Abstract
Allogeneic hematopoietic cell transplantation (HCT) is a well-established therapeutic modality effective for a variety of hematological malignancies but, unfortunately, is associated with significant morbidity and mortality related to cancer relapse as well as to transplant-related complications including graft-versus-host-disease (GvHD). Natural killer (NK) cells are the first donor-derived lymphocyte subset to recover after HCT, and their crucial role in protection against cancer relapse and infections is well established. Conversely, the role played by NK cells in GvHD is still controversial. Early studies suggested a participation of NK cells in GvHD induction or exacerbation. Subsequently, experimental evidence obtained in mice as well observational studies performed in humans led to a model in which NK cells play a regulatory role in GvHD by repressing alloreactive T cell responses. This widely accepted model has been recently challenged by clinical evidence indicating that NK cells can in some cases promote GvHD. In this review, we summarize available knowledge about the role of NK cells in GVHD pathogenesis. We review studies uncovering cellular mechanisms through which NK cells interact with other immune cell subsets during GvHD leading to a model in which NK cells naturally suppress GvHD through their cytotoxic ability to inhibit T cell activation unless exogenous hyperactivation lead them to produce proinflammatory cytokines that can conversely sustain T cell-mediated GvHD induction.
Keywords: natural killer cells, graft-versus-host-disease, bone marrow transplantation, HSCT, tolerance
Introduction
Natural killer (NK) cells are the first donor-derived lymphocyte subsets to recover after hematopoietic cell transplantation (HCT), preceding by several months the reconstitution of adaptive T and B lymphocytes. NK cells have been the focus of significant attention in the HCT field over the last four decades. Studies of the role of NK cells in bone marrow engraftment demonstrated that host NK cells persisting after conditioning can contribute to graft rejection (1) while donor NK cells can promote hematopoietic engraftment (2). At the same time, several preclinical and clinical studies focusing on NK cell alloreactivity in anticancer responses identified donor NK cells as crucial players in preventing cancer relapse after HCT for hematologic malignancies (3, 4). Less well established, however, is the role of NK cells in graft-versus-host-disease (GvHD), a major complication of HCT. While the classical model of GvHD pathogenesis includes, together with donor-derived T cells, donor-derived NK cells in the immune-pathological activation leading to GvHD (5), evidence from preclinical models as well as from studies in human HCT recipients led to a more complex picture where NK cells could either promote or prevent GvHD.
In this review, we summarize the available knowledge about the role of NK cells in GvHD pathogenesis. After reviewing preclinical and clinical studies uncovering cellular mechanisms through which NK cells interact with other immune cell subsets during GvHD, we propose a new model in which distinct effector mechanisms determine the pathogenic or regulatory role of NK cells in promotion or control of GvHD, respectively. Finally, we discuss the impact that GvHD can in turn have on NK cell biology and the potential consequences in the context of HCT.
Early Studies
The first study suggesting a relationship between NK cells and GvHD development was reported by Lopez and coworkers from the Sloan Kettering Cancer Center showing a significant association between GvHD development and pre-transplant levels of NK cell activity, as measured by cytotoxic assays performed using herpes simplex virus type 1-infected fibroblast as target cells, in peripheral blood of a small and heterogeneous cohort of 13 patients undergoing different protocols of HCT (6). Importantly, most of the patients included in the series underwent HCT after myeloablative conditioning, and no information was provided about NK cell activity after transplantation. Shortly thereafter, Livnat et al. (7) and Dokhelar et al. (8) addressed the same issue assessing NK cell activity against the K562 leukemic cell line both before and after HCT and obtained contradictory results finding either no relationship (7) or a positive association (8) between early posttransplant NK cell activity and GvHD development. Despite the contradictory conclusions obtained and the limitations of the studies including the heterogeneity of the patients cohorts as well as of the analytical methods employed, these early studies opened the way to numerous studies addressing the role of NK cells in GvHD.
A first approach has been to investigate the presence of NK cells in GvHD target organs. In the mouse parent-into-F1 (P > F1) model of GvHD, increased NK cell activity measured against YAC lymphoma target cells was detected in spleen (9–11), lymph nodes (9, 10), thymus (9, 12), and intestinal intraepithelial lymphocytes (10) from mice with active GvHD. Similarly, in murine minor mismatch HCT models, large granular lymphocytes displaying an immunophenotype characteristic of NK cells infiltrated the skin (13), liver, and intestine (14) from animals with acute GVHD. Importantly, the use of congenic markers demonstrated that these cells were of donor origin (14). Accordingly, the study of biopsies obtained from skin (15–17), liver (18, 19), and intestinal (20) of patients with acute GvHD showed the presence of NK cells among the lymphoid population infiltrating these GvHD target tissues. The study of biopsies obtained from female patients transplanted with male donor grafts confirmed in humans the donor origin of the NK cells infiltrating tissues during GvHD (16). The target tissues infiltration by NK cells during GvHD, both in mice and humans, supported a model in which NK cells may induce, or at least contribute to, GvHD development. Attempts were, therefore, made to obtain experimental evidence supporting this hypothesis, first by using NK cell depleting antibodies directed against the cell surface glycolipid asialo GM1 or to the cell surface NKR-P1 family receptor NK1.1. However, results from reports employing this approach were inconsistent, few studies suggested a reduction of GvHD upon treatment of recipients (21–23) while most studies employing antibody depletion on donor cells showed only minimal if any impact on GvHD development (23–27). This discrepancy suggested that depleting antibodies exerted their effect through the depletion of an effector cell population appearing after HCT rather then by depleting NK cells contained in the graft. Further, the epitopes recognized by anti-asialo GM1 and anti-NK1.1 antibodies are expressed by several immune cell subsets other than NK cells, including activated T cells involved in GvHD development (28–30), making it impossible to distinguish between an NK and a T cell directed effect. Ghayur et al. used a complimentary approach employing beige mice carrying a homozygous bg mutation that leads to severe deficiency in NK cell function. Adoptive transfer of bg/bg splenocytes failed to induce GvHD in a P > F1 model, while transfer of heterozygous +/bg induced hepatic GvHD, suggesting that donor NK cells were responsible for GvHD induction (31). However, even in this model, a functional deficit in adaptive T cells from beige mice complicates the interpretation of the results (32, 33).
