Abstract
Background and Objective
The oral microbiome may help to maintain systemic health, including how it affects blood glucose levels; however, direct evidence linking the oral microbiome with diabetes is lacking.
Material and Methods
We compared the oral microbiome profiles of 98 participants with incident diabetes, 99 obese non-diabetics, and 97 normal weight non-diabetics, via deep sequencing of the 16S rRNA gene.
Results
We found that the phylum Actinobacteria was present significantly less abundant among diabetes patients than among the controls (P=3.9 × 10−3); the odds ratio (OR) and 95% confidence interval (CI) was 0.27(0.11–0.66) for those individuals who had relative abundance higher than the median value. Within this phylum, five families and seven genera were observed, and most of them were less abundant among diabetes patients. Notably, genera Actinomyces and Atopobium were associated with 66% and 72% decreased risk of diabetes with P values of 8.9 × 10−3 and 7.4 × 10−3, respectively. Stratified analyses by race showed that most taxa in this phylum were associated with diabetes in both black and white participants. This phylum was also less abundant among non-diabetic obese subjects compared to normal weight individuals, especially genera Mobiluncus, Corynebacterium and Bifidobacterium, which showed P <0.05.
Conclusion
Our study revealed that multiple bacteria taxa in the phylum Actinobacteria are associated with risk of type 2 diabetes. Some are also associated with the prevalence of obesity, suggesting that the oral microbiome may play an important role in diabetes etiology.
Keywords: diabetes, oral microbiome, next-generation sequencing
INTRODUCTION
Diabetes is a leading health problem in the US and in many other parts of the world. There is growing evidence suggesting a two-way relationship between diabetes and oral health. Multiple large population-based studies have reported that periodontal diseases were independently associated with an increased risk of diabetes (1,2). Treatment of oral diseases has been shown to be associated with improved glycemic control and a reduction in HbA1C (3,4). On the other hand, individuals with diabetes are more likely to have periodontitis with increased severity when their diabetes is uncontrolled or poorly controlled (5). It has been suggested that systemic inflammation may be the link between the pathogenesis of diabetes and oral diseases (6,7).
The human mouth is heavily colonized by microorganisms, with over 700 species detected (8). The oral microbiome plays a critical role in oral diseases such as dental caries and periodontal diseases. It has been suggested that the oral microbiome also plays a role in systemic health through pathogen inhibition, immune regulation, nutrition absorption and metabolism (9). Increasing evidence suggests that oral bacteria can migrate to extra-oral sites, causing infection and inflammation (8,10). Alteration in the oral microbial community structure has been suggested to be linked to systemic diseases, including cardiovascular disease, stroke, and Alzheimer’s disease (11–15).
There is also growing evidence suggesting that the oral microbiome plays an important role in obesity and diabetes. Studies have suggested a direct link between periodontal pathogenic bacteria (such as P. gingivalis and A. actinomycetemcomitans) and glycemic control and diabetes risk (16–19). However, direct investigations of the relationship between the oral microbiome and diabetes have been very limited. In a study of 29 morbidly-obese subjects, including 13 diabetes patients, the genus Bifidobacteria in the phylum Actinobacteria was shown to have a lower abundance in the diabetes patients (20). In another study including 20 diabetes cases and 11 controls (21), two genera, Streptococci and Lactobacilli, in the phylum Firmicutes were found to be more abundant in the diabetes patients. However, the biological samples used in these two studies were collected after a diabetes diagnosis, which could have led to flawed conclusions because disease status and treatment are known to alter microbial profiles. In addition, these studies were limited by their small (≤20 cases) sample sizes and/or a limited number of bacteria species investigated. Studies are needed to prospectively investigate the associations of oral microbiota in diabetes etiology. In this report, we describe the results from a nested case-control study investigating the associations of the oral microbiome with incident diabetes using data and pre-diagnosis biological samples from a prospective cohort study, the Southern Community Cohort Study (SCCS).
MATERIAL AND METHODS
Study participants and data collection
The SCCS is an ongoing prospective cohort study investigating risk factors for cancer and other chronic diseases including diabetes. Details on the methodology of the study have been described elsewhere (22). In brief, approximately 86,000 adults, two-thirds of whom are African Americans (Blacks), were recruited between 2002 and 2009 from 12 southeastern states. Approximately 86% of them were recruited from community health centers (CHCs), institutions providing basic health care and preventative services in underserved areas, so that the cohort includes large numbers of individuals of low income and educational status. The remaining 14% of the cohort members were recruited through mail-based general population sampling. At the time of enrollment, a mouth rinse sample was collected from ~34,100 participants. The SCCS was reviewed and approved by the institutional review boards at Vanderbilt University Medical Center and Meharry Medical College. Written informed consent was obtained from all study participants.
