Abstract
Achilles tendinopathy is a common overuse condition affecting the adult population. The incidence is on the rise because of greater participation of people in recreational or competitive sporting activities. There are several treatment options available both non-operative and operative. Ultrasound-guided dry needling and high-volume image-guided injection is relatively a new procedure. The aim of this study was to find out the effectiveness of dry needling and HVIGI in the management of mid-portion chronic Achilles tendinopathy by performing a literature review. Search strategy was devised to find the suitable articles for critical appraisal using the electronic databases. Four articles were selected for critical appraisal, and these papers showed good short- to long-term results of image-guided high-volume injection in the management of Achilles tendinopathy. We conclude that high-volume image-guided injection is effective in the management of Achilles tendinopathy. It provides good short- and medium-term relief of symptoms. It should be considered as one of the many options available for this condition.
Keywords: Achilles tendinopathy, High-volume image-guided injection, Dry needling
Introduction
Tendinopathies are soft tissue disorders related to tendons [1]. Non-insertional Achilles tendinopathy occurs because of failure to mediate the repair and degeneration processes, leading to pain and disability [2]. The use of injectable substances such as platelet-rich plasma, autologous blood, polidocanol, corticosteroids and aprotinin in and around tendons is popular for Achilles tendinopathy, but evidence to support their role is minimal [3]. Ultrasound-guided high-volume image-guided injection (HVIGI) is relatively a new technique, and the literature on this technique is limited. This technique can be combined with dry needling.
Background
Achilles tendinopathy is a common overuse condition affecting the adult population. The incidence is on the rise because of greater participation of people in recreational or competitive sports [2]. The general population has an incidence of 2.35 per 1000 people, which is roughly equivalent to more than 150,000 people in the United Kingdom every year [4]. It is a clinical triad of pain, impaired performance and swelling [5]. The main stimulus to cause tendinopathy is excessive repetitive overload; however, about one-third of the studied group did not participate in the vigorous physical activities [6]. During running, forces up to 12.5 times body weight passes through the Achilles tendon which may contribute to tendinopathy [7].
Aetiology
Tendinopathies have been linked to poor vascularity, poor flexibility, genetic make-up, endocrine and metabolic factors [2]. Use of quinolone antibiotic has also been linked [8]. The pathological stimulus is excessive loading of the tendon during physical activity. Various intrinsic factors include poor vascularity, dysfunction of the gastrocnemius-soleus, age, gender, body weight, pes cavus and lateral instability of the ankle [2]. Changes in training pattern, poor technique, previous injuries, footwear and environmental factors such as training on hard, slippery or slanting surfaces are extrinsic factors [2]. The term “tendinopathy” is a description of a clinical condition which includes both pain and pathological process associated with overuse in and around the tendon. “Tendinosis” and “tendonitis” are histopathological descriptive terms and should ideally be used after the histopathological confirmation [9]. The pathological process of Achilles tendinopathy is considered to be a degenerative rather than inflammatory [10].
It is categorised into two types, insertional or non-insertional. Insertional type is less common and affects 20–25% of cases [11]. The predisposing factors for insertional type include increasing age, inflammatory arthropathies, corticosteroid use, diabetes, hypertension, obesity, gout, hyperostotic conditions, lipidaemias and quinolone antibiotics [12]. Symptoms are more proximal in the non-insertional type [13]. It accounts for 70–75% cases presenting, and it affects 9% of recreational runners. It can end the careers of 5% of professional runners [5].
Treatment options
There are many treatment options (Table 1). A recent meta-analysis has advocated that eccentric loading exercises are the gold standard in the management of Achilles tendinopathy [14] although other studies have pointed that there are not enough good-quality randomised controlled trials available in the literature on this subject [3, 12].
Table 1.
