Abstract
Cutaneous adenoid cystic carcinoma (CACC) is an extremely rare neoplasm of head and neck region, and is characterized by propensity for local recurrence and perineural invasion. Late distant metastases occur usually to lungs. Although patients with lung metastases from CACC cannot be cured, long-term survival may be possible due to its slow-growing malignancy. We report a case of a 69-year-old female with lung metastases from CACC 23 years after initial surgery of scalp nodule.
Keywords: Cutaneous adenoid cystic carcinoma, Lung metastases, Thyroid transcription factor 1
1. Introduction
Adenoid cystic carcinoma (ACC) is a rare neoplasm of head and neck region. ACC arises mainly in salivary glands and occasionally in lachrymal glands, ceruminal glands of external ear, nose, paranasal sinuses, palate, and nasopharyngeal spaces [1]. While ACC accounts for approximately 1% of all malignancies in head and neck region, cutaneous ACC (CACC) is rarer and its incidence rate has been estimated at 0.23/1 million person-years [2]. Approximately 41% of CACC arises on scalp, but other locations have been reported, including chest, abdomen, back, eyelid, perineum, and extremities. CACC typically presents as a solitary and slow-growing mass but is characterized by propensity for local recurrence and perineural invasion. Distant metastases occur mostly through haematogenic spread and especially in lungs and bones [3]. Despite of the high rate of distant metastases, long-term survival may be possible [4]. We present a case report of a 69-year-old female with lung metastases from CACC 23 years after initial surgery of scalp nodule.
2. Case presentation
A 69-year-old female was referred to our hospital because of an abnormal shadow on a chest X-ray. The patient underwent extensive local resection of CACC on her scalp at age of 42 years. The extensive local resection was performed with a negative surgical margin, but pathological examination revealed perineural invasion in the specimens. Then the patient stayed asymptomatic.
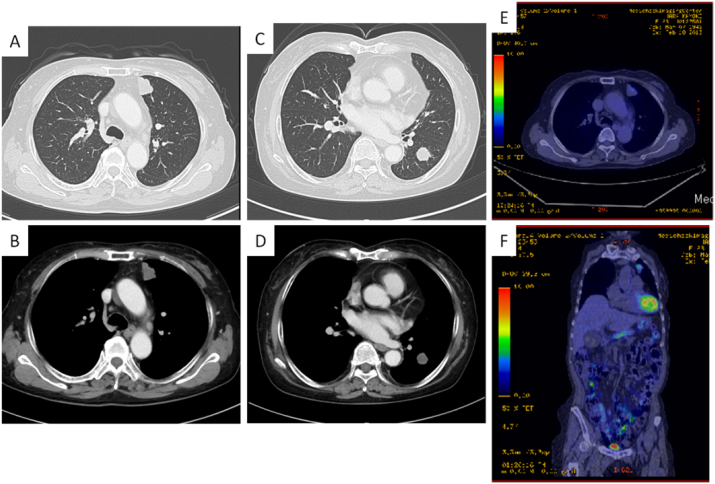
A chest X-ray showed several nodular lesions in bilateral lung fields, and computed tomography revealed multiple nodules at several segments in bilateral lung fields (Fig. 1A–D). A positron emission tomography revealed higher standardized uptake value, ranging 2.6 to 4.2 of maximum value, of pulmonary nodules, suggesting a possibility of malignancy in pulmonary involvement (Fig. 1D and E). Since bronchoscopic study could not confirm pathological findings of the pulmonary nodules, the patient underwent left video-associated thoracic surgery (VATS) and resection of nodule at segment 3 in the left upper lobe.
Fig. 1.
A-D, Chest computed tomography shows well-demarcated nodules at several segments in bilateral lung fields. E and F, A positron emission tomography revealed a higher standardized uptake value of pulmonary nodule at segment 3 in the left upper lobe.
