Abstract
AIM
To evaluate the ability of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 to colonize the intestinal environment of healthy subjects and modify the gut microbiota composition.
METHODS
Twenty healthy Italian volunteers, eight males and twelve females, participated in the study. Ten subjects took a sachet containing 4 × 109 colony-forming units (CFU) of Bifidobacterium longum BB536 and 109 CFU of Lactobacillus rhamnosus HN001, 30 min before breakfast (pre-prandial administration), while ten subjects took a sachet of probiotic product 30 min after breakfast (post-prandial administration). The ability of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 to colonize human gut microbiota was assessed by means of quantitative real-time PCR, while changes in gut microbiota composition were detected by using Ion Torrent Personal Genome Machine.
RESULTS
Immediately after 1-mo of probiotic administration, B. longum BB536 and L. rhamnosus HN001 load was increased in the majority of subjects in both pre-prandial and post-prandial groups. This increase was found also 1 mo after the end of probiotic oral intake in both groups, if compared to samples collected before probiotic consumption. At phyla level a significant decrease in Firmicutes abundance was detected immediately after 1-mo of B. longum BB536 and L. rhamnosus HN001 oral intake. This reduction persisted up to 1 mo after the end of probiotic oral intake together with a significant decrease of Proteobacteria abundance if compared to samples collected before probiotic administration. Whereas, at species level, a higher abundance of Blautia producta, Blautia wexlerae and Haemophilus ducrey was observed, together with a reduction of Holdemania filiformis, Escherichia vulneris, Gemmiger formicilis and Streptococcus sinensis abundance. In addition, during follow-up period we observed a further reduction in Escherichia vulneris and Gemmiger formicilis, together with a decrease in Roseburia faecis and Ruminococcus gnavus abundance. Conversely, the abundance of Akkermansia muciniphila was increased if compared to samples collected at the beginning of the experimental time course
CONCLUSION
B. longum BB536 and L. rhamnosus HN001 showed the ability to modulate the gut microbiota composition, leading to a significant reduction of potentially harmful bacteria and an increase of beneficial ones. Further studies are needed to better understand the specific mechanisms involved in gut microbiota modulation.
Keywords: Probiotics, Gut microbiota, Bifidobacterium, Lactobacillus, Human health
Core tip: Several studies have described the potentially beneficial effects of many probiotic microorganisms belonging to Lactobacillus and Bifidobacterium genera. We evaluated the ability of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001, two probiotic strains used in combination, to colonize the intestinal environment of healthy subjects and modify the gut microbiota composition. We did not observe a negative impact of probiotic on the general health status of the hosts. Contrariwise, the two bacterial strains seemed able to exert a beneficial effect on the bacterial ecology of the gastrointestinal tract, as many significant positive changes in gut microbiota composition have been highlighted.
INTRODUCTION
Probiotics are defined as “non-pathogenic live micro-organisms that, when administered in adequate amounts, confer a health benefit on the host”[1]. In the last years, their numerous beneficial properties and positive impact on human health have deeply been described[2]. Consequently, the global market for probiotics is growing due to the increased demand of consumers who use these products to improve their health and even to prevent some human illnesses such as allergic and gastrointestinal diseases, modulate immune system and ensure the homeostasis of intestinal microbiota[3-5]. Nowadays, hundreds of different bacterial strains are available in the global probiotic market and consequently, the choice of the most suitable probiotic product becomes very difficult and fragmented. For these reasons, safety and efficacy of probiotics are considered the main criteria for using any microorganism in the formulation of probiotic products[6]. Lactobacilli and Bifidobacteria are the main microorganisms used as probiotics; indeed, numerous species belonging to these genera have been reported as safe and effective in improving the host’s health[7]. Interestingly, modulation of intestinal microbiota composition has been proposed as one of the main mechanisms of probiotic activity[8]. Several studies showed that the combination of specific bacterial strains belonging to Lactobacillus and Bifidobacterium species can act in optimal synergy for restoring the intestinal balance[9-11]. In 2015, Drago et al[12], for instance, highlighted the immunomodulatory synergy of L. salivarius LS01 and Bifidobacterium breve BR03 which combination led to an increased immunomodulatory activity if compared to the activity of each single strain. Also Bifidobacterium longum (B. longum) BB536 and Lactobacillus rhamnosus (L. rhamnosus) HN001 are two well-characterized probiotic strains often used in combination which ability to survive the adverse gastrointestinal conditions and adhere to intestinal mucosa has already been demonstrated in a previous study[13]. These probiotic strains possess strong immunomodulatory activities, are able to improve human health, reduce eczema prevalence in children and inhibit adhesion of gram-negative pathogens in intestinal environment[14-21].
