Abstract
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) frequently present with distant metastases at the time of diagnosis and the liver is the most frequent site of spreading. The early identification of metastatic disease represents a major prognostic factor for GEP-NENs patients. Radical surgical resection, which is feasible for a minority of patients, is considered the only curative option, while the best management for patients with unresectable liver metastases is still being debated. In the last few years, a number of locoregional and systemic treatments has become available for GEP-NEN patients metastatic to the liver. However, to date only a few prospective studies have compared those therapies and the optimal management option is based on clinical judgement. Additionally, locoregional treatments appear feasible and safe for disease control for patients with limited liver involvement and effective in symptoms control for patients with diffuse liver metastases. Considering the lack of randomized controlled trials comparing the locoregional treatments of liver metastatic NEN patients, clinical judgment remains key to set the most appropriate therapeutic pathway. Prospective data may ultimately lead to more personalized and optimized treatments. The present review analyzes all the locoregional therapy modalities (i.e., surgery, ablative treatments and transarterial approach) and aims to provide clinicians with a useful algorithm to best treat GEP-NEN patients metastatic to the liver.
Keywords: Gastroenteropancreatic neuroendocrine neoplasms, Liver metastases, Locoregional therapies, Systemic therapies, Ablation, Chemoembolization
Core tip: Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) frequently present with distant metastases. In the last years, a number of treatment has become available for advanced GEP-NENs and the optimal management for these patients remains to be established. While systemic medical therapies and peptide receptor radionuclide therapy represent effective options, they are usually palliative whereas liver-directed treatments often represent the only possibly curative therapy, even if not supported by prospective trials. Considering the lack of randomized trials comparing locoregional treatments in advanced GEP-NEN, clinical judgment remains key to set the most appropriate therapeutic pathway. Prospective data may lead to more personalized and optimized treatments.
INTRODUCTION
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are a heterogeneous group of neoplasms arising from the endocrine cells of the gastrointestinal tract. Although rare, the worldwide incidence of the neuroendocrine tumors is on the rise and ranges from 3.24/100000 in North Europe to 5.25/100000 in the United States[1-3]. GEP-NENs include both functioning tumors, which may secrete different peptide hormones (i.e., serotonin, insulin, gastrin, glucagon, and vasoactive intestinal peptide), and non-functioning tumors, which are often identified at more advanced stages. GEP-NENs are classified as well-differentiated grade 1 and 2 neuroendocrine tumors (NENs G1 and G2), and poorly differentiated grade 3 neuroendocrine carcinomas (NEC G3), according to their Ki-67 proliferation index and mitotic count (Ki-67%)[4].
Up to 65%-95% of GEP-NENs present with distant metastases at the time of diagnosis[5,6] and the liver is the most frequent site of spreading, followed by the lungs and bones[7,8]. Tumor biology (grading) and metastases site represent key prognostic factors and are associated with a significantly reduced overall survival (OS) compared to patients without liver metastases[9]. Experience indicates that the 5-year OS for metastatic intestinal NENs is 56%-83% and 40%-60% for pancreatic NENs[7].
The optimal management for patients with NEN hepatic metastases remains controversial. Systemic treatments [i.e., somatostatin analogs (SSAs), interferon-alpha (IFNα), chemotherapy, targeted therapy and peptide receptor radionuclide therapy (PRRT)] have a limited role to obtain any significant radiological objective response, but have shown to be effective for disease stabilization. On the other hand, surgery and other locoregional treatments (i.e., transarterial and ablative treatments) may obtain significant radiologic response and symptom control but no prospective studies have proved their impact on OS.
The optimal selection of palliative treatments for patients with unresectable liver metastases is crucial to improve their quality of life and prolong their OS. To date, however, there is lack of data comparing the efficacy and safety of these treatments and the best multimodal approach for unresectable liver lesion is still being debated. This study aims to define the state of the art about the locoregional treatments of hepatic metastatic NENs.
AVAILABLE SYSTEMIC TREATMENTS IN METASTATIC DISEASE: OLD, NEW AND EMERGING THERAPIES
Somatostatin analogs and interferon-alpha
The high rate of somatostatin receptor (SSTR) expression in GEP-NENs, in up to 80%-90% of cases, provides the rationale for SSA therapies[10]. Currently, two synthetic analogs (octreotide and lanreotide) are commercially available and are mostly used as monthly depot formulations[11]. SSAs bind with high affinity to SSTR subtype 2 and with moderate affinity to SSTR subtype 5; the somatostatin receptors activation induces multiple intracellular transduction pathways, which exert an inhibitory effect on hormonal secretion and cellular motility[11]. The reduction of hormonal secretion explains the efficacy of SSAs in providing symptomatic relief in patients with functioning GEP-NEN.
