Abstract
Movement defects in obesity are associated with peripheral muscle defects, arthritis, and dysfunction of motor control by the brain. Although movement functionality is negatively correlated with obesity, the brain regions and downstream signaling pathways associated with movement defects in obesity are unclear. A dopaminergic neuronal pathway from the substantia nigra (SN) to the striatum is responsible for regulating grip strength and motor initiation through tyrosine hydroxylase (TH) activity-dependent dopamine release. We found that mice fed a high-fat diet exhibited decreased movement in open-field tests and an increase in missteps in a vertical grid test compared with normally fed mice. This motor abnormality was associated with a significant reduction of TH in the SN and striatum. We further found that phosphorylation of c-Jun N-terminal kinase (JNK), which modulates TH expression in the SN and striatum, was decreased under excess-energy conditions. Our findings suggest that high calorie intake impairs motor function through JNK-dependent dysregulation of TH in the SN and striatum.
Keywords: Dopaminergic neuron, Obesity, Motor abnormality, Tyrosine hydroxylase
INTRODUCTION
Obesity leads to metabolic alterations that cause multiple organ dysfunction, manifesting as hepatosteatosis, increased hepatic glucose production, and defects in insulin secretion by the pancreas [1,2]. An imbalance between energy expenditure and intake leads to dysregulation of energy homeostasis in the brain [3]. Obesity-induced disruption of crosstalk between the periphery and brain causes motor abnormalities [4,5]. For example, both peripheral factors, including osteoarthritis and musculopathy, and a decreased ability of neurocognitive functions to adapt to changes in walking in the context of obesity, aggravate movement impairments [4,5,6]. However, it is not clear which brain areas are associated with obesity-related motor defects.
Movement functionality is negatively correlated with body mass index (BMI) [7,8,9]. Mobility defects observed in obesity are affected by the abnormal distribution of body fat, which is concentrated in the abdomen [4,8]. Motor control including grip strength, voluntary movement, and motor initiation are directly regulated by the dopamine system consisting of tyrosine hydroxylase (TH)-expressing neuron that originate in the substantia nigra (SN) and ventral tegmental area (VTA) and project to the striatum and prefrontal cortex [10,11,12,13]. Defects in the dopamine system lead to Parkinson's disease (PD), and Huntington's disease [12,14]. Recent study reported the correlation of dopamine with obesity [15,16]. Dopamine D2-receptor knockout in striatum increased compulsive eating and access to palatable food [16]. However, the relevance between TH expression in dopamine neuronal system and obesity-related motor defect is still unclear.
The mouse high-fat-diet (HFD)-induced obesity model exhibits features similar to the human obese condition [2,14], including significantly reduced TH mRNA expression in the SN compared with controls, as confirmed by in situ hybridization [17]. The expression and activity of TH, a rate-limiting enzyme in dopamine biosynthesis that mediates the conversion of amino acids to L-DOPA, are indispensable for the regulation of intracellular dopamine levels [11,12,17]. There is also a correlation between an increase in BMI and PD among the elderly [7]. Extreme body weight causes a deterioration in the mobility of PD patients compared with patients with a normal body weight [4,5,8,18]. This decrease in the physical activity of PD patients has been assessed using indirect calorimetry, which revealed a substantial decrease in energy expenditure [7,9,19].
In the current study, we addressed the relationship between high-calorie-intake–induced obesity and defects in movement caused by an associated decrease in TH. TH expression is regulated by SRY (sex-determining region Y), c-Jun N-terminal kinase (JNK), cAMP, and glucocorticoid signaling [20,21]. JNK, which is expressed ubiquitously in neurons, is activated by phosphorylation in response to cellular stress and regulates cell death signaling in the nervous system [22,23]. JNK is necessary for the modulation of neuron-specific synaptic plasticity and cytoskeletal reorganization during neurodevelopment through regulation of proteins associated with microtubules [22,23,24]. As a neurodegeneration mediator, JNK is involved in the loss of TH caused by treatment with the neurotoxin, paraquat [25,26]. However, cystatin C, a cysteine protease inhibitor, enhances TH gene expression in a JNK-dependent manner [27]. In addition, JNK activation is necessary for ATF2 phosphorylation, which increases nicotine-induced TH gene expression [28]. On the basis of these reports, we postulated that chronic energy stress caused by HFD feeding affects movement disorders through a decrease in TH expression and JNK activation in the SN and striatum.
