Abstract
Background
Multiple meta-analyses of diffusion tensor imaging (DTI) studies have reported impaired white matter integrity in patients with major depressive disorder (MDD). However, owing to inclusion of medicated patients in these studies, it is difficult to conclude whether these reported alterations are associated with MDD or confounded by medication effects. A meta-analysis of DTI studies on medication-free (medication-naive and medication washout) patients with MDD would therefore be necessary to disentangle MDD-specific effects.
Methods
We analyzed white matter alterations between medication-free patients with MDD and healthy controls using anisotropic effect size–signed differential mapping (AES-SDM). We used DTI query software for fibre tracking.
Results
Both pooled and subgroup meta-analyses in medication washout patients showed robust fractional anisotropy (FA) reductions in white matter of the right cerebellum hemispheric lobule, body of the corpus callosum (CC) and bilateral superior longitudinal fasciculus III (SLF III), whereas FA reductions in the genu of the CC and right anterior thalamic projections were seen in only medication-naive patients. Fibre tracking showed that the main tracts with observed FA reductions included the right cerebellar tracts, body of the CC, bilateral SLF III and arcuate fascicle.
Limitations
The analytic techniques, patient characteristics and clinical variables of the included studies were heterogeneous; we could not exclude the effects of nondrug therapies owing to a lack of data.
Conclusion
By excluding the confounding influences of current medication status, findings from the present study may provide a better understanding of the underlying neuropathology of MDD.
Introduction
Major depressive disorder (MDD) is a debilitating and pervasive mental illness with a 12-month and a lifetime prevalence of 6.6% and 16.2% respectively.1 The disorder is the third leading cause of global disease burden, with annual total costs to society exceeding $80 billion worldwide,2 and is predicted to be the leading health burden worldwide by 2030.3 Numerous studies have reported cognitive dysregulation and behavioural and physical dysfunction in individuals with MDD.4–6 In order to identify more effective treatment options, greater efforts are needed to elucidate the neurobiological dysfunctions underlying MDD.
Neuroimaging studies have sought to identify the brain abnormalities in individuals with MDD, and a network perspective involving functional and structural connectivity has been widely recognized.7–10 However, structural connectivity with higher within-subject test–retest reliability than functional connectivity offers a more practical approach to characterizing brain abnormalities related to the disease.11 The disruption of cortical-–subcortical neural circuits, including the hypothalamic–pituitary–adrenal (HPA) axis and fronto-limbic circuits, may be involved in the etiology of MDD.12–15 White matter abnormalities have been proposed as potential biomarkers of MDD.11
Diffusion tensor imaging (DTI) is a widely used technique to visualize and measure the diffusion of water in brain tissue, and it is a particularly useful tool for evaluating white matter abnormalities16 and treatment outcomes.17 Fractional anisotropy (FA) is a measure of anisotropic water diffusion that reflects the degree of directionality of cellular structures within the fibre tracts. Generally, it is positively associated with white matter anisotropy, and a loss of anisotropic diffusion is often associated with abnormalities in the cellular microstructure (i.e., the integrity of white matter pathways).18–20 Two whole-brain analysis methods are commonly used. The voxel-based analysis (VBA) technique involves analyzing all white matter voxels and correcting for multiple comparisons and noise by reporting only contiguous clusters of significant voxels. Tract-based spatial statistics (TBSS) isolates the central core of white matter tracts with the highest FA and reports significant clusters within that white matter skeleton.21 Results from whole-brain studies have suggested widespread white matter abnormalities in MDD in regions such as the frontal gyrus,15,22,23 cerebellum,22,24,25 right thalamus,24,25 right parietal lobe,22,23 corpus callosum (CC)26,27 and superior longitudinal fasciculus (SLF).28–30 However, these results are not consistent, probably owing to small or heterogeneous study samples and methodological differences. A whole-brain meta-analysis detecting common regions of white matter abnormality from DTI studies of patients with MDD is therefore of particular importance.
Three recent meta-analyses of DTI studies (Appendix 1, Table S1, available at jpn.ca) found that MDD was characterized by FA alterations in the right inferior fronto-occipital fasciculus,11,31 left SLF,31 CC,11,32 right inferior longitudinal fasciculus and the right posterior thalamic radiation.11 However, previous meta-analyses, particularly the earlier ones, included medicated samples because a subgroup analysis of unmedicated or even drug-free patients would not have been possible in the beginning owing to the small number of published DTI studies. The inclusion of medicated patients may bias meta-analyses and limit the interpretability and generalizability of the results, as a few studies have reported the effects of antidepressant medication on white matter microstructure.33–35 One study reported that the increment in rich club hub connections was greater in the creatine group than in the placebo group, suggesting the effects of creatine administration on brain network organization may partly underlie its efficacy in treating women with MDD.36 Moreover, compared with patients who had current MDD, those with remitted MDD showed an altered pattern of intracommunicability within the default mode network (DMN) and intercommunicability between the DMN and other subnetworks, including the visual recognition network and salience network, after treatment with selective serotonin reuptake inhibitors.37 However, FA reductions in these involved regions (e.g., superior frontal, superior parietal and precentral areas and precuneus) were found in patients with MDD versus controls in some original studies.38–40 It is therefore conceivable that antidepressant medications themselves could result in regional white matter microstructure changes in patients with MDD.
In order to elucidate brain abnormalities directly related to the disease without the confounding effect of current medication, a meta-analysis of studies investigating medication-free patients with MDD who either had never received antidepressant medications (i.e., medication-naive) or who underwent a washout period before MRI scanning would be of great value. Nevertheless, to our knowledge no such meta-analysis has been published to date despite the increase in published DTI studies on medication-free patients with MDD.13,15,22–30,41–44 To this end, the aim of the present meta-analysis is to provide an updated quantitative summary of studies investigating white matter abnormalities in medication-free patients with MDD and the effects of demographics and clinical characteristics on white matter microstructure in individuals with MDD. Differences between the findings of current and previous meta-analyses would provide a clearer picture of the white matter alterations directly related to the illness and those potentially influenced by current antidepressant treatment. We used anisotropic effect size–signed differential mapping (AES-SDM),45 which is the new version of effect size–signed differential mapping (ES-SDM)46 and has been successfully applied to dementia with Lewy bodies,47 childhood maltreatment,48 bipolar disorder and MDD.32 Additionally, fibre tracking was performed to demonstrate the most probable tracts passing through the white matter regions of altered FA identified by AES-SDM, providing a more intuitive visual platform to appreciate the meta-analysis results.
Methods
Inclusion of studies
We used a systematic strategy to identify relevant studies from PubMed, MEDLINE, EMBASE, Web of Science and Science Direct published up to December 2014, using the keywords “depression” or “depressive disorder” or “unipolar depression” or “depress*” plus “diffusion tensor imaging” or “diffusion tensor” or “DTI.” To ensure completeness, we searched within all fields (including titles and abstracts) using those keywords. In addition, we manually searched the reference lists from relevant reviews, meta-analyses and empirical papers found in our original electronic search to identify further studies for inclusion. Studies were included if they used DTI to investigate FA changes in the whole brain (or whole white matter) in patients with MDD, compared patients with MDD and healthy controls using VBA or TBSS approaches, investigated medication-free patients with MDD and if they were written in English. We excluded studies from which peak coordinates could not be retrieved from the published article or after contacting the authors; studies in which different thresholds were used in different regions of the brain; studies whose findings were based on regions of interest or on small volume correction; theoretical papers, case reports and reviews; and studies that reported adolescent or late-life depression and/or included participants with comorbid conditions (e.g., Parkinson disease, mania or traumatic brain injury). We tried our best to contact authors for more information when necessary (e.g., conference papers). Two of us (J.J. and Y.J.Z.) independently conducted the literature search. The results were compared, any inconsistent result was discussed and resolved by consensus.