Although the objective radiographic responses associated with SSAs were rare, multiple phase-II trials and retrospective series observed prolonged OS and disease stabilization in a large proportion of patients[12]. These observations lead to the hypothesis that SSAs exert an inhibitory effect on tumor growth. In 2009 the PROMID study demonstrated that SSAs slow the rate of tumor progression by controlling tumor growth in patients with functionally active and inactive midgut NETs. In this prospective placebo-controlled randomized study the treatment with octreotide LAR showed to significantly increase the time to tumor progression as compared to the placebo group (14.3 mo vs 6 mo)[13]. The recent randomized double-blind placebo-controlled CLARINET trial further extended the indications for SSAs treatment in NEN patients. It demonstrated a significantly prolonged PFS for patients with advanced, well- or moderately differentiated, non-functioning, somatostatin receptor-positive NENs treated with lanreotide as compared to those administered with the placebo (median not reached by the end of the study at 96 wk vs 18 mo). Furthermore, SSAs display a favourable toxicity profile and tolerability, and therefore represent a good first therapeutic option for most patients. The ENETS guidelines suggest the use of SSAs as the first-line therapy for patients with functioning and non-functioning low-grade G1 and G2 GEP-NETs. Alternatively, in case of disease progression to SSAs, second-line treatments, such as IFNα, PRRT, mTOR inhibitors or anti-angiogenic agents in combination with SSAs, have been established[14]. IFNα therapy has been described as a systemic therapy for metastatic GEP-NEN given its anti-proliferative effect and hormonal control. The anti-tumoral effect is attributed to the inhibition of angiogenesis, induction of apoptosis, and interruption of the cell cycle[15]. Faiss et al[16] demonstrated that IFN-α has an anti-proliferative effect comparable to lanreotide in advanced functional and non-functional GEP-NENs. Moreover, the combination of lanreotide plus IFNα had significantly improved symptom control. However, the early combination of SSAs and IFNα or anti-proliferative purposes is not recommended given the IFN-α unfavorable toxicity profile, thus IFNα is primarily indicated for patients with somatostatin-negative tumors[7,16].
Chemotherapy
Systemic chemotherapy using various cytotoxic agents (i.e., streptozotocin, doxorubicin, 5-fluorouracil, cisplatin, etoposide) is indicated for patients with inoperable pancreatic NET, metastatic foregut NET G2, and NEC G3 of any site[17,18]. To date, systemic chemotherapy showed poor results for patients with well-differentiated metastatic midgut NET, therefore on these patients the current cytotoxic regimens are not routinely used[7]. The choice of specific chemotherapeutic agents is primarily based on the degree of tumor differentiation and location of the primary tumor.
In pancreatic NENs streptozocin-based chemotherapy regimens in combination with doxorubicin and/or fluorouracil still represent the gold standard and they are associated with overall tumor response rates of 30%-40%[18]. Recently, promising data on temozolomide use in combination with other chemotherapeutic agents on locally advanced or metastatic pancreatic NENs have resulted[7]. Strosberg et al[19] showed a high partial-remission rate of 70% for metastatic pancreatic NEN treated with a temozolomide-capecitabine combination therapy. In this study a favorable 18-mo PFS and 92% OS at 2 years were observed along with a tolerable toxicity profile. Further prospective comparative studies on this chemotherapy regimen are warranted. In cases of metastatic NEC G3 a combination chemotherapy using cisplatin/etoposide is recommended early, regardless of the site of the primary tumor[20]. So far, there is no established second-line therapy for poorly differentiated endocrine carcinoma.
Targeted therapies
The understanding of the molecular biology of NENs has significantly improved in the last few years and, more recently, a number of novel targeted agents have emerged for the treatment of GEP-NENs patients: everolimus[21], which is an inhibitor of the mammalian target of rapamycin (mTOR), and sunitinib[22], which is an tyrosine kinase inhibitor, are the most promising ones and have been now approved for the treatment of pancreatic NENs. mTOR is a serine/threonine kinase that plays a crucial role in mediating cell growth, proliferation, apoptosis, and angiogenesis; the mTOR pathway has been recently demonstrated to be frequently mutated in patients with pancreatic NET[21]. Everolimus is an orally active mTOR derivated from rampamycin. It was initially evaluated in 30 patients with carcinoid tumors and 30 more with pancreatic NET received doses of 5 mg or 10 mg daily plus depot octreotide. The overall tumor response rate of the evaluable patients was 17% for carcinoid and 27% for pancreatic NEN[21]. Subsequently, the randomized RADIANT 3 trial[21] demonstrated everolimus to improve the median PFS from 4.6 mo in the placebo arm to 11 mo in the treatment arm. The objective response rate on the everolimus arm was 5%. Everolimus has showed an acceptable safety profile. The most common adverse effects among patients treated with everolimus are: stomatitis, diarrhea, fatigue and rash. The most commonly reported grade-3 or 4 adverse events are stomatitis (7%), anemia (6%) and hyperglycemia (5%). In addition, everolimus has been associated with some serious adverse events, albeit rare, including pneumonitis[21]. NENs are usually characterized by abundant vascularization, and different tyrosine kinase inhibitors to be directed against VEGFR have been evaluated in GEP-NENs. To date, sunitinib has shown promising results in patients with advanced pancreatic NENs. In a multinational phase-III study sunitinib has demonstrated a significantly increased median PFS (5.5 mo vs 11.4 mo) as compared to the placebo[22]. The study also demonstrated an improved objective response rate (9.3% vs 0%) and OS rate (90% vs 75%) in the sunitinib arm, but it was terminated early because of serious adverse events and deaths in the placebo group (P < 0.001)[22]. Among the patients treated with everolimus the adverse events were: stomatitis, diarrhea, fatigue and rash. The most frequent side effects were: gastrointestinal symptoms, hypertension, asthenia and fatigue. Severe adverse events (grade 3 or 4) were uncommon and the most frequent were hypertension (10%) and neutropenia (12%)[22].