NK Cell Cytotoxic Functions and GvHD Prevention
While murine models based on antibody depletion or genetic alteration of NK cells failed to provide consistent evidence for a role of NK cells in GvHD pathogenesis, the adoptive transfer of NK cells offered unexpected insights. In an attempt to promote bone marrow engraftment in a major mismatch murine model, Murphy and coworkers adoptively transferred NK cells purified from C.B-17 severe combined immunodeficiency (SCID) (H-2d) mice into lethally irradiated C57BL/6J (H-2b) mice together with non-T-cell depleted bone marrow cells from BALB/cJ (H-2d) mice with or without splenocytes (2). In mice not receiving splenocytes, transferred NK cells did not induce GvHD, thus questioning the NK GvHD-inducing potential suggested by antibody depletion studies. More interestingly, in mice receiving splenocytes, activated NK cells prevented the development of GvHD that invariably lead to death of mice injected with BM cells and splenocytes alone. This unexpected result revealed not only that NK cells can be adoptively transferred safely in this major mismatch model without inducing GvHD but also that they can prevent T cell-mediated GvHD development. The results of this first study were confirmed during the years by several other reports (3, 34–39) and numerous studies in humans suggested that higher numbers of NK cells (40–47) and the presence of NK cell alloreactivity (3, 4, 48–50) reduce GvHD development.
In particular, NK cell alloreactivity has been found to be crucial for NK cell-mediated protection from GvHD. Ruggeri et al. showed in a major mismatch HCT murine model that alloreactive Ly49 ligand-mismatched NK cell infusion prevented T cell-induced GvHD, while administration of even large numbers of non-alloreactive Ly49 ligand-matched NK cells provided no protection (3). These results were subsequently confirmed by Lundqvist et al. who further extended this observation showing that, although inefficient in preventing GvHD, Ly49 ligand-matched NK cells displayed an antitumor activity similar to Ly49 ligand-mismatched NK cells (35). The need of Ly49 ligand-mismatch for GvHD control by NK cells prompted some investigators to silence Ly49C to induce alloreactivity with promising results (51). Alloreactive NK cells were shown to indirectly inhibit T cell proliferation and GvHD induction by depleting antigen-presenting cells (APCs) (3, 38) through their cytolytic activity, the c-Kit−CD27−CD11b+ NK cells being the most potent in this effect (38). In particular, the expression of the activating receptor KIR2DS1, which binds to HLA-C2, seems to contribute to the APCs’ killing and it was even able to override the inhibition mediated by the expression of the inhibitory receptor NKG2A, which binds to HLA-E in humans or Qa-1b in mouse (50). Similarly, proportions of donor-derived NK cells expressing the activating receptor CD94/NKG2C, which recognize as well HLA-E/Qa-1b, were lower in HLA-matched and HLA-mismatched HCT recipients with acute or chronic GvHD compared with patients without GvHD (52). Accordingly, patients with acute or chronic GvHD displayed a lower ratio of CD94/NKG2C to CD94/NKG2A on NK cells suggesting a competition for the same ligands between NKG2C and NKG2A that would result in NK cell activation or suppression, respectively (52). Finally, Ghadially et al. suggested that NK cell-mediated killing of APC during GvHD is mediated by the stimulation of NKp46 receptor by still unknown ligand(s) expressed by dendritic cells (DCs) as the absence of NKp46 on donor NK cells leads to increased stimulation of donor T cells by DCs (53), resulting in increased tissue damage (54).
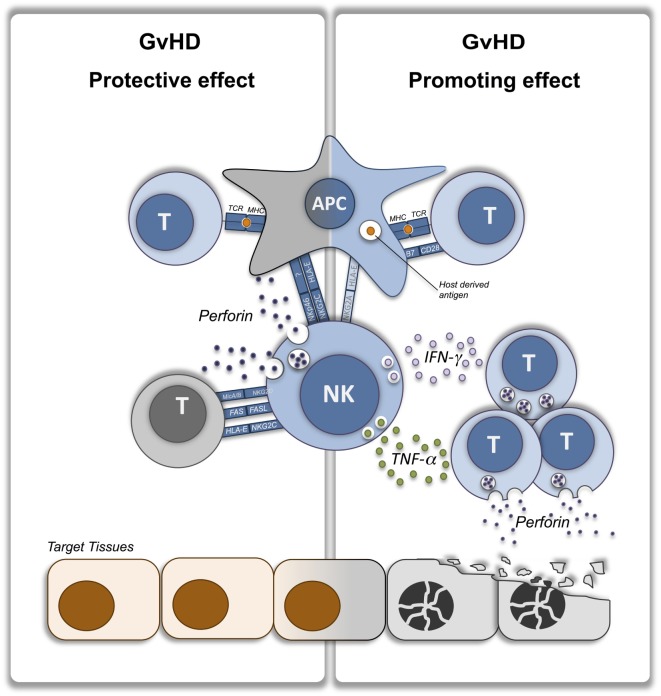
In addition to this indirect, APC-killing mechanism, others and we have shown that NK cells can suppress GvHD by directly lysing activated T cells. In vitro evidence obtained in murine (55) and human (56, 57) cells showed that T cells during activation upregulate stress molecules acting as ligands for the NK activating receptor NKG2D, thus becoming targets of NK cell-mediated killing. In a major mismatch HCT model, we showed that allogeneic T cells upregulate the NKG2D ligand Rae1γ and perhaps other molecules during GvHD and thus become susceptible to NK cell-mediated killing through a NKG2D-dependent cell lysis (37). Noval Rivas and coworkers obtained very similar results in a minor mismatch model of chronic GvHD induced by adoptive transfer of monoclonal anti-male CD4 T cells into lymphopenic male mice (58). Interestingly, we observed in our system an increased ratio of splenic donor regulatory T cells (Treg) to total donor conventional CD4+ and CD8+ T cells (Tcon) in the presence of NK cells, suggesting a differential susceptibility of Treg and Tcon to NK cell-mediated cell lysis leading to an immune-regulatory environment that eventually contributes to GvHD suppression (37). Direct T cell killing by NK cells can, therefore, be considered as a complimentary mechanism of GvHD suppression, in addition to the aforementioned modulation by APC-killing, which can be particularly important at GvHD tissues sites. Accordingly, we have shown that, after transplantation, NK cells traffic to GvHD target organs following a spatial and temporal distribution very similar to T cells (59) offering them the opportunity to target activated T cell at the effector site. However, in contrast to T cells, NK cells have a more limited persistence, which may in part explain their reduced capacity for GVHD induction. Interestingly, GvHD prevention by T cell killing at tissue sites can be exerted as well by residual tissue resident recipient NK cells eventually persisting after conditioning depending on conditioning intensity as it has been recently shown in a minor mismatch murine model (60). T cell killing by NK cells appears to be dependent on both perforin production (37, 60) and FAS-mediated induction of apoptosis (37, 58, 61). Collectively, these models demonstrated that NK cells can suppress GvHD development through their cytotoxic function either directly, by depleting activated alloreactive T cells, or indirectly, by depleting APC and preventing T cell stimulation (Figure 1, left panel).
