Abstract
Platelet-rich plasma (PRP) is blood plasma containing a high number of platelets that release growth factors for wound healing and tissue regeneration. In the present study, the feasibility of improving PRP therapy by using chitosan that exhibits high platelet activation ability was investigated. A total of 13 chitosan samples with different molecular weight (Mw) and degree of deacetylation (DDA) were individually added to blood samples of rats and the amount of growth factors, albumin and fibrinogen in plasma was measured. To examine the influence of plasma activated by chitosan on the proliferation of fibroblasts and adipose tissue-derived stromal cells (ASCs), the plasma was added to the culture medium of human fibroblasts and adipose tissue-derived stromal cells. Chitosan with a DDA of >75% increased the release of platelet factor 4 into the plasma. The amount of growth factors released into the plasma and platelet activation varied depending on the Mw and DDA, while albumin and fibrinogen were hardly affected. The proliferation rate was highest when using plasma activated by chitosan with a DDA of 75–85% and an Mw of 50,000–190,000 Da. These results suggested that the effectiveness of PRP therapy may be improved by using chitosan with a DDA of 75–85% and an Mw of 50,000–190,000 Da.
Keywords: chitosan, platelet-rich plasma, growth factor, cell proliferation, platelet activation, deacetylation
Introduction
Platelet-rich plasma (PRP) is blood plasma with a high number of platelets. As an enriched source of autologous platelets, PRP contains several growth factors that are important for initiating and accelerating tissue repair and regeneration. Given these advantages, PRP therapy has recently emerged as an innovative technique with great potential for healing chronic and acute wounds, including diabetic wounds, bedsores, skin ulcers and thermal burns. Fundamentally, the mechanisms underlying PRP therapy are the molecular and cellular stimulation of normal wound healing responses, similar to those observed during platelet activation (1). However, it is difficult to cure intractable diseases such as diabetic ulcers and decubitus, since the therapeutic effect tend to differ among individuals (2,3). Reviving a wound with impaired healing is unmanageable because standard wound healing methods do not always provide improved healing results. This often demands more advanced therapies (4–6). Platelets can be activated upon two different types of stimuli: Physical stimuli, including heat, cold and vibration, and chemical stimuli, including collagen, lipopolysaccharide and chitosan (7–11). The activated platelets release biologically active substances and growth factors, such as platelet factor 4 (PF4), von Willebrand factor, platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF) and vascular endothelial growth factor (12–14).
Chitosan is a polysaccharide derived from chitin, which is a compound of natural origin obtained from the shell of crabs and shellfish. Chitosan prepared from alkaline N-deacetylation is composed of β-(1–4)-linked D-glucosamine and N-acetyl-D-glucosamine, which are randomly distributed. It carries a positive charge, as the free amino groups of β-(1–4)-linked D-glucosamine are protonated at physiological pH. Chitosan is being extensively used as a potential biomaterial in several medical devices and health care products owing to its biodegradability and advantageous biological properties, including hemostatic activity (15,16), biodegradability (15,17), antibacterial activity (18,19) and its ability to serve as a wound dressing agent to accelerate wound healing (16,20,21). Given these advantages, the use of chitosan as a stimulant for platelet activation can be highly effective in PRP therapy. However, depending on the methods adopted for purifying chitosan from chitin, the molecular weight (Mw) and degree of deacetylation (DDA) vary. In other words, the function, properties and performance of chitosan are associated with their DDA and Mw.
Given these considerations, the present study proposed the concept of effective PRP therapy using chitosan. This strategy relies on the fact that chitosan activates platelets and enhances the release of growth factors into the plasma. To further enhance the effectiveness of PRP, a basic study using 13 different types of chitosan with varying Mw and DDA was performed.