The aim of the present study was to investigate the effects of B. longum BB536 and L. rhamnosus HN001 on the gut microbiota composition of healthy subjects after one month of probiotic oral intake, also evaluating the potential impact of pre- and post-prandial probiotic administration on the colonization ability of B. longum BB536 and L. rhamnosus HN001.
MATERIALS AND METHODS
Experimental design
Twenty healthy Italian subjects, eight males and twelve females, participated in the study. They were all volunteers who were informed in detail about the aim of the study. Baseline characteristics for each subject are summarized in Table 1. All individuals personally delivered fecal samples to the Laboratory of Clinical Microbiology (University of Milan, Milan). The experimental protocol was approved by the Scientific Direction of IRCCS Galeazzi Orthopaedic Institute in the Current Research 2015.
Table 1.
Baseline characteristics of health volunteers
| Subject | Gender | Age | Weight (kg) | Height (m) | Body mass index (kg/m2) |
| Pre-prandial group | |||||
| 1 | Female | 40 | 70.0 | 1.80 | 21.6 |
| 2 | Female | 32 | 65.0 | 1.65 | 23.9 |
| 3 | Male | 45 | 57.0 | 1.64 | 21.2 |
| 4 | Male | 42 | 73.0 | 1.68 | 25.9 |
| 5 | Male | 35 | 85.0 | 1.90 | 23.5 |
| 6 | Male | 30 | 82.0 | 1.83 | 24.5 |
| 7 | Male | 31 | 71.0 | 1.68 | 25.2 |
| 8 | Male | 43 | 82.0 | 1.82 | 24.8 |
| Post-prandial group | |||||
| 9 | Male | 28 | 71.0 | 1.70 | 24.6 |
| 10 | Male | 29 | 64.0 | 1.80 | 19.8 |
| 11 | Male | 33 | 81.0 | 1.79 | 25.3 |
| 12 | Male | 43 | 72.0 | 1.73 | 24.1 |
| 13 | Male | 41 | 81.0 | 1.79 | 25.3 |
| 14 | Male | 35 | 71.0 | 1.77 | 22.7 |
| 15 | Female | 35 | 61.0 | 1.72 | 20.6 |
| 16 | Female | 33 | 60.0 | 1.62 | 22.9 |
Exclusion criteria included antibiotic treatment within the previous 2 mo or suffering from any acute or chronic cardiovascular, gastrointestinal or immunological conditions. Furthermore, probiotics and yogurt have been excluded from the diet during the study period. Volunteers were randomized in two groups. The probiotic product was provided by Alfa Wassermann S.p.a. (Milan, Italy) that also supplied evidence about its safety for human consumption. Ten subjects took a sachet containing 4 × 109 colony-forming units (CFU) of B. longum BB536 and 109 colony-forming units (CFU) of L. rhamnosus HN001 30 minutes before breakfast (pre-prandial administration), while ten subjects took a sachet of probiotic product 30 minutes after breakfast (post-prandial administration). During the probiotic resuspension and before oral intake, some volunteers described the formation of lumps inside the solution. Moreover, the product was tasteless and odorless, favoring the daily oral intake. Fecal samples were collected from each participant one week before the probiotic oral intake, after one month of probiotic administration and one month after the end of probiotic consumption. Two subjects were excluded from the study following an antibiotic therapy, while 2 individuals were excluded from the data analysis as they did not strictly follow the diet.
DNA extraction
Total DNA was extracted from fecal samples using the QIAamp DNA Stool Mini Kit following the manufacturer’s instructions (Qiagen, Italy).
Quantitative PCR
The ability of B. longum BB536 and L. rhamnosus HN001 to colonize the human gut microbiota was assessed by means of a quantitative real-time PCR carried out using a Rotor Gene 3000 system (Diatech), in order to evaluate the potential increase of the lactobacilli and bifidobacteria load following the probiotic oral intake. For B. longum DNA amplification the following primers were used: B. longum (forward) 5’-TTCCAGTTGATCGCATGGTC-3’ and B. longum (reverse) 5’-GGGAAGCCGTATCTCTACGA-3’ (Eurofin, Vimodrone, Italy). The reaction conditions for DNA amplification were 94 °C for 5 min, 35 cycles of 94 °C for 20 s, 55 °C for 20 s, 72 °C for 20 s and 72 °C for 5 min. Differently, for L. rhamnosus DNA amplification the PCR reaction was performed using the following primers: L. rhamn (forward) 5’-TGCATC TTGATTTAATTTTG-3’ and L. rhamn (reverse) 5’-CCACT GCTGCCTCCCGTAGGAGT-3’ (Eurofin, Vimodrone, Italy). The amplification profile was an initial step of 94 °C for 3 min, and then 30 cycles of 94 °C for 45 s, 55 °C for 45 s and 72 °C for 1 min.