Quality assessment
Two of us (J.J. and Y.J.Z.) independently rated each included study for quality and completeness using a 12-point checklist that was adapted from previous meta-analyses.49–52 We modified this checklist to reflect critical variables that were important in assessing DTI studies. The checklist was divided into 3 categories: participants (items 1–4), methods for image acquisition and analysis (items 5–10), and results and conclusions (items 11 and 12; summarized in Appendix 1, Table S2). Each item received a score of 1, 0.5 or 0 if the criteria were fully, partially or not met, respectively.49
Recorded variables
For each included study, we recorded the following variables: sample size, sex and mean age of participants, illness duration, depression symptom severity, mean number of episodes, percentage of patients who ever received psychotherapy, drug and nondrug therapy status, statistical threshold of main findings, method used to correct whole-brain results for multiple comparisons, magnetic field strength, channels of head coil, acquisition voxel size, number of diffusion directions and type of analysis.
Meta-analysis of regional FA differences in white matter
We analyzed FA differences in white matter between patients with MDD and healthy controls using AES-SDM (www.sdmproject.com),32 a voxel-based meta-analytic approach that uses the reported peak coordinates to recreate original maps of the effect size of FA differences in white matter between patients and controls rather than just assessing the probability or likelihood of a peak.53 The AES-SDM method incorporates the positive features of earlier methods (e.g., activation likelihood estimation, multilevel kernel density analysis), reconstructs positive and negative differences in the same signed differential map to avoid any voxel appearing significant in opposite directions, and uses effect size to combine reported peak coordinates with statistical parametric maps.46 Complementary analyses, such as jackknife, subgroup and meta-regression analyses, can help us assess the robustness and heterogeneity of the results.46 The kernels of the previous versions of ES-SDM are isotropic (i.e., the effect size of a voxel close to a peak would depend only on the effect size of the peak and the Euclidian distance between the voxel and the peak).45 Based on ES-SDM, AES-SDM introduces a novel improvement: it adopts anisotropic kernels to assign different values to different neighbouring voxels based on the spatial correlation between them (i.e., highly correlated voxels are estimated to have larger effect sizes, whereas uncorrelated voxels are estimated to have smaller or null effect sizes). To our knowledge, the correlated voxels are more likely to be from the same brain region. The recreation of effect size maps using anisotropic kernels can be more accurate than using isotropic kernels, thus allowing more exhaustive and accurate meta-analyses.45
The AES-SDM method has been described in detail elsewhere.45,46 Therefore, we restrict our summary to its main features. First, peak coordinates and effect sizes (e.g., derived from t values) of white matter FA differences between patients with MDD and healthy controls were extracted from each data set. Notably, to avoid biases toward liberally thresholded brain regions, we excluded those peaks that did not appear statistically significant at the whole brain level, ensuring that the same threshold was applied to each included study.54 Second, a standard Montreal Neurological Institute (MNI) map of differences in FA was separately recreated for each study by means of an anisotropic Gaussian kernel, which assigns higher effect sizes to the voxels more correlated with peaks.45 Third, we obtained a map of the effect size variance for each study from its effect size map and sample size. Fourth, the mean map was derived by voxel-wise calculation of the random-effects mean of the study maps, weighted by the sample size and variance of each study, and accounting for interstudy heterogeneity. We assessed statistical significance using standard randomization tests, thus creating null distributions from which p values can be directly obtained.46 To allow combination of VBA and TBSS studies, we adopted the TBSS template included in AES-SDM, which has been used and described by Wise and colleagues32 as supplementary methods. We analyzed results from VBA studies only if they fell within the same areas used by TBSS studies.32 We used default AES-SDM thresholds (voxel p = 0.005, peak height z = 1, cluster extent = 10 voxels).46 The AES-SDM results are represented on a 3-dimensionally rendered brain, removing part of the left or right hemisphere and highlighting areas of the brain where FA alterations reached significant values.55
Sensitivity analysis
To test the replicability of the results, we used a systematic whole-brain voxel-based jackknife sensitivity analysis. This consists of repeating the main statistical analysis 15 times, discarding a different study each time. If a brain region remains significant in all or most of the combinations of studies, the finding is considered highly replicable.54
Fibre tracking
We used DTI query software56 to explore and display the most probable white matter tracts passing through clusters of voxels that showed significant FA group differences with reference to an atlas of human white matter anatomy.57 The white matter tracts were mapped using streamline tracking techniques and filtered by tract length and region of interest (ROI) centred on the coordinates of significant clusters.56,58 As a novel set of interaction techniques, DTI query allows placement and interactive manipulation of box-shaped ROIs to display pathways passing through specific anatomic areas, making it easier to explore and interpret white matter pathways.11 Numerical path properties, such as average FA, ROI volume and average curvature, can be further explored.56,59
Subgroup analyses
Two subgroup analyses were performed on the basis of medication status (medication washout and medication-naive). Strategically, the subgroup analysis of medication-naive patients with first-episode MDD could help reduce confounders, such as illness duration and previous antidepressant treatment, that may dilute the neuropathological findings in patients with MDD. We compared these results with the subgroup analysis of patients who underwent a washout period before MRI scanning to investigate the regional alterations that may be associated with a long disease process or previous treatment with antidepressant medication. Moreover, we performed a subgroup analysis of these studies with corrected results to throw light upon the possible effects of these uncorrected results on the overall conclusions.
Analysis of heterogeneity and publication bias
We examined the statistical (between-studies) heterogeneity of individual clusters using a random-effects model with Q statistics (χ2 distribution converted to z values) and tested with a permutation approach (uncorrected p < 0.005, peak height z = 1, cluster extent = 10 voxels). We created funnel plots of the peaks of the main findings in order to discard gross abnormalities, such as findings that were driven by only 1 study. We examined the possibility of publication bias for white matter regions showing altered FA using the Egger test in Stata software version 12.0 (www.stata.com/).
Meta-regression analysis
To characterize the potential effects of key demographic and clinical variables on white matter, we explored the following variables by means of simple linear regression: mean age of patients, percentage of female patients in the whole MDD group in each study, illness duration and depression symptom severity (Hamilton Rating Scale for Depression [HAM-D]) of patients with MDD. The mean number of illness episodes, nondrug therapy status and percentage of patients who had ever received psychotherapy could not be studied owing to a lack of data. As described in previous meta-analyses, we decreased the probability threshold to 0.000546,54,60 and discarded findings in regions other than those detected in the main analyses to minimize the detection of spurious associations. Furthermore, regression plots were visually inspected to discard fittings driven by too few studies.46,54
Results
Included studies and sample characteristics
The search strategy identified 4046 studies, 15 of which met our inclusion criteria,13,15,22–30,41–44 with 1 study reporting a significant difference in age between the MDD and control groups.44 They included a combined total of 434 medication-free patients with MDD (183 men and 251 women with a mean age of 34 [range 18–67] yr) and 429 controls (196 men and 233 women with a mean age of 33 [range 18–67] yr). These included studies provided 43 coordinates in total of altered FA in patients with MDD versus healthy controls. Two of the studies used overlapping samples, so we included that with the most participants43 after a discussion over email with the authors. The flow diagram of the identification and attrition of studies is shown in Appendix 1, Figure S1. The clinical and demographic data, scanning methods and altered FA regions from all included studies are summarized in Table 1 and Table 2. The quality scores, ranging from 10.5 to 12 (mean score 11.4), shown in Table 1 demonstrated that the included studies were of high quality, ensuring a more exhaustive and accurate meta-analysis.
Table 1.