Materials and methods
Animals and chitosan
The present study was approved by the Ethics Committee of Animal Care and Use, National Defense Medical College (Saitama, Japan) on July 28, 2014 (approval no., 14040) and the protocol was in accordance with the committee's guidelines for the care of animal subjects. Male Sprague-Dawley rats (28–48 weeks old; weight, 500–700 g; n=4) were obtained from Japan SLC (Hamamatsu, Japan). Following anesthesia with 3% sevoflurane (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) inhalation, each 15-ml blood sample collected from the tail vein was mixed with 3.13% sodium citrate solution (10% v/v) to inhibit coagulation. The blood sample was used for the examination as soon as it was collected. The Mw and DDA of each chitosan sample are listed in Table I. The samples with a DDA (in %)/Mw (in Da) of 84.2/8,600, 85.7/15,900, 50.3/28,800, 48.7/57,700, 35.5/30,000 and 33.6/57,300 (Yaizu Suisankagaku Industry Co., Ltd, Tokyo, Japan) were purified according to a previously described method (22). Chitosan oligomers (dimer, trimer, tetramer, pentamer and hexamer) were purchased from Seikagaku Co. (Tokyo, Japan), chitosan with a DDA of 87.6% and a Mw of 247,000 Da was obtained from Primex ehf (Siglufjordur, Iceland) and chitosan with a DDA of 75–85% and a Mw of 50,000–190,000 Da was from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). As an adjustment for the chitosan solution, 40 mg of each chitosan powder was dissolved in 15 ml 2% acetic acid, the pH was adjusted to 4.0 with 1 M sodium bicarbonate, 2 ml of 10X concentrated Dulbecco's phosphate-buffered saline (PBS) without calcium and magnesium was added to adjust to the osmotic pressure of blood, and the solution was topped up to 20 ml with distilled water. Blank sample was adjusted as follows: 15 ml 2% acetic acid was adjusted to pH 4.0 with 1 M sodium bicarbonate, 2 ml of 10X concentrated PBS without calcium and magnesium was added and the solution was topped up to 20 ml with distilled water.
Table I.
Chitosan samples used in the present study.
| Number | Degree of deacetylation (%) | Molecular weight (Da) |
|---|---|---|
| 1 | 84.2 | 8,600 |
| 2 | 85.7 | 15,900 |
| 3 | 75–85 | 50,000–190,000 |
| 4 | 87.6 | 247,000 |
| 5 | 50.3 | 28,800 |
| 6 | 48.7 | 57,700 |
| 7 | 35.5 | 30,000 |
| 8 | 33.6 | 57,300 |
| II (dimer) | 100 | 413.25 |
| III (trimer) | 100 | 610.87 |
| IV (tetramer) | 100 | 808.49 |
| V (pentamer) | 100 | 1,006.11 |
| VI (hexamer) | 100 | 1,203.72 |
Determination of protein in the plasma
In a typical process, 500 µl of blood was added to 500 µl of 0.2% chitosan solution and 25 µl of 200 mM calcium chloride solution. The solution was gently mixed and incubated at room temperature for 1 h. Subsequently, the mixture was centrifuged at 10,000 × g for 15 min and the plasma was collected. The plasma samples were used at once without freezing. The levels of albumin, fibrinogen, PF4, PDGF-AB, PDGF-BB, IGF and HGF in the plasma were measured using ELISA kits as follows: Rat Albumin ELISA kit (E-25AL) and Rat Fibrinogen ELISA kit (E-25FIB), both from Immunology Consultants Laboratory (Portland, OR, USA); ELISA kit for Platelet Factor 4 (SEA172Ra; USCN Life Science, Wuhan, China); Mouse/Rat PDGF-AB Quantikine ELISA kit (MHD00), Mouse/Rat PDGF-BB Quantikine ELISA kit (MBB00) and Mouse/Rat IGF-I Quantikine ELISA kit (MG100), all from R&D Systems, Inc. (Minneapolis, MN, USA); and Rat HGF EIA (1Z81; Institute of Immunology, Tokyo, Japan).
Cell proliferation assay
Plasma was sterilized using a 0.2-µm filter (EMD Millipore, Billerica, MA, USA). Human fibroblasts (NHDF-Ad) and adipose tissue-derived stromal cells (ASCs; cat. no. PT-5006) (Lonza Japan, Tokyo) were plated at a density of 1.0×104 cells/well in 96-well culture plates (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) and were cultured with Dulbecco's modified Eagle's medium including 5% plasma. Cell proliferation was examined using a Cell Counting kit (Dojindo Co., Kumamoto, Japan).
