Abstract
Background
Growing evidence indicates that major depressive disorder (MDD) is characterized by accelerated biological aging, including greater age-related changes in physiological functioning. The disorder is also associated with abnormal neural reward circuitry, particularly in the basal ganglia (BG). Here we assessed age-related changes in BG volume in both patients with MDD and healthy control participants.
Methods
We obtained whole-brain T1-weighted images from patients with MDD and healthy controls. We estimated grey matter volumes of the BG, including the nucleus accumbens, caudate, pallidum and putamen. Volumes were assessed using multivariate analysis of covariance (MANCOVA) with age as a covariate, followed by appropriate post hoc tests.
Results
We included 232 individuals (116 patients with MDD) in our analysis. The MANCOVA yielded a significant group × age interaction (p = 0.043). Analyses for each region yielded a significant group × age interaction in the putamen (univariate test, p = 0.005; permutation test, p = 0.004); this effect was not significant in the other regions. The negative association between age and putamen volume was twice as large in the MDD than in the control group (−35.2 v. −16.7 mm3/yr), indicating greater age-related volumetric decreases in the putamen in individuals with MDD than in controls.
Limitations
These findings are cross-sectional; future studies should assess the longitudinal impact of accelerated aging on anhedonia and neural indices of reward processing.
Conclusion
Our results indicate that putamen aging is accelerated in patients with MDD. Thus, the putamen may uniquely contribute to the adverse long-term effects of depressive psychopathology and may be a useful target for the treatment of MDD across the lifespan.
Introduction
Major depressive disorder (MDD) is primarily characterized by depressed mood and a blunted experience of pleasure (i.e., anhedonia).1 The disorder is prevalent and chronic and represents a substantial personal, social and economic burden.2–4 Neuroscience promises to elucidate mechanisms underlying depressive pathophysiology, leading to more effective approaches to the prevention and treatment of this debilitating disorder.
Considerable evidence indicates that reward processing is dysfunctional in individuals with MDD.5,6 The basal ganglia (BG) are a set of subcortical structures, including the striatum (caudate and putamen), nucleus accumbens and pallidum, that are critically involved in these reward processing steps.7 Previously research has indicated that depressed individuals have decreased activation in the striatum,5,6 and some investigators have hypothesized that this abnormal activity affects decision-making.5 Research in both animals and humans suggests that the dorsal striatum is involved in action selection and initiation and in encoding and integrating sensorimotor, cognitive and motivational/emotional information.8 Current evidence is mixed regarding specific roles of the putamen and caudate in reward processing. The results of several studies using fMRI suggest that whereas the putamen specifically encodes reward-related feedback, the caudate encodes both reward and punishment feedback.9,10 Finally, researchers have reported that the putamen is activated most strongly during anticipation of reward, whereas the caudate is activated most strongly during the receipt of reward.8,10,11
A growing body of research indicates that adults with MDD have smaller volumes of the caudate12–15 and putamen13,14,16,17 than healthy controls. Postmortem studies have also reported smaller putamen volumes in depressed than in nondepressed adults.18 It is important to note that these studies typically include adult samples with a mean age older than 40 years. Depression-associated differences in BG volumes are less consistent in samples of both younger adults19,20 and adolescents.21 It appears, therefore, that this discrepancy is associated with age differences in the study samples; that is, it is possible that only older individuals with MDD exhibit abnormally reduced putamen and caudate volumes. In this context, it is similarly possible that MDD-related BG volumetric reductions are associated with length of MDD history, a clinical characteristic that is likely to be associated with age. It is also worth noting that many of these studies have used small samples of participants (e.g., patient groups with fewer than 30 individuals).13,18,20,22–25 Recently the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) MDD working group has addressed the issue of underpowered single studies by conducting large-scale meta-analyses of neuroimaging studies of MDD. In a recent meta-analysis from this group that included 1728 individuals with MDD and more than 7199 control participants across 15 samples, only hippocampal volumes differentiated MDD from control participants.26 Importantly, however, age did not interact with diagnostic group to predict regional volumes. Given that ENIGMA used a meta-regression technique to test associations among group differences in summary statistics, it is possible that individual-level associations among age, MDD and subcortical volumes were obscured by the meta-analysis of study averages and that methods that better incorporate individual-level variance are more powerful in characterizing associations among these variables.