Figure 1.
NK cells contribution to graft-versus-host-disease (GvHD) protection or induction. Immunological interactions leading to GvHD protective (left panel) or promoting (right panel) effect of NK cells. Donor immune cells are depicted in blue while host target cells are depicted in orange. NK, natural killer; T, T lymphocyte; APC, antigen-presenting cell; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α.
NK Cell Cytokine Production and GvHD Induction
In addition to their cytolytic potential, NK cell can modulate immune responses through cytokine production. Whether this mechanism can participate in GvHD prevention by NK cells is unclear. One of the early studies showed that administration of anti-TGFβ monoclonal antibody significantly limited the NK cell suppressive effect on GvHD (34). However, no evidence was provided that NK cells were indeed the source of TGFβ and administration of exogenous TGFβ failed to prevent GvHD development, indicating that TGFβ contribution to GvHD suppression is only partial and through a mechanism still to be completely uncovered.
Although it is unclear if NK cells production of immune-suppressive cytokines can prevent GvHD, it is established that pro-inflammatory cytokine production by NK cells can contribute to GvHD development. In a xenogeneic model, Xun et al. showed that in vitro interleukin-2 (IL-2)-activated human NK cells producing interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) were able to induce acute GvHD upon transfer into SCID mice (62, 63). Interestingly, NK cells were found in GvHD target tissues in juxtaposition to damaged cells and produced in situ IFN-γ and TNF-α (62). Although the limitations of the xenogeneic model should be taken into account, the results from the aforementioned experiments suggest that, when pre-activated to produce the pro-inflammatory cytokines IFN-γ and TNF-α, NK cells can indeed promote rather than prevent GvHD development. In accordance, while NK depletion by NK1.1 antibodies had no effect on GvHD when employed on steady-state donor splenocytes (25), it significantly prevented GvHD when employed on splenocytes obtained from donor mice previously treated with the toll-like receptor 3 stimulator polyinosinic:polycytidylic acid (poly I:C) (64, 65) by reducing IFN-γ production (65). Further, higher proportions of IFN-γ-producing NK cells after HCT have been shown to be associated in humans with an increased incidence of acute GvHD (66). Collectively, these studies provide evidence for a promoting role of NK cells in GvHD, opposite from the suppressive role exerted by cytolysis, through the production of pro-inflammatory cytokines that may act directly to induce cell damage or indirectly by increasing T cell-mediated tissue damage through their well-known property to increase MHC expression (Figure 1, right panel). This model can be useful in the interpretation of the otherwise surprising results recently reported by Shah and coworkers (67). Most studies involving adoptive transfer of NK cells into HCT recipients failed to observe GvHD induction after infusion (68–70) (Table 1). Similarly, studies assessing the potential of adoptively transferred allogeneic haploidentical NK cells into lymphodepleted patients in non-allogeneic HCT settings did not observe any cases of acute GvHD (71–75) (Table 1). Few studies reporting the development of acute GvHD after allogeneic NK cell adoptive transfer (76–78) were unable to establish a causative relationship between the NK cell infusion and GvHD development because of other potential contributing factors including immune-suppression discontinuation (76) or residual T cell contamination of the administered cell product (77). Conversely, the report by Shah and coworkers provide some evidence for an NK cell involvement in GvHD development. The authors reported the development of GvHD in five out of nine recipients of HLA-matched, T-cell-depleted peripheral blood HCT upon adoptive transfer of donor-derived IL-15/4-1BBL-activated NK cells (67). The direct involvement of donor NK cells in GvHD was suggested by their presence in the lymphoid infiltrate found in biopsies of GvHD involved tissues (67). However, despite the fact that grafts contained very low numbers of T cells as a result of T cell depletion by CD34 positive selection, several issues suggested that the NK-cell-promoting role on GvHD could have been mediated by an indirect effect on T cells. First, a higher proportion of patients developing GvHD received grafts from unrelated donors, therefore, were provided with a higher alloreactive potential, compared to patients not developing GvHD (67). Second, patients developing GvHD displayed more rapid T-cell engraftment, as revealed by day 14 and day 28 CD3-chimerism, compared with patients not developing GvHD (67). Moreover, it should be noted that patients were free of T-cell directed immune-suppressive treatment at the time of adoptive transfer. Importantly, the timing of the administration of the NK cells could have been another factor pushing the balance toward GvHD induction. Patients from the aforementioned report (67) received the pre-activated NK cells around the time of engraftment. Murine studies have demonstrated the importance of the timing of NK cell administration on GvHD prevention, showing no benefit of delayed treatment (37) and even a potential for GvHD exacerbation when NK cells were administered at later time points (34), although in these latter experiments IL-2 was administered at the same time as the NK cells and could have contributed to the phenomenon. This opposing effect can be related with the production of IFN-γ that has been shown to inhibit GVHD when provided early after HCT and to exacerbate GVHD when acting at a later time (79). Considering all of these factors, it can be speculated that the administration of highly pre-activated NK cells can enhance clinically undetectable T-cell alloreactivity through the production of pro-inflammatory cytokines (Figure 1, right panel) and that this functional aspect can, therefore, prevail on their GvHD-protective cytotoxic activity (Figure 1, left panel), thus promoting GvHD development.
