Abstract
Dourine is caused by Trypanosoma equiperdum via coitus with an infected horse. Although dourine is distributed in Equidae worldwide and is listed as an internationally important animal disease by the World Organization for Animal Health (OIE), no effective treatment strategies have been established. In addition, there are no reports on drug discovery, because no drug screening system exists for this parasite. A new T. equiperdum strain was recently isolated from the genital organ of a stallion that showed typical symptoms of dourine. In the present study, we adapted T. equiperdum IVM-t1 from soft agarose media to HMI-9 liquid media to develop a drug screening assay for T. equiperdum. An intracellular ATP-based luciferase assay using CellTiter-Glo reagent and an intracellular dehydrogenase activity-based colorimetric assay using WTS-8 tetrazolium salt (CCK-8 reagent) were used in order to examine the trypanocidal effects of each compound. In addition, the IC50 values of 4 reference trypanocidal compounds (pentamidine, diminazene, suramin and melarsomine) were evaluated and compared using established assays. The IC50 values of these reference compounds corresponded well to previous studies involving other strains of T. equiperdum. The luciferase assay would be suitable for the mass screening of chemical libraries against T. equiperdum because it allows for the simple and rapid-evaluation of the trypanocidal activities of test compounds, while a simple, inexpensive colorimetric assay will be applicable in developing countries for the evaluation of the drug sensitivity of epidemic trypanosome strains.
Keywords: Colorimetric assay, Drug screening system, Liquid culture, Luciferase assay, Trypanosoma equiperdum
Graphical abstract
1. Introduction
Dourine is caused by Trypanosoma equiperdum of the subgenus Trypanozoon. Unlike other trypanosomes, dourine is transmitted through coitus with an infected horse rather than insect vectors (Brun et al., 1998). Thus, dourine has been found to be distributed worldwide, beyond the tsetse belt in sub-Saharan Africa (Brun et al., 1998). Dourine is listed as an internationally important animal disease by the World Organization for Animal Health (OIE). Evidence from in vitro drug sensitivity assays using laboratory strains of T. equiperdum indicates that suramin, diminazene, quinapyramine and Cymelarsan® (melarsomine) are effective against T. equiperdum (Zhang et al., 1991, Brun and Lun, 1994, Gillingwater et al., 2007). Melarsomine was found to be effective against the T. equiperdum OVI strain in mice, and the T. equiperdum Dodola strain in mice and horses (Hagos et al., 2010, Habte et al., 2015). However, this has not been established as an effective treatment strategy for dourine-infected horses. Moreover, diminazene- and melarsomine-resistant T. equiperdum strains have already been reported (Gillingwater et al., 2007). Thus, an in vitro drug screening assay for T. equiperdum is strongly needed for efforts to seek novel trypanocidal compounds both for evaluating drug sensitivity and for establishing an effective treatment strategy for dourine.
The direct determination of the trypanosome count using a cell counting chamber is a simple method for evaluating trypanocidal activity. This assay is relatively cheap and simple to perform; however, it is time consuming to evaluate large numbers of compounds using this method (Fumarola et al., 2004). The incorporation of a radioactive nucleotide assay has been widely used to analyze the drug sensitivity of trypanosomes in vitro (Brun and Lun, 1994). After the incubation of the trypanosomes with the drugs and 3[H] thymidine, the signals of the radioisotope in the parasite are counted using a liquid scintillation counter. This test has the disadvantage of involving radioactive nucleotides and cumbersome steps. A fluorescence-based screening system using Alamar blue™ has also been developed for an HTS assay for trypanosomes (Sykes and Avery, 2009a). This assay requires expensive equipment, such as a fluorometer for the 96-well plate and is therefore difficult to apply in developing countries, which are most affected by trypanosomosis (Fumarola et al., 2004). Intracellular ATP-based luciferase assays have also been utilized as HTS assays against T. congolense, T. brucei and Leishmania spp. (Sykes and Avery, 2009b, De Muylder et al., 2011, Suganuma et al., 2014). The kits, such as CellTiter-Glo®, are simple, rapid and efficient, with signal detection taking only few minutes. Intracellular ATP-based luciferase assays are more suitable for the rapid-evaluation of trypanocidal activity than Alamar blue™ based fluorescence assays, because the incubation time after the addition of the CellTiter-Glo® reagents is short. However, the expensive equipment and reagents that are necessary for this assay also constrain its application in developing countries. Colorimetric assays using tetrazolium salts, such as MTT, XTT and WST, have also been established for T. brucei and Leishmania spp. (Lu et al., 2013, Ginouves et al., 2014). These methods are based on a color reaction (through the reductive reaction of intracellular dehydrogenase activity), which leads to the formation of a colored chemical product, formazan. The results of the color reaction are measured using a relatively inexpensive and versatile microplate plate reader (ELISA plate reader). In addition, the reagents are affordable in comparison to fluorescence and luciferase assays (Fumarola et al., 2004). Thus, these colorimetric assays are more suitable than other assay systems for evaluating the drug sensitivity of trypanosomes in developing countries.