Peptide receptor radionuclide therapy
The high expression of SSRs in neuroendocrine neoplastic cells also gives the rationale for the use of PRRT in NENs. SSAs are labeled with radioactive peptides to convey radioactivity inside the tumor cell, leading to the internalization of the somatostatin receptor and radio-labeled analog complex. The use of PRRT with the somatostatin analogs yttrium-90-octreotide and 177Lu-octreotate has been explored for NETs for more than a decade and, in the last few years, PRRT has emerged with increasing evidence of efficacy on metastatic disease. For patients that are not eligible for surgery, PRRT is indicated for metastatic or locally advanced, low-grade NETs (G1 or G2) with positive expression of SSTR2, as demonstrated by nuclear imaging techniques using 111In-octreotide (octreoscan) or 68Ga-labeled peptides[23,24]. The two most commonly used radiopeptides for PRRT, i.e., -octreotide and 177Lu-octreotate, produce disease-control rates of 68%-94%, while partial or complete objective responses were observed in up to 30% of patients[25]. In addition to the overt evidence of tumor shrinkage, biochemical and symptomatic responses are commonly observed and promising results have been observed in terms of both progression-free survival (PFS) and OS[23]. The best objective responses have been reported in gastroenteropancreatic NETs with partial responses ranging from 9% to 29% and complete remission from 2% to 6%[24,26]. Recently, Strosberg et al[27] reported similar results from the NETTER-1 trial, the first phase-III multicentric randomized controlled trial evaluating 177Lu-DOTA0-Tyr3-Octreotate (Lutathera®) in patients with inoperable midgut NENs with somatostatin receptor expression. Indeed, the study showed a statistically significant increase in PFS (65.2% vs 10.8%) and an objective response rate (18% vs 3%) in patients treated with Lutathera® plus best supportive care as compared to 60 mg octreotide LAR at 20 mo. In addition the safety profile has been proved acceptable with severe (grade 3 or 4) neutropenia, thrombocytopenia, and lymphopenia occurring in 1%, 2%, and 9%, respectively[28].
LOCOREGIONAL TREATMENTS
As liver metastases are often accounted in the majority of symptoms in NENs and represent a main prognostic factor, therapies directed at metastases are essential to the multi-disciplinary treatment of these patients. However, despite the fact that over the last five decades a number of procedures have been developed to manage them, metastases remain a therapeutic challenge. Liver-directed treatments include: liver surgery (either curative or cytoreductive), ablative techniques (radiofrequency ablation, microwaves) and chemoembolization by employing biological, cytotoxic, or targeted agents either locally or systemically. Three different patterns of liver metastasization may be recognized and they determine the specific therapeutic approach: (1) metastases confined to one liver lobe or limited to two adjacent segments, which can be resected by standard anatomical resection (20%-25% of cases); (2) bilobar metastatic pattern, which can still be approached surgically, including ablative approaches (10%-15% of cases); and (3) diffuse and multifocal (60%-70% of cases)[7,8].
Surgical resection of liver metastases
While surgery is the mainstay of therapy for localized GEP-NENs, surgical management in advanced disease is debated. Grandhi et al[29] showed that the predominant surgical factor impacting on OS was negative surgical margins, thus the surgical option should always aim to accomplish radical excision.
Nowadays, curative surgery (i.e., metastasectomy, partial hepatectomy, and transplantation) is feasible for a minority of patients. Patients are eligible for curative surgery according to a series of indices: (1) tumor biology and timing of metastasis; (2) location, size and number of metastases; (3) absence of extrahepatic disease; and (4) patients’ general health status[30-32].
Although surgery appears to be the most suitable approach for patients with resectable metastases[33], OS has not shown a substantial improvement compared to alternative options in a randomized trial. In 2009, the Hepato-Biliary Cochrane group reviewed the published literature and did not identify any randomized trials, cohort studies, or case-control studies comparing surgical vs non-surgical treatments. Nevertheless the authors concluded that liver resection was the mainstay for the curative treatment of NET patients with resectable liver metastases[34]. The data currently available from some retrospective studies report a 5-year OS of 60%-80% for patients who underwent curative resection as compared to only 30% among GEP-NEN patients with unresected liver metastases[35-37]. Interestingly, the absence of extrahepatic disease seems to be the main requirement to achieve a significantly better prognosis[38].
The timing of the primary NEN excision of patients with both liver metastasis and resectable primary NEN is still being debated. Primary NEN excision prior to liver metastases resection might result in prolonging OS, however this evidence is documented only by a few small uncontrolled retrospective case series. No studies in the literature to date report oncological outcomes for patients who underwent liver metastases resection and subsequently primary NEN excision[39].
For selected patients who had the primary NET successfully excised but with liver metastases not responding to surgery or alternative therapies, liver transplantation can be considered[40].
For patients with unresectable liver metastates, cytoreductive surgery is a relevant option in the spectrum of strategies, even though it is still being discussed. For symptomatic patients cytoreductive surgery may play a role but a resection of at least 90% of the tumor is usually needed to be effective[29]. In these patients, quality of life is substantially affected by endocrinopathies and pain due to the unregulated hormone release and the mass effect on the adjacent structures and organs[41]. Cytoreductive surgery relieves the patients from the burden of the majority of symptoms and prolongs their overall OS with an acceptable degree of morbidity and mortality[42].
Ablative treatments
Ablative treatments include radiofrequency, microwave, cryoablation and alcoholization. Ablative techniques have shown to be effective in both relieving the symptoms of NET liver metastases and achieving local control of the metastases[28]. Radiofrequency ablation (RFA) is a medical procedure by which the liver metastatic tissue is ablated using the heat generated from medium-frequency alternating current (in the range of 350-500 kHz). Thermal ablation can be reached through microwave, a non-ionizing form of radiation that generates very high temperatures in very short timeperiods, potentially leading to improved treatment efficiency and larger ablation zones: thus, microwave ablation is emerging as a valuable alternative to radiofrequency ablation in the treatment of hepatic malignancies[43]. Radiofrequency ablation (RFA) and microwave ablation are the most favored techniques to address neuroendocrine liver metastases and 5-year OS rates of up to 53% have been reported[44]. Radiofrequency ablation and microwave ablation may be performed either percutaneously or laparoscopically as part of a multi-modality treatment (in association of surgery) or as alternative, for patients who are not eligible for major surgical procedure[45]. Ablative techniques are most effective in patients with a low tumor volume (lesions between 1 and 5 cm) and best indicated for a limited number of metastases (< 5-6 lesions)[8]. Liver ultrasonography is carried out to detect the lesions that may be amenable to this treatment. The tip of the radiofrequency thermoablation catheter is placed onto the metastasis and the prongs are set. RFA is based on radiofrequency waves converting into heat. Often, a few sessions are needed to achieve the required temperature and totally ablate the lesion[46]. The size of the tumor is the factor that most affects the effectiveness of the treatment. A study by Akyildiz et al[46] showed that laparoscopic RFA can achieve symptom relief in 97% of patients with a high risk of recurrence of 22% for local liver recurrence and 63% new liver metastases. The median disease-free survival (DFS) and OS were 1.3 years and 6 years respectively with liver tumor volume, symptoms, and extra-hepatic disease as independent predictive OS factors. In addition, the combination of resection and RFA may achieve complete tumor removal. Finally, RFA presents a favorable safety profile given its acceptable morbidity (5%) and no 30-d mortality[47].
