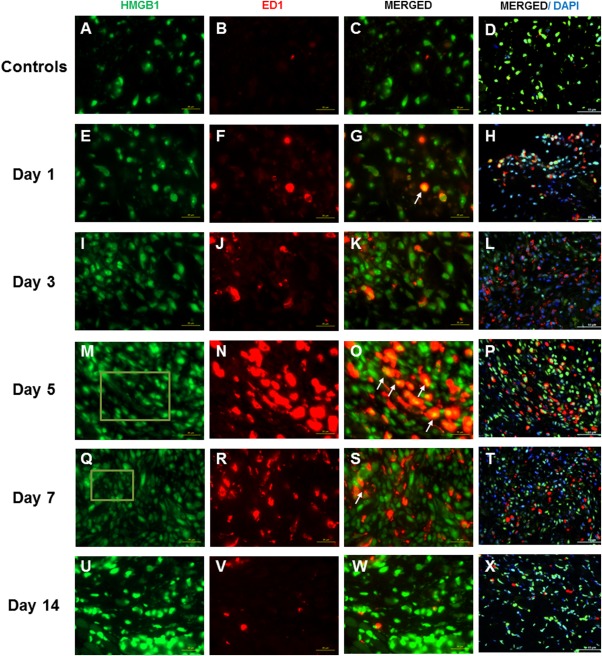
High‐mobility group chromosomal box (HMGB1) and ED1 expression following vocal fold surgical injury. (A, E, I, M, Q, U) Representative immunohistochemical (IHC) staining of HMGB1 in uninjured and injured rat vocal folds over 2 weeks postsurgery. HMGB1 is stained green. (B, F, J, N, R, V) Representative IHC staining of ED1 of the same animals. ED1 is stained red. (C, G, K, O, S, W) Representative IHC staining of merged HMGB1 and ED1 of the same animals. (D, H, L, P, T, X) Representative IHC staining of merged HMGB1, ED1, and 4,6‐diamidino‐2‐phenylindole (DAPI) of the same animals. Yellow cells are costained with HMGB1 (green) and ED1 (red). Examples are indicated with white arrows. In addition to nuclear expression, extracellular HMGB1 staining was evident in injured samples at day 5 and day 7 postsurgery. Examples are indicated in green boxes. At day 14, extracellular HMGB1 deposition became less evident. All images were taken at ×630 original magnification (scale bar = 20 μm), except D, H, L, P, T, and X, which were taken at ×400 original magnification (scale bar = 50 μm) to indicate the relative distribution of ED1‐positive cells to the overall cell population.
Figure 2.
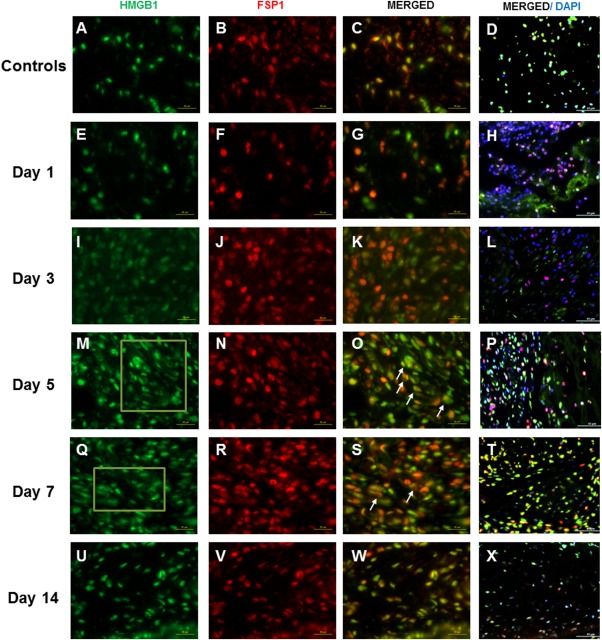
High‐mobility group chromosomal box (HMGB1) and fibroblast‐specific protein 1 (FSP1) expression following vocal fold surgical injury. (A, E, I, M, Q, U) Representative immunohistochemical (IHC) staining of HMGB1 in uninjured and injured rat vocal folds over 2 weeks postsurgery. HMGB1 is stained green. (B, F, J, N, R, V) Representative IHC staining of FSP1 of the same animals. FSP1 is stained red. (C, G, K, O, S, W) Representative IHC staining of merged HMGB1 and FSP1 of the same animals. (D, H, L, P, T, X) Representative IHC staining of merged HMGB1, FSP1, and 4,6‐diamidino‐2‐phenylindole (DAPI) of the same animals. Yellow cells are costained with HMGB1 (green) and FSP1 (red). Examples are indicated with white arrows. In addition to nuclear expression, extracellular HMGB1 staining was evident in