Treatment options
| Non-operative options | Operative options |
|---|---|
| Physical therapies | Tenotomies |
| Eccentric exercises | Multiple percutaneous longitudinal tenotomies |
| Shock wave therapy | Radiofrequency microtenotomy |
| Neovessel destruction | |
| Injectable substances | Arthroscopic |
| High-volume injections | Arthroscopic debridement |
| Corticosteroids | Surgery |
| Local anaesthetic agents | Tendon debridement |
| Platelet-rich plasma | Tendon decompression |
| Autologous blood injection | Tendon transfer |
| LMW heparin | Gastrocnemius lengthening |
| Sclerotherapy | |
| Other | |
| GTN Patches |
What is dry needling and high-volume image-guided injection (HVIGI)
Dry needling involves repeated needling in the abnormal tendon to promote an inflammatory response. Repeated passage of the needle produces physical trauma to the tendon which in turn causes internal haemorrhage leading to an inflammatory response which causes the formation of granulation tissue. This granulation tissue strengthens the tendon [15]. The local anaesthetic is injected around the area before performing the dry needling. There have been early reports of good results of ultrasound-guided needle tenotomy with corticosteroid injection for the treatment of common extensor tendons [16]. Jeffrey et al. evaluated the safety and short-term effectiveness of ultrasound-guided needle tenotomy in other tendons without the use of corticosteroids, because of the fear of tendon rupture [17]. Their study included 14 tendons, but only 4 of them were Achilles tendons.
High-volume image-guided injection (HVIGI) consists of normal saline, local anaesthetic and corticosteroids. It tends to improve amount of pain and improve function in patients suffering with Achilles tendinopathy [20]. The procedure is described as using an aseptic technique, a 21-gauge needle is inserted under real-time ultrasound guidance between the anterior aspect of the Achilles tendon and Kager’s fat pad, targeting the area of maximal neovascularisation. Then a mixture of 10 mL 0.5% bupivacaine hydrochloride and 25 mg of hydrocortisone acetate is injected, followed by 4 × 10 mL of injectable normal saline [20–22]. Some authors have described using more volume and without adding corticosteroids [23].
Other closely related procedures
There are few closely related procedures that are used for this condition. Sclerotherapy is a procedure that involves injecting a chemical into a blood vessel. The theory behind its use is to sclerose the vessels and eradicate the pain generating nerve fibres [2]. Autologous blood injection involves reinjection of patients own blood. The proposed mechanism of action of this is that the cytokines and growth factors within the injected blood help to stimulate tissue healing and production of type 1 collagen, promoting tendon repair [18].
Aim
The aim of this literature review was to find out the evidence for the use of high-volume injections in the treatment of chronic mid-substance Achilles tendinopathy.
Research question
The research question to do the literature review was produced using the PICO model (Table 2).
Table 2.
PICO model
| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
| Adult population with | Dry needling | Other minimally invasive treatment methods | Primary outcome |
| chronic Achilles tendinopathy | |||
| High-volume injection | Pain score | ||
| Secondary outcome | |||
| Functional outcome measure complications |
Materials and methods
A search strategy was devised to answer the research question. The PRISMA guidelines were used to conduct the review [19].
The primary objective was to review the effectiveness of high-volume image-guided injection in the management of Achilles tendinopathy. Therefore, the primary outcome measure was pain scores such as visual analogue score (VAS) and the secondary outcome was functional score such as Victorian institute of sports assessment for Achilles tendon (VISA-A).
Information sources
The articles were identified using the electronic databases of Embase (1980–March 2017), Medline (1950–May 2017) and PubMed. The Ovid search engine was used. The search was carried out on 19 March 2017. An additional search was carried out to find out any unpublished literature using the register of current controlled trials database for recently completed trials (http://controlled-trials.com/isrctn). One study was found, titled the “High Volume Saline Injections for Achilles Tendinopathy (ISRCTN87144429 DOI 10.1186/ISRCTN87144429)” but it has not been published yet. A hand search was also undertaken using the reference lists of review papers that were evaluated to identify any additional relevant articles.
Keywords
The keywords used were Achilles tendinopathy, Achilles tendonitis, high-volume image-guided injection and dry needling. Two searches were performed (Tables 3, 4). The terms were used under mesh headings. “AND” and “OR” terms were used to combine the keywords.