16S gene sequencing
DNA amplification and 16S gene sequencing were performed as previously described[22].
Statistical analysis
The biodiversity index (Shannon, Simpson and Chao) and statistical analyses were carried out using the R Software V.3.3.1, for Windows. Non-parametric Kruskal-Wallis and Mann-Whitney tests were used to find significant differences in α diversity and microbial taxa. Adjustment for multiple testing was evaluated with Dunn’s post-hoc test. P values below 0.05 were considered statistically significant.
RESULTS
In all subjects no side effects were observed following the oral intake of probiotic product.
Colonization ability of B. longum BB536 and L. rhamnosus HN001
After one month of probiotic administration, an increase of B. longum load was detected in 6 of 8 subjects belonging to the pre-prandial group (Figure 1A). This increase was maintained 1 mo after the end of the probiotic consumption. In 2 individuals (subjects 3 and 5) the B. longum load after the end of the treatment was higher than that detected immediately after the probiotic oral intake (Figure 1A). In the post-prandial group, however, we observed an increase of B. longum abundance up to 1 mo after the end of the probiotic in 4 subjects (Figure 1A). Only in one individual (subject 13), 1 mo after the end of the probiotic oral intake the B. longum load was higher than both baseline samples and samples taken immediately after the probiotic oral intake (Figure 1A). Moreover, after 1 mo of probiotic administration an increase of L. rhamnosus load was observed in 5 of 8 subjects belonging to the pre-prandial group (Figure 1B). This increase was also found 1 mo after the end of the probiotic, although L. rhamnosus abundance was slightly lower than that detected immediately after the month of probiotic administration (Figure 1B). In the post-prandial administration group, L. rhamnosus was increased in 4 subjects up to 1 mo after the end of probiotic oral intake.
Figure 1.
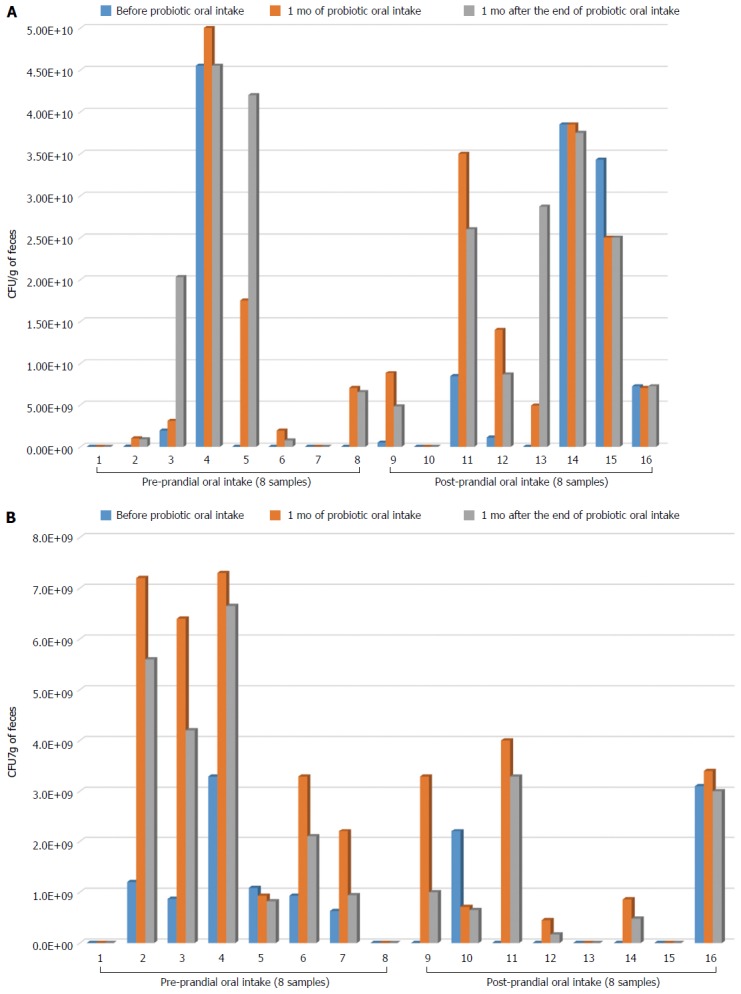
Colonization ability of Bifidobacterium longum BB536 (A) and Lactobacillus rhamnosus HN001 (B). Bar graphs represent the abundance of each bacterial strain, expressed as colony-forming unit per gram of feces (CFU/g feces), recovered from each subject belonging to the pre-prandial group and post-prandial group.