We recently isolated a new strain of T. equiperdum, T. equiperdum IVM-t1 from a horse in Mongolia that was confirmed to have dourine based on clinical symptoms, a positive PCR result, the serological diagnosis, and the microscopic observation of trypanosomes in a genital swab (Suganuma et al., 2016). This trypanosome strain was well adapted and propagated on the soft agarose media. In the present study, we successfully adapted T. equiperdum IVM-t1 that had been cultured in soft agarose media to culture in HMI-9 liquid media in order to develop a drug screening assay for T. equiperdum. In addition, the IC50 values of four trypanocidal drugs were evaluated and compared to the results obtained from established assays.
2. Materials and methods
2.1. In vitro cultivation
The T. equiperdum IVM-t1 strain, which was isolated in Mongolia in 2015, was used in the present study (Suganuma et al., 2016). Soft agarose culture-adapted T. equiperdum IVM-t1 was maintained by continuous sub-culturing once a week at 37 °C in 5% CO2 using soft agarose media (HMI-9 (Hirumi and Hirumi, 1991) with 0.8% low gelling agarose (wt/vol) [Type VII, Sigma-Aldrich Japan, Tokyo, Japan]).
2.2. Adaptation to liquid media
Soft agarose culture-adapted T. equiperdum IVM-t1 parasites were transferred into HMI-9 liquid media supplemented with 0, 0.01, 0.1, 1 and 10% FCeM (vol/vol) (Nissan Chemical Industries, Ltd., Tokyo, Japan) at a parasite concentration of 1 × 104 cells/mL. The number of trypanosomes in each cell culture flask was manually counted every 24 h using a cell counting chamber. After optimizing the FCeM concentration in HMI-9, the liquid culture-adapted T. equiperdum IVM-t1 strain was maintained by replacing the entire culture supernatant with fresh HMI-9 supplemented with 1% FCeM every other day. A one-way ANOVA and Tukey's multiple comparison test were used for the statistical analyses of the doubling time, the daily parasite concentrations and the maximum parasite concentrations with different concentrations of FCeM. The statistical analyses were performed using the GraphPad PRISM 5 software program (GraphPad Software, Inc., CA, USA). The doubling time was calculated by counting the cells in the logarithmic growth phase of growth and using the following equation: Doubling time = (t2 – t1) x log (2)/log (q2/q1). Two measurements were performed: the initial count (q1) at time (t1) and the resultant parasite concentration after 24 h of incubation (q2, t2).
2.3. The optimization of the luciferase assay
To optimize the efficacy of the parasite detection by the luciferase assay using the CellTiter-Glo® Luminescent Cell Viability Assay reagent (Promega Japan, Tokyo, Japan), trypanosomes were prepared by two serial dilutions from 2 × 106 – 1950 cells/mL, and 100 μL aliquots were dispensed into 96-well cell culture plates in duplicate. The luminescence was measured using 12.5, 25, 50 and 100 μL of CellTiter-Glo® reagents at various time intervals after 2 min mixing, in order to evaluate the linearity between the luminescence and the trypanosome concentration.
In order to optimize the initial trypanosome concentration in the 96-well cell culture plates, 100 μL of T. equiperdum at a density of 5 × 104, 2.5 × 104, 1 × 104 and 5 × 103 cells/mL was inoculated on a 96-well cell culture plate and the cells were manually counted every 24 h after inoculation.
Moreover, to optimize the concentration of DMSO (which was used as a solvent) in the culture medium, the inhibitory effect of DMSO was evaluated. One hundred microliters (1 × 104 cells/mL) of T. equiperdum were cultivated for 72 h in HMI-9 supplemented with 1% FCeM and 0, 0.125, 0.25, 0.5, 1, 1.25, 5 and 10% of DMSO. Subsequently, the survival rate of the parasite in each concentration was evaluated by a luciferase assay using 25 μL of CellTiter-Glo® reagent. Student's t-test was used to analyze the differences between the control group (0% DMSO) and DMSO-treated groups.