Table 3.
Search 1 (19.03.2017)
| Searches | Results | Type | |
|---|---|---|---|
| 1 | Achilles tendinopathy.mp. or exp Achilles tendinitis/ | 1486 | Advanced |
| 2 | Exp Achilles tendinitis/Achilles tendinitis.mp. | 1390 | Advanced |
| 3 | 1 or 2 | 1612 | Advanced |
| 4 | Dry needling.mp. | 408 | Advanced |
| 5 | 3 and 4 | 11 | Advanced |
Table 4.
Search 2 (19.03.2017)
| Searches | Results | Type | |
|---|---|---|---|
| 1 | Achilles tendinopathy.mp. or exp Achilles tendinitis | 1486 | Advanced |
| 2 | Exp Achilles tendinitis/Achilles tendinitis.mp. | 1390 | Advanced |
| 3 | 1 or 2 | 1612 | Advanced |
| 4 | High-volume image-guided injections.mp. | 3 | Advanced |
| 5 | 3 and 4 | 3 | Advanced |
Inclusion and exclusion
Studies selected for review were original articles. The inclusion criteria were:
Ultrasound-guided dry needling and high-volume injection studies
At least 10 patients were included in the study
Adult population age more than 18 years
The articles were published in English language
The full text was available for review
The exclusion criteria were:
Systematic reviews or meta-analysis
Animal or experimental studies
Conference abstracts
Studies using other non-operative methods such as eccentric loading exercises, sclerotherapy and prolotherapy.
Article selection
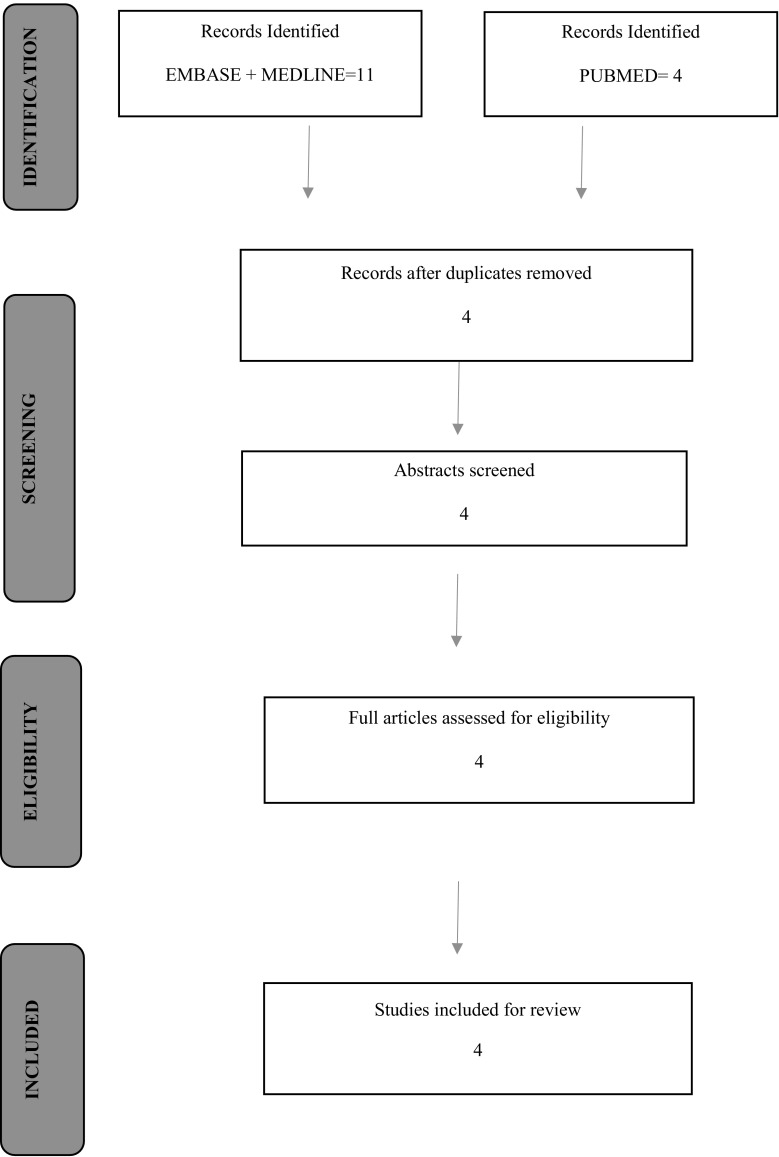
Four records were identified after running searches using Embase, Medline and PubMed. The details of this process are showed on the flow chart (Fig. 1). All 4 articles were found suitable that fulfilled the eligibility criteria and were included for the review (Tables 5, 6).