Demographic and clinical characteristics of participants in the 15 studies included in the meta-analysis
| Study | No. participants (female); group | Age, yr; group | Disease state | Illness duration, yr | Severity (scale type) | Mean no. episodes | Statistical threshold | Fraction of patients receiving psychotherapy | Drug status | Nondrug therapy status | Quality scales (out of 12) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| MDD | Control | MDD | Control | ||||||||||
| Arnold et al.13 | 17 (14) | 21 (14) | 30 | 27 | Remitted | NA | 9 (HAM-D) | NA | pcorr < 0.001 | NA | Medication-free | NA | 11.5 |
| Choi et al.41 | 54 (26) | 54 (26) | 34 | 34 | Depressed | 7.77 | 19 (HAM-D) | NA | pFWE < 0.05 | NA | Medication-free for ≥ 2 wk | NA | 11 |
| Han et al.26 | 20 (15) | 22 (15) | 43 | 44 | Depressed | 0.3667 | 19 (HAM-D) | FE | pFWE < 0.05 | 0 | Naive | No | 12 |
| Hayashi et al.42 | 30 (13) | 30 (13) | 44 | 44 | Depressed | NA | > 14 (HAM-D) | FE | pFWE < 0.05 | 0 | Naive | No | 11.5 |
| Jia et al.22 | 52 (27) | 52 (28) | 35 | 37 | Depressed | 3.2628 | 23 (HAM-D) | NA | pFWE < 0.05 | 0 | Medication-free for ≥ 2 wk | No | 11.5 |
| Korgaonkar et al.44 | 29 (17) | 39 (21) | 41 | 30 | Depressed | NA | 19 (HAM-D) | NA | pFWE < 0.05 | NA | Medication-free | NA | 10.5 |
| Lai and Wu28 | 44 (23) | 27 (15) | 37 | 38 | Depressed | 0.39 | 22 (HAM-D) | FE | pFWE < 0.05 | 0 | Naive | NA | 11.5 |
| Ma et al.23 | 14 (12) | 14 (12) | 29 | 27 | Depressed | 0.8583 | 38 (BDI) | FE | puncorr < 0.001 | NA | Naive | NA | 11.5 |
| Olvet et al.27 | 52 (31) | 46 (21) | 36 | 30 | Depressed | NA | 19 (HAM-D) | 2 | pFWE < 0.05 | 14/52 | Medication-free for ≥ 2 wk | NA | 10.5 |
| Ouyang et al.15 | 18 (9) | 18 (9) | 27 | 27 | Depressed | 1.2583 | 24 (HAM-D) | FE | puncorr < 0.001 | NA | Naive | No ECT | 11.5 |
| Tha et al.24 | 19 (7) | 19 (6) | 39 | 37 | Depressed | 1.5308 | 19 (HAM-D) | NA | puncorr < 0.001 | NA | Medication-free for ≥ 6 mo | No ECT | 11.5 |
| Wang et al.25 | 21 (16) | 22 (14) | 30 | 30 | Depressed | 1.1833 | 20 (HAM-D) | NA | pASC < 0.05 | 0 | Medication-free for ≥ 4 wk | NA | 11.5 |
| Wu et al.29 | 23 (13) | 21 (12) | 31 | 30 | Depressed | 0.1808 | 22 (HAM-D) | FE | puncorr < 0.001 | 0 | Naive | No ECT | 12 |
| Zhu et al.43 | 25 (15) | 25 (15) | 21 | 20 | Depressed | 0.8617 | 35 (CESD) | FE | pcorr < 0.05 | 0 | Naive | No ECT in the past 6 mo | 12 |
| Zuo et al.30 | 16 (13) | 19 (12) | 37 | 37 | Depressed | NA | 30 (HAM-D) | NA | puncorr < 0.005 | NA | Medication-free for ≥ 2 wk | No ECT | 11.5 |
ASC = AlphaSim correction; BDI = Beck Depression Inventory; CESD = Center for Epidemiologic Studies Depression Scale; ECT = electroconvulsive therapy; FE = first episode; FWE = family-wise error correction; HAM-D = Hamilton Rating Scale for Depression; MDD = major depressive disorder; NA = not available.
Table 2.
Scanning methods and altered fractional anisotropy regions of the 15 studies included in this meta-analysis
| Study | Field, T (coil, channels) | Acquisition voxel, mm3 | No. of directions | Coordinate system | No. of coordinates | Type of analysis | Significant change in FA |
|---|---|---|---|---|---|---|---|
| Arnold et al.13 | 1.5 (8) | 2.5 × 2.5 × 2.5 | 30 | — | 0 | VBA | — |
| Choi et al.41 | 3 (12) | 2 × 2 × 2 | 60 | — | 0 | VBA, TBSS | — |
| Han et al.26 | 3 (12) | 1.8 × 1.8 × 3.0 | 20 | MNI | 1 | TBSS | Decrease observed in the body of the CC |
| Hayashi et al.42 | 3 (8) | 1.02 × 1.02 × 4 | 25 | — | 0 | TBSS | — |
| Jia et al.22 | 3 (8) | 1 × 1 × 3 | 15 | MNI | 4 | VBA | Both decreases and increases observed in the bilateral parietal lobe (subgyral), R frontal lobe (paracentral lobule) and R cerebellum (anterior lobe) |
| Korgaonkar et al.44 | 3 (8) | 1.72 × 1.72 × 2.5 | 42 | — | 0 | TBSS | — |
| Lai and Wu28 | 3 (NA) | 2 × 2 × 2 | 30 | MNI | 2 | TBSS | Both decreases and increases observed in the L SLF and R anterior thalamic radiation |
| Ma et al.23 | 1.5 (birdcage) | 1.875 × 1.875 × 4 | 13 | Tal | 4 | VBA | Both decreases and increases observed in the R middle frontal gyrus, L lateral occipitotemporal gyrus, subgyral white matter of the R parietal lobe and R angular gyrus |
| Olvet et al.27 | 3 (8) | 0.95 × 0.95 × 3 | 25 | MNI | 5 | TBSS | Both decreases and increases observed in the R body of the CC, L inferior fronto-occipital fasciculus, R anterior thalamic radiation, R inferior longitudinal fasciculus and R cingulum |
| Ouyang et al.15 | 1.5 (birdcage) | 1.875 × 1.875 × 4 | 13 | MNI | 6 | VBA | Both decreases and increases observed in the bilateral medial frontal gyri, R subgyral frontal and temporal lobes, L middle frontal gyri and L cingulate gyrus |
| Tha et al.24 | 1.5 (NA) | 1.875 × 1.875 × 5 | 12 | MNI | 9 | VBA | Both decreases and increases observed in the bilateral frontal gyrus, bilateral anterior limbs of the internal capsule and L putamen, mediodorsal nucleus of the R thalamus, anterior and superior aspect of bilateral cerebellar hemispheres |
| Wang et al.25 | 3 (NA) | 0.9375 × 0.9375 × 5 | 42 | MNI | 4 | VBA | Decreases observed in the R cuneus gyrus, and increases observed in the R thalamus, R postcentral gyrus and cerebellar vermis |
| Wu et al.29 | 1.5 (NA) | 0.94 × 0.94 × 4 | 13 | MNI | 3 | VBA | Decreases observed in the R SLF within the frontal lobe, R middle frontal and L inferior parietal lobe |
| Zhu et al.43 | 1.5 (NA) | 1.875 × 1.875 × 4 | 13 | MNI | 3 | TBSS | Decreases observed in the L anterior limb of the internal capsule and R parahippocampal gyrus, and both decreases and increases observed in the L posterior cingulate cortex |
| Zuo et al.30 | 1.5 (NA) | 1 × 1 × 4 | 25 | MNI | 2 | TBSS | Decreases observed in the L centre portion of the SLF and L premotor area (BA 6) |
BA = Brodmann area; CC = corpus callosum; DTI = diffusion tensor imaging; FA = fractional anisotropy; L = left; MDD = major depressive disorder; MNI = Montreal Neurological Institute; NA = not available; R = right; Tal = Talairach; SLF = superior longitudinal fasciculus; TBSS = tract-based spatial statistics; VBA = voxel-based analysis.