2.4. The assessment of the trypanocidal activity with optimized assay conditions
Fifty microliters of various concentrations of reference compounds in HMI-9 supplemented with 1% FCeM and 0.5% DMSO were added to a Nunc™ MicroWell™ 96-well optical bottom plate (Thermo Fisher Scientific K.K., Yokohama, Japan) for the luciferase assay, or a 96-well cell culture plate (Corning™ Costar™ Flat Bottom Cell Culture Plates; Thermo Fisher Scientific) for the colorimetric and manual counting assays. Subsequently, 50 μL of liquid culture-adapted- or soft agarose culture-adapted T. equiperdum IVM-t1 in HMI-9 supplemented with 1% FCeM at a density of 2 × 104 cells/mL were aliquoted into each well and mixed by gentle pipetting in the 96-well cell culture plates. The final concentration of trypanosomes used in the assay was 1 × 104 cells/mL in 100 μL of HMI-9 supplemented with 1% FCeM and 0.25% DMSO per well. The trypanosomes were then incubated for 72 h. To evaluate the trypanocidal activity in the luciferase assay, 25 μL of CellTiter-Glo® was added and mixed for 2 min using an MS3 basic plate shaker (IKA® JAPAN K.K., Osaka, Japan). Subsequently, the plates were kept for 5 min in room temperature. The luminescence was then measured using a GloMax®-Multi + Detection System plate reader (Promega Japan). To evaluate the trypanocidal activity using the colorimetric assay, 10 μL of CCK-8 reagent was added, mixed for 2 min using an MS3 basic plate shaker and incubated in an incubator for 4 h in accordance with the manufacturer's protocol. Subsequently, the absorbencies in 450 nm were measured using an MTP-500 microplate reader (Corona Electric, Ibaraki, Japan). In addition, the trypanocidal activity was evaluated based on the number of living trypanosomes, which were manually counted using a cell counting chamber.
The trypanocidal activity of four commercial trypanocidal compounds (pentamidine [Sigma-Aldrich], diminazene aceturate [Sigma-Aldrich], suramin [Wako Pure Chemical Industries, Ltd., Osaka, Japan] and melarsomine [Merial, Lyon, France]) against T. equiperdum IVM-t1 was evaluated. These compounds were stored as 1 mg/mL (diminazene and suramin) or 40 μg/mL (pentamidine and melarsomine) stock solutions in DMSO. Five-fold serial dilutions were made in duplicate from 2500 to 0.032 ng/mL (diminazene and suramin) and 100–0.00128 ng/mL (pentamidine and melarsomine) in HMI-9 with 0.25% DMSO. The IC50 values of each reference compound were calculated based on the results of the luciferase assay, the colorimetric assay and manual counting by plotting the % inhibition (0% inhibition = the luminescence, absorbance or trypanosome concentration of the trypanosome culture wells with 0.25% DMSO as a solvent control without reference compounds [negative control wells], 100% inhibition = the luminescence, absorbance or trypanosome concentration of trypanosome culture wells with 25 μg/mL of diminazene [positive control wells]) in the GraphPad PRISM 5 software program using nonlinear regression (curve fit). A one-way ANOVA and Tukey's multiple comparison tests were used for the statistical analyses of the results. The statistical analyses were performed using the GraphPad PRISM 5 software program.
The reproducibility of these assays was validated using the coefficient of variation (%CV), signal/background ratio (S/B), signal/noise ratio (S/N) and Z’-factor values, which were calculated using the following equations.
Z’: 1 – ([3xSD of negative control wells + 3xSD of positive control wells]/[mean of negative control wells – mean of positive control wells]) as described by Sykes and Avery (2009a).
3. Results
3.1. The optimization of the T. equiperdum liquid culture conditions
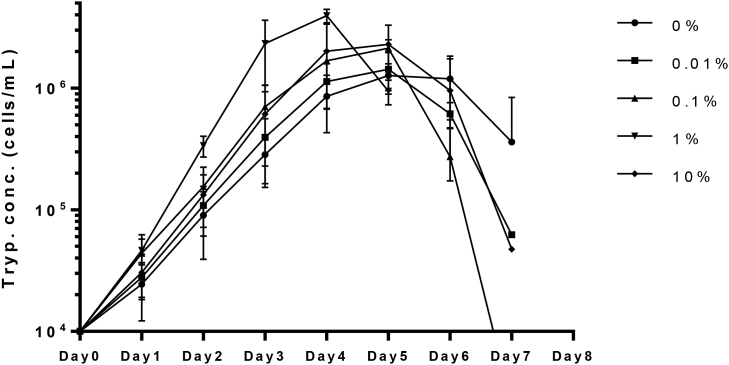
Soft agarose media-adapted T. equiperdum IVM-t1 transferred into HMI-9 without FCeM reached a maximum cell concentration of 1.54 ± 0.32 × 106 cells/mL (Fig. 1). The doubling time in the logarithmic growth phase in HMI-9 without FCeM was determined to be 16.36 ± 1.02 h. The doubling time was shortened by FCeM in a dose-dependent manner and the peak T. equiperdum concentration was increased by FCeM in a dose-dependent manner by supplementation with up to 1% FCeM. The doubling time of 1% FCeM culture (11.15 ± 0.23 h) was significantly shorter than that of other FCeM concentrations (p < 0.05). In addition, the peak trypanosome concentration of the 1% FCeM culture (3.95 ± 0.43 × 106 cells/mL; Day 4) was significantly higher than that of the other concentrations (Day 5, Fig. 1). These results suggested that HMI-9 with 1% FCeM was the most suitable media for the induction of liquid culture-adapted T. equiperdum.