Table 1.
Acute graft-versus-host-disease (GvHD) development reported in published natural killer (NK) cells adoptive transfer clinical trials.
| Reference | N | Age | Disease | Donor type | Conditioning | Time from allo-hematopoietic cell transplantation (HCT) | Cell isolation | NK cells preparation | Cell dose (106/kg) | Combined therapy | Acute GvHD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Passweg et al. (68) | 5 | Adult | AML | Haploidentical | Etoposide | Post allo-HCT (day +3 to +26) | CD3 depletion | Fresh | 6.9–14.1 | – | 0/5 (0%) |
| CML | Cy/TBI/ATG | CD56 selection | |||||||||
| Miller et al. (71) | 19 | Adult | AML | Haploidentical | Cy/Flu | No allo-HCT | CD3 depletion | Interleukin-2 (IL-2) activated | 0.1–20 | IL-2 | 0/43 (0%) |
| Solid tumors | |||||||||||
| Rubnitz et al. (72) | 10 | Ped | AML | Haploidentical | Cy/Flu | No allo-HCT | CD3 depletion | Fresh | 5–8 | IL-2 | 0/10 (0%) |
| CD56 selection | |||||||||||
| Yoon et al. (76) | 14 | Adult | AML | Haploidentical | Bu/Flu/ATG | Post allo-HCT (day +43 to +50) | CD34 selection | In vitro differentiated | N/A | – | 1/14 (7%) (1 grade II) |
| MDS | HLA-mismatched | In vitro differentiation | |||||||||
| ALL | |||||||||||
| Curti et al. (73) | 13 | Adult | AML | Haploidentical | Cy/Flu | No allo-HCT | CD3 depletion | Fresh | 1.11–5 | IL-2 | 0/13 (0%) |
| CD56 selection | |||||||||||
| Stern et al. (77) | 16 | Adult | AML | Haploidentical | MAC/ATG or OKT3 | Post allo-HCT (day +3 to +40) | CD3 depletion | Fresh | 8–76 | – | 4/16 (25%) (1 grade II, 2 grade III, 1 grade IV) |
| Ped | ALL | CD56 selection | Cryopreserved | ||||||||
| Solid tumors | |||||||||||
| Klingemann et al. (74) | 13 | Adult | HL | Haploidentical | None | No allo-HCT | CD3 depletion | IL-2 activated | 0.1–20 | – | 0/13 (0%) |
| NHL | |||||||||||
| MM | |||||||||||
| Bachanova et al. (75) | 57 | Adult Ped |
AML | Haploidentical | Cy/Flu | No allo-HCT (n = 53) Post allo-HCT (n = 4) |
CD3 depletion | IL-2 activated | 3.4–15 | IL-2 | 0/57 (0%) |
| ±CD19 depletion | IL2DT | ||||||||||
| ±CD56 selection | |||||||||||
| Choi et al. (69) | 41 | Adult | AML/MDS | Haploidentical | Bu/Flu/ATG | Post allo-HCT (day +14 to +21) | CD3 depletion | Ex vivo expanded | 20–500 | – | 9/41 (21%) (2 grade I, 2 grade II, 5 grade III–IV) |
| ALL | |||||||||||
| Lymphoma | |||||||||||
| Shah et al. (67) | 9 | Adult | Sarcomas | HLA-matched sibling/unrelated donor | Cy/Flu/Melph | Post allo-HCT (day +7 to +35) | CD3 depletion | IL-15/4-1BBL activated | 0.1–1 | – | 5/9 (55%) (1 grade II, 3 grade IV, 1 non-gradable) |
| Ped | CD56 selection | ||||||||||
| Lee et al. (78) | 21 | Adult Ped |
AML | Haploidentical | Bu/Flu | Post allo-HCT (day −8) | CD3 depletion | IL-2 activated | 0.02–8.32 | IL-2 | 7/21 (33%) (5 grade II, 2 grade III) |
| MDS | CD56 selection | ||||||||||
| CML | |||||||||||
| Jaiswal et al. (70) | 10 | Adult | AML | Haploidentical | Treo/Flu/TBI | Post allo-HCT (day +7) | CD56 selection | Fresh | 1.7–17.7 | – | 0/10 (0%) |
| Ped | CML | PTCy |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BM, bone marrow; Bu, busulfan; CML, chronic myeloid leukemia; Cy, cyclophosphamide; Flu, fludarabine; HL, Hodgkin’s lymphoma; MDS, myelodysplastic syndrome; Melph, melphalan; NHL, non-Hodgkin lymphoma; MAC, myeloablative conditioning; MM, multiple myeloma; PTCy, posttransplant cyclophosphamide; TBI, total body irradiation; Treo, treosulfan.
GvHD Modulation of NK Cells
While NK cells may positively or negatively participate in GvHD development, the GvHD process can in turn affect NK cell biology. Pattengale and coworkers were the first to demonstrate in murine models that acute but not chronic GvHD induce a marked decrease in NK cell activity associated with an impaired production of IFN-γ (80). NK cell reconstitution appears to be significantly delayed by acute GvHD in mice (81) and by acute and chronic GvHD in humans (42, 82–85). Recent evidence from a murine model of GvHD suggest that activated T cells could limit NK cell access to IL-15 through direct competition for this cytokine necessary for NK cell development and homeostasis, administration of exogenous IL-15 being able to restore NK cell reconstitution (81). In addition to its quantitative effect, GvHD induces qualitative defects on NK cells ultimately leading to impaired function. Bunting and coworkers recently showed in mice that, during GvHD, donor NK cells display a hyperactivated phenotype associated with increased signs of apoptosis and autophagy (81). Importantly, they showed that GVHD-induced alterations in NK cells resulted in defective in vivo cytotoxicity resulting in a reduction of graft-versus-leukemia effect and an impaired control of cytomegalovirus infection (81). This dysfunctional status induced by GvHD is reminiscent of the NK cell exhaustion phenomenon we observed upon chronic proliferation, characterized by an impaired transcriptional machinery as revealed by the downregulation of the Tbox transcription factors Eomesodermin and Tbet (86). Accordingly, we reported in humans that exhaustion is increased in NK cells after HCT and is further exacerbated in NK cells from patients with acute GvHD (87).
