Abstract
Endometrial adenocarcinoma (EC) is one of the most frequently diagnosed types of endometrial cancer and is typically a consequence of continuous estrogen receptor stimulation. Erythropoietin-producing hepatocyte receptor B4 (EphB4) and its ligand ephrin-B2 have been reported to be overexpressed in EC cells; however, the function in EC remains unclear. The present study aimed to elucidate the role of EphB4 and ephrin-B2 in EC. The protein expression pattern of EphB4 and ephrin-B2 was analyzed through immunohistochemistry and western blot analysis in endometrium with adenomyosis or simple endometrial hyperplasia, atypical endometrial hyperplasia, double-positive estrogen receptor (ER)/progesterone receptor (PR) EC and double-negative ER/PR EC. The expression of EphB4 and ephrin-B2 was demonstrated to be increased in atypical EH and ER/PR-positive EC, but not ER/PR-negative EC. Furthermore, EphB4 and ephrin-B2 expression was positively associated with ER expression in EC tissue. The results of the present study suggest that the overexpression of EphB4 and ephrin-B2 in the endometrium serves a role in the pathogenesis of EC, in addition to being associated with ER expression.
Keywords: endometrial adenocarcinoma, erythropoietin-producing hepatocyte receptor B4, ephrin-B2, estrogen receptor, progesterone receptor
Introduction
Endometrial adenocarcinoma (EC) is one of the most frequently diagnosed types of endometrial cancer and accounts for >80% of all endometrial carcinoma cases (1). Despite its low mortality rate, EC causes >8,000 mortalities/year in the United States (2). The incidence rate of EC in China has increased and the age of onset has decreased (3). Although patients with early-stage EC who receive surgery have a good chance of survival, younger patients frequently become infertile following treatment. The pathogenesis of EC is frequently associated with continuous estrogen receptor stimulation (4); however, the mechanisms underlying EC pathogenesis remain a major area of investigation in gynecology research. Elucidating the underlying mechanism of endometrial carcinogenesis may aid in the development of novel approaches to prevent endometrial tumorigenesis.
Erythropoietin-producing hepatocyte receptor B4 (EphB4) and its ligand ephrin-B2 belong to the receptor tyrosine kinase (RTK) family (5). RTKs are transmembrane proteins that can be phosphorylated and activated by each other, enabling the transmission of signals to the inside of the cell in order to control cell aggregation, migration, development, maturation, angiogenesis and vascular remodeling (6,7). Previously, overexpression of EphB4 and ephrin-B2 has been demonstrated in numerous types of cancer, including that of the digestive (8–10), respiratory (11), reproductive (12–15), urinary (16) and endocrine (17,18) systems. These studies suggest that overexpression of EphB4 and ephrin-B2 is associated with tumor development and progression. Similarly, associations between EphB4 and ephrin-B2 overexpression, and EC development and progression have been identified (19,20). However, the mechanism of EphB4 and ephrin-B2 overexpression and their exact role in EC remains unclear. Expression of EphB4 is hormone-dependent in the mammary glands of mice (21). Estrogen and progesterone are the primary hormones responsible for the pathogenesis and prognosis of EC, respectively (22,23). The present study aimed to investigate whether EphB4 and ephrin-B2 expression in EC is associated with estrogen receptor (ER) and progesterone receptor (PR) expression status, in order to determine the role of EphB4 and ephrin-B2 in EC.
Materials and methods
Patients and tissue samples
The present study was approved by the Medical Ethics Review Board of the First Affiliated Hospital of Yangtze University (Jingzhou, China) in accordance with the guidelines for the protection of human subjects (24). Informed consent was obtained from all patients (or from relatives if the patients were unavailable). Control endometrial tissue samples (n=12; mean age, 42.3 years old) were collected from patients who had undergone a diagnostic curettage and were subsequently diagnosed with uterine adenomyosis (n=10) or simple endometrial hyperplasia (EH) (n=2). EC (endometrioid type) tissue samples were obtained from patients who underwent a hysterectomy at the Department of Gynecology of The First Affiliated Hospital of Yangtze University (n=44; mean age, 54.9 years old) between January 2009 and March 2015. None of the patients had received pre-operative chemotherapy, radiotherapy or endocrine therapy. Patients were evaluated in accordance with the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system (25). The number of patients classified as FIGO stage I, II, III and IV were 20, 12, 2 and 0, respectively. In addition, atypical EH tissue samples were collected for immunohistochemical staining (n=20; mean age, 44.5 years old).