Fig. 1.
Optimization of the FCeM concentration for liquid culture adaptation. Optimization of the FCeM concentration. Each plot shows the mean value from four independent experiments. Each column shows the mean value ± standard deviation.
3.2. The optimization of the in vitro trypanocidal activity evaluation system using CellTiter-Glo® reagent
The optimization of the volume of CellTiter-Glo® reagent and the time point for the measurement of luminescence was carried out by evaluating the correlation between the luminescence and the trypanosome concentration. This was achieved by adding various volumes of CellTiter-Glo® reagent and measuring the luminescence at different time points. The results showed that the coefficient of determination (R2) values of 100 μL (the volume recommended by the manufacturer), 50 μL and 25 μL of CellTiter-Glo® reagent at 15 min after mixing were still ≥0.99 from the lowest concentration (1950 cells/mL) to the highest concentration (2 × 106 cells/mL) (Supplemental Fig. 1-A–C) Fig. 2-A shows the optimized condition, in which measurement was performed after the addition of 25 μL of CellTiter-Glo®, mixing for 2 min, and standing for 5 min at room temperature. From 7.5 min after mixing, the R2 of 12.5 μL of the reagent was found to gradually decrease from 0.99 (Supplemental Figs. 1–D).
Fig. 2.
The optimization of the conditions of the assay using CellTiter-Glo®reagent. (A) The correlation between the number of viable T. equiperdum IVM-t1 and luminescence was measured using the following optimized conditions: 25 μL of reagent at 5 min after mixing. All of the plots were calculated from four independent experiments and are shown as the mean luminescence signal ± standard deviation. The scale of the X and Y axes is log-10. (B) The optimization of the initial concentration for T. equiperdum IVM-t1 culture using 96-well cell culture plates. Each plot was calculated from three independent experiments and shows the mean value ± standard deviation. (C) The optimization of the DMSO concentration in the medium. All bars were calculated from three independent experiments and are shown as the mean survival rate ± standard deviation. Student's t-test was used to analyze the differences between 0% DMSO (negative control) and each of DMSO concentrations. ***p < 0.001.
Next, the condition of the trypanosome culture in the 96-well plate that led to a final concentration of under 2 × 106 cells/mL in the logarithmic growth phase on day 3 was optimized by the cultivation of various concentrations of trypanosomes from day 0 to day 3 (Fig. 2-B). We cultured that were started with a concentration of <2.5 × 104 cells/mL yielded <2 × 106 cells/mL on day 3 while still in the logarithmic grow phase. The doubling time in the logarithmic phase and the peak of trypanosome concentration did not differ to a statistically significant extent among the initial trypanosome concentrations. Thus, a starting T. equiperdum concentration of 1 × 104 cells/mL in HMI-9 supplemented with 1% FCeM was determined to be the optimal condition for our trypanocidal screening assay using 96-well cell culture plates.
Finally, to optimize the DMSO (as a solvent of the reference trypanocidal compounds) concentration in the experiments, we evaluated the survival rate of T. equiperdum cultivated with various concentrations of DMSO. The supplementation of DMSO at concentrations of >1.0% significantly inhibited the growth of T. equiperdum (p < 0.001) (Fig. 2-C).
Based on these results, the economic efficiency of the reagents and the handling performance of assay, the optimal conditions for the determination of IC50 values of the reference compounds were as follows: 25 μL of CellTiter-Glo® reagent, 0.25% DMSO, the measurement of luminescence after 2 min mixing and standing for another 5 min at room temperature.