Concluding Remarks
Despite major efforts undertaken during many years to better understand NK cells biology in the context of HCT, the role of NK cells during GvHD remained elusive because of conflicting evidence coming from different experimental approaches. NK cells are capable of both effector and regulatory functions. This pleiotropic nature of NK cells is likely responsible for the variable and even conflicting roles that NK cells can play during GvHD. We hope our model (Figure 1) will help interpret this apparent contradiction. Importantly, clarifying the impact of NK cell activation status on their GvHD induction potential will hopefully contribute to the optimization of cell manufacturing procedures to maximize allogeneic NK cell antitumor potential while preventing GvHD induction.
Author Contributions
FS wrote the manuscript and designed the figure. MA critically revised the work for important intellectual content and edited the manuscript. RN edited the manuscript and provided overall guidance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Program Project Grant CA49605 from the National Institute of Health. FS is supported by the Geneva University Hospitals, the Swiss Cancer League, the Dubois-Ferrière-Dinu-Lipatti Foundation, and the Fondation Genevoise de bienfaisance Valeria Rossi di Montelera. MA is supported by the American Society for Blood and Marrow Transplantation and the American Association for Cancer Research.
References
- 1.Murphy WJ, Kumar V, Bennett M. Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID). Evidence that natural killer cells can mediate the specificity of marrow graft rejection. J Exp Med (1987) 165(4):1212–7. 10.1084/jem.165.4.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy WJ, Bennett M, Kumar V, Longo DL. Donor-type activated natural killer cells promote marrow engraftment and B cell development during allogeneic bone marrow transplantation. J Immunol (1992) 148(9):2953–60. [PubMed] [Google Scholar]
- 3.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (2002) 295(5562):2097–100. 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 4.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood (2007) 110(1):433–40. 10.1182/blood-2006-07-038687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood (2000) 95(9):2754–9. [PubMed] [Google Scholar]
- 6.Lopez C, Sorell M, Kirkpatrick D, O’Reilly R, Ching C, The Bone Marrow Transplant Unit . Association between pre-transplant natural kill and graft-versus-host disease after stem-cell transplantation. Lancet (1979) 314(8152):1103–7. 10.1016/S0140-6736(79)92506-6 [DOI] [PubMed] [Google Scholar]
- 7.Livnat S, Seigneuret M, Storb R, Prentice RL. Analysis of cytotoxic effector cell function in patients with leukemia or aplastic anemia before and after marrow transplantation. J Immunol (1980) 124(1):481–90. [PubMed] [Google Scholar]
- 8.Dokhelar M-C, Wiels J, Lipinski M, Tetaud C, Devergie A, Gluckman E, et al. Natural killer cell activity in human bone marrow recipients: early reappearance of peripheral natural killer activity in graft-versus-host disease. Transplantation (1981) 31(1):61–5. 10.1097/00007890-198101000-00014 [DOI] [PubMed] [Google Scholar]
- 9.Roy C, Ghayur T, Kongshavn PA, Lapp WS. Natural killer activity by spleen, lymph node, and thymus cells during the graft-versus-host reaction1. Transplantation (1982) 34(3):144–6. 10.1097/00007890-198209000-00006 [DOI] [PubMed] [Google Scholar]
- 10.Borland A, Mowat AMI, Parrott DM. Augmentation of intestinal and peripheral natural killer cell activity during the graft-versus-host reaction in mice. Transplantation (1983) 36(5):513–9. 10.1097/00007890-198311000-00009 [DOI] [PubMed] [Google Scholar]
- 11.Ghayur T, Seemayer TA, Lapp WS. Kinetics of natural killer cellcytotoxicity reaction: relationship between natural killer cell activity, T and B cell activity, and development of histopathological alterations. Transplantation (1987) 44(2):254–60. 10.1097/00007890-198708000-00016 [DOI] [PubMed] [Google Scholar]
- 12.Ghayur T, Seemayer TA, Lapp WS. Association between the degree of thymic dysplasia and the kinetics of thymic NK cell activity during the graft-versus-host reaction. Clin Immunol Immunopathol (1988) 48(1):19–30. 10.1016/0090-1229(88)90153-5 [DOI] [PubMed] [Google Scholar]
- 13.Guillen F, Ferrara J, Hancock W, Messadi D, Fonferko E, Burakoff S, et al. Acute cutaneous graft-versus-host disease to minor histocompatibility antigens in a murine model. Evidence that large granular lymphocytes are effector cells in the immune response. Lab Invest (1986) 55(1):35–42. [PubMed] [Google Scholar]
- 14.Ferrara JL, Guillen FJ, van Duken PJ, Marion A, Murphy GF, Burakoff SJ. Evidence that large granular lymphocytes of donor origin mediate acute graft-versus-host disease. Transplantation (1989) 47(1):50–4. 10.1097/00007890-198901000-00012 [DOI] [PubMed] [Google Scholar]
- 15.Acevedo A, Aramburu J, López J, Fernández-Herrera J, Fern JM, López-Botet M. Identification of natural killer (NK) cells in lesions of human cutaneous graft-versus-host disease: expression of a novel NK-associated surface antigen (Kp43) in mononuclear infiltrates. J Invest Dermatol (1991) 97(4):659–66. 10.1111/1523-1747.ep12483724 [DOI] [PubMed] [Google Scholar]
- 16.Horn TD, Haskell J. The lymphocytic infiltrate in acute cutaneous allogeneic graft-versus-host reactions lacks evidence for phenotypic restriction in donor-derived cells. J Cutan Pathol (1998) 25(4):210–4. 10.1111/j.1600-0560.1998.tb01721.x [DOI] [PubMed] [Google Scholar]
- 17.Takata M, Imai T, Hirone T. Immunoelectron microscopy of acute graft versus host disease of the skin after allogeneic bone marrow transplantation. J Clin Pathol (1993) 46(9):801–5. 10.1136/jcp.46.9.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dilly S, Sloane J. An immunohistological study of human hepatic graft-versus-host disease. Clin Exp Immunol (1985) 62(3):545. [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M, Umihara J, Shimmoto K, Cui S, Sata H, Ishikawa T, et al. The pathogenesis of graft-versus-host reaction in the intrahepatic bile duct. Pathol Int (1989) 39(10):648–55. 10.1111/j.1440-1827.1989.tb02412.x [DOI] [PubMed] [Google Scholar]