injured samples at day 5 and day 7 postsurgery. Examples are indicated in green boxes. At day 14, extracellular HMGB1 deposition became less evident. All images were taken at ×630 original magnification (scale bar = 20 μm), except D, H, L, P, T, and X, which were taken at ×400 magnification (scale bar = 50 μm) to indicate the relative distribution of FSP1‐positive cells to the overall cell population.
Positively stained α‐SMA cells were primarily aggregated around blood vessel–like structures. α‐SMA–positive stains were not found in other regions of the lamina propria, as shown by the minimum number of cells costained with DAPI and α‐SMA (Fig. 3H, L, and P). Accumulation of α‐SMA–positive cells was first seen on day 1 (Fig. 3J) and became more evident from day 5 to day 14 postsurgery (Fig. 3P, T, and X). Minimal colocalization of HMGB1‐stained and α‐SMA–stained cells were observed at all postsurgical time points.
Figure 3.
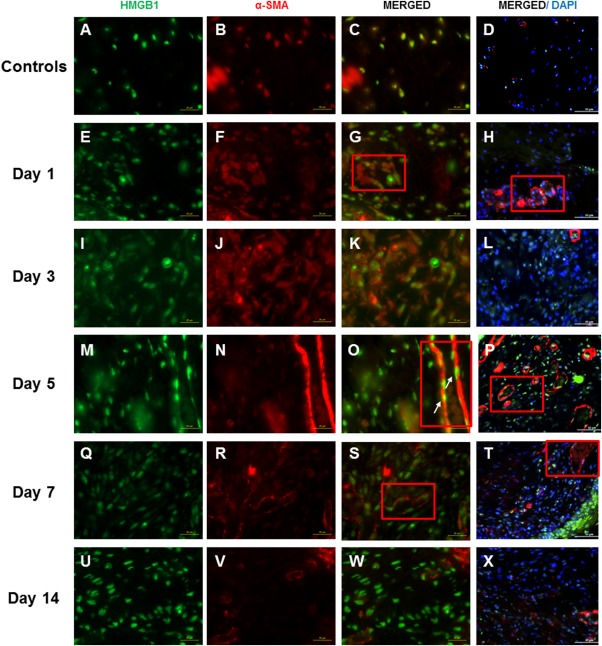
High‐mobility group chromosomal box (HMGB1) and alpha smooth muscle actin (α‐SMA) expression following vocal fold surgical injury. (A, E, I, M, Q, U) Representative immunohistochemical (IHC) staining of HMGB1 of the uninjured and injured rat vocal folds over 2 weeks postsurgery. HMGB1 is stained green. (B, F, J, N, R, V) Representative IHC staining of α‐SMA in the same animals. α‐SMA is stained red. Most α‐SMA–positive cells were found around the blood vessel walls. (C, G, K, O, S, W) Representative IHC staining of merged HMGB1 and α‐SMA of the same animals. (D, H, L, P, T, X) Representative IHC staining of merged HMGB1, α‐SMA, and 4,6‐diamidino‐2‐phenylindole (DAPI) of the same animals. Yellow cells are costained with HMGB1 (green) and α‐SMA (red). Examples are indicated with white arrows. The blood vessel walls are indicated with red boxes. All images were taken at ×630 magnification (scale bar = 20 μm), except D, H, L, P, T, and X, which were taken at ×400 magnification (scale bar = 50 μm) to indicate the relative distribution of α‐SMA‐positive cells to the overall cell population.