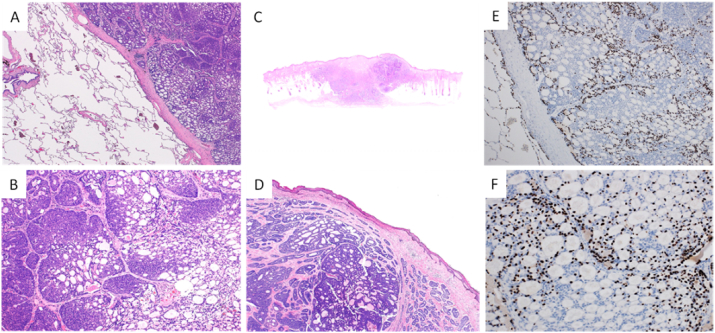
Pathological examination of the specimens from VATS revealed islands of basaloid cells in cribriform patterns in a loose fibromucinous stroma (Fig. 2A and B). The pathological findings of specimens from VATS matched with those of the patient's scalp nodule (Fig. 2D). The patient had no evidence of CACC in other regions except mentioned above. On immunohistochemical staining of thyroid transcription factor 1 (TTF-1), all specimens demonstrated positive immunoreactivity in the present case (Fig. 2E and F). As metastatic but not primary ACC demonstrated positive TTF-1 immunoreactivity in lungs [5], the pulmonary nodules were likely to be metastatic lesions in the present case. Collectively, these pathological findings suggested that lung metastases arose from CACC 23 years after the initial surgery of scalp nodule.
Fig. 2.
A and B, Specimen form video-associated thoracic surgery (VATS) shows islands of basaloid cells in cribriform patterns in a loose fibromucinous stroma. (Hematoxylin and eosin (HE) stain, x20 for A, and x40 for B). C, Well-defined basophilic tumor nests infiltrating into the dermis and subcutaneous tissue in specimen from resection of the patient's scalp nodule. (HE stain, x10). D, Pathological findings of patient's scalp nodule match with those of specimens from VATS. (HE stain, x40). E and F, Specimen form VATS shows focal nuclear expression of thyroid transcription factor 1 (TTF-1), (x40 for E and x100 for F).
Since the patient stayed asymptomatic and refused chemotherapy, she was followed up without any therapeutic intervention and still stayed asymptomatic. No overt progressive disease was seen in follow-up CT 1 year later.
3. Discussion
CACC is an extremely rare neoplasm and arises mainly on scalp at a mean age of 59 years with the male to female ratio of 1:1.1 [2], [3], [6]. CACC is characterized by propensity for local recurrence and perineural invasion. While the rate of local recurrence and perineural invasion has been reported to be as high as 30%, perineural invasion was seen in 59–76% of CACC in several studies [3], [5]. Distant metastases occur as high as 35% usually to lungs through haematogenic spread, and are more common compared with regional lymph node spread. Because remnant perineural invasion may contribute to distant metastases through a slow dissemination, metastatic lesions can manifest as a disease 10–15 years later initial treatment [6]. Despite of the high rate of distant metastases, long-term survival may be possible because of the slow progression of metastases [1], [4].
Pathological findings of CACC may be classified into 3 different histological variants, including cribriform, tubular, and solid. CACC with a solid growth pattern exhibits worse prognosis than those with a ciribirform or tubular growth pattern [3]. Clinicopathologic and immunohistochemical study of 27 cases reported by Ramakrishnan R et al. demonstrated 5-year survival rate of 39%, 26%, and 5%, respectively, for tubular, cribriform, and solid growth pattern [6].
In the present case, the patient caused lung metastases from CACC 23 years after initial treatment. Although the extensive local resection of CACC on her scalp was performed with the negative surgical margin, lung metastases could arise lately through haematologenic spread from the remnant perineural invasion. The pathological findings of specimens from both scalp nodule and VATS demonstrated the cribriform growth pattern of CACC, and may also explain the patient's long-term survival.
Chemotherapy in CACC usually confined to advanced disease with nonresectable, recurrent, or metastatic condition. In addition, chemotherapy is conditionally started when patients with CACC exhibit rapidly progressive disease or are symptomatic. While chemotherapy is studied as single-agent therapy or as combination therapy, no phase III studies have been published because of rarity of CACC [1]. Regression of lung metastases from CACC with combination therapy, such as cisplatin + 5-fluorouracil or cisplatin + doxorubicin, has been demonstrated in several case reports [4]. However, because only small studies were performed, it is difficult to interpret efficacy of chemotherapy. On the other hand, determination of progression-free survival of CACC cannot be easily drawn because of the slow progression of metastases. Moreover, better prognosis of CACC patients with solely lung metastases than those with metastases to bone or other viscera has been reported [1].