- 20.Roy J, PLATT JL, Weisdorf DJ. The immunopathology of upper gastrointestinal acute graft-versus-host disease lymphoid cells and endothelial adhesion molecules. Transplantation (1993) 55(3):572–7. 10.1097/00007890-199303000-00022 [DOI] [PubMed] [Google Scholar]
- 21.Charley M, Mikhael A, Bennett M, Gilliam J, Sontheimer R. Prevention of lethal, minor-determinate graft-host disease in mice by the in vivo administration of anti-asialo GM1. J Immunol (1983) 131(5):2101–3. [PubMed] [Google Scholar]
- 22.Varkila K. Depletion of asialo-GM1 + cells from the F1 recipient mice prior to irradiation and transfusion of parental spleen cells prevents mortality to acute graft-versus-host disease and induction of anti-host specific cytotoxic T cells. Clin Exp Immunol (1987) 69(3):652. [PMC free article] [PubMed] [Google Scholar]
- 23.Ghayur T, Seemayer TA, Lapp WS. Prevention of murine graft-versus-host disease by inducing and eliminating ASGM1 + cells of donor origin. Transplantation (1988) 45(3):586–90. 10.1097/00007890-198803000-00017 [DOI] [PubMed] [Google Scholar]
- 24.Varkila K, Hurme M. Natural killer (NK) cells and graft-versus-host disease (GVHD): no correlation between the NK cell levels and GVHD in the murine P—F1 model. Immunology (1985) 54(1):121. [PMC free article] [PubMed] [Google Scholar]
- 25.Blazar BR, Soderling CC, Koo GC, Vallera DA. Absence of a facilitory role for NK 1.1-positive donor cells in engraftment across a major histocompatibility barrier in mice1, 2. Transplantation (1988) 45(5):876–82. 10.1097/00007890-198805000-00007 [DOI] [PubMed] [Google Scholar]
- 26.Johnson BD, Truitt RL. A decrease in graft-vs.-host disease without loss of graft-vs.-leukemia reactivity after MHC-matched bone marrow transplantation by selective depletion of donor NK cells in vivo. Transplantation (1992) 54(1):104–12. 10.1097/00007890-199207000-00019 [DOI] [PubMed] [Google Scholar]
- 27.Xenocostas A, Ghayur T, Setrakian J, Lapp W, Osmond D. A donor-derived asialo-GM1 + cell induces depression of B-cell genesis during systemic graft-versus-host disease. Blood (1994) 84(11):3965–73. [PubMed] [Google Scholar]
- 28.Charley MR, Mikhael A, Hoot G, Hackett J, Bennett M. Studies addressing the mechanism of anti-asialo GM 1 prevention of graft-versus-host disease due to minor histocompatibility antigenic differences. J Invest Dermatol (1985) 85:121s–3s. 10.1111/1523-1747.ep12275630 [DOI] [PubMed] [Google Scholar]
- 29.Charley MR, Mikhael A, Hackett J, Kumar V, Bennett M. Mechanism of anti-asialo GM1 prevention of graft-vs-host disease: identification of allo-antigen activated T cells. J Invest Dermatol (1988) 91(3):202–6. 10.1111/1523-1747.ep12464858 [DOI] [PubMed] [Google Scholar]
- 30.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, García-Ojeda M, Sibley R, et al. Bone marrow NK1. 1− and NK1. 1 + T cells reciprocally regulate acute graft versus host disease. J Exp Med (1999) 189(7):1073–81. 10.1084/jem.189.7.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghayur T, Seemayer TA, Kongshavn PA, Gartner JG, Lapp WS. Graft-versus-host reactions in the beige mouse: an investigation of the role of host and donor natural killer cells in the pathogenesis of graft-versus-host disease. Transplantation (1987) 44(2):261–6. 10.1097/00007890-198708000-00017 [DOI] [PubMed] [Google Scholar]
- 32.Carlson GA, Marshall ST, Truesdale AT. Adaptive immune defects and delayed rejection of allogeneic tumor cells in beige mice. Cell Immunol (1984) 87(2):348–56. 10.1016/0008-8749(84)90004-2 [DOI] [PubMed] [Google Scholar]
- 33.Halle-Pannenko O, Bruley-Rosset M. Decreased graft-versus-host reaction and T cell cytolytic potential of beige mice. Transplantation (1985) 39(1):85–7. 10.1097/00007890-198501000-00013 [DOI] [PubMed] [Google Scholar]
- 34.Asai O, Longo DL, Tian Z-G, Hornung RL, Taub DD, Ruscetti FW, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest (1998) 101(9):1835. 10.1172/JCI1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood (2007) 109(8):3603–6. 10.1182/blood-2006-05-024315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G, Wu D, Wang Y, Cen J, Feng Y, Sun A, et al. Expanded donor natural killer cell and IL-2, IL-15 treatment efficacy in allogeneic hematopoietic stem cell transplantation. Eur J Haematol (2008) 81(3):226–35. 10.1111/j.1600-0609.2008.01108.x [DOI] [PubMed] [Google Scholar]
- 37.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood (2010) 115(21):4293–301. 10.1182/blood-2009-05-222190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meinhardt K, Kroeger I, Bauer R, Ganss F, Ovsiy I, Rothamer J, et al. Identification and characterization of the specific murine NK cell subset supporting graft-versus-leukemia- and reducing graft-versus-host-effects. Oncoimmunology (2015) 4(1):e981483. 10.4161/2162402X.2014.981483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber CM, Doisne JM, Colucci F. IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model of mismatched hematopoietic cell transplantation. Eur J Immunol (2015) 45(6):1727–35. 10.1002/eji.201445200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soiffer RJ, Gonin R, Murray C, Robertson MJ, Cochran K, Chartier S, et al. Prediction of graft-versus-host disease by phenotypic analysis of early immune reconstitution after CD6-depleted allogeneic bone marrow transplantation. Blood (1993) 82(7):2216–23. [PubMed] [Google Scholar]