In this context, recent research suggests that MDD is characterized by accelerated biological aging. For example, individuals with MDD have been found to have shorter telomeres,27 to be more likely to experience poor cardiac outcomes28 and to exhibit dysfunction of the limbic– hypothalamic–pituitary–adrenal axis.29 Neurobiologically, aging is associated with reductions in grey matter volumes,30,31 and there is evidence that depressed individuals are characterized by accelerated aging of total grey matter volumes.29,32 A 7-year longitudinal study used morphometric analysis to assess age-related abnormalities of grey and white matter in individuals with melancholic MDD.33 Results from that study indicate that several structures exhibit accelerated volumetric reductions in individuals with melancholic MDD, including periventricular white matter near the striatum and the right lingual and left caudal middle temporal gyrus. The study included older participants (mean age > 60 yr) with a relatively late age at onset of melancholic MDD (mean age at onset > 50 yr). To our knowledge, no study has yet examined accelerated neural aging in individuals with MDD not limited to the melancholic subtype and across a wider age range. Furthermore, to our knowledge, no study has specifically examined accelerated aging of the BG.
In the present study we examined whether MDD is characterized by accelerated aging of the BG. Specifically, we assessed volumes of the nucleus accumbens, caudate, pallidum and putamen in relation to age in a large cohort of patients with MDD and healthy controls. Based on the literature mentioned previously, we predicted that depressed individuals would exhibit significantly smaller BG volumes than controls, particularly smaller caudate and putamen volumes, and that this difference would be greater in older than in younger individuals.
Methods
Participants and clinical information
Study participants were physically healthy adults aged 18–60 years with diagnoses of MDD as well as matched healthy controls. We collected demographic data, including highest achieved education level (ranging from high school diploma to graduate or professional degree) and income (in categories from < $10 000 to > $100 000). Clinical diagnoses were established using the Structured Clinical Interview for DSM-IV.34 Participants in the MDD group did not meet diagnostic criteria for current or lifetime bipolar disorder or psychosis, or for substance abuse or dependence. Participants in the control group did not meet criteria for any Axis I disorder or substance abuse/dependence at any point in their lives. For the MDD participants, we assessed the duration of the current episode, the number of previous depressive episodes and the length of time since the first depressive episode. To assess the severity of the depressive episode we administered the Beck Depression Inventory (BDI)35,36 at the scanning session. We obtained written informed consent from each participant. The Stanford University Institutional Review Board approved the study.
MRI data acquisition
We used T1-weighted 3D gradient echo imaging (spoiled gradient recalled acquisition; SPGR) to collect whole brain structural images for each participant. All available participants with MDD were identified first, and then an equal number of control participants were pseudorandomly selected to be matched to the MDD participants on the scan parameters. These parameters included slice collection orientation, head coil, scanner and sequence parameters (i.e., repetition time [TR], echo time [TE], inversion time [TI], flip angle and voxel volume). We acquired T1-weighted images using 3 separate 3 T General Electric MR750 Discovery scanners at Stanford University, with axial or sagittal slice orientation, using either an 8-channel or 32-channel head coil. The TRs ranged from 5.9 to 9.6 ms, TEs from 1.4 to 3.4 ms and TIs from 300 to 500 ms. Flip angle was 11°, 12°, or 15°. Through-plane resolution ranged from 0.9 to 1.5 mm, in-plane resolution from 0.9 × 0.9 mm to 1.0 × 1.0 mm and voxel volume from 0.7 to 1.1 mm3.
Subcortical segmentation
We measured volumes of BG structures using the reliable and well-validated FreeSurfer (version 5.3) automatic segmentation tool.37 Each segmentation was visually inspected for quality assurance. Volumes that passed initial visual inspection were z-scored separately for each region, hemisphere and group. Volumes with a z score greater than 2.5 or less than −2.5 were double-checked for high-quality BG segmentation. We excluded poorly segmented images from further analysis. Volumes of the caudate nucleus, putamen, pallidum and nucleus accumbens were extracted for each hemisphere from each individual. Using linear modelling in MATLAB r2015a (The MathWorks, Inc.), we determined which scan parameters were significantly associated with estimated volumes for each region (p < 0.05). Only the TE parameter was associated with volume of the nucleus accumbens, thus only this parameter was regressed on nucleus accumbens volumes. Complete results from the linear modelling of scan parameters predicting BG volumes are included in Appendix 1, Table S1, available at jpn.ca. Sex and intracranial volume were regressed from all subcortical volumes. We conducted all further analyses on the residuals from these multiple linear regression models. We repeated the same residualization procedure for BG volumes averaged across hemisphere.