The present case should be diagnosed differentially between primary and metastatic ACC in lungs because of the long period of 23 years. Primary ACC in trachea and lungs is uncommon, accounting for less than 0.2% of lung carcinomas, and classified into 4 types, semipedunculated type (type I), intra-extra luminal type (type II), expansive infiltrating type (type III), and peripheral type (type IV) [7]. The relatively low incidence of primary ACC type IV in lungs may be associated closely with distribution of glandular cells, which decline from proximal to distal airways [8]. The present case appeared to be type IV if the lesions were primary ACC in lungs. Immunohistochemical staining of TTF-1 is helpful for differential diagnosis between primary and metastatic ACC in lungs. An J et al. evaluated the expression of TTF-1 between primary and metastatic ACC in lungs, and demonstrated that TTF-1 was expressed in metastatic ACC but not in primary ACC [5]. Furthermore, clinicopathologic and immunohistochemical study of 21 cases reported by Huo Z et al. demonstrated the negative immunoreactivity of TTF-1 in primary ACC from lungs [9]. As mentioned above, the patient may develop lung metastases from CACC 23 years after the surgery of scalp nodule.
4. Conclusion
We present here a case report of lung metastases from CACC 23 years after the initial treatment. CACC is an extremely rare neoplasm of head and neck region, and develops late distant metastases to lungs. Although patients with lung metastases from CACC cannot be cured, long-term follow-up may be necessary for the possible longevity of clinical course.
Conflict of interest
The authors have no conflict of interest to declare.
Contributor Information
Naoko Arano, Email: narano@juntendo.ac.jp.
Yoshiteru Morio, Email: ymorio@juntendo.ac.jp.
Toshiro Futagawa, Email: futagawa@juntendo-urayasu.jp.
Akane Hashizume, Email: akane@juntendo-urayasu.jp.
Osamu Nagashima, Email: nagashim@juntendo.ac.jp.
Shin-Ichi Sasaki, Email: ssasaki@juntendo-urayasu.jp.
Shigeru Tominaga, Email: s-tomi@juntendo-urayasu.jp.
Kazuhisa Takahashi, Email: kztakaha@juntendo.ac.jp.
References
- 1.Papaspyrou G., Hoch S., Rinaldo A. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck. 2011;33(6):905–911. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- 2.Dores G.M., Huycke M.M., Devesa S.S. Primary cutaneous adenoid cystic carcinoma in the United States: incidence, survival, and associated cancers, 1976 to 2005. J. Am. Acad. Dermatol. 2010;63(1):71–78. doi: 10.1016/j.jaad.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naylor E., Sarkar P., Perlis C.S. Primary cutaneous adenoid cystic carcinoma. J Am Acad Dermatol. 2008;58(4):636–641. doi: 10.1016/j.jaad.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Singh A., Ramesh V. Primary cutaneous adenoid cystic carcinoma with distant metastasis: a case report and brief literature review. Indian J Dermatol. Venereol Leprol. 2010;76(2):176–179. doi: 10.4103/0378-6323.60573. [DOI] [PubMed] [Google Scholar]
- 5.An J., Park S., Sung S.H., Cho M.S., Kim S.C. Unusual expression of thyroid transcription factor 1 and napsin A in metastatic adenoid cystic carcinoma of extrapulmonary origin in the lung. Am J Clin Pathol. 2014;141(5):712–717. doi: 10.1309/AJCPB0YIY6LQLQTG. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan R., Chaudhry I.H., Ramdial P. Primary cutaneous adenoid cystic carcinoma: a clinicopathologic and immunohistochemical study of 27 cases. Am. J. Surg. Pathol. 2013;37(10):1603–1611. doi: 10.1097/PAS.0b013e318299fcac. [DOI] [PubMed] [Google Scholar]
- 7.Sato K., Takeyama Y., Kato T., Hashimoto H., Fukui Y., Gonda H., Suzuki R. Tracheal adenoid cystic carcinoma treated by repeated bronchoscopic argon plasma coagulation as a palliative therapy. Ann. Thorac. Cardiovasc Surg. 2014;20(Suppl):602–605. doi: 10.5761/atcs.cr.12.02156. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu J., Oda M., Matsumoto I., Arano Y., Ishikawa N., Minato H. Clinicopathological study of surgically treated cases of tracheobronchial adenoid cystic carcinoma. Gen. Thorac. Cardiovasc Surg. 2010;58(2):82–86. doi: 10.1007/s11748-009-0467-4. [DOI] [PubMed] [Google Scholar]
- 9.Huo Z., Meng Y., Wu H., Shen J., Bi Y., Luo Y., Cao J., Liang Z. Adenoid cystic carcinoma of the tracheobronchial tree: clinicopathologic and immunohistochemical studies of 21 cases. Int. J. Clin. Exp. Pathol. 2014;7(11):7527–7535. [PMC free article] [PubMed] [Google Scholar]