MATERIALS AND METHODS
Immunofluorescence staining
At the conclusion of experiments, NCD- and HFD-fed C57BL/6 mice were perfused with a 4% paraformaldehyde solution. Whole brains were removed, then stored in 4% paraformaldehyde for 3 days. After transferring brains to a 30% sucrose solution, coronal sections (25 µm thick) were prepared, washed with PBS, and then blocked by incubating for 1 hour with 3% donkey serum (Dako, Glostrup, Denmark) and 0.3% Triton X-100 in the dark. Thereafter, sections were washed with PBS and incubated overnight at 4℃ with anti-TH (1:500; Millipore, MA, USA) and anti-pJNK (1:500; Invitrogen, Carlsbad, CA, USA) antibodies, diluted in blocking solution. Sections were then washed with PBS and incubated with fluorescently labeled secondary antibody for 1 hour at room temperature. Sections from SN was obtained through -2.60 mm to -3.90 mm relative to bregma [39]. Sections from striatum was obtained through -1.08 mm to -0.84 mm relative to bregma [40]. TH and pJNK fluorescence (n=10 slides per condition, n=6 each groups) were visualized using an IX70 confocal microscope (Olympus, Tokyo, Japan).
Animals
Male C57BL/6 mice, purchased from Harlan Teklad (Madison, WI, USA), were maintained at a temperature of 22℃ with 12-h light-dark cycle. For HFD-induced obesity, 5-week-old C57BL/6 mice were fed a 60% fat diet (Research Diets Inc., NJ, USA) and body weight was monitored (n=6, each group of feeding condition). Animal experiments were approved by the Institutional Animal Care and Use Committee of Chungnam National University (ethical approval number, CNU-00356).
Open-field test
Each mouse was placed in 40×40×40 cm box, and movement was recorded for 60 minutes. Movement distance of HFD-fed or NCD-fed mice (n=6, each groups) were analyzed using EthoVision XT 11.5 software (Noldus, Wageningen, The Netherlands).
Vertical grid test
The apparatus for the vertical grid test is an open box (8×55×5 cm) with a back wall made of 0.8×0.8 cm wire mesh. HFD and NCD-fed mouse (n=6, each groups of feeding) were adapted to the vertical grid test by placing inside the apparatus for 3 days and allowing them to turn and climb down. Experiments were performed by recording and analyzing time to climb, time to turn, time to climb down, and missteps [11].
Measurement of plasma leptin levels
The plasma leptin concentration in NCD- and HFD-fed mice (n=6, each groups) was measured using an ELISA kit (Enzo Life Science, Farmingdale, NY, USA).
Glucose tolerance test and insulin tolerance test
For glucose tolerance tests, 6 of HFD-fed and NCD-fed mice were fasted for 24 hours and then administered 2 g/kg glucose by intraperitoneal injection. Blood glucose levels were measured using a glucometer (Roche, Basel, Switzerland). For insulin tolerance tests, mice were fasted for 6 hours and then injected with 0.75 U/kg insulin (Eli Lilly, Indianapolis, IN, USA). Insulin tolerance was tested using a mouse insulin ELISA kit (ALPCO, Salem, NH, USA).
Statistical analysis
All results are presented as means ± standard error of the mean (SEM) of triplicate experiments. Statistical analyses were performed using Prizm version 5 software (GraphPad, San Diego, CA, USA). The significance of differences between two groups (n=6, each groups) was analyzed using a one-tailed Student's t-test. A p-value<0.05 was considered statistically significant.