Immunohistochemistry
Formalin-fixed paraffin-embedded control, atypical EH and EC samples were cut with a microtome (Leica Microsystems GmbH, Wetzlar, Germany) into 4-µm-thick sections. Samples were immunohistochemically stained as described previously (26). Briefly, formalin-fixed paraffin-embedded sectioned samples were dried on a slide at 72°C for 1 h. Subsequently, sections were deparaffinized in xylene at room temperature for30 min, rehydrated with graded concentrations of ethanol and washed in PBS. The samples were placed in 10 mM EDTA buffer (pH 8.0) and boiled in a microwave at 110°C for 10 min to allow for epitope retrieval. Endogenous peroxidase activity was quenched through incubating tissue sections in 3% H2O2 at 24°C for 10 min. Sections were partially stained with hematoxylin and eosin to confirm the presence of cancerous cells, and sections adjacent to these were used for immunohistochemical staining. Subsequent to blocking in 10% normal donkey serum (cat. no. 017-000-121; Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA) at 24°C for 1.5 h, sections for immunohistochemical analysis were incubated with the following primary antibodies under different conditions: Polyclonal rabbit anti-human EphB4 (dilution, 1:100; cat. no. sc-5536; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and polyclonal rabbit anti-human ephrin-B2 (dilution, 1:75; cat. no. sc-1010; Santa Cruz Biotechnology, Inc.) overnight at 4°C; monoclonal mouse anti-human ER-α (dilution, ready-to-use; cat. no. GT201701; Gene Company, Ltd., Hong Kong, China) and monoclonal mouse anti-human PR (dilution, ready-to-use; cat. no. GT216002; Gene Company, Ltd.) for 2 h at 4°C. Subsequently, the slides were washed with PBS, incubated with a ready-to-use HRP-conjugated polymer with anti-mouse/rabbit serum immunoglobulin, included in a GTVision™ Detection kit that reacts with rabbit and mouse primary antibodies (this polymer acted as the secondary antibody; dilution, ready-to-use; cat. no. GK500510A; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), at 24°C for 1 h and then incubated with 3,3′-diaminobenzidine chromogenic agent (dilution, 1:50; cat. no. GK346810; Dako; Agilent Technologies, Inc.) at 24°C for 5–15 min. Slides were counterstained with Mayer's hematoxylin (1:10) for 35–60 sec and mounted with Entellan® new mounting medium (Merck Millipore, Darmstadt, Germany). The negative control was treated with rabbit serum (cat. no. X090210-8; Dako; Agilent Technologies, Inc.) instead of the primary antibodies.
Western blot analysis
Western blot analysis was performed as previously described, but with several modifications (27). Total protein was acquired through ultrasonication of samples in RIPA lysis buffer [50 mM Tris-Cl, 150 mM NaCl, 1% Triton X-100, 0.1% aprotinin, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate and 25 mM sodium fluoride (pH7.4)], supplemented with a PhosStop cocktail (cat. no. 4906845001; Roche Applied Science, Mannheim, Germany). Protein concentration was determined using the BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total protein (30 µg) was separated using SDS-PAGE on a 7.5% gel and subsequently electro blotted onto Immobilon®-P polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA) using the Mini-PROTEAN® 3 Electrophoresis system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following a blocking step in 5% bovine serum albumin (cat. no. GA0334; Generay Biotech Co., Ltd., Shanghai, China) at room temperature for 1 h, the membranes were incubated overnight at 4°C with polyclonal rabbit anti-EphB4 (dilution, 1:1,000; cat. no. ABC257) and monoclonal mouse anti-ephrin-B2 (dilution, 1:2,000; cat. no. MABC127) (both from EMD Millipore, Billerica, MA, USA), and monoclonal mouse anti-β-actin (dilution, 1:3,000; cat. no. A2228; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) primary antibodies. Subsequent to membrane washing in TBS with Tween-20, membranes were incubated with horseradish peroxidase-conjugated donkey anti-mouse (cat. no. 715-036-150) or donkey anti-rabbit (cat. no. 711-036-152) secondary antibody (both at 1:10,000; Immuno Research Laboratories, Inc.) at room temperature for 1.5 h. Protein bands were visualized through incubating the blots with enhanced chemifluorescent reagent (cat. no. 34080; Pierce; Thermo Fisher Scientific, Inc.) and exposed to X-ray film in the dark. Experiments were performed in triplicate, and protein bands were quantitatively analyzed using ImageJ software (version 1.38; National Institutes of Health, Bethesda, MD, USA) and SigmaPlot (version 10.0; Systat Software, Inc., San Jose, CA, USA). Images were processed with Photoshop (version CS4; Adobe Systems, Inc., San Jose, CA, USA) and CorelDRAW® version X4 (Corel Corporation, Ottawa, Ontario, Canada).
