Abstract
Background
The aim of this study was to compare the therapeutic efficacy of two different activity levels of the 213Bi-labeled monoclonal antibody MX35 in an ovarian cancer model. Sixty female BALB/c (nu/nu) mice were inoculated intraperitoneally with human ovarian cancer cells (OVCAR-3). Two weeks later, 40 mice were injected intraperitoneal (i.p.) with 1 ml of 213Bi-MX35, 3 MBq/mL (n = 20), or 9 MBq/mL (n = 20). An additional 20 mice received unlabeled MX35. Incidence of tumors and ascites was investigated 8 weeks after therapy. Body weight and white blood cell counts were monitored after treatment for possible signs of toxicity.
Results
The tumor-free fraction of the animals treated with 3 MBq/mL of 213Bi-MX35 was 0.55, whereas that of animals treated with 9 MBq/mL of 213Bi-MX35 was 0.78. The control group treated with unlabeled MX35 had a tumor-free fraction of 0.15. No significant reduction in white blood cell counts or weight loss was observed.
Conclusions
Tumor growth after i.p. treatment with 213Bi-MX35 was significantly reduced compared to treatment with unlabeled MX35. Treatment with 9 MBq/mL of 213Bi-MX35 resulted in higher tumor-free fraction compared with 3 MBq/mL of 213Bi-MX35, but this difference was not statistically significant. No signs of toxicity were observed in the treated animals.
Keywords: Radioimmunotherapy, Dosimetry, High LET radiation, Monoclonal antibodies (mAb), Radiopharmaceuticals
Background
The current therapy for ovarian cancer includes surgery and systemic chemotherapy, and although this approach is usually initially successful, a majority of the patients suffer from recurrence. Tumors occur mainly within the peritoneal cavity, which at late stages leads to ascites production, bowel obstruction, and eventual death. Only 45% have a 5-year relative chance of surviving ovarian cancer [1]. Consequently, additional therapy methods are urgently needed to increase the survival rate.
In an effort to meet this need, our research group has focused on intraperitoneal adjuvant alpha-particle radioimmunotherapy (α-RIT) with 211At and 213Bi [2–9]. Successful preclinical studies with 211At have already led to a clinical phase I study [10, 11], and additional clinical trials are planned. While most of our efforts have been spent on development and evaluation of 211At radiopharmaceuticals, model calculations [12] show that 213Bi might be preferential for intraperitoneal (i.p.) radioimmunotherapy (RIT) of disseminated ovarian cancer. The short half-life in combination with relatively long retention time in the peritoneal cavity of the patient allows administration of higher activities, and the resulting irradiation from unbound 213Bi-antibodies in the surrounding i.p. fluid could substantially contribute to a high absorbed dose to microtumors.
We have previously compared, head-to-head, the therapeutic efficacy and biodistribution of 213Bi- and 211At-labeled monoclonal antibodies (mAbs) in an ovarian xenograft cancer mouse model [13]. In that study, the administered activity concentrations of 213Bi and 211At were chosen to translate to approximately equal absorbed doses to the peritoneal lining in a corresponding patient situation, i.e., 7–8 Gy. The therapeutic results between the 213Bi-RIT and 211At-RIT could not be significantly distinguished. We have, however, also previously shown that the peritoneal lining in mice may tolerate absorbed doses as high as 30–50 Gy [13], implying the possibility of administering higher activity concentration levels in humans.
The current study was designed to evaluate if higher administered activity concentrations of 213Bi-mAb could improve the therapeutic efficacy of 213Bi-RIT in an ovarian cancer mouse model. A second aim was to evaluate if such improvement could be explained by the calculated absorbed dose to microtumors when including both cell-bound and free 213Bi-mAb irradiation.
The therapeutic efficacy was analyzed by rigorous examination of tumor incidence in the mice 8 weeks after treatment, to evaluate the tumor-free fraction for the different activity concentration levels. The highest activity concentration level administered in this study was calculated to be a safe absorbed dose to the bone marrow in mice.
Methods
Radionuclide production
The 213Bi was produced by a 225Ac/213Bi generator as described previously [14, 15] and was eluted from the generator according to the standard protocol provided by Institute for Transuranium Elements (ITU) in Karlsruhe, Germany. In short, 600 μL of a 0.1 M HCl/0.1 M NaI solution was pumped through the generator column to elute the 213Bi. Subsequently, the 213Bi solution was pH-adjusted by addition of 4 M sodium acetate and a 20% L-ascorbic acid solution (the ascorbic acid additionally protects the antibody conjugate from radiolysis) [16].