RESULTS
HFD leads to a general decrease in movement accompanied by abnormal motor behavior
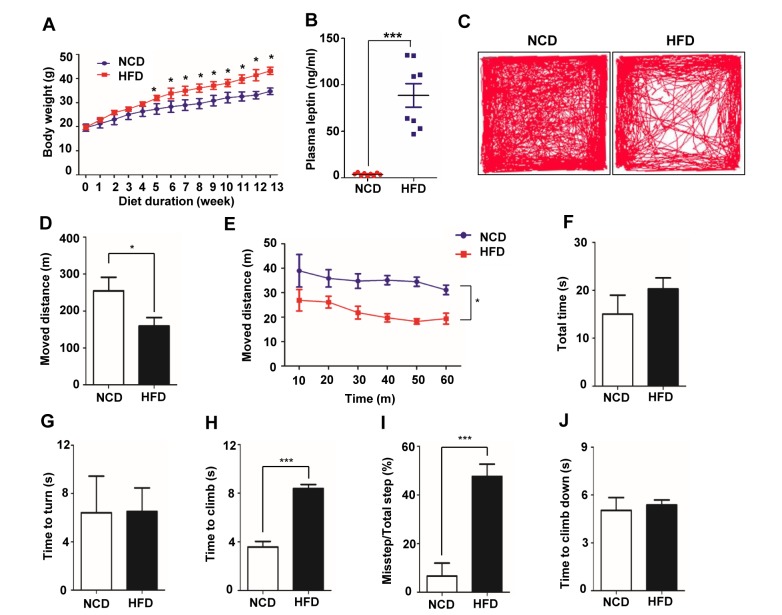
As an experimental model, we used HFD-induced obese mice, which exhibit increased adiposity and metabolic alterations [2]; mice fed a normal diet (NCD) were used as controls. As shown in Fig. 1A, HFD-fed mice showed a 14.5% greater body weight gain than NCD-fed mice after 5 weeks on the diet, and the difference in body weight between groups increased until 13 weeks (Fig. 1A). In obesity, plasma levels of leptin, which is produced by adipocytes, are elevated owing to increases in adipose tissue deposits [29,30,31]. Consistent with this, we found that the plasma leptin concentration, measured after 16 hours of fasting, was ~17.7 ng/ml in the HFD-fed group after 20 weeks, but only ~0.76 ng/ml in the NCD-fed group (Fig. 1B). To verify peripheral metabolic alterations caused by HFD feeding, we performed intraperitoneal glucose tolerance (Table 1) and insulin tolerance (Table 2) tests. Impaired glucose tolerance and decreased insulin sensitivity were observed in the HFD group. These data suggest that consumption of a HFD for 20 weeks enhanced body weight gain predominantly by increasing lipid deposits.
Fig. 1. HFD-induced obesity in C58BL/6 mice and decrease of general and motor behavior in HFD-fed mice. (A) HFD-fed, 5-week-old C57BL/6 mice (n=6/group) showed a significant increase in body weight gain compared with NCD-fed mice. (B) Plasma leptin levels in NCD- or HFD-fed mice was measured by ELISA. (C) Traces of mouse movements during open-field tests are presented. (D) The total moved distance of HFD-fed mice decreased compared with that of NCD-fed mice. (E) Movement was analyzed as distance per time. (F~J) A mouse was placed 3 cm from the bottom of the apparatus and allowed to climb upward, turn around, and climb down during recordings. (F) Total time is the sum of time to climb, make turn and climb down. (G) Time taken for the mouse to turn. (H) Time taken for the mouse to climb before it turned. (I) During the climb down, the number of failed hindlimb steps was measured and converted to a percentage of total steps. (J) Time taken for the mouse to climb down after is turned. In HFD-fed mice, the time to climb and percentage of missteps increased. There were no differences in the total time, time to turn, or time to climb down. *p<0.05, **p<0.01, ***p<0.001.
Table 1. Impaired glucose tolerance in intraperitoneal glucose tolerance tests.
| Time (min) | Blood glucose (mg/dL) | |||
|---|---|---|---|---|
| NCD | HFD | |||
| Mean | SD | Mean | SD | |
| 0 | 82.80 | 10.26 | 133.00 | 15.11 |
| 15 | 379.33 | 24.80 | 455.75 | 18.82 |
| 30 | 290.00 | 36.13 | 393.33 | 28.67 |
| 60 | 200.00 | 14.08 | 321.00 | 28.38 |
| 90 | 156.20 | 16.38 | 247.33 | 24.05 |
| 120 | 137.80 | 14.46 | 235.20 | 24.53 |
Table 2. Impaired insulin sensitivity, confirmed by insulin tolerance tests.