Effects of B. longum BB536 and L. rhamnosus HN001 on gut microbiota biodiversity
To evaluate the bacterial diversity and richness in the gut microbiota before and after the intake of B. longum BB536 and L. rhamnosus HN001 we calculated, for each time point, Shannon’s, Simpson’s and Chao’s indices reported in Figure 2. Although no significant differences in gut microbiota biodiversity were observed after B. longum BB536 and L. rhamnosus HN001 oral intake, a slight reduction of Chao’s index was detected one month after the end of probiotic administration.
Figure 2.
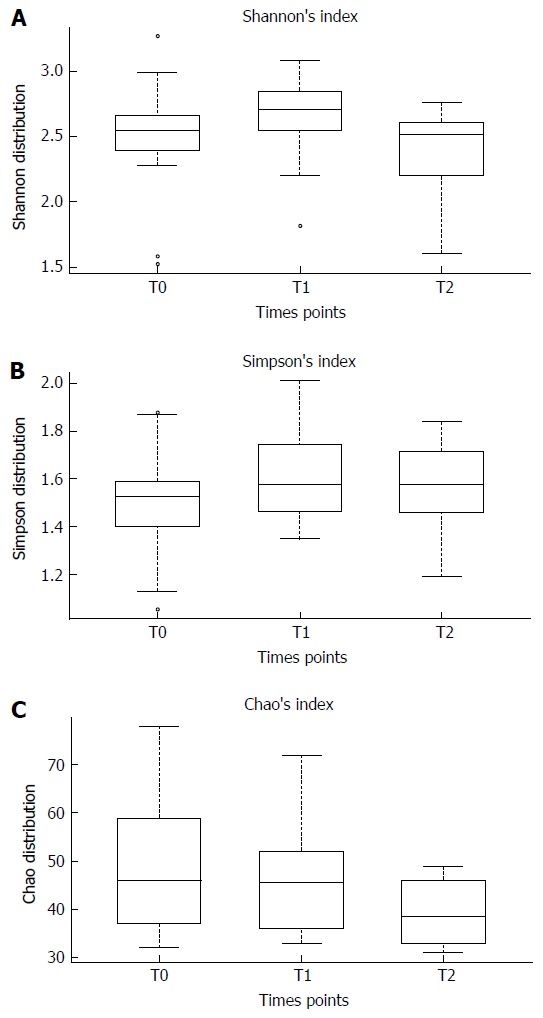
The Shannon (A), Simpson (B) and Chao’s (C) indices before probiotic oral intake (T0), one month after probiotic administration (T1) and one month after the end (T2) of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 consumption. Mann-Whitney tests were performed for each pair wise comparison and the Kruskal-Wallis test across all three groups.
Bacterial changes at phylum level in the gut microbiota composition after the probiotic consumption and one month after the end of probiotic oral intake
Distribution of main bacterial phyla characterizing the gut microbiota of individuals enrolled in the study is shown in Figure 3. Firmicutes were subjected to a significant reduction (about 50%) after one month of B. longum BB536 and L. rhamnosus HN001 oral intake (Figure 3). Moreover, one month after the end of probiotic administration, a further reduction of Firmicutes (about 20%) was observed, together with a significant decrease of Proteobacteria abundance (about 58%), if compared to samples collected before probiotic administration.
Figure 3.
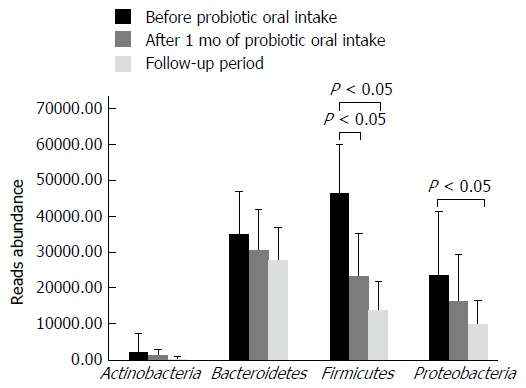
Distribution of the main bacterial phyla detected in fecal samples before Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 consumption, after one month of probiotic administration and one month after the end of probiotic oral intake. Any significant reduction is indicated by an asterisk (P < 0.05).