Assessment of immunohistochemical analysis score
All sections stained for EphB4 and ephrin-B2 were semiquantitatively evaluated by two pathologists using the method described by McCarty et al (28), which considers the intensity of staining and the percentage of cells stained at each intensity. Staining intensity was scored as follows: 0, no staining; 1, weak staining; 2, distinct staining; 3, strong staining; and 4, very strong staining. For each section stained, the percentage of positively stained cells (intensity grade ≥2) was scored as described previously (29), which is as follows: -, 0–5%; +, 6–50%; ++, 51–75%; and +++, >75%.
Statistical analysis
For western blot analysis the averaged data are presented as the mean ± standard error of the mean. The level of a protein was first normalized to its corresponding actin for each sample, and the relative levels were then averaged for all the samples to the control sample. A Mann-Whitney test (comparisons between two groups) was used for analysis and P<0.05 was considered to indicate a statistically significant difference using SigmaPlot version 10.0 (Systat Software, Inc., San Jose, CA, USA).
Results
Overexpression of EphB4 and ephrin-B2 proteins in ER- and PR-positive EC
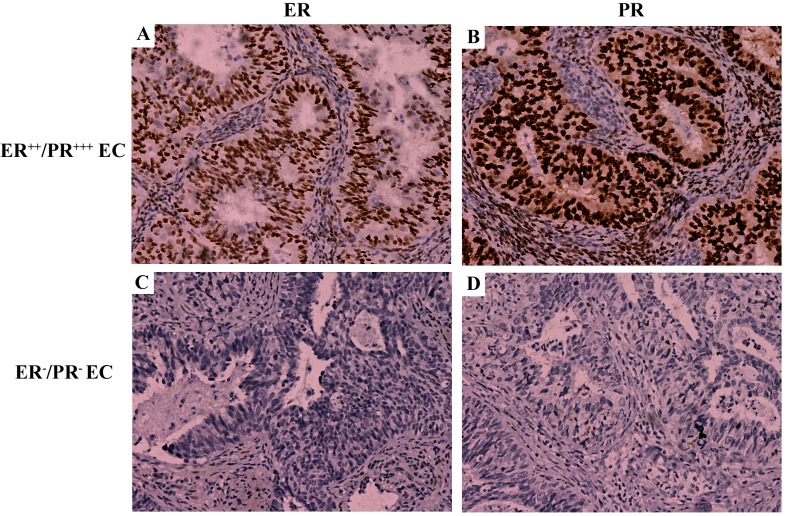
EC tissue was categorized according to immunohistochemical analysis of PR and ER expression(Fig. 1), as PR expression is an important indicator for the prognosis of patients with EC and estrogen signaling has been associated with EC development (22,23). A total of 1/12 samples were identified as ER-positive/PR-negative and no samples were identified as ER-negative/PR-positive. Therefore, the remaining EC tissue samples were categorized into the following two groups: Double ER/PR-positive (n=11) and double ER/PR-negative (n=33). Fig. 1 illustrates the typical expression pattern of ER and PR in the endometrial tissue tested. In the double ER/PR-positive group, staining for ER (Fig. 1A) and PR (Fig. 1B) was identified in the nuclei of the majority of uterine gland cells and a number of stromal cells. By contrast, no staining for ER (Fig. 1C) or PR (Fig. 1D) was identified in the double ER/PR-negative group.
Figure 1.