3.3. The evaluation and comparison of the IC50 values of the reference compounds against T. equiperdum IVM-t1
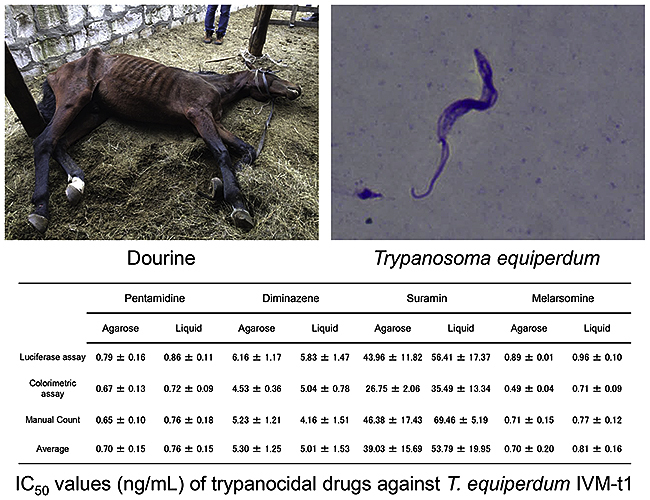
The IC50 values against T. equiperdum were calculated by three different methods using T. equiperdum from two different origins: directly collected from soft agarose culture (soft agarose culture-adapted T. equiperdum); and collected from HMI-9 liquid culture supplemented with 1% FCeM (liquid culture-adapted T. equiperdum). The IC50 value range of pentamidine was 0.65–0.86 ng/mL and no significant differences were observed among the methods and T. equiperdum from the two different origins (Table 1 and Supplemental Fig. 3). Furthermore, there were no significant differences in the IC50 values of diminazene aceturate (IC50 values, 4.16–6.16 ng/mL) (Table 1 and Supplemental Fig. 4). However, the IC50 value of suramin, as evaluated by the colorimetric assays (26.75–35.49 ng/mL) was significantly lower than that determined by manual counting using liquid culture-adapted T. equiperdum (69.46 ± 5.19 ng/mL, p < 0.05) (Table 1 and Supplemental Fig. 5). Some of the IC50 values of melarsomine also showed significant differences among the three methods and between the two different origins of T. equiperdum (p < 0.05) (Table 1 and Supplemental Fig. 6). The results of these drug screening assays were validated using the %CV, S/B, S/N and Z’ values, as shown in Supplemental Table 1.
Table 1.
The IC50 values of the reference trypanocidal compounds.
| Pentamidine |
Diminazene |
Suramin |
Melarsomine |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Agarose | Liquid | Agarose | Liquid | Agarose | Liquid | Agarose | Liquid | ||
| Luciferase assay | ng/mL | 0.79 ± 0.16 | 0.86 ± 0.11 | 6.16 ± 1.17 | 5.83 ± 1.47 | 43.96 ± 11.82 | 56.41 ± 17.37 | a,c,d 0.89 ± 0.01 | c,e 0.96 ± 0.10 |
| (nM) | (1.32 ± 0.27) | (1.46 ± 0.19) | (11.94 ± 2.27) | (11.30 ± 2.85) | (30.76 ± 8.27) | (39.47 ± 12.16) | (1.77 ± 0.22) | (1.91 ± 0.20) | |
| Colorimetric assay | ng/mL | 0.67 ± 0.13 | 0.72 ± 0.09 | 4.53 ± 0.36 | 5.04 ± 0.78 | a 26.75 ± 2.06 | a,c 35.49 ± 13.34 | b 0.49 ± 0.04 | a,b 0.71 ± 0.09 |
| (nM) | (1.13 ± 0.21) | (1.21 ± 0.17) | (8.78 ± 0.69) | (9.77 ± 1.52) | (18.72 ± 1.43) | (24.83 ± 9.34) | (0.98 ± 0.08) | (1.42 ± 0.18) | |
| Manual Count | ng/mL | 0.65 ± 0.10 | 0.76 ± 0.18 | 5.23 ± 1.21 | 4.16 ± 1.51 | 46.38 ± 17.43 | b 69.46 ± 5.19 | a,b 0.71 ± 0.15 | a,c 0.77 ± 0.12 |
| (nM) | (1.09 ± 0.17) | (1.29 ± 0.31) | (10.12 ± 2.34) | (8.06 ± 2.93) | (32.45 ± 12.20) | (48.60 ± 3.63) | (1.41 ± 0.29) | (1.52 ± 0.24) | |
| Average | ng/mL | 0.70 ± 0.15 | 0.76 ± 0.15 | 5.30 ± 1.25 | 5.01 ± 1.53 | 39.03 ± 15.69 | 53.79 ± 19.95 | 0.70 ± 0.20 | 0.81 ± 0.16 |
| (nM) | (1.18 ± 0.25) | (1.29 ± 0.25) | (10.29 ± 2.42) | (9.71 ± 2.98) | (27.31 ± 10.98) | (37.63 ± 13.96) | (1.39 ± 0.40) | (1.62 ± 0.31) | |
Each value shows the mean IC50 value ± standard deviation from four independent experiments by ng/mL and nM.
Agarose, soft agarose culture adapted-T. equiperdum IVM-t1; Liquid, liquid culture-adapted T. equiperdum IVM-t1.
The different letters indicate that a significant difference was observed among the three methods and two origins in each reference drug (p < 0.05 Tukey's multiple comparison test).