Time‐Varying IL‐1β and TNF‐α Quantities in Injured Rat Vocal Folds
Both IL‐1β and TNF‐α showed significant overall differences (P ≤ .05) in expression across time points (Figs. 4 and 5). Compared to uninjured controls, significantly increased IL‐1β expression was observed at the 2 days (P = .007) postinjury time point. TNF‐α protein levels significantly increased at 1 day (P = .007) and 2 days (P = .001) postinjury time points. Both IL‐1β and TNF‐α was not significantly different from their uninjured controls at 3 days postinjury onward.
Figure 4.
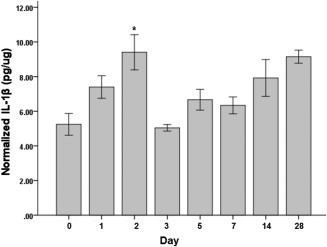
Interleukin (IL)‐1β protein levels normalized to the total protein level in the tissue samples following vocal fold surgical injury. IL‐1β concentrations are in picograms per microgram. Bars and error bars represent mean and standard error of the data (n = 32), respectively. Asterisk denotes that data for that time point are statistically significant compared to uninjured controls (Bonferroni‐corrected alpha: .05/7 = .007).
Figure 5.
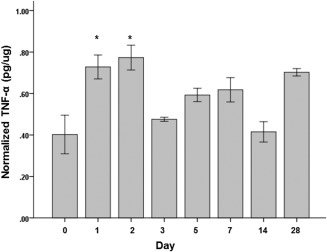
Tumor necrosis factor (TNF)‐α protein levels normalized to the total protein level in the tissue samples following vocal fold surgical injury. TNF‐α concentrations are in picograms per microgram. Bars and error bars represent mean and standard error of the data (n = 32), respectively. Asterisks denote that data for that time point are statistically significant compared to the uninjured controls (Bonferroni‐corrected alpha: .05/7 = .007).
DISCUSSION
The objective of this study was to characterize the cell source and the roles of extracellular HMGB1 in vocal fold injury using a common rat injury model. Our IHC results showed that ED1‐positive and FSP1‐positive cells represented the major cell populations in postsurgical rat vocal folds (Figs. 1 and 2). Concurrently, an active cytoplasmic‐to‐extracellular translocation of HMGB1 was observed between day 5 and day 7 that was similarly seen in our previous HMGB1 IHC results.14 Vocal fold macrophages and fibroblasts were a major source of extracellular HMGB1 in wounded vocal folds.
Conversely, minimal colocalization of HMGB1‐positive and α‐SMA–positive cells was observed in this study, indicating that this group of cells did not strongly secrete HMGB1. α‐SMA is known to stain myofibroblasts and smooth muscle cells in blood vessel walls.20, 21 A myofibroblast is a morphologically and functionally distinctive form of a fibroblast such that during tissue repair, a fibroblast differentiates partially into a smooth muscle cell.22 In this study, cells stained with α‐SMA were mostly observed around capillary‐like structures but not in the lamina propria, suggesting that those α‐SMA–positive cells were vascular smooth muscle cells (Fig. 3G, H, O, P, S, and T). Previous in vitro studies suggested a strong expression of α‐SMA in vocal fold fibroblast–myofibroblast differentiation.23, 24 Interestingly, α‐SMA–expressed myofibroblasts were barely observed in the lamina propria in the literature of in vivo vocal fold scarring.16, 25 In general, stromal cells including fibroblasts express diffuse α‐SMA.26 The phenotype of myofibroblast is defined when the α‐SMA is incorporated into the actin stress fibers and the cytoskeleton microfilament structure becomes visible.27, 28 One possibility is that the α‐SMA is not yet fully incorporated into the actin stress fibers at day 14, and thus the fibroblast–myofibroblast differentiation may only be detectable at a very late stage of tissue repair, beyond the time point of the current study. In addition, at day 14 a cell population of HMGB1‐positive cells that were ED1‐negative, FSP1‐negative, or α‐SMA‐negative were observed. Those cells may be the macrophages or fibroblasts that are in the transition of their phenotypes during this stage of tissue remodeling and they thus did not express a high level of classical IHC cell markers. Further study is required to understand the heterogeneity of macrophages and fibroblasts in vocal fold wound healing.