Very few studies are available for cryoablation and alcoholization in NENs. Cryoablation is an alternative thermoablation technique. The necrosis of neoplastic tissue is obtained through direct and indirect mechanisms, such as mechanical injury caused by ice crystals disrupting the cellular membranes and organelles or as a consequence of vascular supply disruption or due to cold-activated endonucleases triggering apoptotic response[48]. Only one study reported a series of 13 patients with NETs who underwent hepatic cryotherapy: 12 patients had the complete ablation of all the visible tumors, with 2 recurrences at the ablation sites and 12 survivors at 1-year follow-up[49].
The ultrasound-guided injection of ethanol, otherwise known as percutaneous alcohol injection (PAI), performed into neuroendocrine metastases has been described in some series[50,51], even if histology was not exclusive to NENs. In these studies the lesions chosen for ethanol ablation are necessarily less than 3-5 cm in diameter and the cubic volume of alcohol injected requires modeling the target tumor as a sphere, but these estimations are more likely to be accurate when the radius is shorter, and very small metastases can be ablated with minimal collateral damage to the surrounding liver. Therefore, PAI is best used not as mono-therapy but rather as an adjunct to newer ablative techniques when approaching tiny or inauspiciously located metastases.
Transarterial treatments
The rationale of ablative treatments is based on the observation that GEP-NEN metastases are usually highly vascular and are supplied by the hepatic artery while the normal liver parenchyma receives the majority of its blood supply from the portal vein (Figure 1). The arterial occlusion to induce ischemia and necrosis of metastatic liver lesions may be obtained percutaneously through the femoral artery and the subsequent transarterial embolization (TAE) of the hepatic artery, or with the intra-arterial administration of bland chemotherapeutics (transarterial chemoembolization, TACE) (Figure 2), or chemotherapeutic drugs eluting beads (TACE-DEB). Transarterial radioembolization (TARE) with yttrium-90 (Y-90) microspheres represents a further viable strategy to delivery targeted radiation therapy to liver metastases. The Y-90 microspheres are injected through the hepatic artery to the pre-capillary level of the liver metastases, where they become trapped and release internal radiation. This concept minimizes the amount of radiation towards the normal hepatocytes[52].
Figure 1.
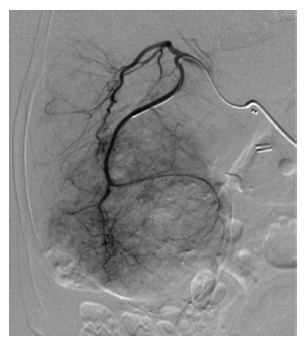
Arteriography of voluminous liver metastases secondary to an ileal neuroendocrine neoplasms.
Figure 2.

Computed tomography scan showing massive liver metastases in a patient with an ileal primary NET before (A) and after (B) transarterial chemoembolization procedure.
Transarterial treatments are indicated when surgery is not feasible for tumor reduction in functioning and non-functioning NENs. These procedures can be repeatedly performed until satisfactory disease control is achieved[7].
The main contraindications to transarterial treatments are: portal vein thrombosis, liver failure and/or severe co-morbidities[53]. Others contraindications are the Whipple procedure and hepato-pulmonary shunts due to the increased risk of morbidity (e.g., liver abscess) and mortality[7]. The most common side effects include: nausea and vomiting, right-upper-quadrant pain, fever, and elevation of transaminases[54]. In addition, post-embolization syndrome is often observed. Major side effects are: gallbladder necrosis, hepato-renal syndrome, pancreatitis, liver abscess, and formation of aneurysms. However, in an experienced center the mortality rate is acceptable (0%-3.3%).
The complete or partial response for symptoms and imaging occurred in 73%-100% and 33%-50% of the patients respectively[54,55]. The 5-year OS rates from several studies using TACE were 50%-83%, and similar outcomes were reported for TAE with 5-year OS rates between 40% and 67%[55]. For TARE a response rate was reported as high as 70%-90%[56].
Three different patterns of liver metastasization may be recognized and they determine the specific therapeutic approach: (1) metastases confined to one liver lobe or limited to two adjacent segments, which can be resected by standard anatomical resection (20%-25% of cases); (2) bilobar metastatic pattern, which can still be approached surgically, including ablative approaches (10%-15% of cases); and (3) diffuse and multifocal (60%-70% of cases)[7,8].
DISCUSSION
Over the last few years, alongside surgery and SSAs, different locoregional and systemic therapies have been developed for advanced GEP-NENs (Table 1). However, given the novelty of these new therapies, only a few comparative studies have been carried out to date and no randomized clinical trials are yet available. Thus, the appropriate selection and sequencing of treatment approaches depends mostly on clinical judgment. For metastatic NEN patients the best approach should take in consideration several factors, including the tumor features (i.e., tumor grading and staging), liver metastasization patterns (mono or bilobar disease, size and number of lesions), the presence of extrahepatic disease, patient characteristics (i.e., performance status, age and co-morbidities) as well as the patient’s perspective. In general, the choice of different treatments should take in account the long-term aim, i.e. curative/cytoreductive intent vs disease control.