4. Discussion
Drug screening assay systems using liquid culture-adapted T. equiperdum are important for the development of novel trypanocidal compounds with in vitro activity against T. equiperdum. Bassarak et al. succeeded in establishing an efficient in vitro T. equiperdum culture system in a method that focused on the trypanosome metabolism by supplementation with a trace additive (Bassarak et al., 2016). Recently, a new T. equiperdum strain, T. equiperdum IVM-t1, was established using a soft agarose culture system that was described in our previous study (Suganuma et al., 2016). This strain of T. equiperdum was characteristically well propagated with adherence on the surface of soft agarose, while the primary culture of this T. equiperdum strain could not be adapted to HMI-9 liquid media (Suganuma et al., 2016). In natural infection, T. equiperdum in the genital organs proliferates on the surface of the genital mucosa and invades tissues upon attachment (Brun et al., 1998). Thus, it was hypothesized that cellular adhesion is a key factor for adaptation and proliferation in in vitro culture. FCeM is a three-dimensional culture medium that contains FP001 polymer (Otsuji et al., 2014). Cells cultivated with media containing 0.015% FCeM (vol/vol) could maintain independence, with cells floating in a spheroid formation (Otsuji et al., 2014). Unlike other three-dimensional polymer, such as methylcellulose, the viscosity of the medium supplemented with FCeM is almost the same as water (Otsuji et al., 2014). Thus, we can handle trypanosome culture using HMI-9 supplemented with FCeM in the same way as normal HMI-9 medium for daily culture maintenance and further experiments. In FCeM supplemented medium, some T. equiperdum grow in a “cloudlike polymer-adherent colony” formation. The proliferation of T. equiperdum IVM-t1 with 1% FCeM supplementation was significantly better than that at lower concentrations of FCeM in HMI-9 media on days 3 and 4. In addition, the doubling time in the 1% FCeM-supplemented media was significantly shorter than in media supplemented with other concentrations of FCeM in the logarithmic growth phase. The peak trypanosome concentration with 1% FCeM supplementation (Day 4, 3.95 ± 0.43 × 106 cells/mL) was significantly higher in comparison to the peak concentrations with the supplementation of other concentrations of FCeM on day 5 (0% FCeM, 1.54 ± 0.32 × 106; 0.01% FCeM, 1.57 ± 0.34 × 106; 0.1%, 2.19 ± 0.23 × 106; and 10%, 2.23 ± 0.93 × 106 cells/mL) (Fig. 1), and was almost the same as the cell concentration reported in a previous study (Bassarak et al., 2016). These results suggested that 1% FCeM supplementation in HMI-9 media was also the optimum media for the liquid culture of T. equiperdum.
The results of the optimization of conditions of the drug screening assay using CellTiter-Glo® reagents suggested that 1 × 104 cells/mL trypanosome as an initial culture concentration, and the measurement of luminescence using 25 μL of CellTiter-Glo® after 2 min mixing and 5 min standing at room temperature were suitable for the current study. Previous reports showed that the IC50 values of diminazene, suramin and melarsomine (Cymelarsan®) against various strains of T. equiperdum (except for the drug-resistant OVI strain) were <0.1–19.2 ng/mL, 18.0–87.5 ng/mL and <0.1–2.0 ng/mL, respectively (Gillingwater et al., 2007, Brun and Lun, 1994). The IC50 values of pentamidine and diminazene did not differ to a statistically significant extent among the methods and trypanosome origins (Table 1). Some statistically significant differences were observed among the IC50 values of suramin and melarsomine that were obtained by different methods and in trypanosomes of the different origins; however, our IC50 values mostly corresponded with those of previous reports (Table 1). In addition, the %CV, S/B, S/N and Z′ values of the current assays were sufficient for a drug screening assay (Supplement Table 1). These results suggest that the established intracellular ATP-based luciferase assay using the CellTiter-Glo® reagent is suitable for high throughput screening (HTS) assays for T. equiperdum, if these optimized assay conditions can be applied in an automated screening system. Meanwhile, WTS-8 tetrazolium salt could be reduced and the color could react with the extracellular reductant in media. The HMI-9 media was supplemented with β-mercaptoethanol (as a reductant) to support trypanosome growth (Baltz et al., 1985). When following the manufacturer's protocol the linearity between the trypanosome concentration and absorbance was high (R2 ≥ 0.99) (Supplemental Fig. 2); however, a color reaction with the CCK-8 tetrazolium salt by β-mercaptoethanol caused high background noise and resulted in a low S/B value (1.61–1.90) (Supplemental Table 1). Moreover, if the test compounds have high reduction activity, the colorimetric assay using CCK-8 might not be applicable for an HTS assay because of the high background noise. Thus, it is strongly recommended that the trypanosomes be observed in 96-well cell culture plates by microscopy before evaluating the trypanocidal activity in an assay. Otherwise, the reference drug IC50 values calculated by colorimetric assays mostly corresponded with those of previous reports (Table 1). Thus, in developing countries, the inexpensive colorimetric assay using CCK-8 might be more appropriate than the luciferase assay for evaluating the drug selectivity of epidemic trypanosomes.