Statistical analysis
Values are expressed as the mean ± standard deviation. Multiple comparisons were evaluated using analysis of variance, as well as Tukey's and Dunnet's tests as appropriate. Statistical analysis was conducted using JMP® 11 software (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Platelet activation
The platelet activation test was performed by measuring PF4 secretion in plasma. Chitosan with a high DDA (>75%) including oligomer induced a higher release of PF4 in plasma than chitosan with a DDA of 50.3% and below (Fig. 1). In particular, chitosan with a Mw of 50,000–190,000 Da and a DDA of 75–85% exhibited the best activating efficiency among the 13 types of chitosan tested.
Figure 1.

Amount of PF4 in plasma. Values are expressed as the mean ± standard deviation (n=4). *P<0.0001, **P<0.001 vs. b. #P<0.05 vs. all other samples. Each number corresponds to a chitosan sample defined in Table I. PF4, platelet factor 4; b, blank.
Growth factors induced by chitosan
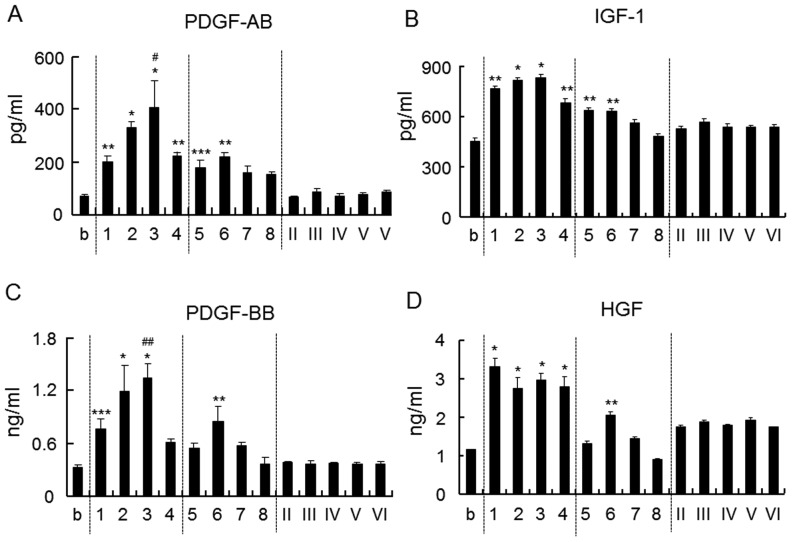
The amounts of growth factors such as PDGF-AB, PDGF-BB, IGF-1 and HGF in plasma were measured (Fig. 2). The amount of PDGF-AB and IGF-1 increased upon the addition of chitosan with a DDA of >75% and a Mw of >8,600 Da, as well as chitosan with a DDA of ~50% and a Mw of 28,800 or 57,700 Da (Fig. 2A and B). The amount of PDGF-BB and HGF was increased upon addition of chitosan with a DDA of >75% and a Mw of >8,600 Da as well as with a DDA of 48% and a Mw of 57,700 Da (Fig. 2C and D). Chitosan oligomer (100% DDA) did not have any influence on the release of these growth factors.
Figure 2.
Amount of (A) PDGF-AB, (B) IGF-1, (C) PDGF-BB and (D) HGF in plasma. Each number corresponds to a chitosan sample defined in Table I. Values are expressed as the mean ± standard deviation (n=4). *P<0.0001, **P<0.0005, ***P<0.001 vs. b. #P<0.0005, ##P<0.05 vs. all other samples. PDGF, platelet-derived growth factor; IGF, insulin growth factor; HGF, hepatocyte growth factor; b, blank.
Effect on plasma protein
The effect of chitosan on plasma albumin, which accounts for ~60% of all plasma protein, and on fibrinogen, which has an important role in secondary hemostasis, was measured. However, chitosan was found to have no effect on the levels of albumin and fibrinogen in plasma (Fig. 3).
Figure 3.