Assessing group × age interactions in subcortical volumes
We performed a repeated-measures multivariate analysis of covariance (MANCOVA) to assess group (MDD, control) × age interactions associated with BG volumes. We performed this analysis with hemisphere as a repeated measure and age as a covariate (between-subjects model: volume ~intercept + diagnosis + age + diagnosis × age; within-subjects model: 4 regions × 2 hemispheres). Significant multivariate effects were followed up using appropriate univariate tests (e.g., analysis of covariance; ANCOVA) to assess interactions in specific BG volumes (between-subjects model: volume ~intercept + diagnosis + age + diagnosis × age; within-subjects model: 2 hemispheres). We accounted for false-positive inflation as a result of multiple comparisons using Bonferroni correction; therefore, the corrected α level for the region-specific ANCOVAs was 0.0125 (i.e., 0.05 ÷ 4). We further assessed age × group interactions in a specific region by comparing the slopes of lines of best fit for each group using a permutation test. For each group we identified the slope term of the model and calculated the difference between these 2 values. Next, group labels were permuted to create 1 000 000 randomized data sets. The slope term of the lines of best fit were identified for each permuted data set in the same manner as for the original data. We then assessed the slope difference between groups for each permuted data set. The original slope difference was then compared with the distribution of slope differences from the permuted data sets. A p value was calculated as the number of permuted data sets that exhibited smaller or larger slope differences (whichever was smaller), divided by the number of permutations and multiplied by 2 (for a 2-tailed test). We performed ANCOVAs using SPSS 22 Statistics software (IBM Corporation). Permutation testing was conducted in MATLAB using in-house programs.
Association between subcortical volumes and clinical characteristics
We used linear models to assess associations between abnormal age-related volumetric effects and clinical characteristics in the MDD group (e.g., by assuming volume residual is an additive function of age and clinical characteristic). Clinical characteristics included depression severity (BDI), duration of current episode in months and length of total depression history in years. Forty-four of the depressed participants indicated that they have experienced too many episodes to count accurately; therefore, we assigned a value of 10 to those participants and conducted a median split of the MDD group on this measure. This resulted in a “fewer” depressive episodes group (i.e., 6 or fewer episodes) and a “more” depressive episodes group (i.e., 7 or more episodes). We conducted subsequent analyses assessing the effect of total number of depressive episodes on BG volumes using a multivariate analysis of variance (MANOVA) with a group term with levels of “fewer” episodes and “more” episodes. We used Bonferroni correction to account for multiple statistical tests (i.e., α = 0.0125; 0.05 ÷ 4).
We conducted exploratory analyses to assess the potential influence of medication status and comorbid anxiety on BG volumes. Specifically, we conducted an ANCOVA to test age × group interactions (unmedicated v. medicated; with comorbid anxiety v. without comorbid anxiety).
Results
Demographic and clinical characteristics of the sample
We included 232 physically healthy adults aged 18–60 years in our analysis: 116 patients with MDD and 116 matched healthy controls. The demographic and clinical characteristics of the MDD and control groups are presented in Table 1 and Table 2. The MDD and control groups did not differ in sex distribution (χ21 = 1.14, p = 0.29) or education level (rank sum statistic = 13 198.0, p = 0.86). There was a statistical trend toward older participants in the MDD compared with the control group (t230 = 1.945, p = 0.05). Participants in the MDD group had significantly lower incomes than participants in the control group (rank sum statistic = 8728.5, p = 0.003), which is not surprising given the social and economic burden of MDD. In neither group, however, was income correlated significantly with any of the BG volumes (all p ≥ 0.29). As expected, the MDD group reported greater severity of depression as measured by the BDI (t210 = −30.291, p < 0.001) than the control group.
Table 1.