Pooled meta-analysis of all included studies with medication-free patients
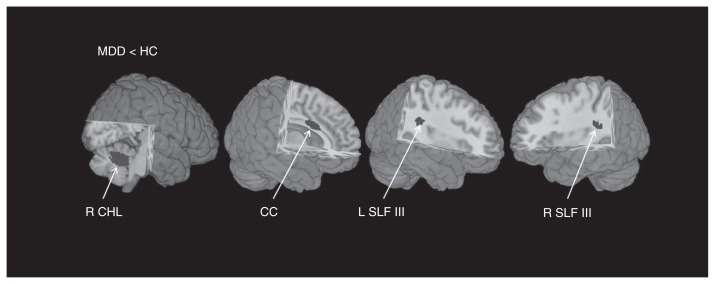
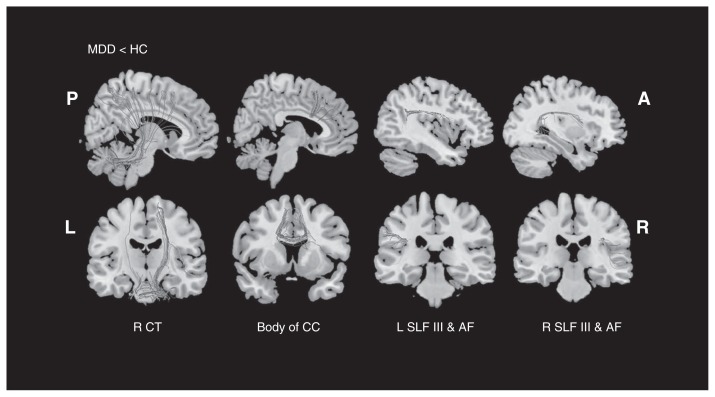
Our meta-analysis of reported coordinates of all included studies with medication-free patients mainly revealed 4 regions of decreased FA in patients with MDD compared with controls: the white matter of the right cerebellum hemispheric lobule, body of the CC, bilateral SLF III and the long segment of the arcuate network (Table 3, Fig. 1). No significant FA increase was found in patients with MDD compared with controls. The main white matter tracts traversing these regions of decreased FA were the right cerebellar tracts, body of the CC, bilateral SLF III and the long segment of the arcuate fascicle (AF), as shown in Figure 2. Previous research in nonhuman primates has shown that the SLF is composed of 4 separate components: SLF I, SLF II, SLF III and AF.61 A study by Makris and colleagues62 identified and segmented the 4 subdivisions in humans using DTI. The SLF III is the ventral component that originates in the supramarginal gyrus and terminates in the ventral premotor and prefrontal cortex.62,63 The AF is the component that originates in the superior temporal gyrus, curves around the caudal tip of the Sylvian fissure and terminates in the dorsal prefrontal cortex.62,63
Table 3.
Regional FA differences between patients with MDD and healthy controls identified by the present meta-analysis
| Brain region (MDD < control) | MNI coordinates | SDM z score | p value, uncorrected | No. voxels* | Cluster breakdown (no. voxels) | Fibre tracking | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| Pooled meta-analysis of all included studies | ||||||||
| R cerebellum hemispheric lobule | 26 | −56 | −28 | −1.598 | 0.00004 | 220 | R cerebellum hemispheric lobule; BA 37, 19, 18 (183) M cerebellar peduncle (12) Undefined (25) |
R cerebellar tracts |
| Corpus callosum | 14 | 10 | 28 | 1.151 | 0.00234 | 36 | Corpus callosum (36) | Body of corpus callosum |
| L superior longitudinal fasciculus III | −36 | −36 | 26 | −1.332 | 0.00047 | 18 | L superior longitudinal fasciculus III (15) Left arcuate network, long segment (3) |
L superior longitudinal fasciculus III L arcuate fascicle, long segment |
| R superior longitudinal fasciculus III | 36 | −36 | 22 | −1.320 | 0.00054 | 16 | R superior longitudinal fasciculus III (14) R arcuate network, long segment (2) |
R superior longitudinal fasciculus III R arcuate fascicle, long segment |
| Subgroup meta-analysis of medication-naive studies | ||||||||
| Corpus callosum | 18 | 40 | 26 | −1.057 | 0.00018 | 51 | Corpus callosum (52) | Genu of corpus callosum |
| R anterior thalamic projections | 28 | 42 | 10 | −1.242 | 0.00001 | 54 | R superior longitudinal fasciculus III (26) Corpus callosum (19) R anterior thalamic projections (9) |
R superior longitudinal fasciculus III Genu of corpus callosum R anterior thalamic projections |
| Subgroup meta-analysis of medication wash-out studies | ||||||||
| R cerebellum hemispheric lobule | 14 | −56 | −28 | −1.566 | 0.00006 | 247 | R cerebellum, hemispheric lobule; BA 37, 19, 18 (202) R middle cerebellar peduncle (13) Undefined (32) |
R cerebellar tracts |
| Corpus callosum | 14 | 8 | 30 | −1.140 | 0.00261 | 19 | Corpus callosum (19) | Body of corpus callosum |
| R superior longitudinal fasciculus III | 38 | −38 | 20 | −1.272 | 0.00069 | 17 | R superior longitudinal fasciculus III (14) R arcuate network, long segment (3) |
R superior longitudinal fasciculus III R arcuate fascicle, long segment |
| L superior longitudinal fasciculus III | −38 | −40 | 28 | −1.253 | 0.00098 | 19 | L superior longitudinal fasciculus III (13) L arcuate network, long segment (6) |
L superior longitudinal fasciculus III L arcuate fascicle, long segment |
| Subgroup meta-analysis of studies with corrected results | ||||||||
| R cerebellum hemispheric lobule | 14 | −52 | −22 | −1.240 | 0.00027 | 153 | R cerebellum hemispheric lobule; BA 37, 19, 18 (142) M cerebellar peduncle (1) Undefined (10) |
R cerebellar tracts |
| Corpus callosum | 14 | 10 | 28 | −1.297 | 0.00016 | 49 | Corpus callosum (49) | Body of corpus callosum |
| L superior longitudinal fasciculus III | −36 | −34 | 26 | −1.231 | 0.00046 | 22 | L superior longitudinal fasciculus III (17) Left arcuate network, long segment (5) |
L superior longitudinal fasciculus III L arcuate fascicle, long segment |
| R superior longitudinal fasciculus III | 34 | −34 | 24 | −1.243 | 0.00025 | 17 | R superior longitudinal fasciculus III (15) R arcuate network, long segment (2) |
R superior longitudinal fasciculus III R arcuate fascicle, long segment |
BA = Brodmann area; FA = fractional anisotropy; L = left; M = middle; MDD = major depressive disorder; MNI = Montreal Neurological Institute; R = right; SDM = signed differential mapping.
All voxels with p < 0.005 uncorrected.
Fig. 1.
Regions showing reduced fractional anisotropy (FA) in medication-free patients with major depressive disorder (MDD) compared with healthy controls (HC). Fractional anisotropy changes in patients are displayed on a 3-dimensionally rendered brain, with part of the left or right hemisphere removed. CC = corpus callosum; CHL = cerebellum hemispheric lobule; L = left; R = right; SLF = superior longitudinal fasciculus.
Fig. 2.
White mater diffusion tensor tracts traversing reduced fractional anisotropy (FA) clusters. A = anterior; AF = arcuate fascicle; CC = corpus callosum; CT = cerebellar tracts; HC = healthy controls; L = left; MDD = major depressive disorder; P = posterior; R = right; SLF = superior longitudinal fasciculus.
Jackknife sensitivity analysis
Whole-brain jackknife sensitivity analysis showed that FA decreases in the white matter regions of the right cerebellum hemispheric lobule, bilateral SLF III and the long segment of the arcuate network were relatively replicable, being preserved throughout all but 1 combination;22 FA decreases in the body of the CC remained significant in all but 2 combinations27,28 (Appendix 1, Table S3).