| Time (min) | % Initial glucose | |||
|---|---|---|---|---|
| NCD | HFD | |||
| Mean | SD | Mean | SD | |
| 0 | 100 | 6.11 | 100 | 7.2 |
| 15 | 47.51 | 2.68 | 60.5 | 4.26 |
| 30 | 33.48 | 3.01 | 41.83 | 3.04 |
| 60 | 24.56 | 1.92 | 29.5 | 1.88 |
| 90 | 25.54 | 1.97 | 31 | 2.02 |
| 120 | 29.24 | 1.2 | 34.17 | 2 |
Because obesity affects motor behaviors, such as walking, standing and grabbing an object while standing [5], we compared the movement of NCD-fed and HFD-fed mice. The distance moved by HFD-fed mice over the course of 60 minutes in an open-field test was approximately 60% that of NCD-fed mice (Fig. 1C~E). To further assess motor deficits, we subjected HFD- and NCD-fed mice to a vertical grid test, which measures time to climb up, turn and climb down, as well as missteps [11]. Although time to turn and total time were not different between NCD-fed and HFD-fed mice (Fig. 1F, G), the time to climb was ~2-times longer for HFD-fed mice than for NCD-fed mice (Fig. 1H). Moreover, the percentage of missteps during the climb down increased by ~5-fold in HFD-fed mice (Fig. 1I). This latter finding indicates that HFD-fed mice slipped more because their grip strength for grabbing the wire was reduced. This slippage increased the speed of the HFD-fed mice as they climbed down, such that their descent time was only slightly longer than that of the NCD-fed mice even though their movement was substantially impaired (Fig. 1J). Taken together, these results show that HFD-induced obesity caused motor dysfunction and decreased overall movement.
Decreased TH in the SN and striatum of HFD-fed mice
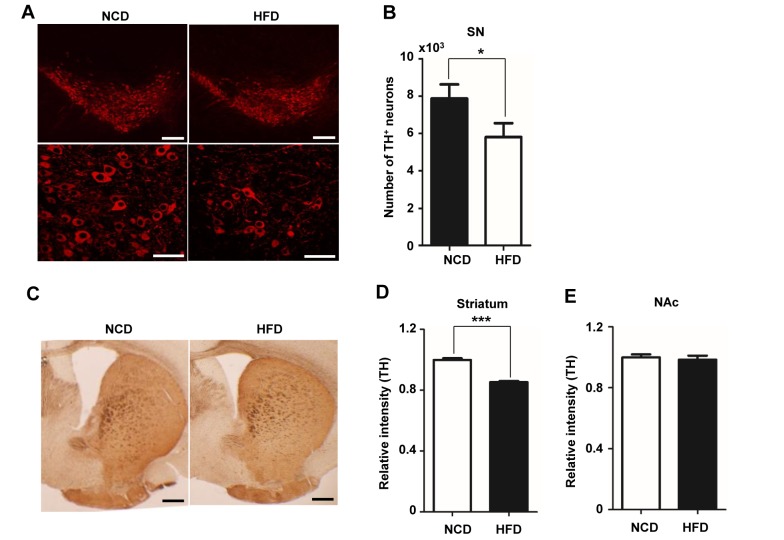
Under conditions of obesity, mRNA expression of TH, a key enzyme in dopamine biosynthesis, is reduced in the SN region [17]. TH-positive dopaminergic neurons in the SN project to the striatum and modulate motor function and mobility [13,32,33]. Because behavioral defects, including delayed climbing time on the vertical grid test and decreased movement in the open-field test, was observed in HFD-fed mice (Fig. 1), we investigated TH expression in the SN and striatum region by immunohistochemistry. Consistent with the decrease in motor function, TH expression was significantly decreased (Fig. 2A). A count of the number of TH-positive neurons showed a ~26.3% decrease in the proportion of cells expressing TH in HFD-fed mice compared with NCD-fed mice (Fig. 2B). In the striatum, TH staining intensity in the HFD group was decreased by ~14.63% compared with the NCD group (Fig. 2C, D). By contrast, TH immunostaining in the nucleus accumbens (NAc) was comparable in HFD- and NCD-fed mice (Fig. 2E). These data suggest that the loss of TH in the SN and striatum is predominantly responsible for the impaired motor function in HFD-fed mice.