Participant demographic and clinical characteristics
| Characteristic | Group; mean ± SD or no. (%)* | Statistic | p value | |
|---|---|---|---|---|
|
| ||||
| MDD, n = 116 | Control, n = 116 | |||
| Age, yr | 36.5 ± 11.6 | 33.7 ± 10.5 | t = 1.95 | 0.05 |
| MDD history, yr | 19.5± 13.0 | — | ||
| Current MDE duration, mo | 25.9 ± 56.6 | — | ||
| Total no. MDEs, median [IQR] | 6.5 [3–10] | — | ||
| Male sex | 25 (21.6) | 32 (27.6) | χ2 = 1.14 | 0.29 |
| Current psychotropic medication use | 39 (33.6) | 0 (0) | χ2 = 45.06 | < 0.001 |
| GAF score | 54.4 ± 7.8 | 87.3 ± 5.9 | t = 8.36 | 0.004 |
| BDI scores at scan | 30.9 ± 9.2 | 2.2 ± 3.4 | t = −30.30 | < 0.001 |
| Annual Income | Rank sum = 8728.50 | 0.003 | ||
| < $10 000 | 22 (19.0) | 5 (4.3) | ||
| $10 000–$25 000 | 17 (14.7) | 11 (9.5) | ||
| $25 000–$50 000 | 18 (15.6) | 15 (12.9) | ||
| $50 000–$75 000 | 11 (9.5) | 24 (20.7) | ||
| $75 000–$100 000 | 12 (10.3) | 12 (10.3) | ||
| > $100 000 | 15 (12.9) | 18 (15.5) | ||
| Unknown/decline to state | 21 (18.1) | 31 (26.7) | ||
| Highest level of education | Rank sum = 13 198.0 | 0.86 | ||
| High school | 7 (6.0) | 2 (1.7) | ||
| Some college | 20 (17.2) | 27 (23.3) | ||
| Tech school | 0 (0) | 3 (2.6) | ||
| Junior college | 14 (12.1) | 5 (4.3) | ||
| Four-year college | 45 (38.8) | 49 (42.2) | ||
| Graduate or professional degree | 29 (25.0) | 28 (24.1) | ||
| Unknown/decline to state | 1 (0.9) | 2 (1.7) | ||
BDI = Beck Depression Inventory; GAF = Global Assessment of Functioning; IQR = interquartile range; MDD = major depressive disorder; MDE = major depressive episode; SD = standard deviation.
Unless indicated otherwise.
Table 2.
Current and lifetime Axis I comorbidities for participants in the MDD group
| Comorbidity | No. (%) of patients |
|---|---|
| Current | |
| None | 40 (34.48) |
| Agoraphobia | 4 (3.45) |
| Anorexia | 0 (0) |
| Binge eating | 3 (2.59) |
| Bulimia | 1 (0.86) |
| Dysthymic disorder | 9 (7.76) |
| Generalized anxiety disorder | 26 (22.41) |
| Obsessive compulsive disorder | 5 (4.31) |
| Panic disorder | 8 (6.90) |
| Posttraumatic stress disorder | 13 (11.21) |
| Social phobia | 35 (30.17) |
| Specific phobia | 11 (9.48) |
| Lifetime | |
| None | 33 (28.45) |
| Agoraphobia | 5 (4.31) |
| Anorexia | 9 (7.76) |
| Binge eating | 7 (6.03) |
| Bulimia | 9 (7.76) |
| Dysthymic disorder | 9 (7.76) |
| Generalized anxiety disorder | 26 (22.41) |
| Obsessive compulsive disorder | 5 (4.31) |
| Panic disorder | 11 (9.48) |
| Posttraumatic stress disorder | 27 (23.28) |
| Social phobia | 38 (32.76) |
| Specific phobia | 14 (12.07) |
MDD = major depressive disorder.
Age × group interactions in BG volumes
The repeated-measures MANCOVA did not yield a significant main effect of hemisphere or significant hemisphere × group or hemisphere × age × group interactions (all F < 1.7; all p > 0.15). Thus, models were analyzed with BG volumetric data averaged across hemispheres. Raw BG volumes were averaged across hemispheres and new residuals were extracted for subsequent analyses. This analysis yielded significant multivariate effects of age (F4,225 = 12.466, p < 0.001) and a trend-level multivariate effect of group (F4,225 = 1.971, p = 0.10), which were qualified by a significant group × age interaction (F4,225 = 2.466, p = 0.043). These results are reported comprehensively in Table 3.
Table 3.
Repeated-measures MANCOVA results
| Term | F statistic | p value |
|---|---|---|
| Between subjects* | ||
| Diagnosis | 1.98 | 0.10 |
| Age | 11.21 | < 0.001 |
| Diagnosis × age | 2.51 | 0.043 |
| Within subjects† | ||
| Hemisphere | 1.57 | 0.18 |
| Hemisphere × diagnosis | 0.34 | 0.85 |
| Hemisphere × age | 1.70 | 0.15 |
| Hemisphere × diagnosis × age | 0.15 | 0.96 |
MANCOVA = multivariate analysis of covariance.
Volume ~ intercept + diagnosis + age + diagnosis × age.
Four regions × 2 hemispheres.