Antibody
MX35, a murine IgG1 mAb, was used in the experiments. The MX35 mAb was developed at the Sloan-Kettering Cancer Center (New York, NY, USA) [17, 18] and was produced from hybridoma cells obtained from the Ludwig Institute for Cancer Research, Zürich, Switzerland. It recognizes the transport protein NaPi2b, which is homogeneously expressed in approximately 90% of human epithelial ovarian cancer cells and to only a small degree in normal tissues [19], making it suitable for epithelial ovarian cancer therapy.
Cell line
The ovarian carcinoma cell line OVCAR-3 (American Type Culture Collection, Rocksville, MD, USA), was used in all in vitro and in vivo experiments. The cells were cultured in T-75 culture flasks in RPMI 1640 medium supplemented with 10% fetal calf serum, 1% L-glutamine, and 1% penicillin-streptomycin and were grown in a humidified atmosphere of 95% air/5% CO2 at 37 °C.
Animals
Female immunodeficient BALB/c (nu/nu) mice (Charles River Laboratories International, Freiburg, Germany), 5–6 weeks of age, were used in the in vivo experiments. The housing of the animals was as previously described [13]. The animal study was performed according to the guidelines from the ethical committee and the legislations for animal research in Sweden and with approval from the Committee on the Ethics of Animal Experiments of the University of Gothenburg, Sweden.
Antibody conjugation
The antibody-chelator conjugation was performed essentially as described previously [13], at room temperature (RT), overnight, in 0.2 M carbonate buffer, pH ~8.5. The chelator [(R)-2-amino-3-(4-isothiocyanatophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-pentaacetic acid (CHX-A′′-DTPA) in dimethyl sulfoxide (0.018 M) was added to 2–4 mg/mL of mAb in 15 × molar excess. Subsequently, the buffer was changed to 0.1 M citrate, pH 5.5, using a NAP-5 column (GE Healthcare, Buckinghamshire, UK). For long-term storage, the buffer of the antibody conjugate was changed to phosphate buffered saline (PBS). The equipment was essentially kept metal-free, and the buffers included were made metal-free by incubation with Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA, USA) as described previously [13]. The number of available chelators attached after labeling was estimated to be two per antibody according to evaluation by an arsenazo III spectrophotometric assay [20].
Radiolabeling of the antibody conjugate
The 213Bi-labeling of the MX35 conjugate was performed and evaluated as previously described, [13] but with some modifications. A total of 0.1 mg of the antibody conjugate was added to the pH-adjusted 213Bi eluate, and the reaction was continued for 5 min. After quenching with 10 μl of 1.5 mg/mL 2-[Bis[2-bis(carboxymethyl)amino] ethyl]amino]acetic acid (DTPA), the labeled product was purified using a NAP-10 column (GE Healthcare) and eluted with PBS.
Immunoreactivity of the 213Bi-labeled antibody conjugates
Prior to the in vivo experiments, the immunoreactivity of the radiolabeled MX35 was evaluated in vitro using suspensions of live OVCAR-3 cells as previously described [13]. Briefly, 10 ng of the labeled antibodies were added to duplicates of 0.5 mL of serially diluted cell suspensions (maximum 5 × 106 cells/mL). The samples were incubated for 3 h at RT with vigorous agitation, centrifuged for 5 min, and washed twice with 1 mL of PBS. Following the last wash and centrifugation, the cell pellets were measured in a γ counter (Wallac, Finland) and compared with reference solutions of radiolabeled MX35. The fraction of bound activity over the total applied activity was calculated, as well as the immunoreactive fraction according to the Lindmo assay [21].
Therapeutic efficacy and toxicity study
Therapeutic efficacy was investigated for two different activity levels of 213Bi-labeled MX35. Sixty mice were inoculated i.p. with 1 × 107 OVCAR-3 cells. Two weeks after cell inoculation, 40 mice were injected i.p. with 213Bi-MX35: 20 were given 9 MBq/mL, equal to 30 μg of 213Bi-MX35 in 1 mL of PBS, and 20 were given 3 MBq/mL, equal to 10 μg of 213Bi-MX35 in 1 mL of PBS. A control group of mice received unlabeled MX35, 30 μg, in 1 mL PBS.