Subgroup meta-analysis of studies with medication-naive patients with first-episode MDD
The first-episode medication-naive subgroup analysis included 7 data sets15,23,26,28,29,42,43 comprising 174 patients with MDD and 157 healty controls. The analysis revealed FA decreases in the genu of the CC and right anterior thalamic projections extending to the right SLF III (Table 3 and Appendix 1, Fig. S2).
Subgroup meta-analysis of medication washout studies
The medication washout subgroup analysis included 8 data sets13,22,24,25,27,30,41,44 that compared 260 patients with MDD and 272 healthy controls. Fractional anisotropy reductions were identified in the white matter of the right cerebellum hemispheric lobule, body of the CC, bilateral SLF III and the long segment of the arcuate network (Table 3 and Appendix 1, Fig. S2).
Subgroup meta-analysis of studies with corrected results
The subgroup analysis of studies with corrected results included 10 data sets13,22,25–28,41–44 comprising 344 patients with MDD and 338 healthy controls. These results were consistent with the pooled meta-analysis (Table 3), showing few effects of uncorrected results on the overall conclusions.
Analysis of heterogeneity and publication bias
Analysis of heterogeneity revealed that the right cerebellum hemispheric lobule and the body of the CC with altered FA had significant statistical heterogeneity among studies (p < 0.005; Appendix 1, Table S4). Funnel plots demonstrated that the main findings were driven by at least 9 studies, shown in Appendix 1, Figure S3. Analysis of publication bias showed that the Egger test was nonsignificant for the right cerebellum hemispheric lobule (p = 0.57), body of the CC (p = 0.39), left SLF III (p = 0.28) and right SLF III (p = 0.28).
Meta-regression analysis
The percentage of women with MDD (i.e., in the whole MDD group in each study), the mean age of patients, illness duration and depression symptom severity (HAM-D) of patients with MDD were not significantly associated with MDD-related white matter FA changes, at least linearly.
Discussion
To our knowledge, this is the first meta-analysis of DTI studies in medication-free patients with MDD examining MDD-related white matter abnormalities without the interference of current medication effects. Medication-free patients showed significantly decreased FA in the white matter of the right cerebellum hemispheric lobule, body of the CC, bilateral SLF III and the long segment of the arcuate network compared with healthy controls. To some extent, our findings are not completely consistent with those of previous meta-analyses, which may be a result of inclusion of both medicated and medication-free patients with MDD in previous studies. Results from the subgroup meta-analysis of medication washout studies were consistent with those of the pooled meta-analysis. However, FA reductions identified in the genu of the CC and right anterior thalamic projections were seen only in the studies of first-episode medication-naive patients, which might suggest some traits for MDD (i.e., white matter abnormalities directly associated with MDD).
Sensitivity analysis showed our findings were relatively replicable. Findings of altered FA in the right cerebellum hemispheric lobule and the body of the CC had significant statistical heterogeneity among studies, which may be owing to the unavoidable heterogeneity in the acquisition parameters of raw diffusion data, patient characteristics and clinical variables of the included studies. The results of meta-regression analysis showed that there was no significant association between white matter microstructure alterations and relevant sociodemographic and clinical variables. The results, however, should be interpreted with some caution in consideration of the small sample size in the present study and the limited variability in the data, since some studies investigating white matter abnormalities in patients with MDD have provided evidence that decreased FA in the SLF and CC were associated with the depressive symptoms14,39,44,64–66 and negatively correlated with depression severity28,65,67,68 and illness duration.14,28 Moreover, patient characteristics of the included studies were heterogeneous, which may result in the negative results. Supporting our hypothesis, a recent study showed specific abnormalities of the brain circuitry in patients with early-versus late-onset MDD, as demonstrated by distinct abnormal FA clusters with opposite correlations with clinical symptoms.69
Fractional anisotropy reductions in the white matter of the right cerebellum hemispheric lobule
The present meta-analysis revealed significantly decreased FA in the white matter of the right cerebellum hemispheric lobule in medication-free patients with MDD compared with healthy controls. The cerebellar tracts traversing this cluster mainly included the long afferent cortico–ponto–cerebellar tract.70 As we know, MDD has traditionally been viewed as an affective disorder characterized by strong feelings of sadness, guilt, worthlessness and hopelessness.13,15,25,43 Furthermore, studies have shown that MDD is associated with significant disturbances in cognitive functioning, including executive functions, attention, memory and psychomotor speed.13,30,43,71 Some investigations have provided evidence for the hypothesis that the cerebellum is an essential node in the distributed neural circuitry subserving cognitive and affective functions.72–74 Further, a range of remarkable tasks associated with cerebellar activation have been designed to assess attention, executive control, language, working memory, emotion and addiction, generating compelling support for the view that the cerebro–cerebellar circuitry provides the cerebellum with the anatomic substrate to influence the control of movement and cognition.75
Reviews regarding the functional role for the cerebellum indicated that an integrated model of neuropsychiatric disorders should include a role for the cerebellum and its relevant neural connections.72,76 Disruption of the functional connections between the cerebellum and frontal lobes impairs expression of emotion.74 Studies reported that FA reductions in the cerebellar hemisphere exhibited a positive correlation with depression severity.24,77 In addition, significant FA differences in the white matter of the frontal lobe, limbic lobe and cerebellum between patients with treatment-resistant depression (TRD) and controls suggest that abnormalities of cortical–limbic–cerebellar white matter networks may contribute to TRD in young patients.77 In sum, our finding of decreased FA in the white matter of the right cerebellar hemispheric lobule is supportive of implications that the cerebellum may play a critical role in the neuropathology of MDD.
Fractional anisotropy reductions in the corpus callosum
The CC was found to have decreased FA in medication-free patients with MDD compared with healthy controls in our study, which is consistent with the findings of previous meta-analyses.11,32 The CC is the largest fibre bundle of the human brain connecting the left and right cerebral hemispheres. The genu of the CC connects the prefrontal and orbitofrontal regions, and the body connects precentral frontal regions and parietal lobes.11 Numerous fMRI studies have revealed that the frontal lobe is associated with motor, perceptual and cognitive functions.78–82 The CC modulates cognitive processes and information transfer between hemispheres.83 One DTI study demonstrated that abnormalities in the structural integrity of the anterior genu of the CC may contribute to impairment of interhemispheric connectivity in medication-naive patients with MDD.84 Therefore, FA reductions in the genu and body of the CC may indicate the functional impairment of frontal lobe information transfer between hemispheres and may be associated with impairment of working memory and emotion processing in patients with MDD.83
A recent DTI study investigating the association between catechol-O-methyltransferase gene polymorphisms and white matter tract integrity in patients with MDD has found that MDD may be associated with the dysfunctional white matter changes in the CC, and the valine homozygote of catechol-O-methyltransferase gene may enhance these pathological changes.85 Furthermore, studies by Guo and colleagues66,86 have demonstrated that abnormalities of white matter tracts, mainly in the projection fibres and CC, may contribute to the pathogenesis of treatment-responsive MDD,66 and that abnormalities of white matter integrity of neuronal tracts connecting 2 brain hemispheres may play a key role in the pathogenesis of TRD.86 Therefore, decreased FA in the CC observed in our meta-analysis may be disease-related and underlie the deficits in emotional modulation and cognitive processing in patients with MDD.