Bacterial changes at species level in gut microbiota composition after the probiotic consumption and one month after the end of probiotic oral intake
Significant differences at species level detected in gut microbiota are shown in Figure 4. Immediately after the probiotic administration, a significant increase of Blautia producta, Blautia wexlerae and Haemophilus ducrey load was observed, together with a reduction of Holdemania filiformis, Escherichia vulneris, Gemmiger formicilis and Streptococcus sinensis abundance (Figure 4A).
Figure 4.
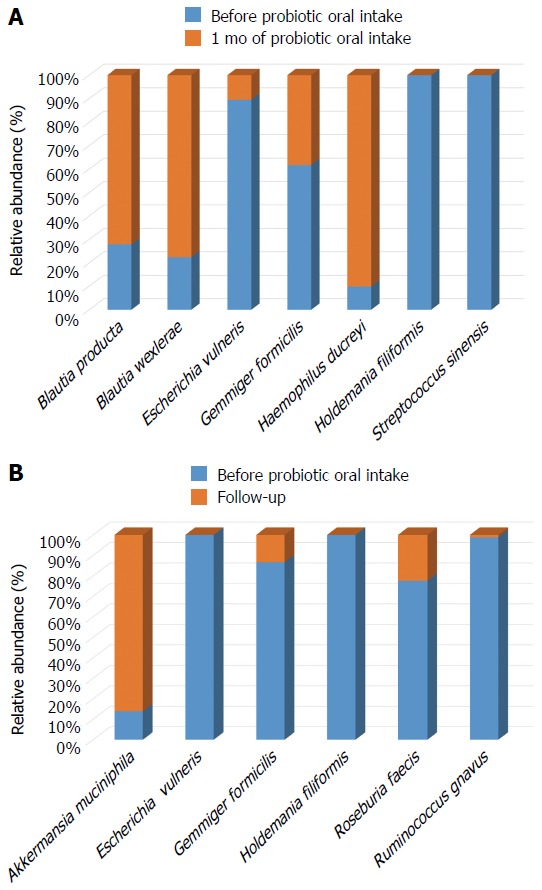
Significant changes in the gut bacterial species composition: (A) bacterial distribution after 1 mo of probiotic oral intake; (B) bacterial distribution one month after the end of probiotic (follow up) (P < 0.05).
Interestingly, the reduction of Holdemania filiformis remained stable up to one month after the end of probiotic intake (P < 0.05) (Figure 4B).
In addition, during the follow-up period a further decrease in Escherichia vulneris and Gemmiger formicilis load, together with a reduction of Roseburia faecis and Ruminococcus gnavus abundance was observed (Figure 4B). Differently, an opposite trend was highlighted for Akkermansia muciniphila which abundance was increased if compared to samples collected at the beginning of the experimental time course (P < 0.05) (Figure 4B).
DISCUSSION
To date, there is little evidence about the ability of probiotics to influence the gut microbiota composition and many factors can negatively influence conclusions drawn from different studies. The administration of different probiotic species or strains, the duration of probiotic administration, the use of monostrain or multistrains products and the presence of numerous variables related to the host’s lifestyle are all elements which can lead to different scientific conclusions[23].