Table 1.
Main indications for locoregional treatments with associated data on safety and survival
| Treatment | Indication | Safety | Survival1 |
| Surgery | Simple pattern of liver metastases, G1/G2 neoplasms, no or minimal extrahepatic disease, preserved liver function, absence of right heart insufficiency, PS 0-1. | Mortality rate 0%-5%, morbidity close to 30%[7] | 5-yr survival of 60%-80%[36,37] |
| Curative Intent: | |||
| Unilobar metastases or limited to two adjacent segments. | |||
| Cytoreductive: | |||
| Bilobar metastatic pattern < 25% (90% of disease resectable, symptomatic patients). | |||
| Ablative treatments | Patients not eligible for major surgery, preserved liver function, simple pattern of liver metastases, lesions between 1 and 5 cm, limited number of metastases < 5-6 lesions. | Morbidity 5%, no 30-d mortality[47] | 5-yr survival up to 53%[44] |
| Transarterial techniques: transarterial embolization, transarterial chemoembolization transarterial radioembolization | Patients not eligible for major surgery, preserved liver function, diffuse pattern of liver metastases > 25%, symptoms. | Mortality rate of 0%-3.3%[55] | 5-yr survival 40%-83%[55] |
Data based on retrospective studies.
To date, a few treatments (i.e. surgery, RFA, PRRT and chemotherapy) have proved to achieve a significant radiological objective response in GEP-NENs, thus these treatments should be preferred for a curative intent. In contrast, SSAs, IFN-α, and targeted therapies have showed to obtain disease stabilization and to improve PFS in phase-III clinical trials. In this evolving setting the role and timing of locoregional liver-directed therapies is still under debate, as to date no prospective clinical trials have demonstrated any significant OS improvement.
SSAs are usually recognized as the first line of therapy in advanced GEP-NENs due to their favorable safety profile and established benefit on PFS[13]. In case of stable disease and localized hepatic metastases, surgical resection with radical intent (R0) should be advised[57,58]. Complete resection (R0) for both mid- and hindgut tumors is associated with better long-term OS[59], with reported 5-year OS and PFS of 70.5% and 29% respectively in retrospective studies[60]. However, GEP-NEN liver metastases have a high rate of recurrence after hepatic resection, up to 70%-94% at 5 years[5,61]. The impact of hepatic resection on OS remains difficult to assess because of the potential selection bias, as the patients with a more extensive burden of disease, a worst performance status, advanced age and with severe co-morbidities tend to be conservatively managed[62]. Although surgery appears to improve OS, the data to avail are mostly from retrospective studies and thus the clinical level of evidence is low.
Both systemic and locoregional therapies are now available to patients in progression after SSAs. However, considering the often long life expectancy of patients with GEP-NEN, the optimal timing of treatments is crucial. Thus, the locoregional therapies should be considered as second-line therapy for patients with metastatic disease limited to the liver (or when the liver represents the main site of disease) or in case of evidence of disease progression limited to the liver[7], while systemic therapy should be reserved for patients with extrahepatic disease progression. In addition, locoregional therapies including cytoreductive surgery have been recognized to be very effective in symptom control, whereas, in contrast, second-line systemic treatments have a limited role in symptomatic relief.
In the absence of randomized trials comparing the OS impact of the different locoregional therapies, or locoregional therapies and medical treatments or PRRT[34], a possible therapeutic algorithm may be based on liver disease extension and safety profile (Figure 3).
Figure 3.
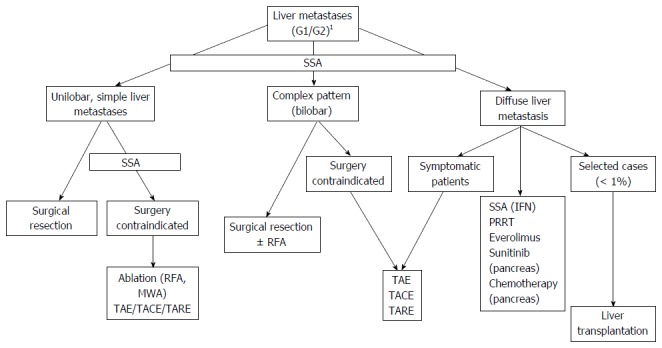
Treatment algorithm for advanced gastroenteropancreatic neuroendocrine tumors. 1Resection of primary, no (or limited) extrahepatic disease. SSA: Somatostatin analog; IFN: Interferon; PRRT: Peptide receptor radionuclide therapy; TAE: Transarterial embolization; TACE: Transarterial chemoembolization; TARE: Transarterial radioembolization; RFA: Radiofrequency ablation.
De-bulking surgery (R2) may have a role in case of low-grade (G1/G2) NENs with limited burden of disease, when > 90% of the tumor can be resected[7]. Symptomatic patients benefit from cytoreductive procedures, whereas their role in asymptomatic patients is still debated[62]. Improvements of specific symptoms after cytoreductive surgery have been reported with a median duration of 19.3-45.5 mo[63]. Moreover, a recent meta-analysis based on retrospective studies comparing radical and palliative surgery outcomes showed a non-significant difference in OS HR = 0.40 (95%CI: 0.14-1.11), suggesting that both the approaches may lead to relevant results in NENs liver metastases[62]. In addition, a paper by Osborne et al[41] suggested the possible superiority of palliative resection of hepatic metastases to embolization.