Representative images of ER and PR expression in EC tissue samples. (A) ER/PR-positive EC sample stained for ER. (B) ER/PR-positive EC sample stained for PR. (C) ER/PR-negative stained for ER. (D) ER/PR-negative stained for PR. Magnification, ×200. ER, estrogen receptor; PR, progesterone receptor; EC, endometrial adenocarcinoma.
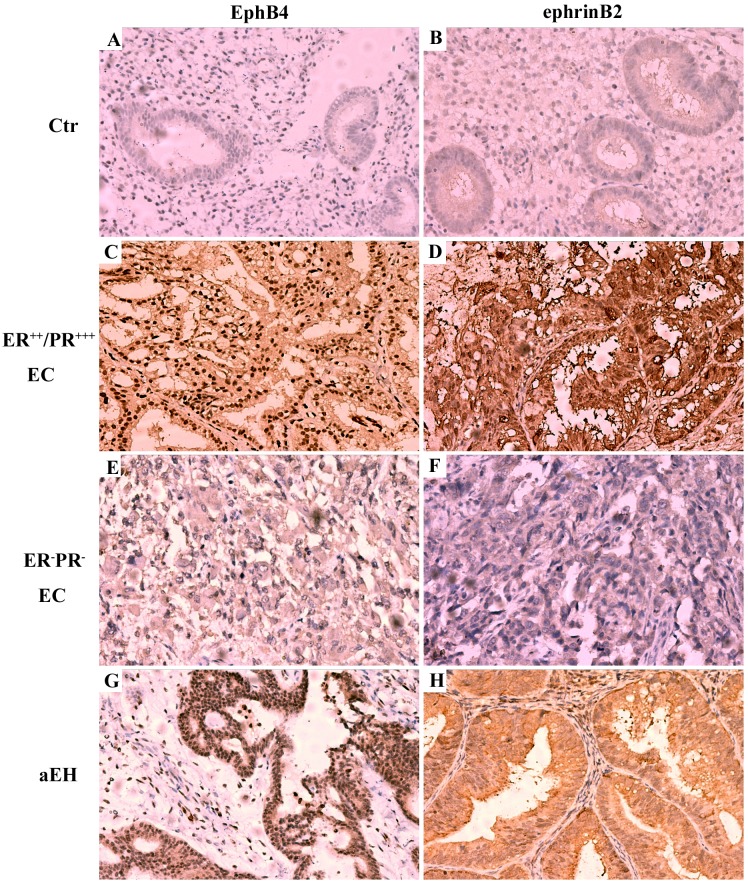
In the control group staining for EphB4 and ephrin-B2 was relatively low (Fig. 2A and B), whilst in the ER/PR-positive group, staining for EphB4 and ephrin-B2 was increased compared with the control group (Fig. 2). Expression of EphB4 protein was typically located in the nuclei of uterine gland cells and stromal cells (Fig. 2C), similar to the expression pattern of ER and PR. The majority of ephrin-B2 protein was expressed on the membrane of uterine gland cells and in the nuclei of stromal cells (Fig. 2D). Notably, it was demonstrated that EphB4 (Fig. 2E) and ephrin-B2 (Fig. 2F) protein was not overexpressed in the double ER/PR-negative group. Estrogen serves an essential role in the development of atypical EH, a lesion that frequently precedes EC (30). Since the expression pattern of EphB4 protein was similar to the expression pattern of ER and PR in the ER/PR-positive group, the expression of EphB4 and ephrin-B2 in EH tissue was also analyzed using immunohistochemistry. In all 20 atypical EH tissue samples, the expression of EphB4 (Fig. 2G) and ephrin-B2 (Fig. 2H) protein was increased compared with the control group.
Figure 2.
Expression pattern of EphB4 and ephrin-B2 in the endometrium of different EC groups using immunohistochemistry. (A) EphB4 and (B) ephrin-B2 expression in Ctr group. (C) EphB4 and (D) ephrin-B2 expression in ER++/PR+++ EC group. (E) EphB4 and (F) ephrin-B2 expression in ER-/PR- EC group. (G) EphB4 and (H) ephrin-B2 expression in aEH group. Magnification, ×200. EphB4, erythropoietin-producing hepatocyte receptor B4; ER, estrogen receptor; PR, progesterone receptor; EC, endometrial adenocarcinoma; aEH, atypical endometrial hyperplasia; Ctr, control.