According to the ethical license, the mice were weighed prior to treatment and then every 7 to 10 days during treatment to monitor for any ascites discomfort. To monitor for acute myelotoxicity, white blood cell (WBC) counts and platelet counts in the blood from the tail vein of 5 mice from each group were analyzed at 6 and 14 days after treatment. The samples were analyzed in a microcell counter (Sysmex F-820; Toa Medical Electronics Co., Ltd., Kobe, Japan). The animals were sacrificed 8 weeks after therapy, and the abdominal cavity was opened to investigate any presence of ascites, microscopic, and/or macroscopic tumors. Microscopic tumor was defined as tumor sizes ranging from single cells to clusters of million cells not detected by the naked eye at autopsy, whereas macroscopic tumors are visible and detectable at autopsy, often more than 1 mm with or without ascites. To investigate occurrence of microscopic tumors, tissues from the abdominal wall, mesentery, and spleen were taken from all animals for paraffin sectioning and hematoxylin and eosin (H&E) staining. Additional samples were taken from suspected lesions at other locations. Between 10 and 30 sections at 50 μm distance were processed for microscopy. To support the H&E-stained slides in microscopic tumor identification, immunohistochemistry (IHC) for the MX35 antigen was also performed in representative cases. Both the animal dissection and the histological analysis were blinded, i.e., performed without any knowledge of the treatment received.
Statistical analyses
Analyses were performed to evaluate possible statistical significance between the two treated groups and the control group. Calculations were performed for the two-sided alternative hypothesis using Fisher’s exact test.
Dosimetric calculations
In the mouse model, the dose-limiting organ could possibly be the bone marrow. Previously presented biodistribution data for i.p. injected 213Bi-MX35 were used for bone marrow dosimetry [12, 13]. The time-integrated activity per unit mass, Ã/m (Bq s kg−1) was calculated from the area under the activity concentration curve for blood. The absorbed dose D was calculated as:
| 1 |
where Δ is the mean energy of the α particles per nuclear transformation for 213Bi (1.33 × 10−12 J), and Φ is the absorbed fraction of the α particles (assumed to be 1). A previously obtained bone marrow-to-blood ratio of 0.58 (for 211At-labeled MX35) [22] was then applied to estimate the resulting absorbed dose to the bone marrow of i.p. injected 213Bi-MX35.
The absorbed dose to the peritoneum was calculated as 50% of the equilibrium dose of the injected fluid. The antibody concentration in the i.p. fluid was assumed to be equal to the antibody concentration in the injected solution during the time of the 213Bi decay.
Biokinetic modeling and microtumor dosimetry was performed as previously described by Palm et al. [12]. The modeling was adjusted for mouse kinetics, for the specific activity achieved in the study and the activity concentrations used, i.e., 3 and 9 MBq/mL. The number of antigen per cell was set to 700 000. Microdosimetry was performed for single cells and clusters with radii 9, 30, and 50 μm.
Results
Antibody labeling and immunoreactivity
The radiochemical yield after the 213Bi-labeling was 53 ± 12% (mean value ± standard deviation; without correction for decay), and the radiochemical purity was 91 ± 6.0%. The cell-binding assay showed that the fraction of bound activity after 3 h was 0.83 for the highest concentration of cells in the cell suspensions. The immunoreactive fraction of the radiolabeled mAbs in vitro, i.e., the calculated fraction of conjugated antibodies able to bind to the cells in a situation of infinite antigen access, was plotted and calculated to be 0.91. The specific activity at the time of injection was on average 45.6 GBq/μmol, which corresponds approximately a radionuclide:antibody ratio of 1:3000.
Therapeutic efficacy
The tumor-free fractions (TFFs) of the groups treated with 3 or 9 MBq/mL of 213Bi-labeled MX35 were 0.55 and 0.78, respectively, (Table 1). No animals in the groups receiving 213Bi-MX35 developed ascites. However, four animals in the control group receiving cold MX35 had obvious ascites production. The control group also had the largest fraction of animals with macroscopic tumor incidence. The overall TFF of the control group was 0.15 (Table 1). The samples taken for microscopy evaluation from the peritoneal cavity showed a wide range of tumor progression, from complete absence of visual tumor cells to large tumors. Examples of visualizations of macroscopic and microscopic tumors by H&E staining and IHC are shown in Fig. 1.