In conclusion, we evaluated the methods of an HTS assay to determine the trypanocidal activity of different agents against T. equiperdum. Although there were some differences between the methods in terms of the IC50 values of some of the reference compounds, both established assays were suitable for HTS assays against T. equiperdum. This offers a great advantage and an efficient tool for screening and determining novel dourine drugs for the treatment of dourine in future studies, not only in developed countries but also in developing countries.
Acknowledgements
We thank Ms. Yoko Matsushita for her excellent technical assistance. This study was financially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 16K18793 (Grants-in-Aid for Young Scientists [B]), and the AMED/JICA SATREPS. This research was partially supported by the “International Collaborative Research Program for Tackling the NTDs (Neglected Tropical Diseases) Challenges in African Countries” from the Japan Agency for Medical Research and Development, AMED.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2017.04.002.
Contributor Information
Keisuke Suganuma, Email: k.suganuma@obihiro.ac.jp.
Shino Yamasaki, Email: yamasakishino0824@yahoo.co.jp.
Nthatisi Innocentia Molefe, Email: nthatisimolefe@gmail.com.
Peter Simon Musinguzi, Email: pmusinguzi@yahoo.com.
Batdorj Davaasuren, Email: davlag_mgl@yahoo.com.
Ehab Mossaad, Email: ehabmssd7@gmail.com.
Sandagdorj Narantsatsral, Email: naran69@gmail.com.
Banzragch Battur, Email: bat912b@yahoo.com.
Badgar Battsetseg, Email: bata07@gmail.com.
Noboru Inoue, Email: ircpmi@obihiro.ac.jp.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Fig. 1. The optimization of the amount CellTiter-Glo® reagent and the time point for the measurement of luminescence. The correlation between the number of viable T. equiperdum IVM-t1 and luminescence measured using 100 (A), 50 (B), 25 (C) and 12.5 μL (D) of the reagent at various time points (0, 2.5, 5, 7.5, 10 and 15 min after mixing). All of the plots were calculated from four independent experiments and are shown as the mean luminescence signal. The scale of the X and Y axes is log-10.
Supplemental Fig. 2. The correlation between the number of viable T. equiperdum IVM-t1 and absorbance. The correlation between the number of viable T. equiperdum IVM-t1 and absorbance was measured after four hours of incubation using 10 μL of CCK-8 reagent in accordance with the manufacturer's protocol. The scale of the X and Y axes is a linear scale.
Supplemental Fig. 3. The sigmoidal dose–response plots and curves of pentamidine. A–E show the dose–response plots for the following evaluations: (A) a luciferase assay using soft agarose-adapted trypanosomes; (B) a luciferase assay using liquid culture-adapted trypanosomes; (C) a colorimetric assay using soft agarose-adapted trypanosomes; (D) a colorimetric assay using liquid culture-adapted trypanosomes; (E) manual counting using soft agarose-adapted trypanosomes; (F) manual counting using liquid culture-adapted trypanosomes. All of the plots were calculated from four independent experiments and are shown as the mean luminescence signal ± standard deviation.
Supplemental Fig. 4. The sigmoidal dose–response plots and curves of diminazene. A–E show the dose–response plots for the following evaluations: (A) a luciferase assay using soft agarose-adapted trypanosomes; (B) a luciferase assay using liquid culture-adapted trypanosomes; (C) a colorimetric assay using soft agarose-adapted trypanosomes; (D) a colorimetric assay using liquid culture-adapted trypanosomes; (E) manual counting using soft agarose-adapted trypanosomes; (F) manual counting using liquid culture-adapted trypanosomes. All of the plots were calculated from four independent experiments and are shown as the mean luminescence signal ± standard deviation.
Supplemental Fig. 5. The sigmoidal dose–response plots and curves of suramin. A–E show the dose–response plots for the following evaluations: (A) a luciferase assay using soft agarose-adapted trypanosomes; (B) a luciferase assay using liquid culture-adapted trypanosomes; (C) a colorimetric assay using soft agarose-adapted trypanosomes; (D) a colorimetric assay using liquid culture-adapted trypanosomes; (E) manual counting using soft agarose-adapted trypanosomes; (F) manual counting using liquid culture-adapted trypanosomes. All of the plots were calculated from four independent experiments and are shown as the mean luminescence signal ± standard deviation.
Supplemental Fig. 6. The sigmoidal dose–response plots and curves of melarsomine. A–E show the dose–response plots for the following evaluations: (A) a luciferase assay using soft agarose-adapted trypanosomes; (B) a luciferase assay using liquid culture-adapted trypanosomes; (C) a colorimetric assay using soft agarose-adapted trypanosomes; (D) a colorimetric assay using liquid culture-adapted trypanosomes; (E) manual counting using soft agarose-adapted trypanosomes; (F) manual counting using liquid culture-adapted trypanosomes. All of the plots were calculated from four independent experiments and are shown as the mean luminescence signal ± standard deviation.