EphB4 and ephrin-B 2protein expression was significantly increased in ER/PR-positive EC
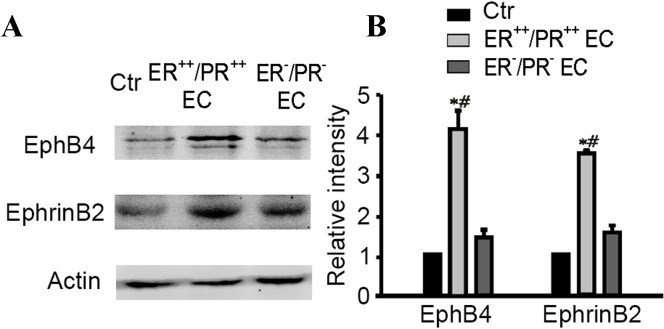
Western blot analysis was performed to confirm that overexpression of EphB4 and ephrin-B2 protein was present in the double ER/PR-positive group and absent in the double ER/PR-negative group (Fig. 3). Compared with the control group, expression of EphB4 and ephrin-B2 protein in the double ER/PR-positive group was significantly increased (4.11±0.46 and 3.46±0.10 relative to the control group; n=4; P=0.0022 for EphB4 and P=0.0001 for ephrin-B2; Fig. 3B). In addition, no significant differences were identified in the EphB4 and ephrin-B2 protein expression between the double ER/PR-negative group and the control group (Fig. 3B).
Figure 3.
Determination of EphB4 and ephrin-B2 expression in different EC groups using western blot analysis. (A) Representative immunoblots illustrating changes in EphB4 and ephrin-B2 expression. (B) Densitometric quantification of immunoreactive bands of EphB4 and ephrin-B2immunoblot. *P<0.01 vs. the control group; #P<0.01 vs. double ER/PR-negative EC group. EphB4, erythropoietin-producing hepatocyte receptor B4; ER, estrogen receptor; PR, progesterone receptor; EC, endometrial adenocarcinoma; EH, atypical endometrial hyperplasia; Ctr, control.
EphB4 and ephrin-B2 overexpression is associated with ER expression in EC
EphA2 overexpression has been associated with a lack of PR and poor patient outcome in EC (29); however, in the present study, EphB4 and ephrin-B2 overexpression was not revealed to be associated with a lack of PR expression. Since EphB4 and ephrin-B2 have been demonstrated to be overexpressed in EH tissue samples (30), the association between EphB4 and ephrin-B2 overexpression and ER expression in EC and EH was investigated in the present study. EphB4 and ephrin-B2 expression in tissue samples was analyzed according to the grading of their ER and PR staining intensity (Table I). In the ER−PR− group, 9/11 samples were EphB4- and ephrin-B2-negative, and 2/11 samples were EphB4+ and ephrin-B2+. In the ER+PR+/+++ group, all 13 samples were EphB4-positive, 6 were EphB4+ and 7 were EphB4++/+++. Regarding ephrin-B2, 8 samples showed no ephrin-B2 expression, 4 were ephrin-B2+ and 1 sample was ephrin-B2++/+++ in the ER+PR+/+++ group. In the ER++/+++PR+/+++ group, EphB4 and ephrin-B2 were expressed in all 20 samples (1 sample was EphB4+/ephrin-B2+ and 19 were EphB4++/+++/ephrin-B2++/+++). These results indicate that there is an association between ER expression and EphB4/ephrin-B2 expression in EC.
Table I.
EC samples immunohistochemically stained for EphB4 and ephrin-B2 according to ER/PR expression level.
| ER/PR expression EC group, no. of patients (%) | ||||||
|---|---|---|---|---|---|---|
| IHC staining | ER−/PR− (n=11) | ER+/PR+/+++ (n=13) | ER++/+++/PR+/+++ (n=20) | |||
| EphB4 | ||||||
| − | 9 (81.82) | 0 (0) | 0 (0) | |||
| + | 2 (18.18) | 6 (46.15) | 1 (5) | |||
| ++/+++ | 0 (0) | 7 (53.85) | 19 (95) | |||
| Ephrin-B2 | ||||||
| − | 9 (81.82) | 8 (61.54) | 0 (0) | |||
| + | 2 (18.18) | 4 (30.77) | 1 (5) | |||
| ++/+++ | 0 (0) | 1 (7.69) | 19 (95) | |||
EphB4, erythropoietin-producing hepatocyte receptor B4; ER, estrogen receptor; PR, progesterone receptor; EC, endometrium carcinoma; IHC, immunohistochemistry.