For the baseline survey, participants completed a comprehensive questionnaire, which collected information on anthropometric characteristics, lifestyle factors, disease history, medication use, and other characteristics. Passive cohort follow-up by record linkage to state tumor registries and the National Death Index registry started immediately after the completion of the baseline survey. Active follow-up surveys started in 2008. In the active follow-up surveys, participants were asked about their personal medical history and medication use. Information on diabetes was obtained from the baseline and follow-up surveys with the question: “Has a doctor ever told you that you have had diabetes or high blood sugar?” For those who responded “yes,” follow-up questions were asked regarding age at diagnoses and use of diabetes medications. In the SCCS, 70% of the surviving cohort participants successfully completed the first round follow-up survey.
In the research presented here, we conducted a nested case-control study that included 300 participants grouped into three sets of 100 individuals, with each set comprising an incident diabetes case, a non-diabetic obese participant, and a normal weight non-diabetic control. All individuals in the present study were selected only from those who completed active follow up. Diabetes cases were defined as incident cases (for those with no prior history of diabetes and had never used diabetic medication at the time of the baseline survey) and a diagnosis of diabetes by a physician, or the reported use of diabetes medication on the follow-up survey. For each incident diabetes case, we selected one control (normal weight group) and one non-diabetes obese group by doing individual matching. The non-diabetes obesity group was selected to investigate whether the associations between bacteria taxa with diabetes were through association with obesity. Normal weight controls were selected from participants who had a normal weight of 25>BMI>18.5 at cohort entry and follow-up, did not have a history of diabetes, and did not use diabetic medication. The 100 non-diabetic obese participants were selected from those participants who had a BMI ≥30, did not have a prior history of diabetes, and did not use diabetic medication at the time of the baseline, during follow-up, or up to the time when the index diabetes case was diagnosed. We individually matched controls and obesity cases to index diabetes cases by age (± 2 years), race, sex, smoking status (current, former, or never), date of mouth rinse sample collection (± 90 days), recruitment source, and the CHC recruitment site. Participants who used antibiotics during the year prior to sample collection were excluded. Participants with a history of HIV infection, cancer (except skin cancer), stroke, or myocardial infarction were excluded as well. To minimize batch effects during the experiment, we included three samples from each set in the same 96-well plate during DNA preparation and sequencing.
DNA extraction and 16S rRNA gene sequencing
DNA was isolated from mouth rinse samples using Qiagen’s QIAmp DNA kit. Sequencing libraries were prepared using the NEXTflex 16S V4 Amplicon-Seq Kit (Bioo Scientific 4201-05), following the manufacturer’s protocol. This kit was designed to sequence approximately 253bp of the fourth hypervariable (V4) domain of the bacterial 16S rRNA gene (23,24). Sequencing was performed at pair-end 150bp using Illumina MiSeq 300 at the VANderbilt Technologies for Advanced Genomics (VANTAGe) Core. Each 96-well plate was sequenced on one MiSeq run with two duplicated quality controls (QCs) and one negative control sample included.
Sequence data processing and QC
Sequencing data were processed using QIIME (Quantitative Insights Into Microbial Ecology) package v1.8. The pair-end reads were stitched together using join_paired_ends.py with parameters of -j 45 –p 5 (≥45bp overlapped and ≤5% unmatched bp between the paired reads). Sequencing reads were then de-multiplexed, with barcode and primers removed using split_libraries_fastq.py. Low-quality reads with phred score <30 were filtered out. Chimeric sequences were removed using ChimeraSlayer (25) and implemented in identify_chimeric_seqs.py. Sequence reads were then clustered into Operational Taxonomic Units (OTUs) at 97% sequence identity by using uclust (26) against the GreenGenes database release gg_13_5 (27). OTUs with very low abundance (fraction <0.0005%) were discarded using filter_otus_from_otu_table.py. OTU tables were rarefied to 5,000 sequences per sample to account for variations in sequencing depth. The most abundant sequence in each OTU was selected as a representative. Taxonomy was then assigned for each OTU using the GreenGenes taxonomy map (28). Samples with sequencing reads <5,000 were removed. A total of 98 incident diabetes cases, 99 obesity cases and 97 normal weight controls were included in the final analyses.
Statistical analysis
Associations between bacteria taxa and diabetes risk or obesity prevalence were identified through conditional logistic regression analyses in each matched set. Subjects were categorized into two groups according to the median value of relative abundance for each taxon among control subjects. Conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CIs) for measuring the association of taxon abundance with diabetes risk or obesity prevalence. The group with relative abundance lower than the median values was the reference. Potential confounder factors that were not matched between cases and controls, such as physical exercise, total energy intake, oral health condition (such as tooth loss) and BMI (when investigating associations between microbiome and diabetes risk) were adjusted.