Alternatively, locoregional interventional radiology techniques (TACE, TAE, TARE, RFA and MWA) are indicated for patients who are not eligible for surgery[64]. A relevant role of RFA and MWA in symptom control in presence of unresectable GEP-NEN liver metastases has been reported[47]. In addition, a recent systematic review analyzing 8 studies and 301 patients, observed symptoms control in 92% of cases after RFA lasting a median of 14-27 mo[44]. Similarly, TACE and TAE appear able to control symptoms in 73%-100% of patients and the duration of symptomatic response varied between 14 and 22 mo[55]. Both techniques are feasible and safe, however TACE/TAE may have some significant morbidity[7].
While the role of the interventional techniques in symptom control has been demonstrated, their effect on OS is still a matter of debate. Indeed, the published studies are mostly retrospective and include a small number of patients[7]. A retrospective study, comparing 103 patients who underwent surgical treatment (liver RFA/resection) vs 273 patients who did not, showed no differences in OS and disease-specific survival at 5 years, while the proportion of patients with progressive disease was lower in the surgical group after 5 years[65]. However, the benefit of ablation on OS remains difficult to demonstrate for patients who underwent several subsequent lines of treatment.
Selective hepatic TAE or TACE can achieve complete or partial objective responses in 33%-50% of the patients. The 5-year OS rates from several studies using TACE were 50%-83%, and similar outcomes have been reported for TAE with 5-year OS rates between 40% and 67%[55]. In particularly, Mayo et al[65] demonstrated no difference in long-term outcomes between surgery vs intra-arterial therapies for asymptomatic patients with large liver tumor (> 25%), suggesting that intra-arterial therapies may be the more appropriate therapy.
The data comparing intra-arterial therapies are currently limited. TAE has most often been compared to TACE, but no statistically significant differences have been drawn[29,45]. The TACE-DEB outcomes seem promising but serious complications, such as bilomas and abscess formation, have been reported[66] and they led to the premature discontinuation of a phase-II trial as serious adverse events. No studies in the literature comparing TARE with Y-90 and intra-arterial therapies are available. However, the main advantage of TARE as enjoying the shortest hospital stay compared to TAE and TACE, is established. Additionally, Y-90 has the advantage of being delivered in a bilobar fashion, which is helpful for patients with a more disseminated disease.
An additional application of locoregional treatments may be towards the downsizing of the tumor and making it amenable to systemic treatments in order to optimize the treatment effect on the remaining neoplastic tissue. Finally, little evidence-based data is currently available to guide the integration of all these various treatment modalities.
CONCLUSION
Overall, locoregional treatments appear feasible and safe for disease control in patients with limited liver involvement and effective in symptoms control in patients with diffuse liver metastases (Table 1). Additionally, liver-directed therapies are curative in contrast to systemic treatments, which are palliative.
Considering the lack of randomized and controlled trials comparing locoregional treatments of liver metastatic NEN patients, clinical judgment remains key to set the most appropriate therapeutic pathway. Prospective data may ultimately lead to a more personalized and optimized treatment. The present review has analyzed all the available locoregional therapy modalities (i.e., surgery, ablative treatments and transarterial approach) with the aim to provide clinicians with a useful algorithm to best treat GEP-NEN patients metastatic to the liver.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflict of interest related to this publication.
Peer-review started: February 10, 2017
First decision: February 23, 2017
Article in press: March 20, 2017
P- Reviewer: Gong ZJ S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Stump R, Haueis S, Kalt N, Tschuor C, Limani P, Raptis DA, Puhan MA, Breitenstein S. Transplantation and surgical strategies in patients with neuroendocrine liver metastases: protocol of four systematic reviews. JMIR Res Protoc. 2013;2:e58. doi: 10.2196/resprot.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rindi G, Arnold R, Bosman F, Capella C, Kilmstra D, Kloppel G. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT et al, editor. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press, 2010: 13-14 In: Bosman FT et al., editor. [Google Scholar]
- 5.Saxena A, Chua TC, Sarkar A, Chu F, Liauw W, Zhao J, Morris DL. Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery. 2011;149:209–220. doi: 10.1016/j.surg.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Pape UF, Berndt U, Müller-Nordhorn J, Böhmig M, Roll S, Koch M, Willich SN, Wiedenmann B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 7.Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 8.Steinmüller T, Kianmanesh R, Falconi M, Scarpa A, Taal B, Kwekkeboom DJ, Lopes JM, Perren A, Nikou G, Yao J, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87:47–62. doi: 10.1159/000111037. [DOI] [PubMed] [Google Scholar]
- 9.Tomassetti P, Campana D, Piscitelli L, Casadei R, Nori F, Brocchi E, Santini D, Pezzilli R, Corinaldesi R. Endocrine tumors of the ileum: factors correlated with survival. Neuroendocrinology. 2006;83:380–386. doi: 10.1159/000096053. [DOI] [PubMed] [Google Scholar]