In addition to the cell source of HMGB1, the synergy of HMGB1 and proinflammatory cytokine IL‐1β and TNF‐α was examined. Compared to the uninjured controls, protein levels of IL‐1β and TNF‐α significantly increased at day 1 and day 2, respectively. Concurrently, our previous ELISA results indicated that the nuclear–cytoplasmic translocation of HMGB1 peaked at day 3 and then the translocation activity diminished from day 5 postinjury onward.14 The 3‐day peak of cytoplasmic HMGB1 may be an active response to IL‐1β and TNF‐α stimulation in addition to the release by necrotic cells. In addition to acting as an early trigger of inflammation, HMGB1 is also reported to be a late‐acting, downstream modulator of tissue repair.29, 30 In vitro studies have shown that exogenous HMGB1 stimulates the synthesis of proinflammatory cytokines from human monocytes31 as well as the proliferation and migration of 3T3 fibroblasts.32 The role of HMGB1 as a late mediator of inflammation and repair has opened a new avenue for the development of HMGB1‐target therapeutics for fibrotic diseases.33
If vocal fold HMGB1 also has aforesaid dual roles, temporal expression of cytoplasmic HMGB1, IL‐1β, and TNF‐α should be bimodal in our animal data. We found protein levels of cytoplasmic HMGB1, IL‐1β, and TNF‐α peaked within the first 3 days but then started to decline to levels comparable to uninjured controls. An apparent rebound of cytoplasmic HMGB1 at day 7, IL‐1β at day 14, and TNF‐α at day 28 postinjury was observed. The amount of the increase, however, was not statistically significant compared to those of uninjured controls.
A similar rebound of IL‐1β protein level has been reported in a rabbit vocal fold study,34 in which the level of IL‐1β peaked at day 1 and rebounded at day 14 following surgery. One speculation is that a high‐level HMGB1 secretion is necessary to trigger a full‐blown inflammatory response at the acute phase of injury. Alternatively, low‐level HMGB1 secretion may be beneficial and necessary to sustain the synthesis of proinflammatory cytokines for the ongoing tissue repair process. Nevertheless, the interactions of HMGB1 and other proinflammatory cytokines will need further in vitro and in vivo studies to systematically block HMGB1 and other cytokine functions to decipher their regulatory relationships. Small interfering RNAs, for instance, were used to knock down HMGB1 or TNF‐α functions in mice with acute liver failure. Results showed that a synergistic relationship of TNF‐α and HMGB1 and the positive feedback loop of these two cytokines further amplified the downstream proinflammatory signals in acute liver injury.35
This investigation has a limitation that warrants discussion. Cell surface marker FSP1 was used to identify the fibroblast phenotype in the present study. FSP1 has been widely accepted as a fibroblast‐specific marker.19, 36 However, monocytes and macrophages can also be positively stained with FSP in other soft tissues.34, 37 In the vocal fold literature, macrophages were reported to be minimally migrated or proliferated in injured rat and porcine vocal folds using IHC staining.16, 38 Given that fibroblasts are known to dominantly present over the course of inflammation and healing,16 we thus assumed that macrophages constituted a negligible population of FSP1‐positive cells in this study.39 We are, however, cautious that a subpopulation of macrophages might express FSP1 in acute inflamed vocal folds. Further work is currently being undertaken to use multiparameter flow cytometry with better sensitivity and specificity to characterize cell subpopulations in surgically injured vocal folds. Computational models are concomitantly used to numerically simulate the cellular and biochemical dynamics of vocal fold injury and repair.40, 41 Simulation results will enable a deeper mechanistic understanding of the cytokine rebound phenomenon. Further investigation determining which specific receptor(s) is (are) activated by HMGB1 in vocal fold macrophages or fibroblasts, as HMGB1 interacts with multiple surface receptors in triggering the production of cytokines, is warranted. The work will elucidate the roles of HMGB1 in vocal fold repair and direct the design of HMGB1‐targeted therapies for vocal fold injury and fibrosis.