Fractional anisotropy reductions in the bilateral SLF
The present meta-analysis identified significantly decreased FA in the bilateral SLF III and the long segment of the AF in medication-free patients with MDD compared with controls, which is partially consistent with the previous meta-analysis showing decreased FA in the left SLF.31 The possible connections between the supramarginal gyrus that receives information from the ventral precentral and the prefrontal gyrus via the SLF III suggest that the SLF III may play a role in transferring somatosensory information, such as language articulation and working memory.62,87 By connecting the superior temporal gyrus with the dorsal prefrontal cortex, the AF may be viewed as an auditory spatial bundle providing a means by which the prefrontal cortex can receive and modulate audiospatial information.62,87
Psychomotor retardation, characterized by changes in speech, motility and cognition, is common in individuals with major depression.88 Furthermore, psychomotor retardation may worsen over the course of depression: Talarowska and colleagues89 reported that the first-episode MDD group recorded better results than the recurrent depressive disorders group with respect to the speed of information processing, visuospatial and auditory–verbal memory and executive functions, auditory–verbal immediate and delayed memory, ability to learn and verbal fluency. The functions of the SLF III and AF may help elucidate the correlations between FA reductions in the SLF and functional impairments (e.g., deficient working memory, impaired maintenance of attention, speech articulation and cognitive impairments).62,87,90,91 To date, many DTI studies on patients with MDD have reported that decreased FA in the SLF was associated with depressive symptoms.39,64 For example, a recent study on medication-naive individuals with a single short-duration episode of MDD revealed lower FA in the right SLF within the frontal lobe in the MDD group than in the healthy group.29 Furthermore, there is evidence that reduced white matter integrity in the SLF and the body of the CC can predict future depressive symptoms, suggesting disruption to white matter integrity may be a biomarker to predict late-life depression.39 Therefore, our findings of decreased FA in the SLF are supportive of implication of the SLF in the neuropathology of MDD.
Differential findings between the subgroup analyses
We conducted 2 subgroup analyses on the basis of medication status. Consistent with the pooled meta-analysis, the subgroup meta-analysis of medication washout studies revealed FA reductions in the right cerebellum hemispheric lobule, body of the CC, and bilateral SLF, while the first-episode medication-naive subgroup analysis identified FA reductions in the genu of the CC, right SLF III and right anterior thalamic projections. Patients with long disease duration are exposed to chronic stress, which has been demonstrated to contribute to frontostriatal degeneration and reductions in the length and branch numbers of apical dendrites,92 and these changes could in turn present with a relative increase in the FA.34,93 Without the confounding influences of illness duration and previous antidepressant treatment, we suggest that FA reductions in the genu of the CC and right anterior thalamic projections are more likely to be directly associated with the disease. Supporting our findings, some investigations have shown that FA abnormalities in genu of the CC and anterior thalamic projections might represent important markers of early-onset depression.68,94 Hence, we hypothesize that the distinctions between our subgroup findings may be associated with the effects of long disease duration and previous antidepressant treatment, which calls for further investigation.
Directions for future studies
Increasing evidence has shown that MDD-related FA changes are correlated with particular biological and clinical characteristics, such as gene regulation and suicidality. As for gene regulation, some studies have reported its effects on white matter FA in patients with MDD: an association between the catechol-O-methyltransferase gene Val158Met polymorphism and white matter abnormalities42,85 and a higher remission rate in brain-derived neurotrophic factor (met) carriers than brain-derived neurotrophic factor (val/val) homozygotes.95 Furthermore, medication-free patients with MDD who have a history of suicide attempts have exhibited significant white matter differences: decreased FA in the left anterior limb of the internal capsule,22 right lentiform nucleus22 and white matter adjacent to the right dorsomedial prefrontal cortex27 in suicide attempters compared with nonattempters.
Aside from antidepressant medications, treatment for depression can involve nondrug therapies, such as electroconvulsive therapy (ECT),96,97 deep brain stimulation (DBS)98,99 and transcranial magnetic stimulation (TMS),100,101 which may have effects on white matter. One study investigating young patients with TRD showed increased FA in the left middle frontal gyrus after high-frequency repetitive TMS.102 Moreover, a longitudinal DTI study of patients with depression found a reduction of FA in the right frontal white matter and genu of the CC 6 months after ECT treatment, and the values returned to the pre-ECT values after 1 year.96 This is an interesting finding given evidence that ECT may mediate the neuroplasticity of white matter microstructure in patients with major depression.103 Furthermore, a recent review has reported that although the mechanisms of its action are still not completely understood, DBS for TRD has shown encouraging therapeutic effects, demonstrating significant and sustained improvements in depressive symptomatology, with remission in a large percentage of cases.98 To our knowledge, only a limited number of studies of nondrug therapies involving a small patient sample size have been conducted so far.98 Future DTI studies on medication-free patients with MDD may focus on the therapeutic effects of nondrug therapies, benefitting from homogeneous samples grouped by the type of therapy as well as illness duration, drug status, gene polymorphism and history of suicide attempts.
Limitations
As the present study used a relatively robust approach to identify related studies, the results should be representative of medication-free patients with MDD. However, the study still has several limitations. First, the main meta-analysis included primary studies enrolling medicated patients with MDD who underwent a washout period before MRI scanning. Clearly, the best way to minimize the effects of illness duration and antidepressant treatment is to focus on medication-naive patients; however, the size of our medication-naive subgroup analysis is small, as direct study of unmedicated depressed is associated with substantial practical and ethical problems,104 which limits the generalizability of the results. In addition, we could not exclude the effects of nondrug therapies owing to the lack of data. Once there are sufficient numbers of published DTI studies on medication-naive patients with first-episode MDD without nondrug therapies, meta-analyses will be needed to investigate the white matter abnormalities directly associated with the disease. Second, the acquisition parameters of raw diffusion data, patient characteristics and clinical variables of the included studies were heterogeneous. As the number of studies included in our meta-analysis was small, we were not able to perform separate subgroup meta-analyses for clinical variables, such as illness duration and depression severity, that would likely diversify the results. Third, the voxel-wise meta-analysis was based on peak coordinates and effect sizes from published studies rather than raw statistical brain maps, and this approach may result in less accurate results.46
Conclusion
The present meta-analysis revealed significant FA reductions in the white matter of the right cerebellum hemispheric lobule, CC and bilateral SLF, which are involved in cognition, memory function and emotional processing, in medication-free patients with MDD. The results of our subgroup analyses suggest that FA changes in the genu of the CC, right SLF III and right anterior thalamic projections in medication-naive patients with first-episode MDD may be disease-related, which may help to parse out the effects of long disease duration and previous antidepressant treatment. These findings may contribute to a better understanding of the underlying neuropathology of MDD. Given that the pooled meta-analysis results were mainly affected by the medication washout subgroup, which had a relatively large sample size, and that the small medication-naive subgroup contributed less to the analysis results, future studies with much larger and homogeneous samples may be more robust in delineating disease-related white matter abnormalities in patients with MDD as well as the therapeutic effects of some nondrug treatments.
Acknowledgements
The authors thank all authors of the included studies and especially thank Dr. Joaquim Radua for his kind help and suggestions in using TBSS template of AES-SDM to combine VBA and TBSS studies in our meta-analysis. This study was supported by the National Natural Science Foundation (Grant Nos. 81000605, 81220108013 and 0040205401A59). Q. Gong also acknowledges his Visiting Professor appointment in the Department of Psychiatry at the Yale School of Medicine, Yale University, USA.
Footnotes
Competing interests: None declared.
Contributors: J. Jiang, Y.-J. Zhao, K.-M. Li, H.-Y. Zhu, P. Kumar and Q.-Y. Gong designed the study. X.-Y. Hu, M.-Y. Du, Z.-Q. Chen and M. Wu acquired the data, which J. Jiang and Y.-J. Zhao analyzed. J. Jiang, Y.-J. Zhao, K.-M. Li and P. Kumar wrote the article, which all authors reviewed and approved for publication.