RESULTS
Characteristics of the study subjects
Table 1 presents the distribution of selected baseline demographic characteristics for participants. Diabetes cases, non-diabetic obese participants, and normal weight controls were matched with respect to race, sex, age, and smoking status. Control participants had higher education levels (with an especially high percentage of participants with education ≥ college), higher annual household income, better oral health (with a higher proportion of people having a full set of teeth), and were more likely to be drinkers than in diabetes cases. The above characteristics for the non-diabetic obese cases resembled the diabetes group more closely than the controls except that non-diabetic obese cases were more likely to be drinkers than diabetes cases.
Table 1.
Characteristics of the study participants in the Southern Community Cohort Studya
| Chacteristic | Group | Diabetes (N=98) | Normal weight non-diabetics (N=97) | Obese non-diabetics (N=99) |
|---|---|---|---|---|
| Race | ||||
| Whites | 51.0 | 50.5 | 50.5 | |
| Blacks | 49.0 | 49.5 | 49.5 | |
| Sex | ||||
| Male | 51.0 | 50.5 | 50.5 | |
| Female | 49.0 | 49.5 | 49.5 | |
| Age | ||||
| 40–49 | 40.8 | 37.1 | 39.4 | |
| 50–59 | 44.9 | 48.5 | 45.5 | |
| 60–69 | 14.3 | 14.4 | 15.2 | |
| BMI | ||||
| Underweight, <18.5 | 1.0 | |||
| Normal, 18.5–24.9 | 14.4 | 100.0 | ||
| Overweight, 25–29.9 | 28.9 | |||
| Obese, >=30 | 55.7 | 100 | ||
| Education | ||||
| <high school | 11.2 | 7.2 | 11.1 | |
| High/vocational school | 30.6 | 27.8 | 24.2 | |
| Some college | 25.5 | 25.8 | 26.3 | |
| >=college | 32.7 | 39.2 | 38.4 | |
| Annual household income ($) | ||||
| <15,000 | 24.5 | 26.3 | 21.4 | |
| 15,000–24,999 | 18.1 | 12.6 | 13.3 | |
| 25,000–49,999 | 22.3 | 21.1 | 28.6 | |
| >=50,000 | 35.1 | 40.0 | 36.7 | |
| Tobacco smoking | ||||
| Current | 27.6 | 24.7 | 26.3 | |
| Former | 24.5 | 25.8 | 25.3 | |
| Never | 48.0 | 49.5 | 48.5 | |
| Alcohol drinking | ||||
| None | 44.2 | 28.7 | 31.3 | |
| Light, <1 drink per day | 33.7 | 41.5 | 40.6 | |
| Moderate, 1–2 drink/day | 13.7 | 16.0 | 13.5 | |
| Heavy, >2 drinks per day | 8.4 | 13.8 | 14.6 | |
| Tooth loss | ||||
| None | 24.0 | 38.9 | 30.5 | |
| 1 to 10 | 53.1 | 47.4 | 53.7 | |
| >10, not all | 13.5 | 9.5 | 8.4 | |
| All | 9.4 | 4.2 | 7.4 | |
Percentage was presented in the table
Sequencing summary
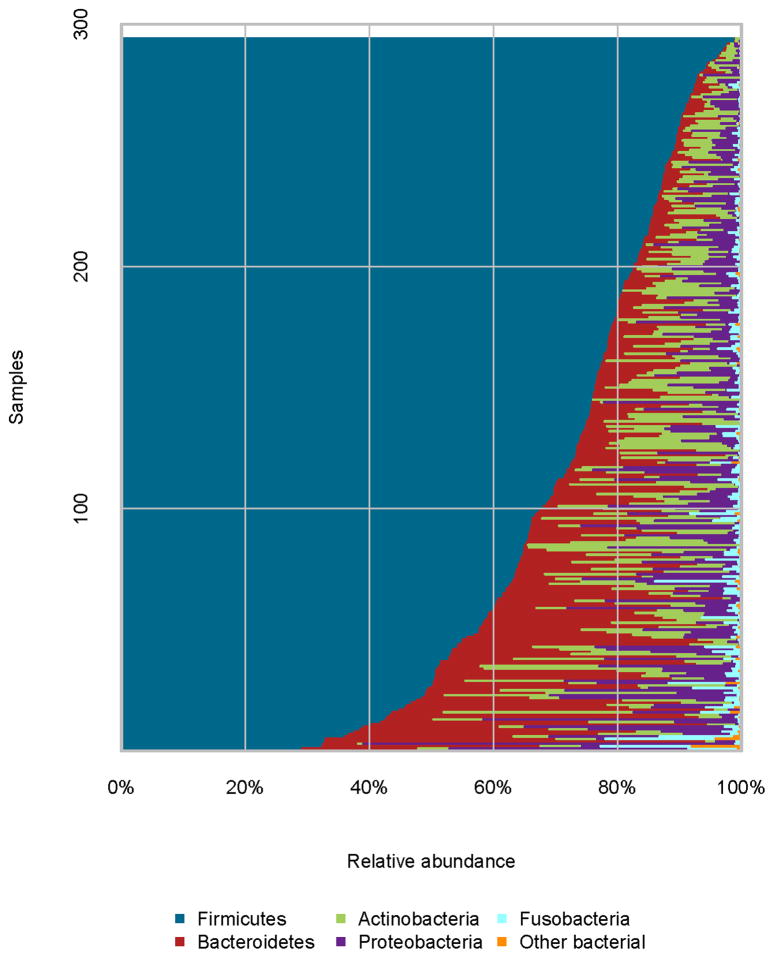
Among the 294 samples, we obtained 22.8 million sequence reads, with an average of 77,473 reads per sample. A total of 682 OTUs were observed, with a mean of 140 OTUs and a range of 60–269 OTUs per sample. Most of these OTUs were rare, with only 10% having a relative abundance >0.1%. These OTUs were classified into 11 phyla and 95 genera. The phylum Firmicutes predominated, representing approximately 73% of the bacteria. The other three common phyla were Bacteroidetes, Actinobacteria, and Proteobacteria, with average relative abundances of 11.8%, 7.2%, and 6.5%, respectively (Figure 1).