CONCLUSION
In surgically injured vocal folds, the danger signal HMGB1 was secreted by the native and infiltrated macrophages and fibroblasts and was likely related to the stimulation of IL‐1β and TNF‐α during the acute phase of wound healing. In addition, HMGB1 may act as a late mediator to incite ongoing vocal fold repair by inducing a low level of IL‐1β and TNF‐α during the subacute phase of the injury. Determining mechanisms of HMGB1 release and signal pathways for HMGB1‐mediated inflammatory responses is warranted for further studies.
Acknowledgment
The authors thank Kendra Browne and Melanie Peragine for assistance with animal surgery.
The study was funded by the National Institute on Deafness and Other Communication Disorders (R01DC4336, RO1DC9600, and R03DC012112).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med 2008;14:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol 2007;3:382–390. [DOI] [PubMed] [Google Scholar]
- 3. Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 2004;255:320–331. [DOI] [PubMed] [Google Scholar]
- 4. Yang H, Tracey KJ. High mobility group box 1 (HMGB1). Crit Care Med 2005;33:S472–S474. [DOI] [PubMed] [Google Scholar]
- 5. Peltz ED, Moore EE, Eckels PC, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock 2009;32:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 2011;29:139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lotze MT, Tracey KJ. High‐mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev 2005;5:331–342. [DOI] [PubMed] [Google Scholar]
- 8. Lawrence T. The nuclear factor NF‐kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Branski RC, Zhou H, Sandulache VC, Chen J, Felsen D, Kraus DH. Cyclooxygenase‐2 signaling in vocal fold fibroblasts. Laryngoscope 2010;120:1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King SN, Berchtold CM, Thibeault SL. Lipopolysaccharide responsiveness in vocal fold fibroblasts. J Inflamm (Lond) 2014;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Yu L, Zhang T, et al. HMGB1 enhances embryonic neural stem cell proliferation by activating the MAPK signaling pathway. Biotechnol Lett 2014;36:1631–1639. [DOI] [PubMed] [Google Scholar]
- 12. Hayakawa K, Pham LD, Arai K, Lo EH. High‐mobility group box 1: an amplifier of stem and progenitor cell activity after stroke. Acta Neurochir Suppl 2013;118:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Straino S, Di Carlo A, Mangoni A, et al. High‐mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol 2008;128:1545–1553. [DOI] [PubMed] [Google Scholar]
- 14. Li NY, Lee BJ, Thibeault SL. Temporal and spatial expression of high‐mobility group box 1 in surgically injured rat vocal folds. Laryngoscope 2012;122:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Catten M, Gray SD, Hammond TH, Zhou R, Hammond EH. Analysis of cellular location and concentration in vocal fold lamina propria. Otolaryngol Head Neck Surg 1998;118:663–667. [DOI] [PubMed] [Google Scholar]
- 16. Tateya I, Tateya T, Lim X, Sohn JH, Bless DM. Cell production in injured vocal folds: a rat study. Ann Otol Rhinol Laryngol 2006;115:135–143. [DOI] [PubMed] [Google Scholar]
- 17. Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen 2010;18:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res 2009;52:1008–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 1995;130:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rockey DC, Weymouth N, Shi Z. Smooth muscle alpha actin (Acta2) and myofibroblast function during hepatic wound healing. PLoS One 2013;8:e77166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherng S, Young J, Ma H. Alpha‐smooth muscle actin (α‐SMA). J Am Sci 2008;4:7–9. [Google Scholar]