Table 1.
Results after treatment with 213Bi-MX35 and unlabeled MX35
| Group | Treatment | Activity concentration | Macroscopic tumor (no. of animals) | Microscopic tumor (no. of animals) | Ascites (no. of animals) | TFF |
|---|---|---|---|---|---|---|
| 1 | 213Bi-MX35 | 3 MBq/mL | 4/20 | 9/20 | 0 | 0.55 |
| 2 | 213Bi-MX35 | 9 MBq/mL | 1/18a | 4/18a | 0 | 0.78 |
| 3 | Ref. group (cold MX35) | – | 7/20 | 17/20 | 4 | 0.15 |
TFF tumor-free fraction, i.e., fraction of the mouse groups with no macroscopic or microscopic tumors and no ascites
aTwo animals were excluded from the mouse group receiving 9 MBq/mL; one was sacrificed earlier because of atypical mouse behavior (no tumor tissue could be detected), and one was excluded because of a sole subcutaneous tumor outside of the peritoneum which was suspected to be the result of cell leakage from the peritoneal cavity post inoculation
Fig. 1.
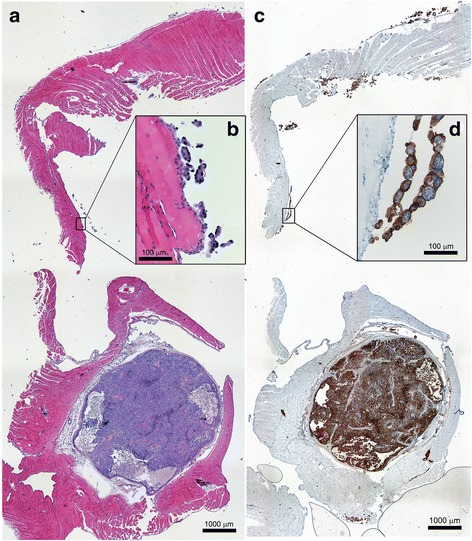
Tissue sections of the abdominal wall from a reference animal treated with unlabeled MX35 visualizing a macroscopic tumor (bottom of a and c) and microscopic tumors (b and d). a and b are stained with H&E while c and d show the dense distribution (in brown) of the MX35 antigen on tumor cells using IHC
Statistical analyses
The statistical analysis performed using Fisher’s exact test showed that the difference in TFF between the mouse group receiving 3 MBq/mL of 213Bi-MX35 and the control group and between the group receiving 9 MBq/mL of 213Bi-MX35, and the control group was statistically significant (p = 0.02) and (p = 0.0002) respectively. The difference between the group receiving 3 MBq of 213Bi-MX35 and the group receiving 9 MBq/mL of 213Bi-MX35 was, however, not statistically significant (p = 0.18).
Toxicity
In the group treated with 3 MBq/mL of 213Bi-MX35, the WBC counts were on average 4.3 × 109/L 6 days after treatment, as shown in Fig. 2. In the 9 MBq/mL group, the WBC counts were slightly lower with an average of 3.9 × 109/L. However, the WBC counts of the control animals were 3.4 × 109/L, i.e., lower than in both of the treated groups. Fourteen days after treatment, the WBC counts had increased by 22% on average in all animals. The increase in WBC counts was lowest in the control group (8%). Thus, no bone marrow toxicity could be observed from the WBC levels of the animals in any of the treated groups.
Fig. 2.
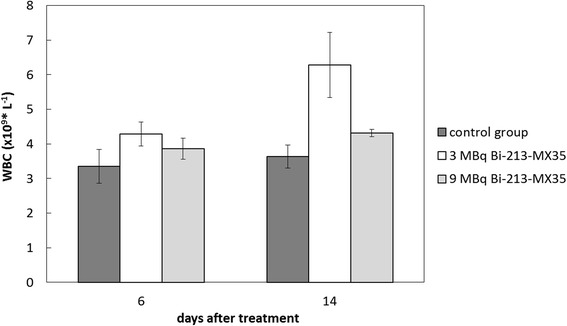
White blood cell (WBC) counts measured in the blood samples taken from the tail vein 6 and 14 days after treatment. Each group consisted of five animals. Means and standard error of the mean (SEM; error bars) are depicted
Neither could we demonstrate significant hematological toxicity from the platelet counts after any of the treatments, as shown in Fig. 3. The group receiving 9 MBq/mL of 213Bi-MX35 had the lowest platelet counts 6 days after treatment (mean value = 777 × 109/L). However, the variation between the animals was large, and the mean value for the group receiving 9 MBq/mL was similar to the value of the control group 14 days after treatment.