- 41.Yamasaki S, Henzan H, Ohno Y, Yamanaka T, Iino T, Itou Y, et al. Influence of transplanted dose of CD56 + cells on development of graft-versus-host disease in patients receiving G-CSF-mobilized peripheral blood progenitor cells from HLA-identical sibling donors. Bone Marrow Transplant (2003) 32(5):505–10. 10.1038/sj.bmt.1704165 [DOI] [PubMed] [Google Scholar]
- 42.Abrahamsen IW, Somme S, Heldal D, Egeland T, Kvale D, Tjonnfjord G. Immune reconstitution after allogeneic stem cell transplantation: the impact of stem cell source and graft-versus-host disease. Haematologica (2005) 90(1):86–93. [PubMed] [Google Scholar]
- 43.Kim DH, Sohn SK, Lee NY, Baek JH, Kim JG, Won DI, et al. Transplantation with higher dose of natural killer cells associated with better outcomes in terms of non-relapse mortality and infectious events after allogeneic peripheral blood stem cell transplantation from HLA-matched sibling donors. Eur J Haematol (2005) 75(4):299–308. 10.1111/j.1600-0609.2005.00514.x [DOI] [PubMed] [Google Scholar]
- 44.Larghero J, Rocha V, Porcher R, Filion A, Ternaux B, Lacassagne MN, et al. Association of bone marrow natural killer cell dose with neutrophil recovery and chronic graft-versus-host disease after HLA identical sibling bone marrow transplants. Br J Haematol (2007) 138(1):101–9. 10.1111/j.1365-2141.2007.06623.x [DOI] [PubMed] [Google Scholar]
- 45.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia (2007) 21(10):2145–52. 10.1038/sj.leu.2404892 [DOI] [PubMed] [Google Scholar]
- 46.Tanaka M, Kobayashi S, Numata A, Tachibana T, Takasaki H, Maruta A, et al. The impact of the dose of natural killer cells in the graft on severe acute graft-versus-host disease after unrelated bone marrow transplantation. Leuk Res (2012) 36(6):699–703. 10.1016/j.leukres.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 47.Kim SY, Lee H, Han M-S, Shim H, Eom H-S, Park B, et al. Post-transplantation natural killer cell count: a predictor of acute graft-versus-host disease and survival outcomes after allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk (2016) 16(9):527.–535. 10.1016/j.clml.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 48.Ludajic K, Balavarca Y, Bickeböller H, Rosenmayr A, Fae I, Fischer G, et al. KIR genes and KIR ligands affect occurrence of acute GVHD after unrelated, 12/12 HLA matched, hematopoietic stem cell transplantation. Bone Marrow Transplant (2009) 44(2):97–103. 10.1038/bmt.2008.432 [DOI] [PubMed] [Google Scholar]
- 49.Clausen J, Kircher B, Auberger J, Schumacher P, Ulmer H, Hetzenauer G, et al. The role of missing killer cell immunoglobulin-like receptor ligands in T cell replete peripheral blood stem cell transplantation from HLA-identical siblings. Biol Blood Marrow Transplant (2010) 16(2):273–80. 10.1016/j.bbmt.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 50.Sivori S, Carlomagno S, Falco M, Romeo E, Moretta L, Moretta A. Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: implications in haploidentical HSCT. Blood (2011) 117(16):4284–92. 10.1182/blood-2010-10-316125 [DOI] [PubMed] [Google Scholar]
- 51.Cao D, Hu L, Wang Y, Wang L, Zheng W, Ma W. Suppression of graft-versus-host disease after adoptive infusion of alloreactive NK cells induced by silencing Ly49C gene in mice. Transpl Immunol (2009) 20(4):243–8. 10.1016/j.trim.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 52.Kordelas L, Steckel N-K, Horn PA, Beelen DW, Rebmann V. The activating NKG2C receptor is significantly reduced in NK cells after allogeneic stem cell transplantation in patients with severe graft-versus-host disease. Int J Mol Sci (2016) 17(11):1797. 10.3390/ijms17111797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghadially H, Horani A, Glasner A, Elboim M, Gazit R, Shoseyov D, et al. NKp46 regulates allergic responses. Eur J Immunol (2013) 43(11):3006–16. 10.1002/eji.201343388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghadially H, Ohana M, Elboim M, Gazit R, Gur C, Nagler A, et al. NK cell receptor NKp46 regulates graft-versus-host disease. Cell Rep (2014) 7(6):1809–14. 10.1016/j.celrep.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, et al. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol (2003) 170(7):3572–6. 10.4049/jimmunol.170.7.3572 [DOI] [PubMed] [Google Scholar]
- 56.Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK-cell lysis. Blood (2007) 110(2):606–15. 10.1182/blood-2006-10-052720 [DOI] [PubMed] [Google Scholar]
- 57.Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML, et al. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK–T cell interaction. Blood (2011) 117(18):4778–86. 10.1182/blood-2010-08-300954 [DOI] [PubMed] [Google Scholar]
- 58.Noval Rivas M, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol (2010) 184(12):6790–8. 10.4049/jimmunol.0902598 [DOI] [PubMed] [Google Scholar]
- 59.Olson JA, Zeiser R, Beilhack A, Goldman JJ, Negrin RS. Tissue-specific homing and expansion of donor NK cells in allogeneic bone marrow transplantation. J Immunol (2009) 183(5):3219–28. 10.4049/jimmunol.0804268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nalle SC, Kwak HA, Edelblum KL, Joseph NE, Singh G, Khramtsova GF, et al. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Science translational medicine (2014) 6(243):243ra87. 10.1126/scitranslmed.3008941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvarez M, Bouchlaka MN, Sckisel GD, Sungur CM, Chen M, Murphy WJ. Increased antitumor effects using IL-2 with anti-TGF-β reveals competition between mouse NK and CD8 T cells. J Immunol (2014) 193(4):1709–16. 10.4049/jimmunol.1400034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xun C, Brown SA, Jennings CD, Henslee-Downey PJ, Thompson JS. Acute graft-versus-host-like disease induced by transplantation of human activated natural killer cells into SCID mice. Transplantation (1993) 56(2):409–16. 10.1097/00007890-199308000-00031 [DOI] [PubMed] [Google Scholar]