Figure 1.
Relative abundance of bacterial phyla in individual samples.
Associations of microbiome composition with diabetes
At the phylum level, Actinobacteria was significantly associated with a decreased risk of diabetes (P=3.9 × 10−3). This phylum was common, observed among all participants, with a median abundance of 5.1% in diabetes cases and 6.8% in controls. Compared with the reference group (participants with relative abundance lower than the median value), the OR and 95% CI was 0.5127(0.11–0.66) for participants with relative abundance higher than median value (Table 2). Within this phylum, five families were observed, Actinomycetaceae, Bifidobacteriaceae, Coriobacteriaceae, Corynebacteriaceae, and Micrococcaceae, and all of them were less abundant among diabetes cases compared to normal weight controls, with ORs (95% Cis) being 0.29(0.10 – 0.83), 0.87(0.35 – 2.13), 0.17(0.02 – 1.34), 0.73(0.38 – 1.40) and 0.56(0.29 – 1.07), respectively (Table 2). At the genus level, a total of seven taxa were clearly defined. With the exception of Scardovia, all the other 6 genera were less abundant among diabetes cases than among controls. Notably, genera Actinomyces and Atopobium were significantly associated with a decreased risk of diabetes with ORs (95% CIs) being 0.34(0.15 – 0.76) and 0.28(0.11 – 0.71), and P values of 8.9 × 10−3 and 7.4 × 10−3, respectively (Table 2). In addition to the relative abundance, genera Mobiluncus, Atopobium and Corynebacterium were less prevalent among diabetes cases than among controls, with 7.1% vs. 11.3%, 65.3% vs. 74.2% and 79.6% vs. 83.5%, respectively. Stratified analyses by race showed that most taxa in the phylum Actinobacteria were associated with diabetes in both black and white participants (Table 3); however, the associations did not reach statistical significance with the exception of the phylum Actinobacteria among Blacks with OR (95% CI) being 0.39(0.16 – 1.00) and a P value of 0.05.
Table 2.
Associations between Actinobacteria and diabetes risk
| Taxa | % Positive (Carriage)a | Abundanceb | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Case | Control | OR(95% CI) | P value | OR(95% CI) | P value | |
| Phylum Actinobacteria | 100.0 | 100.0 | 0.27(0.11 – 0.66) | 3.9E-03 | ||
| Family Actinomycetaceae | 100.0 | 100.0 | 0.29(0.10 – 0.83) | 0.02 | ||
| Genus Actinomyces | 100.0 | 100.0 | 0.34(0.15 – 0.76) | 8.9E-03 | ||
| Genus Mobiluncus | 7.1 | 11.3 | 0.48(0.16 – 1.48) | 0.20 | 0.48(0.16 – 1.48) | 0.20 |
| Family Bifidobacteriaceae | 52.0 | 48.5 | 1.05(0.53 – 2.10) | 0.88 | 0.87(0.35 – 2.13) | 0.76 |
| Genus Bifidobacterium | 33.7 | 34.0 | 0.91(0.46 – 1.80) | 0.79 | ||
| Genus Scardovia | 25.5 | 21.6 | 1.16(0.53 – 2.54) | 0.70 | 1.25(0.48 – 3.22) | 0.65 |
| Family Coriobacteriaceae | 68.4 | 75.3 | 0.42(0.17 – 1.02) | 0.05 | 0.17(0.02 – 1.34) | 0.09 |
| Genus Atopobium | 65.3 | 74.2 | 0.43(0.19 – 0.96) | 0.04 | 0.28(0.11 – 0.71) | 7.4E-03 |
| Family Corynebacteriaceae | 79.6 | 83.5 | 0.60(0.15 – 2.35) | 0.46 | 0.73(0.38 – 1.40) | 0.34 |
| Genus Corynebacterium | 79.6 | 83.5 | 0.60(0.15 – 2.35) | 0.46 | 0.73(0.38 – 1.40) | 0.34 |
| Family Micrococcaceae | 100.0 | 100.0 | 0.56(0.29 – 1.07) | 0.08 | ||
| Genus Rothia | 100.0 | 100.0 | 0.56(0.29 – 1.07) | 0.08 | ||
Note
For those taxa present in all participants, no statistical analyses regarding carriage were conducted.