- 22. Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 2011;57:376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Branco A, Bartley SM, King SN, Jette ME, Thibeault SL. Vocal fold myofibroblast profile of scarring. Laryngoscope 2016;126:E110–E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vyas B, Ishikawa K, Duflo S, Chen X, Thibeault SL. Inhibitory effects of hepatocyte growth factor and interleukin‐6 on transforming growth factor‐beta1 mediated vocal fold fibroblast‐myofibroblast differentiation. Ann Otol Rhinol Laryngol 2010;119:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johns MM, Kolachala V, Berg E, Muller S, Creighton FX, Branski RC. Radiation fibrosis of the vocal fold: from man to mouse. Laryngoscope 2012;122(suppl 5):S107–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha‐smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001;12:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darby I, Skalli O, Gabbiani G. Alpha‐smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990;63:21–29. [PubMed] [Google Scholar]
- 28. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano‐regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363. [DOI] [PubMed] [Google Scholar]
- 29. Lutz W, Stetkiewicz J. High mobility group box 1 protein as a late‐acting mediator of acute lung inflammation. Int J Occup Med Environ Health 2004;17:245–254. [PubMed] [Google Scholar]
- 30. Czura CJ, Tracey KJ. Targeting high mobility group box 1 as a late‐acting mediator of inflammation. Crit Care Med 2003;31:S46–S50. [DOI] [PubMed] [Google Scholar]
- 31. Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG‐1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000;192:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ranzato E, Patrone M, Pedrazzi M, Burlando B. Hmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE‐dependent ERK1/2 activation. Cell Biochem Biophys 2010;57:9–17. [DOI] [PubMed] [Google Scholar]
- 33. Li LC, Gao J, Li J. Emerging role of HMGB1 in fibrotic diseases. J Cell Mol Med 2014;18:2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Branski RC, Rosen CA, Verdolini K, Hebda PA. Biochemical markers associated with acute vocal fold wound healing: a rabbit model. J Voice 2005;19:283–289. [DOI] [PubMed] [Google Scholar]
- 35. Wang W, Sun L, Deng Y, Tang J. Synergistic effects of antibodies against high‐mobility group box 1 and tumor necrosis factor‐alpha antibodies on D‐(+)‐galactosamine hydrochloride/lipopolysaccharide‐induced acute liver failure. FEBS J 2013;280:1409–1419. [DOI] [PubMed] [Google Scholar]
- 36. Lawson WE, Polosukhin VV, Zoia O, et al. Characterization of fibroblast‐specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:899–907. [DOI] [PubMed] [Google Scholar]
- 37. Osterreicher CH, Penz‐Osterreicher M, Grivennikov SI, et al. Fibroblast‐specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A 2011;108:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King SN, Guille J, Thibeault SL. Characterization of the leukocyte response in acute vocal fold injury. PLoS One 2015;10:e0139260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawai Y, Kishimoto Y, Suzuki R, et al. Distribution and characteristics of slow‐cycling cells in rat vocal folds. Laryngoscope 2016;126:E164–E170. [DOI] [PubMed] [Google Scholar]
- 40. Li NYK, Abbott KV, Rosen C, An G, Hebda PA, Vodovotz Y. Translational systems biology and voice pathophysiology. Laryngoscope 2010;120:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li NYK, Vodovotz Y, Hebda PA, Abbott KV. Biosimulation of inflammation and healing in surgically injured vocal folds. Ann Otol Rhinol Laryngol 2010;119:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]