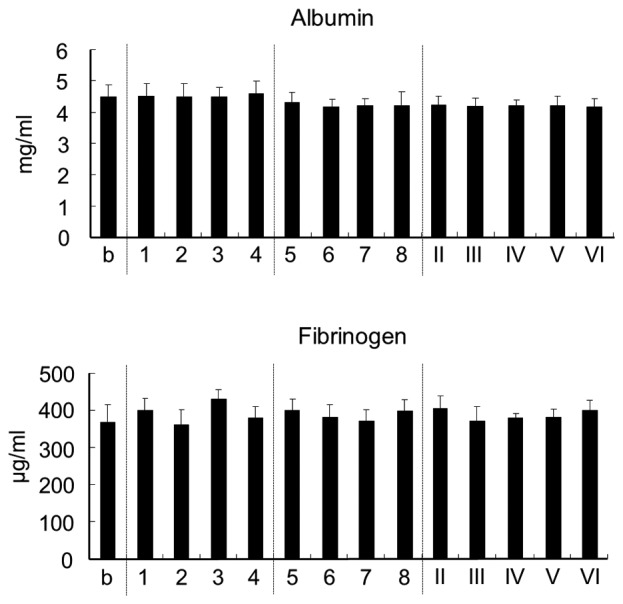
Amount of albumin and fibrinogen in plasma. Each number corresponds to a chitosan sample defined in Table I. Values are expressed as the mean ± standard deviation (n=4). b, blank.
Cell proliferation
To analyze the effect of chitosan-treated plasma on cell proliferation, human fibroblasts and ASCs were cultured using plasma activated by chitosan (Fig. 4). Based on the results of growth factors induced by chitosan (Fig. 2), the effects of plasma individually treated with 7 different chitosan samples on cell proliferation was examined. The use of plasma activated by chitosan with a DDA of 75–85% and a Mw of 50,000–190,000 Da resulted in the highest increase in the proliferation of fibroblasts and ASCs. The second-highest increase was achieved using chitosan with a DDA of 85.7 % and a Mw of 15,900 Da. However, the chitosan oligomer did not increase the cell proliferation when compared to other chitosan samples.
Figure 4.

Proliferation of human fibroblasts and ASCs. Each number corresponds to a chitosan sample defined in Table I. Values are expressed as the mean ± standard deviation (n=4). *P<0.0005, **P<0.001 vs. b. ASC, adipose tissue-derived stromal cell; OD, optical density; b, blank.
Discussion
PRP therapy is a treatment process that uses a patient's own blood to activate and release of growth factor-rich granules at the wound site. Chitosan has been reported to improve the efficiency of PRP therapy (23–25). PRP-loaded chitosan scaffolds may be an appropriate carrier for PRP applications that facilitate the sustained release of growth factors (23). Chitosan is used as a functional delivery aid to simultaneously support PRP, stem cells and growth factors (24). Platelets contain negatively charged membranes due to the presence of negatively charged sugars, negatively charged phospholipids such as phosphatidylethanolamine phosphatidylcholine, carbohydrates such as sialic acid, and, to a much lesser extent, negatively charged amino acids such as aspartate and glutamate (26–29). By contrast, chitosan is positively charged due to the existence of free amino groups derived from the deacetylation of N-acetyl-D-glucosamine. Chitosan is highly positively charged, and strongly attracts and binds to negatively charged molecules. These interactions potentially induce platelet activation. Normally, non-activated platelets store CD62P in the alpha-granule membranes, but several stimulators rapidly translocate to the platelet surface (30–33). Platelet activated by these stimulators releases alpha-granule constituents, such as PF4 and growth factors (12,13). Although chitosan has previously been reported to cause platelet activation (34–37), the effect of differences in Mw and DDA has remained elusive. Therefore, basic studies using 13 types of chitosan with varying Mw and DDA were performed in the present study.
As it was assumed possible to produce more effective treatments for intractable diseases by use of PRP in which platelets are activated by chitosan, a preliminary experiment was performed to assess the platelet activation and the release of growth factors in PRP including chitosan; however, no accurate analysis was possible. Due to the platelet activation effect of chitosan, the PF4 levels and growth factors showed large variations due to being affected by slight stimulations occurring during the measurement procedure. Consequently, these factors were assayed in plasma, which was centrifuged after chitosan was added to the whole blood. Chitosan with a DDA of >75%, including chitosan oligomer, significantly enhanced PF4 release; chitosan with a reasonably high DDA therefore increased the platelet activation. This activation can be attributed to the increase in the number of free amino groups in chitosan with a higher DDA. A high DDA is therefore important for high platelet activation, and additionally, a Mw of >8,900 Da was also required for higher activation.