Discussion
The EphB4 receptor has been identified as a frequently overexpressed RTK in numerous types of cancer, including the following: Lung (31), esophagus (32), ovary (14), breast (33), thyroid (18), cervix (13) and prostate (34,35). The results of the present study suggest that overexpression of EphB4 and ephrin-B2 in EC is heterogeneous and hormone-dependent. In the double ER/PR-negative group, there was no significant difference in EphB4 and ephrin-B2expression compared with the control group; however, in the double ER/PR-positive group expression of these proteins was significantly increased compared with the control group. In addition, overexpression of EphB4 and ephrin-B2 in EH was demonstrated.
It has been hypothesized that EphB4 and ephrin-B2serve a role in EC development and are associated with the expression and/or function of ER. Although further research is required to confirm this, the present study demonstrated that high expression levels of ER protein are associated with an increase in EphB4 and ephrin-B2 protein expression in EC. The association between EphB4 and ER expression has been investigated in healthy mammary gland tissue and breast cancer, identifying EphB4 as a regulator of ER-α (36,37). Several studies of ovarian and endometrial cancer have reported that EphB4 and ephrin-B2 are overexpressed and serve as predictors of poor patient prognosis (13,14,20); however, the underlying mechanisms of this observation remain unclear. The results of the present study suggest that there is an interaction between ER and EphB4/ephrin-B2 signaling, providing a direction for future research in this area. In different cancer types, EphB may function through different mechanisms; for example, EphB2/EphB3 signaling has been demonstrated to suppress the progression of colorectal cancer (38,39) and breast cancer (40) through different pathways.
A previous study reported that overexpression of EphA2 is associated with poor patient prognosis and a lack of PR expression in endometrial cancer (29). PR-positive EC cells respond well to progesterone treatment and patients with PR-positive EC have a good prognosis (41). However, patients who are diagnosed with PR-negative EC are more likely to have poorly-differentiated cancer cells, for which there are currently few effective treatments (42,43). The present study revealed that EphB4 and ephrin-B2 were not expressed in any of the PR-negative EC tissues tested. So our results suggest that EphB4 and ephrin-B2 are potential therapeutic biomarkers for drug target or cancer prevention of the double ER/PR-positive EC, rather than prognostic indicators in patients with EC. Identifying the mechanisms separately for different types of EC is the direction for future research, which may individualize treatment.
In conclusion, the results of the current study suggest that EphB4 and ephrin-B2 serve a role in the pathogenesis of EC, in addition to being associated with ER signaling. The association between EphB4/ephrin-B2 expression and PR-positive EC, in addition to ER expression, and the mechanisms underlying these interactions remain to be investigated. This is important for the development of novel and efficient treatments to inhibit endometrial carcinogenesis.
Acknowledgements
The present study was supported by the Chinese Medical Association 2014 Exchange Fund for Antineoplastic Therapy (grant no. CIMF-F-H001-313).