Fig. 2. TH expression is decreased in mice with HFD-induced obesity. (A) TH immunoreactivity (red) was detected in the SN region of mice fed a NCD or HFD for 20 weeks. Upper panel, low magnification (×100) image; lower panel, higher magnification (×600) image. (B) The number of TH-positive neurons in the SN was counted and graphed (n=10 slides per condition, n=6/group). Sections were incubated at 4℃ with chicken anti-TH antibody (1:300), and fluorescence was visualized by confocal microscopy. (C) Immunohistochemical detection of TH in the striatum. Brain slices were incubated with a rabbit anti-TH antibody (1:300). After incubation with secondary antibody, diaminobenzidine staining was performed. (D) The TH-positive area was measured using ImageJ software. (E) The intensity of TH immunostaining in the NAc region was measured using ImageJ. Scale bars: 50 µm. *p<0.05, **p<0.01.
Decrease of phosphorylated JNK in HFD-fed mice
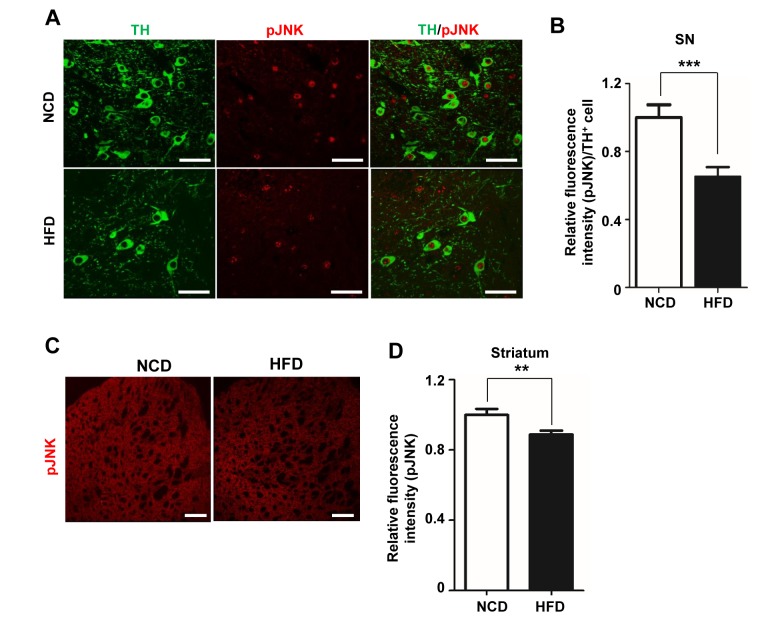
HFD feeding significantly reduced TH mRNA and protein levels in both the SN and striatum. It has also been reported that TH expression is modulated by JNK [27,28,34]. Because JNK is activated by phosphorylation [22,23,27], we hypothesized that the decrease in TH observed in HFD-fed mice was due, at least in part, to a decrease in activated, phosphorylated JNK (pJNK). To test this hypothesis, we performed double immunofluorescence staining for TH and pJNK in the SN of NCD- and HFD-fed mice. As shown in Fig. 3A, pJNK was detected in TH-positive neurons from control mice, which also exhibited a ‘halo’ of pJNK in the cytosol. By contrast, HFD-fed mice exhibited a decrease in the intensity of pJNK and TH staining and lacked the halo of pJNK immunoreactivity. pJNK intensity in TH positive neurons was decreased by ~32.1% in HFD-fed mice compared with NCD-fed mice (Fig. 3B). In the striatum of HFD group, pJNK intensity was decreased by ~11.21% compared with the NCD group (Fig. 3C, D). These results indicate that the decrease in TH in obesity was accompanied by a reduction in pJNK.
Fig. 3. The decrease in TH is associated with a reduction in phosphorylated JNK levels. (A) Brain slices (25 µm thick) were double-immunofluorescence stained by incubating overnight with anti-TH and rabbit anti-pJNK antibodies (1:500). TH (green) and pJNK (red) fluorescence in the SN were visualized by confocal microscopy. (B) Relative intensity of pJNK fluorescence per TH-positive cell in the SN was measured using ImageJ software (n=10 slides per condition, n=6/group). (C) pJNK immunoreactivity was detected in the striatum. (D) The intensity of pJNK fluorescence in the striatum was measured and graphed. Scale bars: 50 µm. *p<0.05, **p<0.01.