Participants with relative abundance lower than the median values among controls were the reference group.
Table 3.
Associations between Actinobacteria and diabetes risk in Blacks and Whites
| Taxa | Race | % Positive (Carriage)a | Abundanceb | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Case | Control | OR(95% CI) | P value | OR(95% CI) | P value | ||
| Phylum Actinobacteria | |||||||
| Black | 100.0 | 100.0 | 0.39(0.16 – 1.00) | 0.05 | |||
| White | 100.0 | 100.0 | 0.34(0.01 – 34.75) | 0.65 | |||
| Family Actinomycetaceae | Black | 100.0 | 100.0 | ||||
| White | 100.0 | 100.0 | 0.33(0.04 – 2.50) | 0.28 | |||
| Genus Actinomyces | Black | 100.0 | 100.0 | ||||
| White | 100.0 | 100.0 | 0.32(0.08 – 1.28) | 0.11 | |||
| Genus Mobiluncus | Black | 8.3 | 14.6 | 0.38(0.06 – 2.65) | 0.33 | 0.38(0.06 – 2.65) | 0.33 |
| White | 6.0 | 8.2 | 0.38(0.01 – 27.95) | 0.66 | 0.38(0.01 – 27.95) | 0.66 | |
| Family Bifidobacteriaceae | Black | 43.8 | 50.0 | 0.76(0.30 – 1.90) | 0.56 | 1.02(0.40 – 2.60) | 0.97 |
| White | 60.0 | 46.9 | 1.60(0.56 – 4.59) | 0.38 | |||
| Genus Bifidobacterium | Black | 29.2 | 37.5 | 0.71(0.28 – 1.85) | 0.49 | 0.90(0.27 – 2.98) | 0.87 |
| White | 38.0 | 30.6 | 1.32(0.46 – 3.78) | 0.61 | |||
| Genus Scardovia | Black | 29.2 | 22.9 | 1.42(0.50 – 4.00) | 0.51 | 0.71(0.18 – 2.83) | 0.63 |
| White | 22.0 | 20.4 | |||||
| Family Coriobacteriaceae | Black | 68.8 | 70.8 | 0.17(0.01 – 2.45) | 0.19 | ||
| White | 68.0 | 79.6 | 0.17(0.03 – 1.14) | 0.07 | 0.27(0.05 – 1.46) | 0.13 | |
| Genus Atopobium | Black | 62.5 | 70.8 | ||||
| White | 68.0 | 77.6 | 0.31(0.06 – 1.51) | 0.15 | |||
| Family Corynebacteriaceae | Black | 66.7 | 77.1 | 0.54(0.15 – 1.87) | 0.33 | ||
| White | 92.0 | 89.8 | 0.49(0.06 – 4.21) | 0.51 | 0.63(0.14 – 2.79) | 0.54 | |
| Genus Corynebacterium | Black | 66.7 | 77.1 | 0.54(0.15 – 1.87) | 0.33 | ||
| White | 92.0 | 89.8 | 0.49(0.06 – 4.21) | 0.51 | 0.63(0.14 – 2.79) | 0.54 | |
| Family Micrococcaceae | Black | 100.0 | 100.0 | 0.58(0.21 – 1.62) | 0.30 | ||
| White | 100.0 | 100.0 | 0.65(0.09 – 4.80) | 0.67 | |||
| Genus Rothia | Black | 100.0 | 100.0 | 0.58(0.21 – 1.62) | 0.30 | ||
| White | 100.0 | 100.0 | 0.65(0.09 – 4.80) | 0.67 | |||
Note
For those taxa present in all participants, no statistical analyses regarding carriage were conducted.
Participants with relative abundance lower than the median values among controls were the reference group.
We found that the family Gemellaceae in the phylum Firmicutes was associated with an increased risk of diabetes. This family was observed to be present in all of the diabetes cases and 99% of the controls. Participants having abundance higher than the median value has a 77% increased risk of developing diabetes. In this family, only one genus, Gemella, was clearly defined. It was associated with an increased risk of diabetes, with OR (95% CI) being 1.77(0.92 – 3.43). In this family, there was one unclearly-defined genus also associated with an increased risk of diabetes, with OR (95% CI) being 2.70(1.26 – 5.79) and having a P value of 0.01.