References
- 1.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Liu Z, Yu X, Zhang X, Lü S, Chen X, Lü B. The association between metabolic abnormality and endometrial cancer: A large case-control study in China. Gynecol Oncol. 2010;117:41–46. doi: 10.1016/j.ygyno.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Kirschner MA, Schneider G, Ertel NH, Worton E. Obesity, androgens, estrogens, and cancer risk. Cancer Res. 1982;42:3281s–3285s. (Suppl 8) [PubMed] [Google Scholar]
- 5.Sakano S, Serizawa R, Inada T, Iwama A, Itoh A, Kato C, Shimizu Y, Shinkai F, Shimizu R, Kondo S, et al. Characteristics of a ligand for receptor protein-tyrosine kinase HTK expressed in haematopoietic stem cells. Oncogene. 1996;13:813–822. [PubMed] [Google Scholar]
- 6.Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19:534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquale EB. Eph receptor signalling casts a wide net on cell behavior. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Zhao ZW, Zhang Y, Xin Y. Over-expression of Ephb4 is associated with carcinogenesis of gastric cancer. Dig Dis Sci. 2011;56:698–706. doi: 10.1007/s10620-010-1346-7. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Zhao Z. Clinical implications of EphB4 receptor expression in pancreatic cancer. Mol Biol Rep. 2013;40:1735–1741. doi: 10.1007/s11033-012-2224-5. [DOI] [PubMed] [Google Scholar]
- 10.Rönsch K, Jäger M, Schöpflin A, Danciu M, Lassmann S, Hecht A. Class I and III HDACs and loss of active chromatin features contribute to epigenetic silencing of CDX1 and EPHB tumor suppressor genes in colorectal cancer. Epigenetics. 2011;6:610–622. doi: 10.4161/epi.6.5.15300. [DOI] [PubMed] [Google Scholar]
- 11.Zheng MF, Ji Y, Wu XB, Ye SG, Chen JY. EphB4 gene polymorphism and protein expression in non-small-cell lung cancer. Mol Med Rep. 2012;6:405–408. doi: 10.3892/mmr.2012.936. [DOI] [PubMed] [Google Scholar]
- 12.Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Coexpression of EphB4 and ephrin B2 in tumour advancement of ovarian cancers. Br J Cancer. 2008;98:845–851. doi: 10.1038/sj.bjc.6604216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Coexpression of EphB4 and ephrin B2 in tumor advancement of uterine cervical cancers. Gynecol Oncol. 2009;4:84–88. doi: 10.1016/j.ygyno.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer. 2007;96:1083–1091. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozgür E, Heidenreich A, Dagtekin O, Engelmann U, Bloch W. Distribution of EphB4 and Ephrin B2 in normal and malignant urogenital tissue. Urol Oncol. 2011;29:78–84. doi: 10.1016/j.urolonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Choi WW, Yan R, Yu H, Krasnoperov V, Kumar SR, Schuckman A, Klumpp DJ, Pan CX, Quinn D, et al. The differential expression of EphB2 and EphB4 receptor kinases in normal bladder and in transitional cell carcinoma of the bladder. PLoS One. 2014;9:e105326. doi: 10.1371/journal.pone.0105326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Q, Suo Z, Risberg B, Karlsson MG, Villman K, Nesland JM. Expression of Ephb2 and Ephb4 in breast carcinoma. Pathol Oncol Res. 2004;10:26–33. doi: 10.1007/BF02893405. [DOI] [PubMed] [Google Scholar]
- 18.Sharma GK, Dhillon VK, Masood R, Maceri DR. Overexpression of EphB4, Ephrin B2, and epidermal growth factor receptor in papillary thyroid carcinoma: A pilot study. Head Neck. 2015;37:964–969. doi: 10.1002/hed.23694. [DOI] [PubMed] [Google Scholar]
- 19.Takai N, Miyazaki T, Fujisawa K, Nasu K, Miyakawa I. Expression of receptor tyrosine kinase EphB4 and its ligand ephrin-B2 is associated with malignant potential in endometrial cancer. Oncol Rep. 2001;8:567–573. doi: 10.3892/or.8.3.567. [DOI] [PubMed] [Google Scholar]
- 20.Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Overexpression of ephrin B2 and EphB4 in tumor advancement of uterine endometrial cancers. Ann Oncol. 2007;18:485–490. doi: 10.1093/annonc/mdl414. [DOI] [PubMed] [Google Scholar]
- 21.Andres AC, Reid HH, Zürcher G, Blaschke RJ, Albrecht D, Ziemiecki A. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene. 1994;9:1461–1467. [PubMed] [Google Scholar]