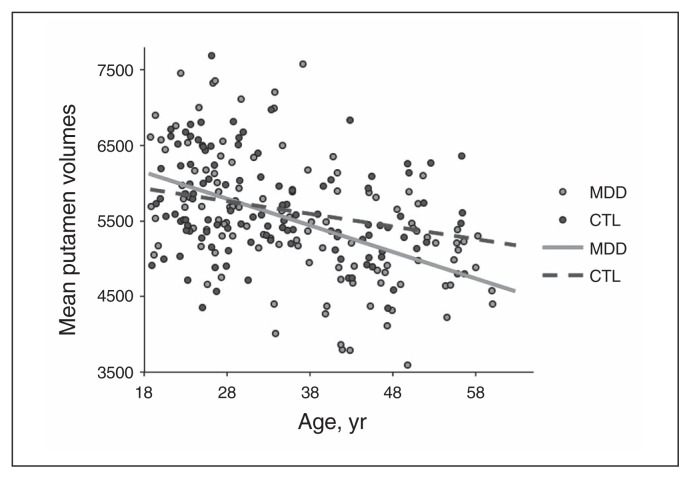
To examine this interaction we conducted ANCOVAs of the BG residualized average volumetric data at the individual region level. These analyses indicated that the multivariate group × age interaction was driven by a group × age interaction in the putamen (F1,230 = 7.536, p = 0.005). This result was still significant after correcting for multiple comparisons (corrected p = 0.013). The full results of these ANCOVAs are summarized in Table 4. Permutation testing of slopes from linear fitting indicated that the age × volume residual slope was significantly more negative in the MDD group than in the control group (p = 0.004). To visualize this association with meaningful units of measurement, we linearly fit the raw, unresidualized data; this analysis indicated that the negative association between age and putamen volume was twice as large in the MDD group as it was in the control group (−35.2 v. −16.7 mm3/yr; Fig. 1), indicating greater age-related volumetric decreases in the putamen in individuals with MDD than in controls. Taken together, although the volume of the putamen decreased with age in both the MDD and the control groups, this volumetric reduction occurred at a faster rate in the MDD group.
Table 4.
ANCOVA results for all BG volumes*
| Age | Diagnosis | Diagnosis × age | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Region | F statistic | p value | F statistic | p value | F statistic | p value |
| Caudate | 4.92 | 0.027 | 0.01 | 0.93 | 0.00 | 0.96 |
| Putamen | 40.07 | < 0.001 | 6.17 | 0.014 | 7.54 | 0.005 |
| Pallidum | 6.16 | 0.014 | 1.07 | 0.30 | 1.75 | 0.19 |
| Nucleus accumbens | 23.51 | < 0.001 | 1.90 | 0.17 | 2.14 | 0.15 |
ANCOVA = analysis of covariance; BG = basal ganglia.
Model design: region ~intercept + diagnosis + age + diagnosis × age.
Fig. 1.
Raw putamen volumes by diagnostic group. Lines of best fit are plotted for each group. The slope of the line of best fit for the residualized putamen volumes in the major depressive disorder (MDD) group was more negative than that of the healthy control (CTL) group (permutation test p = 0.004), indicating accelerated aging of the putamen in the MDD compared with the control group. The slopes of the raw data for were −35.2 and −16.7 mm3/year in the MDD and the control groups, respectively.
Associations between BG regional volumes and clinical characteristics
We assessed associations between putamen volumes and clinical characteristics using linear models. Specifically, we tested models in the MDD group to assess whether clinical characteristics were associated with average volume reductions over and above the contribution of age. No significant associations were found between putamen volume and duration of current episode (n = 111, t110 = −0.81, p = 0.42) or length of MDD history (n = 112, t111 = 0.65, p = 0.51). We found a trend-level association between BDI scores and putamen volumes, such that higher current BDI scores were associated with greater volumes (n = 105, t104 = 1.76, p = 0.08). The MANOVA did not yield a significant group (i.e., “fewer” or “more” episodes) × age interaction (F4,109 = 1.499, p = 0.21). Neither medication status nor comorbid anxiety was associated with age-related acceleration of volumetric reductions in any BG region (all p ≥ 0.12; Appendix 1, Table S2).
Discussion
The present study was conducted to examine whether individuals with MDD are characterized by accelerated aging in the BG compared with healthy individuals. We found accelerated age-related volumetric reduction of the putamen in individuals with MDD compared with controls. There was no evidence of MDD-related accelerated aging of the volumes of the caudate, pallidum or nucleus accumbens. Accelerated aging of putamen volume in individuals with MDD was not associated with clinical characteristics, including severity of depression, duration of current depressive episode, total number of depressive episodes, length of MDD history, medication status, or comorbid anxiety. These results suggest that the putamen is uniquely affected by MDD; given the involvement of the putamen in reward processing, this structure may be an important target for the treatment of MDD across the lifespan.