We further investigated whether those bacterial taxa that were associated with diabetes risk were correlated to obesity, a well-established risk factor for diabetes, by comparing non-diabetic obese and normal weight participants for these bacterial taxa. We found that the phylum Actinobacteria was presented with lower abundance among obese subjects compared to normal weight individuals. Especially, genera Mobiluncus, Corynebacterium and Bifidobacterium showed significant associations with ORs (95% CIs) being 0.15(0.03–0.77), 0.38(0.16–0.93), 0.25(0.07–0.94), and having P values of 0.02, 0.03 and 0.04, respectively. Similarly, the family Gemellaceae and the genus Granulicatella in the phylum Firmicutes were more abundant among non-diabetic obese participants than among normal weight controls; however the difference did not reach significance.
DISCUSSION
Increasing evidence suggests that oral bacteria may play an important role in the pathogenesis of chronic diseases, including diabetes, via its role in regulating systemic inflammation (11–15). In this nested case-control study, we found that higher abundance of the phylum Actinobacteria and almost all taxa in this phylum were associated with a decreased risk of diabetes. At the genus level, Actinomyces was the most abundant with a median abundance >1%, and showed a strong association with diabetes risk. On the other hand, the family Gemellaceae in the phylum Firmicutes showed higher abundance among diabetes patients than among normal weight controls.
The phylum Actinobacteria (a synonym for Actinomycetes) is a group of gram-positive bacteria. These bacteria are the producers of many bioactive metabolites, and are present in two-thirds of the antibiotics used in the world (29). They also produce inhibitors for enzymes, such as Amylase and N-Acetyl-b-D-glucosaminidase. Amylase, present in the saliva, is the major enzyme in catalyzing hydrolysis of dietary starch into disaccharides and trisaccharides. Amylase inhibitors produced by Actinobacteria may be useful in controlling carbohydrate-dependent diseases such as diabetes (30). N-Acetyl-glucosaminidase is higher in diabetes patients and is correlated with glycemic control (31). Inhibitors of this enzyme, produced by Actinobacteria, could potentially be developed as a therapeutic agent for diabetes (32).
The genus Actinomyces, one of the two common genera in the phylum Actinobacteria, showed a strong inverse association with diabetes risk in the present study. It was observed among 100% of participants, with median abundances of 1.9% among diabetes cases, 2.1% among non-diabetic obese and 2.7% among normal weight controls. Similar prevalence and abundance were observed in the oral samples from the human microbiome project (HMP), present among 99.2% subjects with a median abundance of 2.9%. Interestingly, gut microbiome studies have shown that the genus Bifidobacterium in the phylum Actinobacteria was associated with a decreased risk of diabetes and obesity (33,34). Similar but non-significant associations were observed between the oral Bifidobacterium and diabetes in the present study and in another oral microbiome study (20). This genus was only observed among ~33% of subjects in the present study, with an abundance of only ~0.1%. Therefore, the statistical power for assessing this genus in association with diabetes risk in the present study is low. Human intervention studies of probiotic yogurt containing Bifidobacterium lactis resulted in an improvement in glucose-tolerance and glucose-induced insulin secretion, HbA1C, and antioxidant activities, as well as a decrease in inflammation (35–39). These findings highlight the importance of investigating bacteria taxa in the phylum Actinobacteria on the development of diabetes and serving as a potential therapeutic target in diabetes. If the results in the present study can be validated in independent studies, the findings are easy to translate because oral microbiota are easy to assess and modify.
The phylum Firmicutes was the most common phylum in oral samples. In the present study, associations with diabetes were observed for multiple taxa within this phylum. The genus Granulicatella, in the family Gemellaceae, showed higher abundance among diabetic and non-diabetic obese participants than among normal weight controls. The family Gemellaceae has been reported to be more abundant in saliva samples from obese participants than in normal weight individuals (40). This family was also reported to be more prevalent in Crohn’s disease mucosa (41). The genus Gemella has recently been reported to be among the genera most predictive of dysbiosis (42). This genus has been shown to be positively associated with dental cavities (43), but two species within this genus were inversely associated with periodontitis in another study (44).
Limited research has been conducted to investigate the possible etiologic connections between the oral microbiome composition and diabetes (20,21). Although multiple bacteria taxa were suggested to be associated with diabetes, only a few of these were replicated in subsequent studies. As mentioned above, the genus Bifidobacteria in the phylum Actinobacteria showed lower levels in diabetes patients (20), an association that we replicated in the present study. A previous study including 20 prevalent diabetes patients reported that two genera in the phylum Firmicutes, including Streptococcus and Lactobacillus, were more abundant in diabetes patients (21). We did not find these taxa were associated with diabetes.