- 22.Allen NE, Key TJ, Dossus L, Rinaldi S, Cust A, Lukanova A, Peeters PH, Onland-Moret NC, Lahmann PH, Berrino F, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2008;15:485–497. doi: 10.1677/ERC-07-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobel M, Atenafu EG, Rambau PF, Ferguson SE, Nelson GS, Ho TC, Panzarella T, McAlpine JN, Gilks CB, Clarke BA, Bernardini MQ. Progesterone receptor expression is associated with longer overall survival within high-grade histotypes of endometrial carcinoma: A Canadian high risk endometrial cancer consortium (CHREC) study. Gynecol Oncol. 2016;141:559–563. doi: 10.1016/j.ygyno.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Ketefian S. Ethical considerations in research. Focus on vulnerable groups. Invest Educ Enferm. 2015;33:164–172. doi: 10.17533/udea.iee.v33n1a19. [DOI] [PubMed] [Google Scholar]
- 25.Zivanovic O, Leitao MM, Iasonos A, Jacks LM, Zhou Q, Abu-Rustum NR, Soslow RA, Juretzka MM, Chi DS, Barakat RR, et al. Stage-specific outcomes of patients with uterine leiomyosarcoma: A comparison of the international Federation of gynecology and obstetrics and American joint committee on cancer staging systems. J Clin Oncol. 2009;27:2066–2072. doi: 10.1200/JCO.2008.19.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamat AA, Fletcher M, Gruman LM, Mueller P, Lopez A, Landen CN, Jr, Han L, Gershenson DM, Sood AK. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12:1707–1714. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Miao Y, Wang XH, Wang Z. Elevation of p-NR2AS1232 by Cdk5/p35 contributes to retinal ganglion cell apoptosis in a rat experimental glaucoma model. Neurobiol Dis. 2011;43:455–464. doi: 10.1016/j.nbd.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 28.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal anti-receptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 29.Kamat AA, Coffey D, Merritt WM, Nugent E, Urbauer D, Lin YG, Edwards C, Broaddus R, Coleman RL, Sood AK. EphA2 Overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer. 2009;115:2684–2692. doi: 10.1002/cncr.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacey JV, Jr, Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas. 2009;63:39–44. doi: 10.1016/j.maturitas.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson BD, Liu R, Rolle CE, Tan YH, Krasnoperov V, Kanteti R, Tretiakova MS, Cervantes GM, Hasina R, Hseu RD, et al. The EphB4 receptor tyrosine kinase promotes lung cancer growth: A potential novel therapeutic target. PLoS One. 2013;8:e67668. doi: 10.1371/journal.pone.0067668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasina R, Mollberg N, Kawada I, Mutreja K, Kanade G, Yala S, Surati M, Liu R, Li X, Zhou Y, et al. Critical role for the receptor tyrosine kinase EPHB4 in esophageal cancers. Cancer Res. 2013;73:184–194. doi: 10.1158/0008-5472.CAN-12-0915. [DOI] [PubMed] [Google Scholar]
- 33.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, Quinn DI, Henshall SM, Sutherland RL, Pinski JK, et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 35.Lee YC, Perren JR, Douglas EL, Raynor MP, Bartley MA, Bardy PG, Stephenson SA. Investigation of the expression of the EphB4 receptor tyrosine kinase in prostate carcinoma. BMC Cancer. 2005;5:119. doi: 10.1186/1471-2407-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci. 1998;11:2741–2751. doi: 10.1242/jcs.111.18.2741. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt F, Nguyen PH, Gupta N, Mayer D. Eph receptor B4 is a regulator of estrogen receptor alpha in breast cancer cells. J Recept Signal Transduct Res. 2013;33:244–248. doi: 10.3109/10799893.2013.795971. [DOI] [PubMed] [Google Scholar]
- 38.Batlle E, Bacani J, Beghtel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 39.Cortina C, Palomo-Ponce S, Iglesias M, Fernández-Masip JL, Vivancos A, Whissell G, Humà M, Peiró N, Gallego L, Jonkheer S, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 40.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 41.Shabani N, Kuhn C, Kunze S, Schulze S, Mayr D, Dian D, Gingelmaier A, Schindlbeck C, Willgeroth F, Sommer H, et al. Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur J Cancer. 2007;43:2434–2444. doi: 10.1016/j.ejca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Yao YY, Xu WZ, Wang Y, Shen DH, Wang JL, Wei LH. Relationships between the molecular biomarkers and the clinicopathologic features and prognosis in endometrial carcinoma. Beijing Da Xue Xue Bao. 2011;43:743–748. (In Chinese) [PubMed] [Google Scholar]
- 43.Ricciardi E, Maniglio P, Frega A, Marci R, Caserta D, Moscarini M. Fertility-sparing treatment of endometrial cancer precursors among young women: A reproductive point of view. Eur Rev Med Pharmacol Sci. 2012;16:1934–1947. [PubMed] [Google Scholar]