Fig. 3.
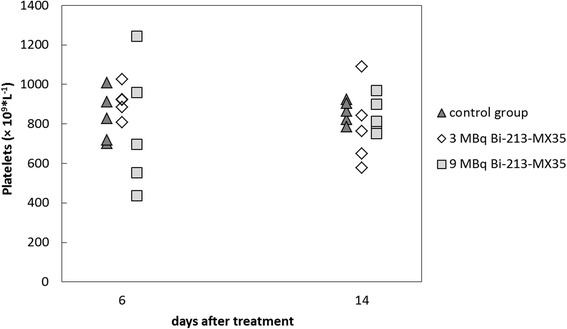
Platelet counts measured in the blood samples taken from the tail vein 6 and 14 days after treatment. Each group consisted of five animals
Dosimetric calculations
When using previous biodistribution data [13] to calculate the absorbed dose in the mice receiving 3 MBq/mL of 213Bi-MX35, the absorbed dose to blood was approximately 1.3 Gy, resulting in an estimated absorbed dose to the bone marrow of 0.8 Gy. Accordingly, the mice administered 9 MBq/mL of 213Bi-MX35 received an absorbed dose to blood of approximately 3.9 Gy, yielding an estimated dose to the bone marrow of 2.3 Gy. The absorbed dose to the kidneys was estimated to be 0.45 and 1.35 Gy for the 3 and 9 MBq/mL level, respectively. The injected activity concentrations of 213Bi-MX35 at 3 and 9 MBq/mL would result in absorbed doses to a human peritoneum of 7.9 and 23.6 Gy, respectively, according to the dosimetric calculations. The calculated dose to microtumors from both non-specific and specific, i.e., cell-bound, irradiation are shown in Table 2.
Table 2.
Absorbed dose to tumors
| Radius (μm) | Specific absorbed dose (Gy) | Unspecific absorbed dose (Gy) | Total absorbed dose (Gy) | |
|---|---|---|---|---|
| 3 MBq/mL | 9 | 3.1 | 7.8 | 10.9 |
| 30 | 3.5 | 8.2 | 11.7 | |
| 50 | 4.1 | 7.2 | 11.3 | |
| 9 MBq/mL | 9 | 3.3 | 23.4 | 26.7 |
| 30 | 3.8 | 24.6 | 28.4 | |
| 50 | 4.3 | 21.6 | 25.9 |
Discussion
The current study was performed to evaluate the therapeutic efficacy of RIT with 213Bi in an ovarian cancer model at two activity concentration levels, by comparing a previously used administered activity concentration with an assumed maximum level tolerated by the mice. The mice were treated following 2–3 weeks post cell injection and were evaluated for tumor occurrence 8 weeks after treatment. The end point, tumor-free fraction at 8 weeks post injection was chosen to avoid debilitating symptoms such as severe ascites before 8 weeks and to be able to compare with previously published results using this endpoint. While the peritoneum might be the critical normal organ in humans, the bone marrow sets the limit for administered activity concentration in mice. This difference is due to the higher transport rate from the peritoneal fluid to the systemic circulation in mice. In humans, this rate is slow compared mice and to the half-life of 213Bi. In our previous studies, we observed that the mice could tolerate 2 Gy to the bone marrow from α radiation, but that 3 Gy could lead to lethal myelotoxicity. For the highest administered activity group in the current study, with an estimated absorbed dose to the bone marrow of 2.3 Gy, the WBC counts did not appear affected to any significant extent. Another potential organ at risk with 213Bi is the kidneys. In the current study, the absorbed dose to the kidneys was 0.45 and 1.35 Gy, which according to previous studies is well below the tolerance dose [23, 24].