To our best knowledge, the present study is the largest study, and the only prospective study, to investigate the role of the oral microbiome in the development of diabetes. Inclusion of both White and Black participants, the ability to match multiple socio-demographic factors and smoking behavior, and the inclusion of both normal weight and non-diabetic obese control subjects are the noticeable strengths of this study. One of the limitations of the present study is that diabetes cases were self-reported. We have previously conducted a study to validate self-reported diabetes in a subset of 800 study participants and found that 94% of self-reported cases were validated (45). This is consistent with prior studies and support that self-reported incident diabetes is largely accurate and can be used in epidemiology studies (46–48).
Another limitation is the lack of a systematic assessment of oral health at the baseline exam when the samples were collected. Data on tooth decay and tooth loss were collected during the follow-up, and were adjusted in the analyses of the association of the microbiome with diabetes and obesity. Previous studies showed that periodontal pathogenic bacteria, such as P. gingivalis, T. denticola and T. forsythia were associated with diabetes risk and glycemic control (16–19,49). Our study was limited because we can only classify the bacteria at the genus level and cannot investigate these specific periodontal pathogenic bacteria species in relation to diabetes risk. Whole metagenome shotgun sequencing is needed for a more comprehensive investigation of the microbiome at the species/strain level. In the present study, most of the associations with diabetes were observed in both Blacks and Whites. However, our study did not have adequate statistical power to detect potential modification effects by race.
In the present study, 11 phyla, 57 families and 95 genera were observed. The significant associations reported above would lose significance if the Bonferroni correction for multiple comparisons were applied. However, these taxa were not independent. The Bonferroni correction is likely to be too conservative and lead to a false negative conclusion. In addition, most of the bacterial taxa had very low prevalence and abundance, and our study does not have sufficient power to carry out a systematic evaluation of this. Further investigation with a large sample size is warranted.
In summary, we found that multiple bacterial taxa were associated with diabetes in this prospective study. Higher abundances of most taxa in the phylum Actinobacteria were associated with a decreased risk of diabetes. These results suggest that the oral microbiome may play an important role in diabetes etiology. A more comprehensive investigation with a larger sample size and with metagenome sequencing is warranted.
Acknowledgments
The authors wish to thank all the individuals who took part in the study, and all the researchers, clinicians, technicians, and administrative staff who have enabled this work to be carried out. We thank Regina Courtney, Jie Wu, Jing He, and Marshal Younger for their help with sample preparation, statistical analysis, and technical support for the project. The data analyses were conducted using the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University.
Sample preparation was conducted at the Survey and Biospecimen Shared Resources, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485). The 16S rRNA sequencing was conducted at the VANderbilt Technologies for Advanced GEnomics (VANTAGE) Core, which is partly supported by NIH/NCRR grant G20 RR030956. The SCCS was supported by NIH grant R01CA92447. This project was also supported by the development fund from Department of Medicine at Vanderbilt University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jung YS, Shin MH, Kweon SS, et al. Periodontal disease associated with blood glucose levels in urban Koreans aged 50 years and older: the Dong-gu study. Gerodontology. 2014 doi: 10.1111/ger.12107. [DOI] [PubMed] [Google Scholar]
- 2.Morita I, Inagaki K, Nakamura F, et al. Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res. 2012;91:161. doi: 10.1177/0022034511431583. [DOI] [PubMed] [Google Scholar]
- 3.Bharti P, Katagiri S, Nitta H, et al. Periodontal treatment with topical antibiotics improves glycemic control in association with elevated serum adiponectin in patients with type 2 diabetes mellitus. Obes Res Clin Pract. 2013;7:e129. doi: 10.1016/j.orcp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, Alizadeh M. Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J. 2012;57:31. doi: 10.1111/j.1834-7819.2011.01652.x. [DOI] [PubMed] [Google Scholar]
- 5.Chee B, Park B, Bartold PM. Periodontitis and type II diabetes: a two-way relationship. Int J Evid Based Healthc. 2013;11:317. doi: 10.1111/1744-1609.12038. [DOI] [PubMed] [Google Scholar]
- 6.Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine RS. Obesity, diabetes and periodontitis--a triangular relationship? Br Dent J. 2013;215:35. doi: 10.1038/sj.bdj.2013.627. [DOI] [PubMed] [Google Scholar]