DISCUSSION
Movement, including walking and postural control, is defective in obesity relative to that in normal-weight individuals [4,5,9]. Indeed, as BMI increases, motor function and mobility decrease [5,8]. The dopamine system, composed of TH-expressing neurons that project from the SN to the striatum, is critical for the initiation and fine modulation of movement [13,32]. TH plays a crucial role in dopamine biosynthesis such that, when dopamine is depleted owing to the loss of TH function, locomotor function is impaired; notably, these phenomena underlie the pathophysiology of PD [11,13]. Importantly, BMI is significantly correlated with PD risk [8]. Therefore, we focused on dopaminergic neuron-related behavioral changes by comparing NCD- and HFD-fed mice.
We found that HFD feeding reduced TH levels in the SN by ~21% compared with that observed in NCD-fed mice. We assessed the impact of a chronic excess of energy on behavior, as well as SN and striatal expression of TH in HFD-fed mice, aged 4 to 20 weeks, which corresponds to juveniles to adults in humans. We found that downregulation of TH in the SN and striatum was associated with defects in motor function such as missteps, reflecting the effect of the dopamine system on grip strength, and decreased movement distance, determined by performing vertical grid tests and open-field tests (Fig. 1C~J). The increased climbing time observed for HFD-fed mice was associated with an increase in abdominally concentrated body fat rather than a defect in the dopamine system, reflecting a change in the animal's center of mass. These findings suggest that a reduction in TH in brain areas related to motor regulation underlie the movement defects that occur in HFD-fed mice. According to the report, C57BL/6 mice had acute MPTP administration showed ~80% decrease of striatal dopamine level. This mice took 2.2 folds longer total time to climb down and 3.29 folds to make turn on the vertical grid test [11]. Unlike MPTP-injected mice, when we performed grid test of HF-fed mice, total time was comparable due to increase of miss steps. Slippage increased the speed of time to climb down and it is associated with a defect in grip strength. Therefore we suggest that high fat diet feeding decreased ~26.3% and ~14.63% of TH neuron in SN and striatum respectively, and this lower degree of TH loss than MPTP-injected mice resulted in mild movement disorder.
We also investigated which signaling pathway led to the decrease in TH in HFD-induced obesity. It has been reported that TH expression is modulated by multiple signaling pathways, including JNK, cAMP, and SRY [20,21,35]. JNK is expressed in neurons and acts as a mediator of neurodegeneration following administration of neurotoxins [25,26]. However, JNK activity is necessary for the induction of TH by nicotine or cystatin C [27,28]. These observations imply that TH expression is differentially regulated in response to extracellular signals. We found that the decrease in TH was associated with a significant reduction in JNK phosphorylation in the SN and striatum. This suggests that loss of JNK activity contributes to the reduction in TH expression in the context of chronic energy excess, but not under normal energy status conditions. There are several reports that the high glucose condition associated with Type 2 dibetes mellitus triggers dopaminergic neuronal degeneration and turn on the apoptotic cell death due to increase of reactive oxygen species (ROS) production, which called glucotoxicity. Although chronic obesity increase the blood glucose level, HFD mice have milder glucose intolerance and can be reversed by a low fat diet comparing with db/db mice [36,37]. These researches supported that HFD mice model can induce the decrease of TH expression without neuronal cell death, but direct relevance need to further investigation. Another signal that could alter TH expression in HFD-induced obesity is leptin, reflecting that fact that plasma levels of leptin, which is produced by adipose tissue, increase as obesity progresses [10,29,38]. Notably, it has been reported in a human study that plasma leptin level and TH expression are negatively correlated; it has also been shown that TH-positive neurons in the SN and striatum express the leptin receptor [10,38]. However, whether leptin signaling is involved in the reduction in TH observed in obesity remains to be determined.
Collectively, our findings suggest that excess energy intake impairs motor function by reducing TH via JNK downregulation. One implication of our results is that L-DOPA treatment might improve movement disorders in cases of extreme obesity. Clearly, strategies for modulating this signaling pathway to increase TH expression in obesity and enhance its efficiency in improving motor function will require further investigation.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Republic of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (MSIP) and by the Ministry of Education (2014R1A1A1037655, 2016R1A2B4010398, 2016R1D-1A1B03932766) and by Chungnam National University Hospital Research Fund and by research fund of Chungnam National University.