Fig. 1.
Flow chart showing search process in identifying suitable papers for analysis
Table 5.
Summary-1
| Authors | Study design | Age | Sex M: F | Previous treatment | Intervention | Pt had surgery | Final analysis | Level of study |
|---|---|---|---|---|---|---|---|---|
| Chan et al. [21] | Case series retrospective | 37.2 (24–58) | 26:4 | Yes | HVIGI 10 mL 0.5% bupivacaine 25 mg hydrocortisone 4 × 10 ml of normal saline |
0 | 21 | IV |
| Humphrey et al. [22] | Case series prospective | 43.5 ± 11.6 | 7:4 | Yes | HVIGI 10 mL 0.5% bupivacaine 25 mg hydrocortisone 4 × 10 mL normal saline |
0 | 11 | IV |
| Maffulli et al. [20] | Case series prospective | 37.5 (2263) | 69:25 | Yes | HVIGI 10 ml 0.25% bupivacaine 62,500 U aprotinin |
8 | 87 | IV |
| Wheeler et al. [23] | Case series retrospective two groups | 50.6 ± 11.3 | 14:20 | Yes | Group 1: 10 ml 1% lidocaine +40 ml N/saline HVIGI Group 2: dry needling + HVIGI. 10 ml 1% lidocaine +20 ml N/saline |
Group 1:3 Group 2:6 |
34 | III. |
Table 6.
Summary-2
| Outcome measures | Strengths/weakness | Conclusion | |
|---|---|---|---|
| Chan et al. | Short-term study-specific questionnaire Long-term VISA-A score |
Strength
Use of validated scores Standardised management Weakness Selection bias Postal questionnaire Recall bias No control group |
HVIGI reduce pain and improve short- and long-term functions |
| Humphrey et al. | VISA-A score Neovascularisation grade |
Strengths
Prospective Use of validated scores Standardised management Weakness Selection bias Small number No randomisation No control group |
HVIGI—an effective treatment to improve symptoms, reduce neovascularisation and decrease maximal tendon thickness |
| Maffulli et al. | Difference in VISA-A score Neovascularisation grade |
Strengths
Prospective Approval of local ethics committee Single radiologist—board certified Independent assessor Standardised management Weakness Selection bias No control group |
HVIGI effective in reducing pain and improve short- and long-term functions in 68% of the patients |
| Wheeler et al. [23] | Difference in VISA-A score |
Strengths
Compared two groups Use of validated scores Weakness Duration of symptoms in Group B not available Post-injection management not standardised |
HVIGI reduces VISA-A score in both groups Symptoms improve more in group 1 (HVIGI using more volume without dry needling) |
Study types
Three articles selected were case series studies [20–22]. Studies were done in a single centre in London hospital. Wheeler et al. [23] compared effect of HVIGI with or without dry needling in two groups.
Population
In the first three studies, the patients were recruited from the same centre, were physically active individuals and were private patients. The sample population in these three papers does not represent the whole population as the general population seeking treatment from NHS hospitals is a mixture of individuals with varying physical activities. The fourth study was carried out in the NHS setting, and therefore, population resemble more closely to the general population.