Our finding of putamen-specific accelerated aging in individuals with MDD may help to elucidate facets of aberrant reward processing in people with the disorder. As discussed earlier, researchers have found that the putamen is activated strongly during the anticipation of reward and punishment.8–10 This function of the putamen is likely to be related to anhedonia (loss of pleasure and reward-seeking behaviour). It is possible that reduced putamen volumes in individuals with MDD represent a neural underpinning for anhedonia. Activity of the BG has been previously related to anhedonia in depressed adolescents and adults.38,39 Future research should examine whether putamen structure is associated with anhedonia and whether putamen function is characterized by age-related interactions with MDD status.
Previous research has found evidence of accelerated biological aging in individuals with related mood disorders and schizophrenia. One study found that those with schizophrenia exhibit accelerated decline of whole brain fractional anisotropy, an index of white matter integrity measured using diffusion-weighted MRI.40 Other studies have reported that individuals with bipolar disorder exhibit accelerated grey matter reduction with age41 and rapid decline of levels of brain-derived neurotrophic factor, an index of neuronal survival and integrity that is associated with aging effects in the brain.42 Given that aberrant reward processing has also been reported in individuals with schizophrenia and bipolar disorder,43–46 researchers might profitably take a transdiagnostic approach to identifying potential protective factors and treatment targets of accelerated cellular aging across psychiatric disorders. Treatments that buffer against accelerated reductions in brain volume aging may improve symptoms and prognoses for people with these disorders.
We should note here that our results do not replicate previously reported differences in caudate volumes between depressed and nondepressed samples. One reason for this may be that previous studies that found MDD-related differences in caudate volumes did not assess age × diagnosis interactions,12–15 and it is possible that the observed group differences were qualified by an interaction with age. Other studies examined a different age group than we did,13 and many had smaller samples (e.g., patient groups of fewer than 30 individuals).13,18,20,22–25 The analysis with the largest sample size to date, the meta-analysis conducted by the ENIGMA MDD working group, also did not find differences in caudate volumes between individuals with MDD and controls.26
Our finding that age and diagnosis interacted to predict putamen volumes is not necessarily inconsistent with findings from the meta-analysis conducted by the ENIGMA MDD working group, which reported that age and diagnostic group did not interact to predict putamen volume.26 This is because the ENIGMA study assessed the effects of age on volume using meta-regression of average volumes on average ages across studies. Thus the association that we observed across 232 individuals would not necessarily be replicated in a meta-regression of 15 samples that used summary statistics for each study. Nevertheless, it will be important to replicate the findings reported here in future large-scale meta-analyses of individual participant data from the ENIGMA or other consortia.
Soriano-Mas and colleagues33 found that periventricular white matter proximal to the putamen exhibited age-related reductions in volume in patients with melancholic MDD. In this context, our results of accelerated putamen grey matter reductions in patients with MDD may be associated with accelerated aging of proximal white matter. It is noteworthy that, owing to large-scale multiple comparison correction, the whole brain approach used by Soriano-Mas and colleagues may not have had the statistical power to detect grey matter abnormalities in their study. Future studies should directly assess whether grey matter abnormalities of the putamen are related to structural properties of proximal white matter. Such studies might consider using diffusion-weighted imaging and tractography and/or quantitative MRI to quantify metrics associated with white matter, including myelin content and axonal degeneration.47
The putamen has also been implicated in psychomotor functioning. For example, neurons in the putamen fire reliably during active motion and during actions specifically associated with reward cues.48 Notably, abnormality in psychomotor functioning (i.e., agitation or retardation) is a criterion symptom of MDD. A prior study found that activity in the cingulate motor area and the putamen were significantly decreased in patients with MDD compared with control participants during a movement task.49 Another study found weaker functional connectivity between the putamen and cortical regions involved in motor functioning in individuals with MDD than in controls.50 Psychomotor disturbance in those with MDD has also been associated with reduced cerebral blood flow in the putamen and caudate during a motor task.51 A study using positron emission tomography found that dopaminergic function of the putamen is reduced in individuals with MDD compared with controls.52 Given these findings, reduced putamen volumes may be associated with the psychomotor disturbance that characterizes many individuals with MDD, and these effects may be more pronounced later in life.