Platelets activated by chitosan released various growth factors, including PDGF-AB, PDGF-BB, HGF and IGF-1. However, the effects on the levels of growth factors were not the same among the different types of chitosan. The release of PDGF-AB and PDGF-BB was the highest in the presence of chitosan with a DDA of 75–85% and a Mw of 50,000–190,000 Da. However, this was not the case for IGF-1 and HGF, whose release was highest in the presence of chitosan with a DDA of >75% and a Mw of 8,600–190,000 Da as well as with a DDA of 48% and a Mw of 57,700 Da. The observed difference in the levels of growth factors may possibly be attributed to the charge balance or interactions of other proteins. Further studies are required to explain for the high platelet activation observed in chitosan with a higher DDA and lower Mw. Overall the results of the present study demonstrated that the addition of chitosan to blood activates platelets to release growth factors in plasma, thereby improving the effectiveness of the PRP therapy.
Growth factors promote cell proliferation, differentiation and angiogenesis (38,39). PRP induces stimulation of cell growth in ASCs, periodontal ligament and mesenchymal stem cells as well as enhancement of cellular adhesion, proliferation and differentiation of human periodontal ligament cells (40). In the present study, plasma with chitosan-induced growth factor enrichment stimulated the growth of fibroblasts and ASCs. In particular, the proliferation was enhanced with the use of plasma containing chitosan with a DDA of 75–85% and a Mw of 50,000–190,000 Da. Recently, Bura et al (41) demonstrated the feasibility and safety of autologous ASC transplantation in patients with objectively proven critical limb ischemia not suitable for revascularization. The use of ASCs and PRP, which is activated by chitosan with a DDA of 75–85% and a Mw of 50,000–190,000 Da for therapeutic application in wound healing and complications in patients with intractable diseases such as diabetic ulcers and decubitus, is expected to be an efficacious approach.
Fibrinogen is an acute-phase protein that is a part of the coagulation cascade, the end result of which is the production of thrombin that converts fibrinogen to fibrin clots. Surfaces of the materials coated with fibrinogen promote platelet adhesion and activation (42). Albumin, which accounts for ~60% of plasma protein, is negatively charged, as are platelets (43). Albumin combines with various internal substrates and functions to transport them to the target tissue. In the present study, chitosan was not found to affect fibrinogen and albumin in plasma.
Of all the 13 chitosan samples tested in the present study, that with a DDA of 75–85% and a Mw of 50,000–190,000 Da showed the highest platelet activation and release of growth factors. Moreover, plasma induced by chitosan stimulated the proliferation of human fibroblasts and ASCs. However, chitosan did not affect the levels of fibrinogen and albumin in plasma. These results suggested that the effectiveness of PRP can be improved by using this type of chitosan.
Acknowledgements
The authors would like to thank Yaizu Suisankagaku Industry Co., Ltd (Tokyo, Japan) for supplying the chitosan, as well as Ms. Keiko Yamazaki and Ms. Reiko Yoshimoto for their research assistance.