In the present study, the timing of probiotic administration did not significantly influence the colonization ability of B. longum BB536 and L. rhamnosus HN001. Indeed, both probiotic strains were capable to colonize the intestinal environment independently of the pre- or post-prandial oral intake. Furthermore, the two probiotic strains did not influence the gut microbiota diversity and richness of healthy individuals, as no significant changes in the Shannon, Simpson and Chao indices were detected. However, the Chao index was slightly decreased one month after the end of probiotic oral intake, suggesting a certain degree of uniformity between the gut microbiota of different subjects analyzed. These results confirmed data of a previous work which highlighted no significant effects of probiotics on the gut microbiota diversity and richness[23]. The lack of activity of certain probiotic strains on the gut microbiota could be related to their inability to colonize the intestinal environment. The ability to adhere to and colonize the gut, indeed, is a fundamental feature for probiotic microorganisms to be effective on the host and is closely species- and strain-dependent [24]. We assume that B. longum BB536 and L. rhamnosus HN001 had a good colonization ability: an increase in B. longum and L. rhamnosus fecal load was detected in the majority of subjects analyzed after the probiotic supplementation. They also had an impact on the gut microbiota composition at phylum level; indeed, a significant reduction of Firmicutes was detected after one month of probiotic oral intake. This result may be of importance since a high abundance of Firmicutes has previously been related to obesity, and with a reduction of Bacteroidetes[25], as obese individuals often show an unbalanced ratio of Firmicutes and Bacteroidetes in their intestinal microbiota. Researchers hypothesized that the Firmicutes phylum contains numerous bacterial species with an increased ability to harvest energy from diet, leading to a large increase in total body fat[25]. Interestingly, the further reduction of Firmicutes we observed after the end of probiotic administration was concomitant to a significant reduction of Proteobacteria, a bacterial phylum often involved in the onset and progression of gastrointestinal diseases[25]. In particular, different microorganisms belonging to Proteobacteria, such as Campylobacter, enterohepatic Helicobacter and Escherichia coli are often associated with the pathogenesis of inflammatory bowel disease (IBD), being able to negatively influence the immune system and enhance intestinal inflammation[26]. Consequently, these changes can be considered positive marks due to B. longum BB536 and L. rhamnosus HN001, which seem to be acting as beneficial biomodulators of gut microbiota. Changes observed at phylum level were then confirmed by analyzing the distribution of intestinal bacterial species after probiotic oral intake; indeed, the Escherichia vulneris, Gemmiger formicilis and Ruminococcus gnavus load was reduced after probiotic administration. These microorganisms have numerous mechanisms, including secretion of toxins and colonization factors, for inducing the imbalance of intestinal homeostasis and leading to the the onset of gastrointestinal diseases[27-29]. In particular, Ruminococcus gnavus may possess different pathogenic traits and virulence factors, as it was already observed being involved in two cases of bacteremia associated with diverticular disease[29]. Similarly, Gemmiger formicilis, together with Ruminococcus lactaris and Enterococcus durans, has been observed to be significantly increased in individuals with Chron’s disease and subjected to recurrence of inflammatory lesions[30]. Probably, these bacteria can act as pro-inflammatory modulators enhancing the inflammatory state and worsening symptomatology associated to the disease. Interestingly, the anti-inflammatory effect B. longum and L. rhamnosus strains was further highlighted by the greater abundance of Blautia producta and Blautia wexlerae during the follow-up period if compared to samples collected at the beginning of the study and after one month of probiotic administration. Blautia spp, indeed, produce short-chain fatty acids, which act as main fuel for enterocytes, and anti-inflammatory compounds involved in the promotion of muscular activity and epithelial cell proliferation and in the enhancement of blood through the colonic vasculature[31,32]. Moreover, several studies have already demonstrated the ability of both B. longum BB536 and L. rhamnosus HN001 to decrease the severity of allergic responses and positively stimulate a host’s immune system[20,33]. The latter, in particular, could be mediated by both a direct interaction between probiotic strains and cells of the immune system, and modulation of intestinal microorganisms able to influence pro- and anti-inflammatory response. Considering this, the regulation of gut microbiota composition by probiotic bacteria takes on an even more important role in maintaining a host’s health. Intestinal microbiota, indeed, participating in the regulation of the immune system, is closely involved in the onset or manifestation of allergic diseases, such as atopic dermatitis[34]. A dysbiotic microbiota can enhance intestinal and cutaneous pro-inflammatory response by production of metabolites and toxins with a strong inflammatory power[34]. Intestinal Staphylococcus aureus, for instance, which was observed to be more abundant in patients affected by moderate/severe atopic dermatitis, is able to produce a toxin with superantigenic properties that exacerbates atopic symptomatology[35].
More interestingly, the beneficial impact that B. longum and L. rhamnosus strains had on intestinal homeostasis is underlined by the significant increase of Akkermansia muciniphila detectable only after the end of probiotic intake. A. muciniphila is closely related with human health and it is inversely associated with body fat mass and glucose intolerance[36]. Moreover, this bacterium seems to be involved in the maintenance of intestinal barrier functions and above all in prevention of intestinal inflammation, playing a pivotal role in the host’s overall health status[37]. Also the persisting reduction of Streptococcus sinensis one month after the probiotic supplementation can be considered a positive mark for probiotic supplementation, as S. sinensis is a potential pathogenic microorganism which could be directly involved in infective endocarditis[38]. Consequently, its decrease can further underline the beneficial impact that B. longum BB536 and L. rhamnosus HN001 administration may have on gut microbiota of healthy individuals.
In conclusion, our preliminary data highlighted the probiotic activity exerted by a B. longum BB536 and L. rhamnosus HN001 combination which influences the intestinal environment. The two probiotic strains have been demonstrated to influence the gut microbiota composition, even if all bacterial changes detected after probiotic intake have not yet been well-characterized.
