Abstract
Osteosarcoma is a primary malignant bone tumour, for which no improvement in survival rate has been made since the nineteen seventies. We set out to systemically identify the in vitro studies performed in the past two decades describing potential future therapies. Strikingly, we obtained a total of 5282 PubMed hits on this subject. The amount of publications has increased almost exponentially over the past few years. Studies from Chinese institutes are mainly responsible for this huge increase, accounting for 53% of the publications in 2015. Approximately 1/3 of all drugs described in the past three years could be classified as traditional medicine. Furthermore, it struck our attention that even though in such studies multiple cell lines are essential to represent the heterogeneity in patients, many studies were performed with only one or two cell lines, i.e. U-2 OS or MG-63. These cells are fast growing, facilitating rapid experimental application but also boosting drug responsiveness. This probably explains why so many in vitro studies have been published for this relatively rare disease. Furthermore, it illustrates the current publication pressure, especially in China.
Keywords: Cell lines, Compounds, In vitro studies, MG-63, Osteosarcoma, U-2 OS
1. Introduction
Osteosarcoma is the most frequent primary malignant bone tumour. It has a peak incidence between 10 and 14 year of age and only 30% of all osteosarcomas occur in individuals aged >40 [1]. Osteosarcoma is a rare disease, with an annual incidence rate of approximately 4.4 per 106 for people aged 0–24 years [1]. Several subtypes of osteosarcoma can be distinguished, of which conventional high-grade central, or intramedullary osteosarcoma, is by far the most common (75% of the cases). Osteosarcoma is characterized by the production of osteoid matrix and is located mostly at the metaphysis of long bones. In addition to surgery patients receive intensive pre- and post-operative chemotherapy [2]. Although neo-adjuvant chemotherapy has markedly improved outcome, since its introduction in the 70ties survival has reached a plateau of about 60–70% [3]. Especially osteosarcoma presenting with metastases at diagnosis has a particular poor outcome. Therefore, new treatment options are needed. As osteosarcoma is a rare disease, international collaborations are essential for the conduction of clinical trials. The European and American osteosarcoma study group (EURAMOS), started its first trial in 2005, in which 2260 patients from 326 centres across 17 countries were enrolled [2]. This largest osteosarcoma study to date could be achieved by a committed collaboration between four well established study groups. However, due to the absence of consensus and regional differences in compound approval, a second study has not emerged yet, which is especially discouraging now this successful worldwide network has been established [2].
2. Almost exponential increase in amount of osteosarcoma in vitro studies
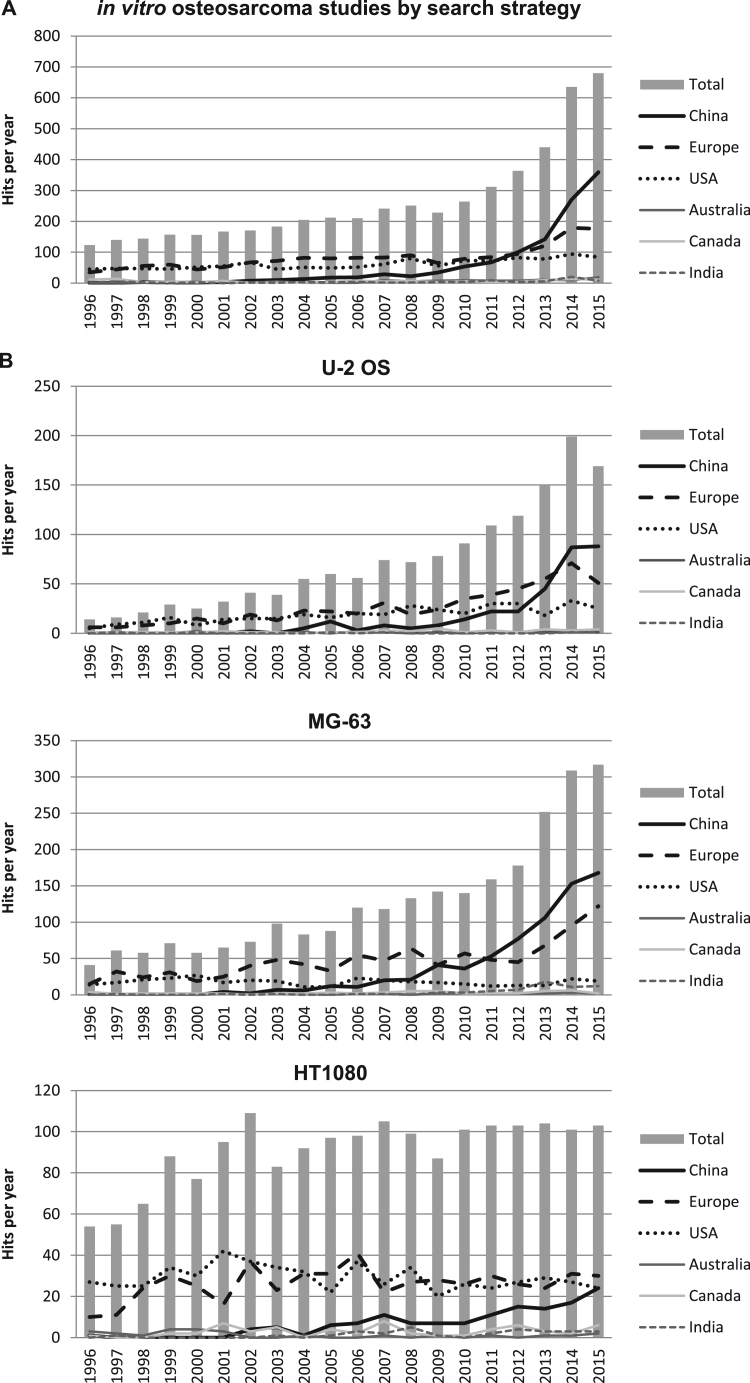
To see how the quantity of osteosarcoma in vitro studies developed overtime, we set out to systematically identify all the drugs that have been tested on osteosarcoma cells in vitro in the past two decades. A PubMed search strategy was compiled which can be found in Supplementary data 1. To our surprise, we got a total of 5282 hits in PubMed. It struck our attention that the number of publications on treatment of osteosarcoma cell lines with various compounds has increased almost exponentially (Fig. 1A). After importing these PubMed hits in EndNote X7, we obtained an indication of how the amount of