The current study showed that both 3 and 9 MBq/mL of 213Bi-MX35 had a significant effect on microscopic tumors in the mouse model. The TFF found for 3 MBq/mL in the current study (0.55) was in good agreement with results from a previous study (0.60) aimed at comparing 213Bi with 211At [13]. The current study also showed that the TFF was further improved (0.78) by increasing the administered activity concentration to 9 MBq/mL, but this difference was not statistically significant.
Calculated dosimetry indicates that the microtumor irradiation from the 213Bi-mAbs with the specific activity obtained in this study will have its main origin from surrounding, non-targeted 213Bi-mAbs in the i.p. fluid. This non-specific irradiation does not depend on tumor cell antigen expression or on the specific activity of the labeled compound. In the current study, a specific activity high enough to result in eradicative doses from cell-bound 213Bi-mAbs was not achieved. The specific irradiation is only slightly higher for the 9 MBq/ml activity concentration as antigen saturation occurs within a few minutes after injection for both activity concentrations Thus, the therapeutic effect is explained mainly by the non-specific α irradiation that linearly increases with activity concentration. However, the actual size of the tumors at the time of the treatment in this tumor model is not known. An indication of the size was obtained from histological samples after treatment, and it describes a large individual variation with sizes between barely visible to large tumor clusters. The therapeutic effect may therefore in this study to some extent also be related to the variation of tumor burden among the mice at the time of treatment [2]. To increase the absorbed dose to tumor clusters larger than the range of the alpha particles, a fractionated treatment schedule could possibly improve the therapeutic outcome.
The half-life of 213Bi results in a low bone marrow dose in humans, allowing administration of high activities. The resulting non-specific irradiation is significant and can be of clinical advantage in e.g., humans where the targeted antigen expression might be low or lacking in a subpopulation of cells. Likewise, in the case of low-specific activity, i.e., a low nuclide to antibody ratio, of the 213Bi-mAb product, a high administered activity concentration could still lead to non-specific irradiation that could eradicate microtumors. However, achieving a high-specific activity product would increase the tumor dose, increasing the probability of cure.
We have previously shown that absorbed doses up to 30–50 Gy [25] results in a very limited effect on the peritoneum in mice. However, translation of the data retrieved from the current and previous animal studies to a human situation is difficult to make and can at this stage only be given as an approximation. Although the peritoneal sensitivity to α radiation needs to be further studied before a tolerance dose can be estimated, it is likely that the highest administered activity concentration in the current study, which translates to 9 GBq in 1 L and 24 Gy to a human peritoneum, would be within a safe interval in a clinical situation. This is due to the distance between the peritoneal lining and the radiosensitive intestinal crypts being greater than the range of the emitted α particles, thereby eliminating the risk of intestinal toxicity. Following administration of 9 GBq/L, the corresponding estimated absorbed dose to the bone marrow in a patient will be very low, about 0.02 Gy [11]. The absorbed dose to other risk organs in the patient, e.g., the kidney will also be very low. This is due do the half-life of 213Bi and the slow clearance from the peritoneal cavity, i.e., there will be a very limited systemic distribution/irradiation following i.p. administration of 213Bi labeled antibodies.
The current study shows some promise for i.p. [213Bi]-RIT treatment of microscopic ovarian cancer. However, obstacles remain including, e.g., availability of the radionuclide in order to achieve activity levels suitable for scale up to clinical treatment and obtaining the required specific activity of the 213Bi-mAb.
Conclusions
Tumor growth after i.p. treatment with 213Bi-MX35 was reduced compared to treatment with unlabeled MX35. Compared with 3 MBq/mL of 213Bi-MX35, treatment with 9 MBq/mL of 213Bi-MX35 resulted in higher non-specific irradiation and a tendency of higher TFF. No signs of toxicity were observed in the treated animals. Although the unspecific irradiation contribute to a high absorbed dose to tumor cells in i.p. therapy, higher specific activity of the 213Bi-mAb is desirable to increase the specific irradiation of the tumor cells and ultimately improve the therapeutic outcome.
Acknowledgements
We thank Helena Kahu for an excellent work with cell culturing and animal experiments.
Funding
This work was financed by grants from the Swedish Cancer Society, Swedish Research Council, Assar Gabrielsson Foundation, Sahlgrenska University Hospitals Clinical Research Foundations (ALF), and King Gustaf V Jubilee Clinic Research Foundation in Gothenburg, Sweden. The funding bodies have taken no part in the preparations of this manuscript or the preceding experiments.