Intervention
Maffulli et al. used aprotinin rather than hydrocortisone in the HVIGI. Currently, there are no trials comparing HVIGI with hydrocortisone and HVIGI with aprotinin. At present, there are some concerns with the use of aprotinin such as post-operative thrombosis, organ dysfunction and allergic reactions. Wheeler et al. used HVIGI with or without dry needling. They also did not use corticosteroids.
Comparison
There were no control groups in the first three papers and, therefore, there was no comparison. Wheeler et al. compared the effect of HVIGI with and without dry needling.
Outcome measures
VAS is easy to use and understand. It seems to assess more closely what patients experience. It is responsive, reliable and valid [24]. The results are recorded in normalised numeric form and, therefore, easy to compare with different patients. It can be sometimes difficult to understand by the users and require time and commitment to explain to them. It has been suggested that mark on the VAS has no interpretable meaning [24]. Chan et al. used the study-specific questionnaire, and VAS was part of it.
VISA-A score is a reliable and validated score. It provides utility in both clinical setting and research. The questionnaire can be self-administered, with minimal investigator assistance, and hence, it avoids the observer bias [25]. The study population in these three studies were all athletes but were in different sports. It is suggested that VISA-A score can be used in intervention studies, but the results would be more specific if used in homogenous group of athletes, such as runners only or volleyball players only [26].
Results
The summary of the results is shown below (Table 7).
Table 7.
Results summary
| VAS score | VISA-A score | USG findings | |
|---|---|---|---|
| Chan et al. | Two weeks | At 30 weeks | × |
| Pre: 76 mm | Pre: 44.8 | ||
| Post: 25 mm | Post: 76.2 | ||
| At 30 weeks | |||
| Pre: 76 mm | |||
| Post: 28 mm | |||
| Humphrey et al. | × | Pre: 46.3 | Thickness |
| Post: 84.1 | Baseline: 8.7 mm | ||
| 3 weeks: 7.6 mm | |||
| Maffulli et al. | × | Baseline: 41.7 | Thickness |
| 12 months: 74.6 | Baseline: 9.1 mm | ||
| 12 months: 7.3 mm | |||
| Wheeler et al. | × | Group A | |
| Pre: 30 ± 21 | |||
| Post: 64 ± 28 | |||
| Group B | |||
| Pre: 31 ± 14 | |||
| Post: 37 ± 20 |
Strengths and weaknesses of the studies
The first three studies were carried out in the same centre, which was a private hospital in London. There is consistency in the papers with similar methodology, intervention and results. The authors have developed tools and protocols to consistently provide treatment for this condition. However, the normal population results cannot be compared with this group of patients. The fourth study was carried out in the general population. It was inspired by an audit. These studies can be used as baseline studies to formulate high-quality randomised controlled trials.
Discussion
These papers have shown promising results of high-volume injection in the management of chronic Achilles tendinopathy. Wheeler et al. [23] has further added that HVIGI without dry needling is more effective than HVIGI with dry needling. Achilles tendinopathy is a difficult condition to treat. The mainstay of treatment is non-operative. Oral NSAIDs are being used, both orally and as local application, but there is no convincing evidence that they are effective [26]. Eccentric loading exercises are most commonly used with good results [19]. It can be used for 6–12 weeks. The role of shockwave therapy (SWT) is controversial, and further evidence is required to justify its use in the Achilles tendinopathy [26].
In the resistant cases, injections have been used with variable results. These include corticosteroids, sclerotherapy, autologous blood, platelet-rich plasma, high-volume injections, hyperosmolar dextrose, and aprotinin and low-dose heparin. Andres et al. [26] have described that sclerotherapy has shown promising results, but most of the papers have come from the same group of authors. However, the same can be said about the ultrasound-guided high-volume image-guided injections, as all the three papers are from the same hospital. The results of platelet-rich plasma do not show expected benefits [27]. A randomised double-blind placebo-controlled study evaluating eccentric exercises and PRP or saline injection showed no difference in improvement in pain and activity at six months. A recent meta-analysis concluded that there was no evidence of any benefit in using PRP in the treatment of Achilles tendinopathy. It may be useful in Achilles tendon repair [19].