publications developed in China, the USA, India, Australia, Canada and Europe (defined as countries that are in the Schengen Area and/or the European Union) in the past few years by searching for these countries in the authors address box. The majority of the publications came from China, Europe or the USA. The amount of publications from the USA showed a minor increase, from 45 in 1996 to 73 in 2015, whereas the amount of publication from Europe increased substantially from 35 in 1996 to 176 in 2015. Strikingly, the amount of publications from China rose from 1 to 359, thereby mainly being responsible for the huge increase in publications observed in the last few years. China has been the leading country since 2012, and 53% of the publications from 2015 involved Chinese institutes; this is a much higher proportion than reported for other research areas such as haematology research [4]. We read all the abstracts of the hits of the past three years (n=1755), and categorized them based on non-osteosarcoma (n=297, many studies on other cancers metastasizing to the bone), no in vitro study (n=179), no drugs study (n=596, many studies performed siRNA knockdown only) and no inhibitory effect (n=126), leaving us with 560 studies from the last three years, a relatively large number when considering the rareness of this disease. Interestingly, approximately 1/3 of the compounds could be classified as traditional medicine, of which over 80% was published by Chinese authors. For example, we encountered several compounds such as Evodiamine, a chemical extracted from the Tetradium genus of plants [5], [6] and crude extracts of Rheum palmatum L., the root of Chinese rhubarb [7].
Fig. 1.
The amount of hits from PubMed searches per year. A: The amount of osteosarcoma in vitro studies greatly increased in the past few years, with an increased proportion of articles with Chinese authors. B: Amount of PubMed hits per year for U-2 OS (osteosarcoma), MG-63 (osteosarcoma) and HT-1080 (fibrosarcoma) demonstrates that the exponential increase in in vitro studies is not identified in other sarcomas.
3. The use of a single cell line
Remarkably, many studies were performed with only one cell line, either U-2 OS or MG-63. Due to the high heterogeneity of osteosarcoma, studying a panel of cell lines instead of a single cell line is essential for eventual clinical applicability. U-2 OS and MG-63 are both ATCC cell lines established in 1964 and 1977 respectively. Searching PubMed for U-2 OS (and U2OS/U2-OS/U-2-OS) and MG-63 (and MG63) identified respectively 1449 and 2564 publications from 1996 to 2015, while the combination only resulted in 163 PubMed hits. Determining the amount of publications per year and per country for these cell lines as described above further demonstrated the almost exponential increase in publications with osteosarcoma cell lines attributable to studies from Chinese institutes (Fig. 1B). To compare these results with another ATCC sarcoma cell line with a comparable amount of total publications, we performed an identical search for HT-1080 (fibrosarcoma), in which we did not see this trend (Fig. 1B), demonstrating that this is not a trend observed in all ATCC sarcoma cell lines.
U-2 OS and MG-63 are ubiquitously applied also outside the osteosarcoma field for general cell biology studies because they are among the few human cell lines that are relatively susceptible to transfection. In addition, these cells grow rapidly facilitating experimental application but also boosting drug responsiveness [8]. This probably explains why so many in vitro studies have been published for this relatively rare disease.