Authors’ contributions
All authors conceived and designed the study, and all authors critically reviewed the manuscript. The animal study was performed by AG, EA, TB, SL, SP, and RH. The main authors responsible for writing the manuscript were AG, SL, SP, LJ, and PA. Important material (the Bi-213 generators were) was contributed by FB and AM. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- H&E
Hematoxylin and eosin
- i.p.
Intraperitoneal
- IHC
Immunohistochemistry
- mAbs
Monoclonal antibodies
- PBS
Phosphate buffered saline
- RIT
Radioimmunotherapy
- RT
Room temperature
- TFF
Tumor-free fraction
- WBC
White blood cell
- α-RIT
Alpha-particle radioimmunotherapy
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Elgqvist J, Andersson H, Back T, Claesson I, Hultborn R, Jensen H, et al. Alpha-radioimmunotherapy of intraperitoneally growing OVCAR-3 tumors of variable dimensions: outcome related to measured tumor size and mean absorbed dose. Journal of Nuclear Medicine. 2006;47:1342–50. [PubMed] [Google Scholar]
- 3.Elgqvist J, Andersson H, Back T, Claesson I, Hultborn R, Jensen H, et al. Fractionated radio immunotherapy of intraperitoneally growing ovarian cancer in nude mice with At-211-MX35 F(ab ’)(2): therapeutic efficacy and myelotoxicity. Nucl Med Biol. 2006;33:1065–72. doi: 10.1016/j.nucmedbio.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Elgqvist J, Andersson H, Back T, Hultborn R, Jensen H, Karlsson B, et al. Therapeutic efficacy and tumor dose estimations in radioimmunotherapy of intraperitoneally growing OVCAR-3 cells in nude mice with (211)At-labeled monoclonal antibody MX35. J Nucl Med. 2005;46:1907–15. [PubMed] [Google Scholar]
- 5.Elgqvist J, Andersson H, Bernhardt P, Back T, Claesson I, Hultborn R, et al. Administered activity and metastatic cure probability during radioimmunotherapy of ovarian cancer in nude mice with 211At-MX35 F(ab’)2. Int J Radiat Oncol Biol Phys. 2006;66:1228–37. doi: 10.1016/j.ijrobp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Elgqvist J, Andersson H, Jensen H, Kahu H, Lindegren S, Warnhammar E, et al. Repeated intraperitoneal alpha-radioimmunotherapy of ovarian cancer in mice. J Oncol. 2010;2010:394913. doi: 10.1155/2010/394913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palm S, Back T, Claesson I, Danielsson A, Elgqvist J, Frost S, et al. Therapeutic efficacy of astatine-211-labeled trastuzumab on radioresistant SKOV-3 tumors in nude mice. Int J Radiat Oncol Biol Phys. 2007;69:572–9. doi: 10.1016/j.ijrobp.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Andersson H, Lindegren S, Back T, Jacobsson L, Leser G, Horvath G. Radioimmunotherapy of nude mice with intraperitoneally growing ovarian cancer xenograft utilizing 211At-labelled monoclonal antibody MOv18. Anticancer Res. 2000;20:459–62. [PubMed] [Google Scholar]
- 9.Andersson H, Palm S, Lindegren S, Back T, Jacobsson L, Leser G, et al. Comparison of the therapeutic efficacy of 211At- and 131I-labelled monoclonal antibody MOv18 in nude mice with intraperitoneal growth of human ovarian cancer. Anticancer Res. 2001;21:409–12. [PubMed] [Google Scholar]