However, a reduction of potential harmful bacteria and an increase of beneficial ones may constitute an important probiotic feature of B. longum BB536 and L. rhamnosus HN001. Of course, we need further clinical and pre-clinical studies to demonstrate a clear application of this probiotics combination in the clinical field.
COMMENTS
Background
Probiotics are live microorganisms able to influence positively host’s health. Today, probiotic bacteria are used to improve and even prevent some human illnesses, such as allergic and gastrointestinal diseases, as they are able not only to inhibit pathogens proliferation but also to stimulate and strengthen host’s immune system. In the present study, Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 were evaluated for their ability to colonize intestinal environment and modify gut microbiota composition.
Research frontiers
In probiotic research there is a high demand for studies to show mechanisms by which probiotic bacteria are able to influence host’s health. In particular, there is a great interest in understanding how probiotic can modify intestinal microbiota composition.
Innovations and breakthroughs
The present study was conducted with high quality and highlighted the positive impact of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on intestinal microbiota, as numerous pathogenic microorganisms were subjected to a significant reduction after probiotic oral intake, while the amount of some beneficial bacteria was observed to be increased up to the end of probiotic administration.
Applications
Probiotics, and in particular Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536, have been demonstrated to influence positively host’s gut microbiota. Consequently, their use may represent a valid help in improving human health and reducing disease-associated symptomatology.
Peer-review
This is a well-written paper to assess the ability of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 to colonize the gut of healthy subjects and to modify intestinal microbiota composition. This paper has potentially important implications.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Scientific Direction of IRCCS Galeazzi Orthopaedic Institute in the Current Research 2015.
Informed consent statement: All subjects enrolled in the study were volunteers who were informed in detail about the aim of the study and they gave informed consent to the study. As declared in “Materials and Methods”, indeed, all individuals personally delivered fecal samples to the Laboratory of Clinical Microbiology were samples were analyzed.
Conflict-of-interest statement: The authors declare no conflicts of interests related to the publication of this study.
Data sharing statement: No additional data was available.
Peer-review started: December 23, 2016
First decision: January 10, 2017
Article in press: March 20, 2017
P- Reviewer: Franceschi F, Serban ED S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
References
- 1.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS. Beneficial Properties of Probiotics. Trop Life Sci Res. 2016;27:73–90. doi: 10.21315/tlsr2016.27.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floch MH. Recommendations for probiotic use in humans-a 2014 update. Pharmaceuticals (Basel) 2014;7:999–1007. doi: 10.3390/ph7100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currò D, Ianiro G, Pecere S, Bibbò S, Cammarota G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br J Pharmacol. 2016 doi: 10.1111/bph.13632. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora M, Baldi A. Regulatory categories of probiotics across the globe: a review representing existing and recommended categorization. Indian J Med Microbiol. 2015;33 Suppl:2–10. doi: 10.4103/0255-0857.150868. [DOI] [PubMed] [Google Scholar]
- 6.Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60 Suppl 2:S129–S134. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11:4745–4767. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khailova L, Petrie B, Baird CH, Dominguez Rieg JA, Wischmeyer PE. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PLoS One. 2014;9:e97861. doi: 10.1371/journal.pone.0097861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tham CS, Peh KK, Bhat R, Liong MT. Probiotic properties of bifidobacteria and lactobacilli isolated from local dairy products. Ann Microbiol. 2012;62:1079–1087. [Google Scholar]
- 11.Sarkar A, Mandal S. Bifidobacteria-Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol Res. 2016;192:159–171. doi: 10.1016/j.micres.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Drago L, De Vecchi E, Gabrieli A, De Grandi R, Toscano M. Immunomodulatory Effects of Lactobacillus salivarius LS01 and Bifidobacterium breve BR03, Alone and in Combination, on Peripheral Blood Mononuclear Cells of Allergic Asthmatics. Allergy Asthma Immunol Res. 2015;7:409–413. doi: 10.4168/aair.2015.7.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inturri R, Stivala A, Blandino G. Microbiological characteristics of the probiotic strains B. longum BB536 and L. rhamnosus HN001 used in combination. Minerva Gastroenterol Dietol. 2015;61:191–197. [PubMed] [Google Scholar]