Limitations
Despite the strengths of using a large sample and a large age-distribution across adulthood, our results need to be interpreted in light of several limitations. First, we did not collect data associated with reward-specific symptoms (e.g., anhedonia); therefore, we cannot draw strong conclusions regarding the association between reduced volumes and reward functioning. Second, our results do not speak to the association between accelerated abnormalities in reward functioning and MDD. Future studies should directly relate putamen volumetric abnormalities to measures of reward function as measured by fMRI (e.g., reward anticipation as modelled by the monetary incentive delay [MID] task).53 Third, the present study is cross-sectional and cannot confirm at an individual participant level whether MDD is associated with accelerated volumetric reduction of the putamen. Frodl and colleagues32 found greater whole brain grey matter decline in adults with MDD in a 3-year longitudinal study, but they did not observe age × group interactions in the BG in whole-brain analyses.32 The discrepancy between those findings and our results may be explained by the fact that Frodl and colleagues did not examine subregions of the BG and by the relatively short longitudinal period studied by these investigators; that is, age × structure interactions may be too subtle to observe within a 3-year interval. Fourth, we used a clinical interview (the SCID) to assess participants’ history of depressive episodes. It will be important in future research to assess depression history more objectively, perhaps by reviewing medical records, and to assess its association with BG volume. Finally, these data were collected across multiple MRI systems using several unique sets of scan parameters. Although we took several steps to account for variability associated with scan parameters (i.e., matching scan parameters across groups and regressing out scan parameters that related to BG volumes), we cannot fully rule out the possibility that scan parameters influenced our results.
Conclusion
Our findings indicate that the putamen is characterized by accelerated aging in individuals with MDD, which may be associated with reward-related abnormalities in individuals with this disorder. Our findings provide further evidence for accelerated biological aging in individuals with MDD and underscore the importance of early detection and treatment of this chronic and prevalent disorder.
Acknowledgements
This study was supported by National Science Foundation Integrative Graduate Education and Research Traineeship (NSF IGERT) Recipient Award 0801700 and National Science Foundation Graduate Research Fellowship Program (NSF GRFP) DGE-1147470 awarded to M. Sacchet, and by a National Institute of Mental Health Grant MH59259 awarded to I. Gotlib.
Footnotes
Competing interests: None declared.
Contributors: M. Sacchet and I. Gotlib designed the study. M. Sacchet, M. Camacho, E. Livermore and I. Gotlib acquired the data, which M. Sacchet, M. Camacho, E. Thomas and I. Gotlib analyzed. M. Sacchet, M. Camacho and I. Gotlib wrote the article, which all authors reviewed and approved for publication.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76:155–62. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Greenberg PE, Mickelson KD, et al. The effects of chronic medical conditions on work loss and work cutback. J Occup Environ Med. 2001;43:218–25. doi: 10.1097/00043764-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Marchand WR, Yurgelun-Todd D. Striatal structure and function in mood disorders: a comprehensive review. Bipolar Disord. 2010;12:764–85. doi: 10.1111/j.1399-5618.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 6.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandel ER, Schwartz JH, Jessell TM, et al., editors. Principles of Neural Science. 5th ed. New York (NY): McGraw-Hill Books; 2013. pp. 1105–8. [Google Scholar]
- 8.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutson B, Adams CM, Fong GW, et al. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Hairston J, Schrier M, et al. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nat Neurosci. 2005;8:1611–8. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan KR, McDonald WM, Escalona P, et al. Magnetic resonance imaging of the caudate nuclei in depression: preliminary observations. Arch Gen Psychiatry. 1992;49:553–7. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan K, McDonald WM, Doraiswamy PM, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243:41–6. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 14.Parashos IA, Tupler LA, Blitchington T, et al. Magnetic-resonance morphometry in patients with major depression. Psychiatry Res Neuroimaging. 1998;84:7–15. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 15.Sacchet MD, Livermore EE, Iglesias JE, et al. Subcortical volumes differentiate major depressive disorder, bipolar disorder, and remitted major depressive disorder. J Psychiatr Res. 2015;68:91–8. doi: 10.1016/j.jpsychires.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreescu C, Butters MA, Begley A, et al. Gray matter changes in late life depression — a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–72. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain MM, McDonald WM, Doraiswamy PM, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–9. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 18.Baumann B, Bogerts B. The pathomorphology of schizophrenia and mood disorders: similarities and differences. Schizophr Res. 1999;39:141–8. doi: 10.1016/s0920-9964(99)00113-9. [DOI] [PubMed] [Google Scholar]