- 10.Andersson H, Cederkrantz E, Back T, Divgi C, Elgqvist J, Himmelman J, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of (211)At-MX35 F(ab’)2—a phase I study. J Nucl Med. 2009;50:1153–60. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 11.Cederkrantz E, Andersson H, Bernhardt P, Back T, Hultborn R, Jacobsson L, et al. Absorbed doses and risk estimates of (211)At-MX35 F(ab′)2 in intraperitoneal therapy of ovarian cancer patients. Int J Radiat Oncol Biol Phys. 2015;93:569–76. doi: 10.1016/j.ijrobp.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Palm S, Back T, Haraldsson B, Jacobsson L, Lindegren S, Albertsson P. Biokinetic modeling and dosimetry for optimizing intraperitoneal radioimmunotherapy of ovarian cancer microtumors. J Nucl Med. 2016;57:594–600. doi: 10.2967/jnumed.115.167825. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson AM, Back T, Elgqvist J, Jacobsson L, Hultborn R, Albertsson P, et al. Comparison of therapeutic efficacy and biodistribution of 213Bi- and 211At-labeled monoclonal antibody MX35 in an ovarian cancer model. Nucl Med Biol. 2012;39:15–22. doi: 10.1016/j.nucmedbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Apostolidis C, Molinet R, Rasmussen G, Morgenstern A. Production of Ac-225 from Th-229 for targeted alpha therapy. Anal Chem. 2005;77:6288–91. doi: 10.1021/ac0580114. [DOI] [PubMed] [Google Scholar]
- 15.Zielinska B, Apostolidis C, Bruchertseifer F, Morgenstern A. An improved method for the production of Ac-225/Bi-213 from Th-229 for targeted alpha therapy. Solvent Extr Ion Exc. 2007;25:339–49. doi: 10.1080/07366290701285108. [DOI] [Google Scholar]
- 16.Nikula TK, McDevitt MR, Finn RD, Wu C, Kozak RW, Garmestani K, et al. Alpha-emitting bismuth cyclohexylbenzyl DTPA constructs of recombinant humanized anti-CD33 antibodies: pharmacokinetics, bioactivity, toxicity and chemistry. J Nucl Med. 1999;40:166–76. [PubMed] [Google Scholar]
- 17.Mattes MJ, Lloyd KO, Lewis JL. Binding parameters of monoclonal antibodies reacting with ovarian carcinoma ascites cells. Cancer Immunol Immun. 1989;28:199–207. doi: 10.1007/BF00204989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattes MJ, Look K, Furukawa K, Pierce VK, Old LJ, Lewis JL, et al. Mouse monoclonal antibodies to human epithelial differentiation antigens expressed on the surface of ovarian carcinoma ascites cells. Cancer Res. 1987;47:6741–50. [PubMed] [Google Scholar]
- 19.Yin BW, Kiyamova R, Chua R, Caballero OL, Gout I, Gryshkova V, et al. Monoclonal antibody MX35 detects the membrane transporter NaPi2b (SLC34A2) in human carcinomas. Cancer Immun. 2008;8:3. [PMC free article] [PubMed] [Google Scholar]
- 20.Pippin CG, Parker TA, McMurry TJ, Brechbiel MW. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconjug Chem. 1992;3:342–5. doi: 10.1021/bc00016a014. [DOI] [PubMed] [Google Scholar]
- 21.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA. Determination of the immunoreactive fraction of radiolabeled monoclonal-antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 22.Elgqvist J, Bernhardt P, Hultborn R, Jensen H, Karlsson B, Lindegren S, et al. Myelotoxicity and RBE of 211At-conjugated monoclonal antibodies compared with 99mTc-conjugated monoclonal antibodies and 60Co irradiation in nude mice. J Nucl Med. 2005;46:464–71. [PubMed] [Google Scholar]
- 23.Back T, Haraldsson B, Hultborn R, Jensen H, Johansson ME, Lindegren S, et al. Glomerular filtration rate after alpha-radioimmunotherapy with At-211-MX35-F(ab ’)(2): a long-term study of renal function in nude mice. Cancer Biother Radio. 2009;24:649–58. doi: 10.1089/cbr.2009.0628. [DOI] [PubMed] [Google Scholar]
- 24.Back T, Elgqvist J, Hultborn R, Kahu H, Lindegren S, Palm S, et al. Irradiation effects on the kidneys after radioinimunotherapy with the alpha emitter astatine-211: report from an on-going long-term study in nude mice. Cancer Biother Radio. 2006;21:396–7. [Google Scholar]
- 25.Cederkrantz E, Angenete E, Back T, Falk P, Haraldsson B, Ivarsson ML, et al. Evaluation of effects on the peritoneum after intraperitoneal alpha-radioimmunotherapy with (211)At. Cancer Biother Radiopharm. 2012;27:353–64. doi: 10.1089/cbr.2012.1184. [DOI] [PubMed] [Google Scholar]