References
- 1.Shalitin S, Battelino T, Moreno LA. Obesity, metabolic syndrome, and nutrition. World Rev Nutr Diet. 2017;116:16–51. doi: 10.1159/000452185. [DOI] [PubMed] [Google Scholar]
- 2.Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 2012;821:421–433. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–R1231. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- 4.Forhan M, Gill SV. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metab. 2013;27:129–137. doi: 10.1016/j.beem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Teasdale N, Simoneau M, Corbeil P, Handrigan G, Tremblay A, Hue O. Obesity alters balance and movement control. Curr Obes Rep. 2013;2:235–240. [Google Scholar]
- 6.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, 4th, Taylor CM, Welsh DA, Berthoud HR. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607–615. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott RD, Ross GW, White LR, Nelson JS, Masaki KH, Tanner CM, Curb JD, Blanchette PL, Popper JS, Petrovitch H. Midlife adiposity and the future risk of Parkinson's disease. Neurology. 2002;59:1051–1057. doi: 10.1212/wnl.59.7.1051. [DOI] [PubMed] [Google Scholar]
- 8.Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67:1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5. [DOI] [PubMed] [Google Scholar]
- 9.Palacios N, Gao X, McCullough ML, Jacobs EJ, Patel AV, Mayo T, Schwarzschild MA, Ascherio A. Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord. 2011;26:2253–2259. doi: 10.1002/mds.23855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiLeone RJ. The influence of leptin on the dopamine system and implications for ingestive behavior. Int J Obes (Lond) 2009;33(Suppl):S25–S29. doi: 10.1038/ijo.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim ST, Son HJ, Choi JH, Ji IJ, Hwang O. Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson’s disease. Brain Res. 2010;1306:176–183. doi: 10.1016/j.brainres.2009.09.103. [DOI] [PubMed] [Google Scholar]
- 12.Kim W, Im MJ, Park CH, Lee CJ, Choi S, Yoon BJ. Remodeling of the dendritic structure of the striatal medium spiny neurons accompanies behavioral recovery in a mouse model of Parkinson's disease. Neurosci Lett. 2013;557 Pt B:95–100. doi: 10.1016/j.neulet.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 13.Bourdy R, Sánchez-Catalán MJ, Kaufling J, Balcita-Pedicino JJ, Freund-Mercier MJ, Veinante P, Sesack SR, Georges F, Barrot M. Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacology. 2014;39:2788–2798. doi: 10.1038/npp.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. doi: 10.1016/j.nbd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen JC, Ali SF, Kosari S, Woodman OL, Spencer SJ, Killcross AS, Jenkins TA. Western diet chow consumption in rats induces striatal neuronal activation while reducing dopamine levels without affecting spatial memory in the radial arm maze. Front Behav Neurosci. 2017;11:22. doi: 10.3389/fnbeh.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, South T, Han M, Chen J, Wang R, Huang XF. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009;1268:181–189. doi: 10.1016/j.brainres.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Guan Z, Wang L, Song G, Ma B, Wang Y. Meta-analysis: overweight, obesity, and Parkinson's disease. Int J Endocrinol. 2014;2014:203930. doi: 10.1155/2014/203930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Willett WC, Ascherio A. Obesity and the risk of Parkinson's disease. Am J Epidemiol. 2004;159:547–555. doi: 10.1093/aje/kwh059. [DOI] [PubMed] [Google Scholar]
- 20.López-Toledano MA, Redondo C, Lobo MV, Reimers D, Herranz AS, Paíno CL, Bazán E. Tyrosine hydroxylase induction by basic fibroblast growth factor and cyclic AMP analogs in striatal neural stem cells: role of ERK1/ERK2 mitogen-activated protein kinase and protein kinase C. J Histochem Cytochem. 2004;52:1177–1189. doi: 10.1369/jhc.3A6244.2004. [DOI] [PubMed] [Google Scholar]
- 21.Tank AW, Curella P, Ham L. Induction of mRNA for tyrosine hydroxylase by cyclic AMP and glucocorticoids in a rat pheochromocytoma cell line: evidence for the regulation of tyrosine hydroxylase synthesis by multiple mechanisms in cells exposed to elevated levels of both inducing agents. Mol Pharmacol. 1986;30:497–503. [PubMed] [Google Scholar]