- 10.Kulke MH, Bendell J, Kvols L, Picus J, Pommier R, Yao J. Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. J Hematol Oncol. 2011;4:29. doi: 10.1186/1756-8722-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaltsas G, Grossman AB. The expanding role of somatostatin analogues in the treatment of neuroendocrine tumours: the CLARINET study. Clin Endocrinol (Oxf) 2015;83:759–761. doi: 10.1111/cen.12831. [DOI] [PubMed] [Google Scholar]
- 12.Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89:471–476. doi: 10.1159/000197899. [DOI] [PubMed] [Google Scholar]
- 13.Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 14.Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Oberg K. Interferon in the management of neuroendocrine GEP-tumors: a review. Digestion. 2000;62 Suppl 1:92–97. doi: 10.1159/000051862. [DOI] [PubMed] [Google Scholar]
- 16.Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B; International Lanreotide and Interferon Alfa Study Group. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 17.Walter T, Brixi-Benmansour H, Lombard-Bohas C, Cadiot G. New treatment strategies in advanced neuroendocrine tumours. Dig Liver Dis. 2012;44:95–105. doi: 10.1016/j.dld.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Delaunoit T, Ducreux M, Boige V, Dromain C, Sabourin JC, Duvillard P, Schlumberger M, de Baere T, Rougier P, Ruffie P, et al. The doxorubicin-streptozotocin combination for the treatment of advanced well-differentiated pancreatic endocrine carcinoma; a judicious option? Eur J Cancer. 2004;40:515–520. doi: 10.1016/j.ejca.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Strosberg JR, Cheema A, Kvols LK. A review of systemic and liver-directed therapies for metastatic neuroendocrine tumors of the gastroenteropancreatic tract. Cancer Control. 2011;18:127–137. doi: 10.1177/107327481101800207. [DOI] [PubMed] [Google Scholar]
- 20.Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 23.Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierckx RA, Paganelli G. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest. 2009;32:360–369. doi: 10.1007/BF03345728. [DOI] [PubMed] [Google Scholar]
- 24.Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, de Jong M, de Herder WW, Krenning EP. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–R73. doi: 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- 25.Bodei L, Kwekkeboom DJ, Kidd M, Modlin IM, Krenning EP. Radiolabeled Somatostatin Analogue Therapy Of Gastroenteropancreatic Cancer. Semin Nucl Med. 2016;46:225–238. doi: 10.1053/j.semnuclmed.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 26.van Essen M, Krenning EP, Bakker WH, de Herder WW, van Aken MO, Kwekkeboom DJ. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging. 2007;34:1219–1227. doi: 10.1007/s00259-006-0355-4. [DOI] [PubMed] [Google Scholar]
- 27.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandhi MS, Lafaro KJ, Pawlik TM. Role of Locoregional and Systemic Approaches for the Treatment of Patients with Metastatic Neuroendocrine Tumors. J Gastrointest Surg. 2015;19:2273–2282. doi: 10.1007/s11605-015-2931-z. [DOI] [PubMed] [Google Scholar]
- 29.Frilling A, Li J, Malamutmann E, Schmid KW, Bockisch A, Broelsch CE. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg. 2009;96:175–184. doi: 10.1002/bjs.6468. [DOI] [PubMed] [Google Scholar]
- 30.Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47:460–466. doi: 10.1016/j.jhep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Reddy SK, Clary BM. Neuroendocrine liver metastases. Surg Clin North Am. 2010;90:853–861. doi: 10.1016/j.suc.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Bacchetti S, Bertozzi S, Londero AP, Uzzau A, Pasqual EM. Surgical treatment and survival in patients with liver metastases from neuroendocrine tumors: a meta-analysis of observational studies. Int J Hepatol. 2013;2013:235040. doi: 10.1155/2013/235040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurusamy KS, Ramamoorthy R, Sharma D, Davidson BR. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database Syst Rev. 2009;(2):CD007060. doi: 10.1002/14651858.CD007060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scigliano S, Lebtahi R, Maire F, Stievenart JL, Kianmanesh R, Sauvanet A, Vullierme MP, Couvelard A, Belghiti J, Ruszniewski P, et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer. 2009;16:977–990. doi: 10.1677/ERC-08-0247. [DOI] [PubMed] [Google Scholar]
- 35.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 36.Cherif R, Gaujoux S, Couvelard A, Dokmak S, Vuillerme MP, Ruszniewski P, Belghiti J, Sauvanet A. Parenchyma-sparing resections for pancreatic neuroendocrine tumors. J Gastrointest Surg. 2012;16:2045–2055. doi: 10.1007/s11605-012-2002-7. [DOI] [PubMed] [Google Scholar]
- 37.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 38.Capurso G, Rinzivillo M, Bettini R, Boninsegna L, Delle Fave G, Falconi M. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg. 2012;99:1480–1486. doi: 10.1002/bjs.8842. [DOI] [PubMed] [Google Scholar]
- 39.Grossman EJ, Millis JM. Liver transplantation for non-hepatocellular carcinoma malignancy: Indications, limitations, and analysis of the current literature. Liver Transpl. 2010;16:930–942. doi: 10.1002/lt.22106. [DOI] [PubMed] [Google Scholar]
- 40.Jensen EH, Kvols L, McLoughlin JM, Lewis JM, Alvarado MD, Yeatman T, Malafa M, Shibata D. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol. 2007;14:780–785. doi: 10.1245/s10434-006-9148-z. [DOI] [PubMed] [Google Scholar]