4. A researcher's perspective
There may be a jewel hidden in this avalanche of studies, but it is difficult to discern due to the huge quantity of papers using questionable designs. If in vitro studies are performed, researchers should always include a panel of cell lines to represent the tumour heterogeneity in osteosarcoma patients. As a researcher, it is impossible to keep track of all the research that is being published, which may lead to missing promising therapeutic targets. The publication of these relatively easy executable studies is obviously fed by the incentive of “publish or perish”, but it keeps scientists occupied with often irrelevant work, it usurps financial budgets and obscures relevant investigations. Other major drivers of the enormous increase in research papers is the substantial increase in the number of academic journals [9]. Aggressive editor's requests to submit manuscripts to new journals keep filling a scientist's mailbox, which can be quite disturbing. This trend to prevail quantity over quality is occupying precious time from editors and reviewers. Currently, the Science Citation Index is used for medical career evaluation in the majority of large Chinese hospitals, resulting in a huge pressure of Chinese medical doctors to publish articles and an increase in number of publishing scientists [10], [11]. Therefore, it is essential that the evaluation system on research performance will be changed with the focus shifted from quantity to quality as was recently again advocated that bibliometrics are warping science [12]. Our analysis of in vitro osteosarcoma studies illustrates the effect of the increased publication pressure since the convenience of osteosarcoma cell lines renders them into low hanging fruit but results in studies with limited scientific value, which constrains solutions for this deadly disease that affects young patients.
Conflict of interest
The authors declare that they have no conflict of interest/competing interests.
Authors’ contributions
EFPP performed the literature study and made the figures. AMCJ supervised the study. EFPP, TNvL and AMCJ wrote the manuscript.
Acknowledgements
The authors would like to thank Claudia F. Pees for establishing the PubMed Search and Prof Dr. Judith V.M.G. Bovée for fruitful discussions.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jbo.2017.04.004.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F. Conventional osteosarcoma. In: Rosenberg A.E., Cleton-Jansen A.-M., de Pinieux G., Deyrup A.T., Hauben E., Squire J., editors. WHO Classification of Tumours of Soft Tissue and Bone. IARC; Lyon: 2013. pp. 282–288. [Google Scholar]
- 2.Whelan J.S., Bielack S.S., Marina N., Smeland S., Jovic G., Hook J.M., Krailo M., Anninga J., Butterfass-Bahloul T., Bohling T., Calaminus G., Capra M., Deffenbaugh C., Dhooge C., Eriksson M., Flanagan A.M., Gelderblom H., Goorin A., Gorlick R., Gosheger G., Grimer R.J., Hall K.S., Helmke K., Hogendoorn P.C., Jundt G., Kager L., Kuehne T., Lau C.C., Letson G.D., Meyer J., Meyers P.A., Morris C., Mottl H., Nadel H., Nagarajan R., Randall R.L., Schomberg P., Schwarz R., Teot L.A., Sydes M.R., Bernstein M., collaborators E. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann. Oncol. 2015;26(2):407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anninga J.K., Gelderblom H., Fiocco M., Kroep J.R., Taminiau A.H., Hogendoorn P.C., Egeler R.M. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur. J. Cancer. 2011;47(16):2431–2445. doi: 10.1016/j.ejca.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Ye X., Sun Y., Deng A.M., Qian B.H. Hematology research output from Chinese authors and other countries: a 10-year survey of the literature. J. Hematol. Oncol. 2015;8:8. doi: 10.1186/s13045-014-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai X., Meng H., Ma L., Guo A. Inhibitory effects of evodiamine on human osteosarcoma cell proliferation and apoptosis. Oncol. Lett. 2015;9(2):801–805. doi: 10.3892/ol.2014.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng Z.J., Wu N., Liu Y., Shu K.J., Zou X., Zhang R.X., Pi C.J., He B.C., Ke Z.Y., Chen L., Deng Z.L., Yin L.J. Evodiamine inhibits the proliferation of human osteosarcoma cells by blocking PI3K/Akt signaling. Oncol. Rep. 2015 doi: 10.3892/or.2015.4084. [DOI] [PubMed] [Google Scholar]
- 7.Lin C.C., Lee M.H., Lin J.H., Lin M.L., Chueh F.S., Yu C.C., Lin J.P., Chou Y.C., Hsu S.C., Chung J.G. Crude extract of Rheum palmatum L. induces cell cycle arrest S phase and apoptosis through mitochondrial-dependent pathways in U-2 OS human osteosarcoma cells. Environ. Toxicol. 2015 doi: 10.1002/tox.22105. [DOI] [PubMed] [Google Scholar]
- 8.Hafner M., Niepel M., Chung M., Sorger P.K. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat. Methods. 2016;13(6):521–527. doi: 10.1038/nmeth.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker P.M., Rifai N. Expanding options for scientific publication: is more always better? Circulation. 2013;127(2):155–156. doi: 10.1161/CIRCULATIONAHA.112.155952. [DOI] [PubMed] [Google Scholar]
- 10.Ye B., Liu A.H. Inadequate evaluation of medical doctors in China. Lancet. 2013;381(9882):1984. doi: 10.1016/S0140-6736(13)61200-3. [DOI] [PubMed] [Google Scholar]
- 11.Tang L., Shapira P., Youtie J. Is there a clubbing effect underlying Chinese research citation increases? J. Assoc. Inf. Sci. Technol. 2015;66(9):1923–1932. [Google Scholar]
- 12.Benedictus R., Miedema F., Ferguson M.W. Fewer numbers, better science. Nature. 2016;538(7626):453–455. doi: 10.1038/538453a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material