- 22.Oliva AA, Jr, Atkins CM, Copenagle L, Banker GA. Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci. 2006;26:9462–9470. doi: 10.1523/JNEUROSCI.2625-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffey ET. Nuclear and cytosolic JNK signalling in neurons. Nat Rev Neurosci. 2014;15:285–299. doi: 10.1038/nrn3729. [DOI] [PubMed] [Google Scholar]
- 24.Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F. Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J Neurosci. 2010;30:7804–7816. doi: 10.1523/JNEUROSCI.0372-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shukla AK, Pragya P, Chaouhan HS, Tiwari AK, Patel DK, Abdin MZ, Chowdhuri DK. Heat shock protein-70 (Hsp-70) suppresses paraquat-induced neurodegeneration by inhibiting JNK and caspase-3 activation in Drosophila model of Parkinson's disease. PLoS One. 2014;9:e98886. doi: 10.1371/journal.pone.0098886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Tiffany-Castiglioni E, Koh HC, Son IH. Paraquat activates the IRE1/ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol Lett. 2009;191:203–210. doi: 10.1016/j.toxlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Liang X, Nagai A, Terashima M, Sheikh AM, Shiota Y, Mitaki S, Kim SU, Yamaguchi S. Cystatin C induces apoptosis and tyrosine hydroxylase gene expression through JNK-dependent pathway in neuronal cells. Neurosci Lett. 2011;496:100–105. doi: 10.1016/j.neulet.2011.03.091. [DOI] [PubMed] [Google Scholar]
- 28.Gueorguiev VD, Cheng SY, Sabban EL. Prolonged activation of cAMP-response element-binding protein and ATF-2 needed for nicotine-triggered elevation of tyrosine hydroxylase gene transcription in PC12 cells. J Biol Chem. 2006;281:10188–10195. doi: 10.1074/jbc.M513806200. [DOI] [PubMed] [Google Scholar]
- 29.El-Haschimi K, Lehnert H. Leptin resistance - or why leptin fails to work in obesity. Exp Clin Endocrinol Diabetes. 2003;111:2–7. doi: 10.1055/s-2003-37492. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Reed DR, Price RA. Leptin resistance is associated with extreme obesity and aggregates in families. Int J Obes Relat Metab Disord. 2001;25:1471–1473. doi: 10.1038/sj.ijo.0801736. [DOI] [PubMed] [Google Scholar]
- 31.Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Crocker AD. The regulation of motor control: an evaluation of the role of dopamine receptors in the substantia nigra. Rev Neurosci. 1997;8:55–76. doi: 10.1515/revneuro.1997.8.1.55. [DOI] [PubMed] [Google Scholar]
- 33.Joshua M, Adler A, Bergman H. The dynamics of dopamine in control of motor behavior. Curr Opin Neurobiol. 2009;19:615–620. doi: 10.1016/j.conb.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Cammarota M, Bevilaqua LR, Rostas JA, Dunkley PR. Histamine activates tyrosine hydroxylase in bovine adrenal chromaffin cells through a pathway that involves ERK1/2 but not p38 or JNK. J Neurochem. 2003;84:453–458. doi: 10.1046/j.1471-4159.2003.01517.x. [DOI] [PubMed] [Google Scholar]
- 35.Milsted A, Serova L, Sabban EL, Dunphy G, Turner ME, Ely DL. Regulation of tyrosine hydroxylase gene transcription by Sry. Neurosci Lett. 2004;369:203–207. doi: 10.1016/j.neulet.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 36.Khang R, Park C, Shin JH. Dysregulation of parkin in the substantia nigra of db/db and high-fat diet mice. Neuroscience. 2015;294:182–192. doi: 10.1016/j.neuroscience.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Song J, Kim J. Degeneration of dopaminergic neurons due to metabolic alterations and Parkinson's disease. Front Aging Neurosci. 2016;8:65. doi: 10.3389/fnagi.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto A, Yamafuji M, Tachibana T, Nakabeppu Y, Noda M, Nakaya H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci Rep. 2013;3:3273. doi: 10.1038/srep03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dautan D, Hacioğlu Bay H, Bolam JP, Gerdjikov TV, Mena-Segovia J. Extrinsic sources of cholinergic innervation of the striatal complex: a whole-brain mapping analysis. Front Neuroanat. 2016;10:1. doi: 10.3389/fnana.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]