- 19.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont RM, Butters N, Schafer K, et al. Diagnostic specificity of focal white matter abnormalities in bipolar and unipolar mood disorder. Biol Psychiatry. 1995;38:482–6. doi: 10.1016/0006-3223(95)00100-u. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Rosenberg DR, Easter PC, et al. Striatal volume abnormalities in treatment-naïve patients diagnosed with pediatric major depressive disorder. J Child Adolesc Psychopharmacol. 2008;18:121–31. doi: 10.1089/cap.2007.0026. [DOI] [PubMed] [Google Scholar]
- 22.Dahabra S, Ashton CH, Bahrainian M, et al. Structural and functional abnormalities in elderly patients clinically recovered from early- and late-onset depression. Biol Psychiatry. 1998;44:34–46. doi: 10.1016/s0006-3223(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 23.Hickie I, Scott E, Mitchell P, et al. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151–60. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- 24.Lacerda ALT, Nicoletti MA, Brambilla P, et al. Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry Res Neuroimaging. 2003;124:129–40. doi: 10.1016/s0925-4927(03)00123-9. [DOI] [PubMed] [Google Scholar]
- 25.Lenze EJ, Sheline YI. Absence of striatal volume differences between depressed subjects with no comorbid medical illness and matched comparison subjects. Am J Psychiatry. 1999;156:1989–91. doi: 10.1176/ajp.156.12.1989. [DOI] [PubMed] [Google Scholar]
- 26.Schmaal L, Veltman DJ, van Erp TGM, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806–12. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon NM, Smoller JW, McNamara KL, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–5. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 29.Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge Y, Grossman RI, Babb JS, et al. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–33. [PMC free article] [PubMed] [Google Scholar]
- 31.Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–8. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frodl TS, Koutsouleris N, Bottlender R, et al. Depression-related variation in brain morphology over 3 years: Effects of stress? Arch Gen Psychiatry. 2008;65:1156–65. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- 33.Soriano-Mas C, Hernandez-Ribas R, Pujol J, et al. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol Psychiatry. 2011;69:318–25. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 34.First MB. User’s guide for the structured clinical interview for DSMIV axis I disorders: SCIDI. Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 35.Beck AT, Steer RA. Manual for the revised Beck depression inventory. San Antonio (TX): Psychological Corporation; 1987. [Google Scholar]
- 36.Beck AT, Steer RA, Brown GK, et al. Manual for the beck depression inventoryII. San Antonio (TX): Psychological Corporation; 1996. [Google Scholar]
- 37.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 38.Gabbay V, Ely BA, Li Q, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52:628–41.e13. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keedwell PA, Andrew C, Williams SCR, et al. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Kochunov P, Glahn DC, Rowland LM, et al. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biol Psychiatry. 2013;73:482–91. doi: 10.1016/j.biopsych.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brambilla P, Harenski K, Nicoletti M, et al. Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology. 2001;43:242–7. doi: 10.1159/000054897. [DOI] [PubMed] [Google Scholar]
- 42.Yatham LN, Kapczinski F, Andreazza AC, et al. Accelerated age-related decrease in brain-derived neurotrophic factor levels in bipolar disorder. Int J Neuropsychopharmacol. 2009;12:137–9. doi: 10.1017/S1461145708009449. [DOI] [PubMed] [Google Scholar]
- 43.Abler B, Greenhouse I, Ongur D, et al. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–27. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adida M, Jollant F, Clark L, et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Nusslock R, Young CB, Damme KSF. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: assessment and treatment implications. Behav Res Ther. 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiskopf N, Mohammadi S, Lutti A, et al. Advances in MRI-based computational neuroanatomy: from morphometry to in-vivo histology. Curr Opin Neurol. 2015;28:313–22. doi: 10.1097/WCO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 48.Connor NP, Abbs JH. Sensorimotor contributions of the basal ganglia: recent advances. Phys Ther. 1990;70:864–72. doi: 10.1093/ptj/70.12.864. [DOI] [PubMed] [Google Scholar]
- 49.Liberg B, Ekman CJ, Sellgren C, et al. Vertex-based morphometry in euthymic bipolar disorder implicates striatal regions involved in psychomotor function. Psychiatry Res. 2014;221:173–8. doi: 10.1016/j.pscychresns.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Marchand WR, Lee JN, Suchy Y, et al. Aberrant functional connectivity of cortico-basal ganglia circuits in major depression. Neurosci Lett. 2012;514:86–90. doi: 10.1016/j.neulet.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 51.Hickie I, Ward P, Scott E, et al. Neo-striatal rCBF correlates of psychomotor slowing in patients with major depression. Psychiatry Res Neuroimaging. 1999;92:75–81. doi: 10.1016/s0925-4927(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 52.Bragulat V, Paillère-Martinot ML, Artiges E, et al. Dopaminergic function in depressed patients with affective flattening or with impulsivity: [18F]fluoro-L-dopa positron emission tomography study with voxel-based analysis. Psychiatry Res. 2007;154:115–24. doi: 10.1016/j.pscychresns.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Knutson B, Westdorp A, Kaiser E, et al. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]