References
- 1.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–55. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu L, Xie J, Long J, et al. Epidemiology of major depressive disorder in mainland china: a systematic review. PLoS One. 2013;8:e65356. doi: 10.1371/journal.pone.0065356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers C. Global burden of disease. In: Heggenhougen HK, editor. International Encyclopedia of Public Health. Oxford (UK): Academic Press; 2008. pp. 59–72. [Google Scholar]
- 4.Baune BT, Fuhr M, Air T, et al. Neuropsychological functioning in adolescents and young adults with major depressive disorder — a review. Psychiatry research. 2014;218:261–71. doi: 10.1016/j.psychres.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 5.Kieseppä T, Mäntylä R, Tuulio-Henriksson A, et al. White matter hyperintensities and cognitive performance in adult patients with bipolar I, bipolar II, and major depressive disorders. European Psychiatry. 2014;29:226–232. doi: 10.1016/j.eurpsy.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Qin J, Wei M, Liu H, et al. Abnormal brain anatomical topological organization of the cognitive-emotional and the frontoparietal circuitry in major depressive disorder. Magn Reson Med. 2014;72:1397. doi: 10.1002/mrm.25036. [DOI] [PubMed] [Google Scholar]
- 7.de Kwaasteniet B, Ruhe E, Caan M, et al. Relation between structural and functional connectivity in major depressive disorder. Biol Psychiatry. 2013;74:40–7. doi: 10.1016/j.biopsych.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Korgaonkar MS, Cooper NJ, Williams LM, et al. Mapping inter-regional connectivity of the entire cortex to characterize major depressive disorder: a whole-brain diffusion tensor imaging tractography study. Neuroreport. 2012;23:566–71. doi: 10.1097/WNR.0b013e3283546264. [DOI] [PubMed] [Google Scholar]
- 9.Korgaonkar MS, Fornito A, Williams LM, et al. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;66:567–574. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biological Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aihara M, Ida I, Yuuki N, et al. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–56. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Arnold JF, Zwiers MP, Fitzgerald DA, et al. Fronto-limbic microstructure and structural connectivity in remission from major depression. Psychiatry Res. 2012;204:40–8. doi: 10.1016/j.pscychresns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 14.de Diego-Adeliño J, Pires P, Gomez-Anson B, et al. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med. 2014;44:1171–82. doi: 10.1017/S003329171300158X. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang X, Tao HJ, Liu HH, et al. White matter integrity deficit in treatment-naive adult patients with major depressive disorder. East Asian Arch Psychiatry. 2011;21:5–9. [PubMed] [Google Scholar]
- 16.Kubicki M, Westin CF, Maier SE, et al. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatry. 2002;10:324–36. doi: 10.1080/10673220216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korgaonkar MS, Williams LM, Song YJ, et al. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br J Psychiatry. 2014;205:321–8. doi: 10.1192/bjp.bp.113.140376. [DOI] [PubMed] [Google Scholar]
- 18.Ahn S, Lee SK. Diffusion tensor imaging: exploring the motor networks and clinical applications. Korean J Radiol. 2011;12:651–661. doi: 10.3348/kjr.2011.12.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 20.Taylor WD, Hsu E, Krishnan KR, et al. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol Psychiatry. 2004;55:201–7. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Jia Z, Huang X, Wu Q, et al. High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry. 2010;167:1381–90. doi: 10.1176/appi.ajp.2010.09101513. [DOI] [PubMed] [Google Scholar]
- 23.Ma N, Li L, Shu N, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164:823–6. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- 24.Tha KK, Terae S, Nakagawa S, et al. Impaired integrity of the brain parenchyma in non-geriatric patients with major depressive disorder revealed by diffusion tensor imaging. Psychiatry Res. 2013;212:208–15. doi: 10.1016/j.pscychresns.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Huang X, Huang P, et al. Early-stage psychotherapy produces elevated frontal white matter integrity in adult major depressive disorder. PLoS One. 2013;8:e63081. doi: 10.1371/journal.pone.0063081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han K-M, Choi S, Jung J, et al. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord. 2014;155:42–8. doi: 10.1016/j.jad.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Olvet DM, Peruzzo D, Thapa-Chhetry B, et al. A diffusion tensor imaging study of suicide attempters. Psychiatry Res. 2014;51:60–7. doi: 10.1016/j.jpsychires.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai CH, Wu YT. Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychol Med. 2014;44:2825–32. doi: 10.1017/S0033291714000440. [DOI] [PubMed] [Google Scholar]
- 29.Wu F, Tang Y, Xu K, et al. Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. 2011;191:80–3. doi: 10.1016/j.pscychresns.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo N, Fang J, Lv X, et al. White matter abnormalities in major depression: a tract-based spatial statistics and rumination study. PLoS One. 2012;7:e37561. doi: 10.1371/journal.pone.0037561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord. 2011;1:3. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise T, Radua J, Nortje G, et al. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biological Psychiatry. 2015;79:293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Sijens PE, Mostert JP, Irwan R, et al. Impact of fluoxetine on the human brain in multiple sclerosis as quantified by proton magnetic resonance spectroscopy and diffusion tensor imaging. Psychiatry Res. 2008;164:274–82. doi: 10.1016/j.pscychresns.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Taylor WD, Macfall JR, Boyd B, et al. One-year change in anterior cingulate cortex white matter microstructure: relationship with late-life depression outcomes. Am J Geriatr Psychiatry. 2011;19:43–52. doi: 10.1097/JGP.0b013e3181e70cec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo SY, Jang JH, Shin YW, et al. White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand. 2007;116:211–9. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoon S, Kim JE, Hwang J, et al. Effects of creatine monohydrate augmentation on brain metabolic and network outcome measures in women with major depressive disorder. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Qin J, Wei M, Liu H, et al. Altered anatomical patterns of depression in relation to antidepressant treatment: evidence from a pattern recognition analysis on the topological organization of brain networks. J Affect Disord. 2015;180:129–37. doi: 10.1016/j.jad.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 38.Taylor WD, MacFall JR, Payne ME, et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–6. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 39.Reppermund S, Zhuang L, Wen W, et al. White matter integrity and late-life depression in community-dwelling individuals: diffusion tensor imaging study using tract-based spatial statistics. Br J Psychiatry. 2014;205:315–20. doi: 10.1192/bjp.bp.113.142109. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Wang Y, Liu H, et al. Diffusion tensor imaging and resting state functional magnetic resonance imaging on young patients with major depressive disorder. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:25–31. doi: 10.3969/j.issn.1672-7347.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Choi KS, Holtzheimer PE, Franco AR, et al. Reconciling variable findings of white matter integrity in major depressive disorder. Neuropsychopharmacology. 2014;39:1332–9. doi: 10.1038/npp.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi K, Yoshimura R, Kakeda S, et al. COMT Val158Met, but not BDNF Val66Met, is associated with white matter abnormalities of the temporal lobe in patients with first-episode, treatment-naive major depressive disorder: a diffusion tensor imaging study. Neuropsychiatr Dis Treat. 2014;10:1183–90. doi: 10.2147/NDT.S61275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Wang X, Xiao J, et al. Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res. 2011;1369:223–9. doi: 10.1016/j.brainres.2010.10.104. [DOI] [PubMed] [Google Scholar]
- 44.Korgaonkar MS, Grieve SM, Koslow SH, et al. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. 2011;32:2161–71. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radua J, Rubia K, Canales-Rodriguez EJ, et al. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhong J, Pan P, Dai Z, et al. Voxelwise meta-analysis of gray matter abnormalities in dementia with Lewy bodies. Eur J Radiol. 2014;83:1870–4. doi: 10.1016/j.ejrad.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry. 2014;171:854–63. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- 49.Baiano M, David A, Versace A, et al. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res. 2007;93:1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Brambilla P, Hardan A, di Nemi SU, et al. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–69. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Shepherd AM, Matheson SL, Laurens KR, et al. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–84. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Strakowski SM, DelBello MP, Adler C, et al. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–64. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 53.Radua J, Via E, Catani M, et al. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41:1539–50. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- 54.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 55.Du M, Liu J, Chen Z, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39:397–406. doi: 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherbondy A, Akers D, Mackenzie R, et al. Exploring connectivity of the brain’s white matter with dynamic queries. IEEE Trans Vis Comput Graph. 2005;11:419–30. doi: 10.1109/TVCG.2005.59. [DOI] [PubMed] [Google Scholar]