- 8.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miles B, Abdel-Ghaffar KA, Gamal AY, Baban B, Cutler CW. Blood dendritic cells: “canary in the coal mine” to predict chronic inflammatory disease? Front Microbiol. 2014;5:6. doi: 10.3389/fmicb.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzo G, Guiglia R, Lo RL, Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21:496. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Zarco MF, Vess TJ, Ginsburg GS. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012;18:109. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahn J, Chen CY, Hayes RB. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23:399. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makiura N, Ojima M, Kou Y, et al. Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol Immunol. 2008;23:348. doi: 10.1111/j.1399-302X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 17.Aemaimanan P, Amimanan P, Taweechaisupapong S. Quantification of key periodontal pathogens in insulin-dependent type 2 diabetic and non-diabetic patients with generalized chronic periodontitis. Anaerobe. 2013;22:64. doi: 10.1016/j.anaerobe.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Hyvarinen K, Salminen A, Salomaa V, Pussinen PJ. Systemic exposure to a common periodontal pathogen and missing teeth are associated with metabolic syndrome. Acta Diabetol. 2015;52:179. doi: 10.1007/s00592-014-0586-y. [DOI] [PubMed] [Google Scholar]
- 19.Thorstensson H, Dahlen G, Hugoson A. Some suspected periodontopathogens and serum antibody response in adult long-duration insulin-dependent diabetics. J Clin Periodontol. 1995;22:449. doi: 10.1111/j.1600-051x.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 20.Shillitoe E, Weinstock R, Kim T, et al. The oral microflora in obesity and type-2 diabetes. J Oral Microbiol. 2012:4. doi: 10.3402/jom.v4i0.19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kampoo K, Teanpaisan R, Ledder RG, McBain AJ. Oral bacterial communities in individuals with type 2 diabetes who live in southern Thailand. Appl Environ Microbiol. 2014;80:662. doi: 10.1128/AEM.02821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Signorello LB, Hargreaves MK, Steinwandel MD, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972. [PMC free article] [PubMed] [Google Scholar]
- 23.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4516. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas BJ, Gevers D, Earl AM, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 27.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baltz RH. Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol. 2008;8:557. doi: 10.1016/j.coph.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Bo-Linn GW, Santa Ana CA, Morawski SG, Fordtran JS. Starch blockers--their effect on calorie absorption from a high-starch meal. N Engl J Med. 1982;307:1413. doi: 10.1056/NEJM198212023072301. [DOI] [PubMed] [Google Scholar]
- 31.Watts GF, Vlitos MA, Morris RW, Price RG. Urinary N-acetyl-beta-D-glucosaminidase excretion in insulin-dependent diabetes mellitus: relation to microalbuminuria, retinopathy and glycaemic control. Diabete Metab. 1988;14:653. [PubMed] [Google Scholar]
- 32.Imada C. Enzyme inhibitors and other bioactive compounds from marine actinomycetes. Antonie Van Leeuwenhoek. 2005;87:59. doi: 10.1007/s10482-004-6544-x. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Ma C, Han L, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61:69. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- 34.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 35.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Stenman LK, Waget A, Garret C, Klopp P, Burcelin R, Lahtinen S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef Microbes. 2014;5:437. doi: 10.3920/BM2014.0014. [DOI] [PubMed] [Google Scholar]
- 37.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. 2011;94:3288. doi: 10.3168/jds.2010-4128. [DOI] [PubMed] [Google Scholar]
- 38.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 39.Mohamadshahi M, Veissi M, Haidari F, Javid AZ, Mohammadi F, Shirbeigi E. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: A randomized controlled clinical trial. J Res Med Sci. 2014;19:531. [PMC free article] [PubMed] [Google Scholar]
- 40.Piombino P, Genovese A, Esposito S, et al. Saliva from obese individuals suppresses the release of aroma compounds from wine. PLoS One. 2014;9:e85611. doi: 10.1371/journal.pone.0085611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haberman Y, Tickle TL, Dexheimer PJ, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124:3617. doi: 10.1172/JCI75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsen PE, Dai Y. Metabolome of human gut microbiome is predictive of host dysbiosis. Gigascience. 2015;4:42. doi: 10.1186/s13742-015-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanasi E, Dewhirst FE, Chalmers NI, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44:485. doi: 10.1159/000320158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lourenco TG, Heller D, Silva-Boghossian CM, Cotton SL, Paster BJ, Colombo AP. Microbial signature profiles of periodontally healthy and diseased patients. J Clin Periodontol. 2014;41:1027. doi: 10.1111/jcpe.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huizinga M, Elasy T, Villegas R, Signorello L, Blot WJ, Cavanaugh K. Validation of diabetes self-report and characteristics of undiagnosed diabetes in the Southern Community Cohort Study. Diabetes. 2009;58:A279. [Google Scholar]
- 46.Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol. 2012;176:738. doi: 10.1093/aje/kws156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huerta JM, Tormo MJ, Egea-Caparros JM, Ortola-Devesa JB, Navarro C. Accuracy of self-reported diabetes, hypertension and hyperlipidemia in the adult Spanish population. DINO study findings. Rev Esp Cardiol. 2009;62:143. doi: 10.1016/s1885-5857(09)71532-4. [DOI] [PubMed] [Google Scholar]
- 48.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schara R, Skaleric E, Seme K, Skaleric U. Prevalence of periodontal pathogens and metabolic control of type 1 diabetes patients. Journal of the International Academy of Periodontology. 2013;15:29. [PubMed] [Google Scholar]