References
- 1.Ferrari M, Zia S, Valbonesi M, Henriquet F, Venere G, Spagnolo S, Grasso MA, Panzani I. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs. 1987;10:47–50. [PubMed] [Google Scholar]
- 2.Greer N, Foman NA, MacDonald R, Dorrian J, Fitzgerald P, Rutks I, Wilt TJ. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: A systematic review. Ann Intern Med. 2013;159:532–542. doi: 10.7326/0003-4819-159-8-201310150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Jones KR, Fennie K, Lenihan A. Evidence-based management of chronic wounds. Adv Skin Wound Care. 2007;20:591–600. doi: 10.1097/01.ASW.0000284936.32707.8d. [DOI] [PubMed] [Google Scholar]
- 4.Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: A systematic review and meta-analysis. Eplasty. 2011;11:e38. [PMC free article] [PubMed] [Google Scholar]
- 5.Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14:680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 6.Bolton LL, van Rijswijk L, Shaffer FA. Quality wound care equals cost-effective wound care: A clinical model. Adv Wound Care. 1997;10:33–38. [PubMed] [Google Scholar]
- 7.Zucker MB, Nachmias VT. Platelet activation. Arteriosclerosis. 1985;5:2–18. doi: 10.1161/01.ATV.5.1.2. [DOI] [PubMed] [Google Scholar]
- 8.Winokur R, Hartwig JH. Mechanism of shape change in chilled human platelets. Blood. 1995;85:1796–1804. [PubMed] [Google Scholar]
- 9.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525–1541. [PubMed] [Google Scholar]
- 10.Brown GT, Narayanan P, Li W, Silverstein RL, McIntyre TM. Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop. J Immunol. 2013;191:5196–5203. doi: 10.4049/jimmunol.1300354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res. 2010;125:292–296. doi: 10.1016/j.thromres.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Burnouf T, Goubran HA, Chen TM, Ou KL, El-Ekiaby M, Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27:77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary culture. Proc Natl Acad Sci USA. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori H, Amano Y, Nogami Y, Takase B, Ishihara M. Hemostasis for severe hemorrhage with photocrosslinkable chitosan hydrogel and calcium alginate. Ann Biomed Eng. 2010;38:3724–3732. doi: 10.1007/s10439-010-0121-4. [DOI] [PubMed] [Google Scholar]
- 16.Ono K, Ishihara M, Ozeki Y, Deguchi H, Sato M, Saito Y, Yura H, Sato M, Kikuchi M, Kurita A, Maehara T. Experimental evaluation of photocrosslinkable chitosan as a biologic adhesive with surgical applications. Surgery. 2001;130:844–850. doi: 10.1067/msy.2001.117197. [DOI] [PubMed] [Google Scholar]
- 17.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J Trauma. 2006;60:655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 18.Sarasam AR, Brown P, Khajotia SS, Dmytryk JJ, Madihally SV. Antibacterial activity of chitosan-based matrices on oral pathogens. J Mater Sci Mater Med. 2008;19:1083–1090. doi: 10.1007/s10856-007-3072-z. [DOI] [PubMed] [Google Scholar]
- 19.Mellegard H, Kovács ÁT, Lindbäck T, Christensen BE, Kuipers OP, Granum PE. Transcriptional responses of Bacillus cereus towards challenges with the polysaccharide chitosan. PLoS One. 2011;6:e24304. doi: 10.1371/journal.pone.0024304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigemasa Y, Minami S. Applications of chitin and chitosan for biomaterials. Biotechnol Genet Eng Rev. 1996;13:383–420. doi: 10.1080/02648725.1996.10647935. [DOI] [PubMed] [Google Scholar]
- 21.Park CJ, Gabrielson NP, Pack DW, Jamison RD, Johnson AJ Wagoner. The effect of chitosan on the migration of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials. 2009;30:436–444. doi: 10.1016/j.biomaterials.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 22.Hattori H, Ishihara M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed Mater. 2015;10:015014. doi: 10.1088/1748-6041/10/1/015014. [DOI] [PubMed] [Google Scholar]
- 23.Kutlu B, Tiğlı Aydın RS, Akman AC, Gümüşderelioglu M, Nohutcu RM. Platelet-rich plasma-loaded chitosan scaffolds: Preparation and growth factor release kinetics. J Biomed Mater Res B Appl Biomater. 2013;101:28–35. doi: 10.1002/jbm.b.32806. [DOI] [PubMed] [Google Scholar]