- 41.Osborne DA, Zervos EE, Strosberg J, Boe BA, Malafa M, Rosemurgy AS, Yeatman TJ, Carey L, Duhaine L, Kvols LK. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol. 2006;13:572–581. doi: 10.1245/ASO.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 42.Lubner MG, Brace CL, Ziemlewicz TJ, Hinshaw JL, Lee FT. Microwave ablation of hepatic malignancy. Semin Intervent Radiol. 2013;30:56–66. doi: 10.1055/s-0033-1333654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohan H, Nicholson P, Winter DC, O’Shea D, O’Toole D, Geoghegan J, Maguire D, Hoti E, Traynor O, Cantwell CP. Radiofrequency ablation for neuroendocrine liver metastases: a systematic review. J Vasc Interv Radiol. 2015;26:935–942.e1. doi: 10.1016/j.jvir.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Fiore F, Del Prete M, Franco R, Marotta V, Ramundo V, Marciello F, Di Sarno A, Carratù AC, de Luca di Roseto C, Colao A, et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine. 2014;47:177–182. doi: 10.1007/s12020-013-0130-9. [DOI] [PubMed] [Google Scholar]
- 45.Cazzato RL, Garnon J, Ramamurthy N, Tsoumakidou G, Imperiale A, Namer IJ, Bachellier P, Caudrelier J, Rao P, Koch G, et al. 18F-FDOPA PET/CT-Guided Radiofrequency Ablation of Liver Metastases from Neuroendocrine Tumours: Technical Note on a Preliminary Experience. Cardiovasc Intervent Radiol. 2016;39:1315–1321. doi: 10.1007/s00270-016-1334-1. [DOI] [PubMed] [Google Scholar]
- 46.Akyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery. 2010;148:1288–1293; discussion 1293. doi: 10.1016/j.surg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Lewis MA, Hubbard J. Multimodal liver-directed management of neuroendocrine hepatic metastases. Int J Hepatol. 2011;2011:452343. doi: 10.4061/2011/452343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seifert JK, Cozzi PJ, Morris DL. Cryotherapy for neuroendocrine liver metastases. Semin Surg Oncol. 1998;14:175–183. doi: 10.1002/(sici)1098-2388(199803)14:2<175::aid-ssu10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 49.Livraghi T, Vettori C, Lazzaroni S. Liver metastases: results of percutaneous ethanol injection in 14 patients. Radiology. 1991;179:709–712. doi: 10.1148/radiology.179.3.2027979. [DOI] [PubMed] [Google Scholar]
- 50.Giovannini M. Percutaneous alcohol ablation for liver metastasis. Semin Oncol. 2002;29:192–195. doi: 10.1053/sonc.2002.31677. [DOI] [PubMed] [Google Scholar]
- 51.Cao CQ, Yan TD, Bester L, Liauw W, Morris DL. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg. 2010;97:537–543. doi: 10.1002/bjs.6931. [DOI] [PubMed] [Google Scholar]
- 52.Roche A, Girish BV, de Baère T, Baudin E, Boige V, Elias D, Lasser P, Schlumberger M, Ducreux M. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur Radiol. 2003;13:136–140. doi: 10.1007/s00330-002-1558-0. [DOI] [PubMed] [Google Scholar]
- 53.O’Toole D, Maire F, Ruszniewski P. Ablative therapies for liver metastases of digestive endocrine tumours. Endocr Relat Cancer. 2003;10:463–468. doi: 10.1677/erc.0.0100463. [DOI] [PubMed] [Google Scholar]
- 54.Vogl TJ, Naguib NN, Zangos S, Eichler K, Hedayati A, Nour-Eldin NE. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol. 2009;72:517–528. doi: 10.1016/j.ejrad.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Memon K, Lewandowski RJ, Riaz A, Salem R. Chemoembolization and radioembolization for metastatic disease to the liver: available data and future studies. Curr Treat Options Oncol. 2012;13:403–415. doi: 10.1007/s11864-012-0200-x. [DOI] [PubMed] [Google Scholar]
- 56.Landry CS, Scoggins CR, McMasters KM, Martin RC. Management of hepatic metastasis of gastrointestinal carcinoid tumors. J Surg Oncol. 2008;97:253–258. doi: 10.1002/jso.20957. [DOI] [PubMed] [Google Scholar]
- 57.Bonaccorsi-Riani E, Apestegui C, Jouret-Mourin A, Sempoux C, Goffette P, Ciccarelli O, Borbath I, Hubert C, Gigot JF, Hassoun Z, et al. Liver transplantation and neuroendocrine tumors: lessons from a single centre experience and from the literature review. Transpl Int. 2010;23:668–678. doi: 10.1111/j.1432-2277.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 58.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 59.Saxena A, Chua TC, Perera M, Chu F, Morris DL. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol. 2012;21:e131–e141. doi: 10.1016/j.suronc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Cho CS, Labow DM, Tang L, Klimstra DS, Loeffler AG, Leverson GE, Fong Y, Jarnagin WR, D’Angelica MI, Weber SM, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer. 2008;113:126–134. doi: 10.1002/cncr.23523. [DOI] [PubMed] [Google Scholar]
- 61.Bacchetti S, Pasqual EM, Bertozzi S, Londero AP, Risaliti A. Curative versus palliative surgical resection of liver metastases in patients with neuroendocrine tumors: a meta-analysis of observational studies. Gland Surg. 2014;3:243–251. doi: 10.3978/j.issn.2227-684X.2014.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 63.de Baere T, Deschamps F, Tselikas L, Ducreux M, Planchard D, Pearson E, Berdelou A, Leboulleux S, Elias D, Baudin E. GEP-NETS update: Interventional radiology: role in the treatment of liver metastases from GEP-NETs. Eur J Endocrinol. 2015;172:R151–R166. doi: 10.1530/EJE-14-0630. [DOI] [PubMed] [Google Scholar]
- 64.Norlén O, Stålberg P, Zedenius J, Hellman P. Outcome after resection and radiofrequency ablation of liver metastases from small intestinal neuroendocrine tumours. Br J Surg. 2013;100:1505–1514. doi: 10.1002/bjs.9262. [DOI] [PubMed] [Google Scholar]
- 65.Mayo SC, de Jong MC, Bloomston M, Pulitano C, Clary BM, Reddy SK, Clark Gamblin T, Celinski SA, Kooby DA, Staley CA, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18:3657–3665. doi: 10.1245/s10434-011-1832-y. [DOI] [PubMed] [Google Scholar]
- 66.Guiu B, Deschamps F, Aho S, Munck F, Dromain C, Boige V, Malka D, Leboulleux S, Ducreux M, Schlumberger M, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol. 2012;56:609–617. doi: 10.1016/j.jhep.2011.09.012. [DOI] [PubMed] [Google Scholar]