Metcalfe et al. [28] in their systematic review have concluded that there is no consensus as to whether local glucocorticoid injections have a therapeutic role in the treatment of Achilles tendinopathy. There is a small chance of tendon damage, and therefore, risks may outweigh the potential benefits. Conversely, Chan et al. and Humphrey et al. used hydrocortisone in their injection, but there was no reported tendon rupture in their study. Wheeler et al. did not use steroids in their technique to avoid the risk of tendon damage [23].
Reviewing these four papers one can say that HVIGI have shown good early results. These injections also reduced neovascularisation in the tendon as shown by Maffulli et al. [20], but it is worth remembering that pain is also possible in the presence of normal imaging [5]. Surgery should remain as last resort and when most of the non-operative methods have failed, and the patient fully understands the risks and benefits of the surgical procedure. There is 60–80% chance of satisfactory results after the surgical procedures [26], and these results are pretty similar to HVIGI as Maffulli et al. [20] showed improvement in pain and function in 68% of the patients.
Conclusion
We, therefore, conclude from the literature review that ultrasound-guided dry needling with high-volume injection provides good short- to medium-term relief of symptoms in the management of chronic mid-substance Achilles tendinopathy. The results are better without dry needling. It can be considered as one of the many options available for the management of this condition. It works well in conjunction with non-operative treatments, especially with eccentric loading exercises. It has low complication rate. However, based on this it is difficult to comment if this is superior to other non-operative options. Even in these four studies, there were so many variables, such as volume and the content of the solution used to achieve desired effects. Therefore, good-quality randomised controlled trials are required to find the best evidence in the management of this condition. But setting up a RCT in this case would not be easy, as there are so many options available. It is not simply comparing two treatment methods. Surgery should remain as the last resort when all the non-operative methods have failed.
Compliance with ethical standards
Conflict of interest
The author declares that he has no competing interests.
References
- 1.Riley G. Tendinopathy-from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4:82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 2.Maffulli N, Kader D. Tendinopathy of tendo Achilles. J Bone Jt Surg Br. 2002;84-B:1–8. doi: 10.1302/0301-620X.84B1.12792. [DOI] [PubMed] [Google Scholar]
- 3.Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. J Bone Joint Surg Am. 2010;92(15):2604–2613. doi: 10.2106/JBJS.I.01744. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge S, Van den Berg C, de Vos R, van der Heide HJL, Weir A, Verhaar JAN, Bierma-Zeinstra SMA. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011;45(13):106–1028. doi: 10.1136/bjsports-2011-090342. [DOI] [PubMed] [Google Scholar]
- 5.Longo UG, Rittweger J, Garau G, Radonic B, Gutwasser C, Gilliver SF, Kusy K, Zielinski J, Felsenber D, Maffulli N. No influence of age, gender, weight, height, and impact profile in Achilles tendinopathy in masters track and field athletes. Am J Sports Med. 2009;37(7):1400–1405. doi: 10.1177/0363546509332250. [DOI] [PubMed] [Google Scholar]
- 6.Rolf C, Movin T. Etiology, histopathology and outcome of surgery in achillodynia. Foot Ankle Int. 1997;18(9):565–569. doi: 10.1177/107110079701800906. [DOI] [PubMed] [Google Scholar]
- 7.Komi PV. Relevance of in vivo force measurements to human biomechanics. J Biomech. 1990;23(Suppl 1):23–34. doi: 10.1016/0021-9290(90)90038-5. [DOI] [PubMed] [Google Scholar]
- 8.Casparian JM, Luchi M, Moffat RE, Hinthorn D. Quinolones and tendon ruptures. South Med J. 2000;93(5):488–491. doi: 10.1097/00007611-200093050-00008. [DOI] [PubMed] [Google Scholar]