- 24.Busilacchi A, Gigante A, Mattioli-Belmonte M, Manzotti S, Muzzarelli RA. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr Polym. 2013;98:665–676. doi: 10.1016/j.carbpol.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Oktay EO, Demiralp B, Demiralp B, Senel S, Akman A Cevdet, Eratalay K, Akincibay H. Effects of platelet-rich plasma and chitosan combination on bone regeneration in experimental rabbit cranial defects. J Oral Implantol. 2010;36:175–184. doi: 10.1563/AAID-JOI-D-09-00023. [DOI] [PubMed] [Google Scholar]
- 26.Bosmann HB. Platelet adhesiveness and aggregation: II. Surface sialic acid, glycoprotein: N-acetylneuraminic acid transferase, and neuraminidase of human blood platelets. Biochim Biophys Acta. 1972;279:456–474. doi: 10.1016/0304-4165(72)90167-5. [DOI] [PubMed] [Google Scholar]
- 27.Lupu C, Calb M. Changes in the platelet surface charge in rabbits with experimental hypercholesterolemia. Atherosclerosis. 1988;72:77–82. doi: 10.1016/0021-9150(88)90065-2. [DOI] [PubMed] [Google Scholar]
- 28.vd Winkel JG, Wetzels JF, van Duijnhoven JL, Koene RA, Capel PJ. Red blood cell surface charge and alcian blue binding. Nephrol Dial Transplant. 1987;2:280–281. [PubMed] [Google Scholar]
- 29.Briedé JJ, Heemskerk JW, Hemker HC, Lindhout T. Heterogeneity in microparticle formation and exposure of anionic phospholipids at the plasma membrane of single adherent platelets. Biochim Biophys Acta. 1999;1451:163–172. doi: 10.1016/S0167-4889(99)00085-3. [DOI] [PubMed] [Google Scholar]
- 30.Mackman N, Tilly RE, Key NS. Role of the extinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 31.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746–756. [PubMed] [Google Scholar]
- 32.Klinger MH. Platelets and inflammation. Anat Embryol (Berl) 1997;196:1–11. doi: 10.1007/s004290050075. [DOI] [PubMed] [Google Scholar]
- 33.Hagberg IA, Lyberg T. Evaluation of circulating platelet-leukocyte conjugates: A sensitive flow cytometric assay well suited for clinical studies. Platelets. 2000;11:151–160. doi: 10.1080/095371000403080. [DOI] [PubMed] [Google Scholar]
- 34.Shen EC, Chou TC, Gau CH, Tu HP, Chen YT, Fu E. Releasing growth factors from activated human platelets after chitosan stimulation: A possible bio-material for platelet-rich plasma preparation. Clin Oral Implants Res. 2006;17:572–578. doi: 10.1111/j.1600-0501.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- 35.Fukusawa M, Abe H, Masaoka T, Orita H, Horikawa H, Campeau JD, Washio M. The hemostatic effect of deacetylated chitin membrane on peritoneal injury in rabbit model. Surg Today. 1992;22:333–338. doi: 10.1007/BF00308742. [DOI] [PubMed] [Google Scholar]
- 36.International committee for standardization in hematology, corp-author. Recommendation of measurement of erythrocyte sedimentation rate of human blood. Am J Clin Pathol. 1977;68:505–507. doi: 10.1093/ajcp/68.4.505. [DOI] [PubMed] [Google Scholar]
- 37.Sugamori T, Iwase H, Maeda M, Inoue Y, Kurosawa H. Local hemostatic effects of microcrystalline partially deacetylated chitin hydrochloride. J Biomed Mater Res. 2000;49:225–232. doi: 10.1002/(SICI)1097-4636(200002)49:2<225::AID-JBM10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 38.Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2):S3–S22. Suppl 1. [PubMed] [Google Scholar]
- 39.Miyazawa K. Hepatocyte growth factor activator (HGFA): A serine protease that links tissue injury to activation of hepatocyte growth factor. FEBS. 2010;277:2208–2214. doi: 10.1111/j.1742-4658.2010.07637.x. [DOI] [PubMed] [Google Scholar]
- 40.Han J, Meng HX, Tang JM, Li SL, Tang Y, Chen ZB. The effect of different platelet-rich plasma concentrations on proliferation and differentiation of human periodontal ligament cells in vitro. Cell Prolif. 2007;40:241–252. doi: 10.1111/j.1365-2184.2007.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 42.He Q, Ao Q, Gong K, Zhang L, Hu M, Gong Y, Zhang X. Preparation and characterization of chitosan-heparin composite matrices for blood contacting tissue engineering. Biomed Mater. 2010;5:055001. doi: 10.1088/1748-6041/5/5/055001. [DOI] [PubMed] [Google Scholar]
- 43.Busher JT. Serum albumin and globulin. In: Walker KH, Hall DW, Hurst WJ, editors. Clinical Methods: The History, Physical and Laboratory Examinations. 3rd. Butterworth Publishers; Boston: 1990. pp. 497–499. [Google Scholar]