References
- Baltz T., Baltz D., Giroud C., Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985;4:1273–1277. doi: 10.1002/j.1460-2075.1985.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassarak B., Moser I., Menge C. In vitro production of Trypanosoma equiperdum antigen and its evaluation for use in serodiagnosis of dourine. Vet. Parasitol. 2016;223:133–140. doi: 10.1016/j.vetpar.2016.04.032. [DOI] [PubMed] [Google Scholar]
- Brun R., Hecker H., Lun Z.R. Trypanosoma evansi and T. equiperdum: distribution, biology, treatment and phylogenetic relationship (a review) Vet. Parasitol. 1998;79:95–107. doi: 10.1016/s0304-4017(98)00146-0. [DOI] [PubMed] [Google Scholar]
- Brun R., Lun Z.R. Drug sensitivity of Chinese Trypanosoma evansi and Trypanosoma equiperdum isolates. Vet. Parasitol. 1994;52:37–46. doi: 10.1016/0304-4017(94)90033-7. [DOI] [PubMed] [Google Scholar]
- De Muylder G., Ang K.K., Chen S., Arkin M.R., Engel J.C., McKerrow J.H. A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011;5:e1253. doi: 10.1371/journal.pntd.0001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumarola L., Spinelli R., Brandonisio O. In vitro assays for evaluation of drug activity against Leishmania spp. Res. Microbiol. 2004;155:224–230. doi: 10.1016/j.resmic.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gillingwater K., Buscher P., Brun R. Establishment of a panel of reference Trypanosoma evansi and Trypanosoma equiperdum strains for drug screening. Vet. Parasitol. 2007;148:114–121. doi: 10.1016/j.vetpar.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Ginouves M., Carme B., Couppie P., Prevot G. Comparison of tetrazolium salt assays for evaluation of drug activity against Leishmania spp. J. Clin. Microbiol. 2014;52:2131–2138. doi: 10.1128/JCM.00201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habte B., Bsrat A., Ashenafi H., Regassa F. Efficacy of some trypanocidal drug against Trypanosoma equiperdum OVI in experimentally infected mice in debre zeit, Ethiopia. EJBS. 2015;7:07–13. [Google Scholar]
- Hagos A., Goddeeris B., Yilkal K., Alemu T., Fikru R., Yacob H., Feseha G., Claes F. Efficacy of Cymelarsan® and Diminasan® against Trypanosoma equiperdum infections in mice and horses. Vet. Parasitol. 2010;171:200–206. doi: 10.1016/j.vetpar.2010.03.041. [DOI] [PubMed] [Google Scholar]
- Hirumi H., Hirumi K. In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology. 1991;102(Pt 2):225–236. doi: 10.1017/s0031182000062533. [DOI] [PubMed] [Google Scholar]
- Lu J., Vodnala S.K., Gustavsson A.L., Gustafsson T.N., Sjoberg B., Johansson H.A., Kumar S., Tjernberg A., Engman L., Rottenberg M.E., Holmgren A. Ebsulfur is a benzisothiazolone cytocidal inhibitor targeting the trypanothione reductase of Trypanosoma brucei. J. Biol. Chem. 2013;288:27456–27468. doi: 10.1074/jbc.M113.495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji T.G., Bin J., Yoshimura A., Tomura M., Tateyama D., Minami I., Yoshikawa Y., Aiba K., Heuser J.E., Nishino T., Hasegawa K., Nakatsuji N. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014;2:734–745. doi: 10.1016/j.stemcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma K., Allamanda P., Hakimi H., Zhou M., Angeles J.M., Kawazu S.I., Inoue N. Establishment of ATP-based luciferase viability assay in 96-well plate for Trypanosoma congolense. J. Vet. Med. Sci. 2014;76:1437–1441. doi: 10.1292/jvms.14-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma K., Narantsatsral S., Battur B., Yamasaki S., Otgonsuren D., Musinguzi S.P., Davaasuren B., Battsetseg B., Inoue N. Isolation, cultivation and molecular characterization of a new Trypanosoma equiperdum strain in Mongolia. Parasit. Vectors. 2016;9:481. doi: 10.1186/s13071-016-1755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes M.L., Avery V.M. Development of an Alamar Blue viability assay in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Am. J. Trop. Med. Hyg. 2009;81:665–674. doi: 10.4269/ajtmh.2009.09-0015. [DOI] [PubMed] [Google Scholar]
- Sykes M.L., Avery V.M. A luciferase based viability assay for ATP detection in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Parasit. Vectors. 2009;2:54. doi: 10.1186/1756-3305-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Q., Giroud C., Baltz T. In vivo and in vitro sensitivity of Trypanosoma evansi and T. equiperdum to diminazene, suramin, MelCy, quinapyramine and isometamidium. Acta Trop. 1991;50:101–110. doi: 10.1016/0001-706x(91)90002-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.