- 9.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. J Arthrosc Relat Surg. 1998;14(8):840–843. doi: 10.1016/S0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 10.Hutchison AM, Pallister I, Evans RM, Bodger O, Topliss CJ, Williams P, Beard DJ. Intense pulsed light treatment of chronic midbody Achilles tendinopathy: a double blind randomised placebo-controlled trial. Bone Jt J. 2013;95-B(4):504–509. doi: 10.1302/0301-620X.95B4.30558. [DOI] [PubMed] [Google Scholar]
- 11.Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19(1):54–64. doi: 10.1097/JSM.0b013e31818ef090. [DOI] [PubMed] [Google Scholar]
- 12.Roche AJ, Calder JDF. Achilles tendinopathty. A review of the current concepts of treatment. Bone Jt J. 2013;95-B(10):1299–1307. doi: 10.1302/0301-620X.95B10.31881. [DOI] [PubMed] [Google Scholar]
- 13.Maffulli N, Kenward MG, Testa V, Capasso G, Regine R, King JB. Clinical diagnosis of Achilles tendinopathy with tendinosis. Clin J Sport Med. 2003;13(1):11–15. doi: 10.1097/00042752-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Sussmilch-Leitch SP, Collins NJ, Bialocerkowski AE, Warden JS, Crossley KM. Physical therapies of Achilles tendinopathy: systematic review and meta-analysis. J Foot Ankle Res. 2012;5(1):15. doi: 10.1186/1757-1146-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James SLJ, Ali K, Pocock C, Robertson C, Walter J, Bell J, Connell D. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med. 2007;41(8):518–522. doi: 10.1136/bjsm.2006.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McShane JM, Nazarian LM, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25(10):1281–1289. doi: 10.7863/jum.2006.25.10.1281. [DOI] [PubMed] [Google Scholar]
- 17.Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187–1192. doi: 10.7863/jum.2009.28.9.1187. [DOI] [PubMed] [Google Scholar]
- 18.Bell KJ, Fulcher ML, Rowlands DS, Kerse N. Impact of autologous blood injections in treatment of mid-portion Achilles tendinopathy: double blind randomised controlled trial. BMJ. 2013;346:f2310. doi: 10.1136/bmj.f2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Atman D. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 20.Maffulli N, Spiezia F, Longo UG, Denaro V, Maffulli GD. High volume image guided injections for the management of chronic tendinopathy of the main body of the Achilles tendon. Phys Ther Sport. 2013;14(3):163–167. doi: 10.1016/j.ptsp.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Chan O, O’Dowd D, Padhiar N, Morrissey D, King J, Jalan R, Maffulli N. High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil. 2008;30(20–22):1697–1708. doi: 10.1080/09638280701788225. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey J, Chan O, Crisp T, Padhiar N, Morrissey D, Twycross-Lewis R, King J, Maffulli N. The short-term effects of high volume image guided injections in resistant non-insertional Achilles tendinopathy. J Sci Med Sport. 2010;13(3):295–298. doi: 10.1016/j.jsams.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler PC, Mahadevan D, Bhatt R, Bhatia M. A comparison of two different high-volume image-guided injection procedures for patients with chronic non insertional Achilles tendinopathy: a pragmatic retrospective cohort study. J Foot Ankle Surg. 2016;55(5):976–979. doi: 10.1053/j.jfas.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Hasson D, Arnetz BB. Validation and findings comparing VAS vs. Likert scales for psychosocial measurements. Int Electron J Health Educ. 2005;8:178–192. [Google Scholar]
- 25.Robinson JM, Cook JL, Purdam C, Visentini PJ, Ross J, Maffulli N, Taunton JE, Khan KM. VISA-A questionnaire: a valid and reliable index of the clinical severity Achilles tendionpathy. Br J Sports Med. 2001;35(5):335–341. doi: 10.1136/bjsm.35.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539–1554. doi: 10.1007/s11999-008-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vos RJ, Weir A, van Schie HTM, Bierma-Zeinstra SMA, Verhaar JAN, Weinans H, Tol JL. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 28.Metcalfe D, Achten J, Costa ML. Glucocorticoid injections in lesions of the Achilles tendon. Foot Ankle Int. 2009;30(7):661–665. doi: 10.3113/FAI.2009.0661. [DOI] [PubMed] [Google Scholar]