- 57.Mori S, Wakana S, Nagae-Poetscher LM, et al. NeuroApps: MRI Atlas of Human White Matter. Revue Francophone des Laboratoires. 2012;(443):19. [Google Scholar]
- 58.Vederine FE, Wessa M, Leboyer M, et al. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1820–6. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Yao L, Lui S, Liao Y, et al. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:100–6. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 60.Iwabuchi SJ, Krishnadas R, Li C, et al. Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev. 2015 doi: 10.1016/j.neubiorev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–16. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 62.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 63.Kamali A, Flanders AE, Brody J, et al. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219:269–81. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou K, Huang X, Li T, et al. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:525–30. [PMC free article] [PubMed] [Google Scholar]
- 65.Cole J, Chaddock CA, Farmer AE, et al. White matter abnormalities and illness severity in major depressive disorder. Br J Psychiatry. 2012;201:33–9. doi: 10.1192/bjp.bp.111.100594. [DOI] [PubMed] [Google Scholar]
- 66.Guo WB, Liu F, Xue ZM, et al. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci Lett. 2012;522:139–44. doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Dalby RB, Frandsen J, Chakravarty MM, et al. Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res. 2010;184:38–48. doi: 10.1016/j.pscychresns.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Henderson SE, Johnson AR, Vallejo AI, et al. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry. 2013;4:152. doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y, Xu J, Yu H, et al. Delineation of early and later adult onset depression by diffusion tensor imaging. PLoS One. 2014;9:e112307. doi: 10.1371/journal.pone.0112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanaan RA, Borgwardt S, McGuire PK, et al. Microstructural organization of cerebellar tracts in schizophrenia. Biol Psychiatry. 2009;66:1067–9. doi: 10.1016/j.biopsych.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 71.Raes F, Hermans D, Williams JM, et al. Is overgeneral autobiographical memory an isolated memory phenomenon in major depression? Memory. 2006;14:584–94. doi: 10.1080/09658210600624614. [DOI] [PubMed] [Google Scholar]
- 72.Konarski JZ, McIntyre RS, Grupp LA, et al. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–86. [PMC free article] [PubMed] [Google Scholar]
- 73.Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. 2000;13:189–214. [Google Scholar]
- 74.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum — insights from the clinic. Cerebellum. 2007;6:254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 75.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 76.Hoppenbrouwers SS, Schutter DJLG, Fitzgerald PB, et al. The role of the cerebellum in the pathophysiology and treatment of neuro-psychiatric disorders: a review. Brain Res Rev. 2008;59:185–200. doi: 10.1016/j.brainresrev.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Peng HJ, Zheng HR, Ning YP, et al. Abnormalities of cortical-limbic-cerebellar white matter networks may contribute to treatment-resistant depression: a diffusion tensor imaging study. BMC Psychiatry. 2013;13:72. doi: 10.1186/1471-244X-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korgaonkar MS, Grieve SM, Etkin A, et al. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38:863–871. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kerestes R, Ladouceur CD, Meda S, et al. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2012;42:29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- 80.Vasic N, Walter H, Sambataro F, et al. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39:977–87. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- 81.Fitzgerald PB, Srithiran A, Benitez J, et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp. 2008;29:490–501. doi: 10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuo K, Glahn DC, Peluso MA, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–66. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- 83.Yamada S, Takahashi S, Ukai S, et al. Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: a tract-specific analysis study. J Affect Disord. 2015;174:542–548. doi: 10.1016/j.jad.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Xu K, Jiang W, Ren L, et al. Impaired interhemispheric connectivity in medication-naive patients with major depressive disorder. J Psychiatry Neurosci. 2013;38:43–8. doi: 10.1503/jpn.110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seok J-H, Choi S, Lim HK, et al. Effect of the COMT Val158Met polymorphism on white matter connectivity in patients with major depressive disorder. Neurosci Lett. 2013;545:35–9. doi: 10.1016/j.neulet.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Guo WB, Liu F, Chen JD, et al. Altered white matter integrity of fore-brain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:201–6. doi: 10.1016/j.pnpbp.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 87.Schmahmann JD, Smith EE, Eichler FS, et al. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flint AJ, Black SE, Campbell-Taylor I, et al. Abnormal speech articulation, psychomotor retardation, and subcortical dysfunction in major depression. J Psychiatr Res. 1993;27:309–19. doi: 10.1016/0022-3956(93)90041-y. [DOI] [PubMed] [Google Scholar]
- 89.Talarowska M, Zajaczkowska M, Galecki P. Cognitive functions in first-episode depression and recurrent depressive disorder. Psychiatr Danub. 2015;27:38–43. [PubMed] [Google Scholar]
- 90.Hatton SN, Lagopoulos J, Hermens DF, et al. White matter tractography in early psychosis: clinical and neurocognitive associations. J Psychiatry Neurosci. 2014;39:417–27. doi: 10.1503/jpn.130280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karlsgodt KH, van Erp TG, Poldrack RA, et al. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–8. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 92.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 93.Baloch S, Verma R, Huang H, et al. Quantification of brain maturation and growth patterns in C57BL/6J mice via computational neuroanatomy of diffusion tensor images. Cereb cortex. 2009;19:675–687. doi: 10.1093/cercor/bhn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bessette KL, Nave AM, Caprihan A, et al. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging Behav. 2014;8:531–41. doi: 10.1007/s11682-013-9274-8. [DOI] [PubMed] [Google Scholar]
- 95.Alexopoulos GS, Glatt CE, Hoptman MJ, et al. BDNF Val66Met polymorphism, white matter abnormalities and remission of geriatric depression. J Affect Disord. 2010;125:262–8. doi: 10.1016/j.jad.2010.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Larsson EM, Steffensen E, Nordanskog P, et al. Does electroconvulsive therapy (ECT) affect white matter integrity? A longitudinal diffusion tensor imaging study of patients with depression. 19th Symposium Neuroradiologicum — The World Congress of Diagnostic and Therapeutic Neuroradiology; Bologna, Italy. Oct. 4–9, 2010. [Google Scholar]
- 97.Nobuhara K, Okugawa G, Minami T, et al. Effects of electroconvulsive therapy on frontal white matter in late-life depression: a diffusion tensor imaging study. Neuropsychobiology. 2004;50:48–53. doi: 10.1159/000077941. [DOI] [PubMed] [Google Scholar]
- 98.Anderson RJ, Frye MA, Abulseoud OA, et al. Deep brain stimulation for treatment-resistant depression: efficacy, safety and mechanisms of action. Neurosci Biobehav Rev. 2012;36:1920–33. doi: 10.1016/j.neubiorev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol psychiatry. 2013;73:1204–12. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 100.Furtado CP, Hoy KE, Maller JJ, et al. An investigation of medial temporal lobe changes and cognition following antidepressant response: a prospective rTMS Study. Brain Stimul. 2013;6:346–354. doi: 10.1016/j.brs.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 101.Kozel FA, Johnson KA, Nahas Z, et al. Fractional anisotropy changes after several weeks of daily left high-frequency repetitive transcranial magnetic stimulation of the prefrontal cortex to treat major depression. J ECT. 2011;27:5–10. doi: 10.1097/YCT.0b013e3181e6317d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peng H, Zheng H, Li L, et al. High-frequency rTMS treatment increases white matter FA in the left middle frontal gyrus in young patients with treatment-resistant depression. J Affect Disord. 2012;136:249–257. doi: 10.1016/j.jad.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Lyden H, Espinoza RT, Pirnia T, et al. Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry. 2014;4:e380. doi: 10.1038/tp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ambrosi E, Chiapponi C, Sani G, et al. White matter microstructural characteristics in bipolar I and bipolar II disorder: a diffusion tensor imaging study. J Affect Disord. 2016;189:176–183. doi: 10.1016/j.jad.2015.09.035. [DOI] [PubMed] [Google Scholar]