- 63.Xun CQ, Thompson JS, Jennings CD, Brown SA. The effect of human IL-2-activated natural killer and T cells on graft-versus-host disease and graft-versus-leukemia in SCID mice bearing human leukemic cells. Transplantation (1995) 60(8):821–7. 10.1097/00007890-199510000-00011 [DOI] [PubMed] [Google Scholar]
- 64.MacDonald GC, Gartner JG. Prevention of acute lethal graft-versus-host disease in F1 hybrid mice by pretreatment of the graft with anti-NK-1.1 and complement. Transplantation (1992) 54(1):147–51. 10.1097/00007890-199207000-00026 [DOI] [PubMed] [Google Scholar]
- 65.Ellison CA, HayGlass KT, Fischer JM, Rector ES, MacDonald GC, Gartner JG. Depletion of natural killer cells from the graft reduces interferon-γ levels and lipopolysaccharide-induced tumor necrosis factor-α release in F1 hybrid mice with acute graft-versus-host disease1. Transplantation (1998) 66(3):284–94. 10.1097/00007890-199808150-00002 [DOI] [PubMed] [Google Scholar]
- 66.Cooley S, McCullar V, Wangen R, Bergemann TL, Spellman S, Weisdorf DJ, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood (2005) 106(13):4370–6. 10.1182/blood-2005-04-1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell–depleted stem cell transplantation. Blood (2015) 125(5):784–92. 10.1182/blood-2014-07-592881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia (2004) 18(11):1835–8. 10.1038/sj.leu.2403524 [DOI] [PubMed] [Google Scholar]
- 69.Choi I, Yoon SR, Park S-Y, Kim H, Jung S-J, Jang YJ, et al. Donor-derived natural killer cells infused after human leukocyte antigen–haploidentical hematopoietic cell transplantation: a dose-escalation study. Biol Blood Marrow Transplant (2014) 20(5):696–704. 10.1016/j.bbmt.2014.01.031 [DOI] [PubMed] [Google Scholar]
- 70.Jaiswal SR, Zaman S, Nedunchezhian M, Chakrabarti A, Bhakuni P, Ahmed M, et al. CD56-enriched donor cell infusion after post-transplantation cyclophosphamide for haploidentical transplantation of advanced myeloid malignancies is associated with prompt reconstitution of mature natural killer cells and regulatory T cells with reduced incidence of acute graft versus host disease: a pilot study. Cytotherapy (2017) 19(4):531–42. 10.1016/j.jcyt.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 71.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood (2005) 105(8):3051–7. 10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 72.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol (2010) 28(6):955–9. 10.1200/jco.2009.24.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood (2011) 118(12):3273–9. 10.1182/blood-2011-01-329508 [DOI] [PubMed] [Google Scholar]
- 74.Klingemann H, Grodman C, Cutler E, Duque M, Kadidlo D, Klein AK, et al. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell–enriched infusions. Transfusion (2013) 53(2):412–8. 10.1111/j.1537-2995.2012.03764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood (2014) 123(25):3855–63. 10.1182/blood-2013-10-532531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoon S, Lee Y, Yang S, Ahn K, Lee J-H, Lee J-H, et al. Generation of donor natural killer cells from CD34+ progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant (2010) 45(6):1038–46. 10.1038/bmt.2009.304 [DOI] [PubMed] [Google Scholar]
- 77.Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant (2013) 48(3):433–8. 10.1038/bmt.2012.162 [DOI] [PubMed] [Google Scholar]
- 78.Lee DA, Denman CJ, Rondon G, Woodworth G, Chen J, Fisher T, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant (2016) 22(7):1290–8. 10.1016/j.bbmt.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y-G, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest (1998) 102(12):2126. 10.1172/JCI4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pattengale PK, Ramstedt U, Gidlund M, Örn A, Axberg I, Wigzell H. Natural killer activity in (C57BL/6 × DBA/2) F1 hybrids undergoing acute and chronic graft-vs.-host reaction. Eur J Immunol (1983) 13(11):912–9. 10.1002/eji.1830131110 [DOI] [PubMed] [Google Scholar]
- 81.Bunting MD, Varelias A, Souza-Fonseca-Guimaraes F, Schuster IS, Lineburg KE, Kuns RD, et al. GVHD prevents NK-cell-dependent leukemia and virus-specific innate immunity. Blood (2017) 129(5):630–42. 10.1182/blood-2016-08-734020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kheav VD, Busson M, Scieux C, de Latour RP, Maki G, Haas P, et al. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica (2014) 99(12):1860–7. 10.3324/haematol.2014.108407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Podgorny PJ, Liu Y, Dharmani-Khan P, Pratt LM, Jamani K, Luider J, et al. Immune cell subset counts associated with graft-versus-host disease. Biol Blood Marrow Transplant (2014) 20(4):450–62. 10.1016/j.bbmt.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 84.Ullrich E, Salzmann-Manrique E, Bakhtiar S, Bremm M, Gerstner S, Herrmann E, et al. Relation between acute GVHD and NK cell subset reconstitution following allogeneic stem cell transplantation. Front Immunol (2016) 7:595. 10.3389/fimmu.2016.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huenecke S, Cappel C, Esser R, Pfirrmann V, Salzmann-Manrique E, Betz S, et al. Development of three different NK cell subpopulations during immune reconstitution after pediatric allogeneic hematopoietic stem cell transplantation: prognostic markers in GvHD and viral infections. Front Immunol (2017) 8:109. 10.3389/fimmu.2017.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gill S, Vasey AE, De Souza A, Baker J, Smith AT, Kohrt HE, et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood (2012) 119(24):5758–68. 10.1182/blood-2012-03-415364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simonetta F, Pradier A, Bosshard C, Masouridi-Levrat S, Chalandon Y, Roosnek E. NK cell functional impairment after allogeneic hematopoietic stem cell transplantation is associated with reduced levels of T-bet and eomesodermin. J Immunol (2015) 195(10):4712–20. 10.4049/jimmunol.1501522 [DOI] [PubMed] [Google Scholar]