- 14.Cross ML, Mortensen RR, Kudsk J, Gill HS. Dietary intake of Lactobacillus rhamnosus HNOO1 enhances production of both Th1 and Th2 cytokines in antigen-primed mice. Med Microbiol Immunol. 2002;191:49–53. doi: 10.1007/s00430-002-0112-7. [DOI] [PubMed] [Google Scholar]
- 15.Inturri R, Stivala A, Furneri PM, Blandino G. Growth and adhesion to HT-29 cells inhibition of Gram-negatives by Bifidobacterium longum BB536 e Lactobacillus rhamnosus HN001 alone and in combination. Eur Rev Med Pharmacol Sci. 2016;20:4943–4949. [PubMed] [Google Scholar]
- 16.Wickens K, Stanley TV, Mitchell EA, Barthow C, Fitzharris P, Purdie G, Siebers R, Black PN, Crane J. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization? Clin Exp Allergy. 2013;43:1048–1057. doi: 10.1111/cea.12154. [DOI] [PubMed] [Google Scholar]
- 17.Shu Q, Gill HS. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157: H7 infection in mice. FEMS Immunol Med Microbiol. 2002;34:59–64. doi: 10.1111/j.1574-695X.2002.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 18.Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr. 2001;20:149–156. doi: 10.1080/07315724.2001.10719027. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sheraji SH, Amin I, Azlan A, Manap MY, Hassan FA. Effects of Bifidobacterium longum BB536 on lipid profile and histopathological changes in hypercholesterolaemic rats. Benef Microbes. 2015;6:661–668. doi: 10.3920/BM2014.0032. [DOI] [PubMed] [Google Scholar]
- 20.Akatsu H, Iwabuchi N, Xiao JZ, Matsuyama Z, Kurihara R, Okuda K, Yamamoto T, Maruyama M. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. JPEN J Parenter Enteral Nutr. 2013;37:631–640. doi: 10.1177/0148607112467819. [DOI] [PubMed] [Google Scholar]
- 21.Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, Tominaga T, Togashi H, Benno Y, Xiao JZ. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012;18:14–18. doi: 10.1016/j.anaerobe.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Drago L, Toscano M, De Grandi R, Grossi E, Padovani EM, Peroni DG. Microbiota network and mathematic microbe mutualism in colostrum and mature milk collected in two different geographic areas: Italy versus Burundi. ISME J. 2017;11:875–884. doi: 10.1038/ismej.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Møller PL, Michaelsen KF, Paerregaard A, Sandström B, Tvede M, Jakobsen M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol. 1999;65:4949–4956. doi: 10.1128/aem.65.11.4949-4956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- 27.Guentzel MN. Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 26. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8035/ [PubMed]
- 28.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen SG, Skov MN, Justesen US. Two cases of Ruminococcus gnavus bacteremia associated with diverticulitis. J Clin Microbiol. 2013;51:1334–1336. doi: 10.1128/JCM.03382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondot S, Lepage P, Seksik P, Allez M, Tréton X, Bouhnik Y, Colombel JF, Leclerc M, Pochart P, Doré J, et al. Structural robustness of the gut mucosal microbiota is associated with Crohn’s disease remission after surgery. Gut. 2016;65:954–962. doi: 10.1136/gutjnl-2015-309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eren AM, Sogin ML, Morrison HG, Vineis JH, Fisher JC, Newton RJ, McLellan SL. A single genus in the gut microbiome reflects host preference and specificity. ISME J. 2015;9:90–100. doi: 10.1038/ismej.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topping DL. Short-chain fatty acids produced by intestinal bacteria. Asia Pac J Clin Nutr. 1996;5:15–19. [PubMed] [Google Scholar]
- 33.Thomas DJ, Husmann RJ, Villamar M, Winship TR, Buck RH, Zuckermann FA. Lactobacillus rhamnosus HN001 attenuates allergy development in a pig model. PLoS One. 2011;6:e16577. doi: 10.1371/journal.pone.0016577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drago L, Toscano M, Pigatto PD. Probiotics: immunomodulatory properties in allergy and eczema. G Ital Dermatol Venereol. 2013;148:505–514. [PubMed] [Google Scholar]
- 35.Drago L, Toscano M, De Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. J Clin Gastroenterol. 2012;46 Suppl:S56–S63. doi: 10.1097/MCG.0b013e318265ef38. [DOI] [PubMed] [Google Scholar]
- 36.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 37.Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo PC, Tam DM, Leung KW, Lau SK, Teng JL, Wong MK, Yuen KY. Streptococcus sinensis sp. nov., a novel species isolated from a patient with infective endocarditis. J Clin Microbiol. 2002;40:805–810. doi: 10.1128/JCM.40.3.805-810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


