Abstract
Background & Aims
Previous studies have suggested that iron absorption in suckling mammals is refractory to stimuli that normally would decrease absorption in adults. To better understand the regulation of iron absorption during suckling, we have characterized the relationship between hepcidin, ferroportin, and iron absorption at this crucial stage of life.
Methods
To determine whether ferroportin is involved in iron absorption during suckling, absorption was measured in intestine-specific ferroportin knockout mice. The effect of constitutive hepcidin overexpression on intestinal iron absorption also was investigated in suckling transmembrane serine protease 6 knockout mice. Finally, suckling mice were injected with lipopolysaccharide to induce hepcidin expression. Blood was collected for serum iron analysis, and liver tissue and duodenal enterocytes were collected for gene and protein expression profiles.
Results
Iron absorption was very low in suckling ferroportin knockout mice, indicating that ferroportin is responsible for the majority of the iron absorbed at this time. However, increases in hepcidin during suckling, as seen in transmembrane serine protease 6 knockout mice and in mice injected with lipopolysaccharide, did not affect enterocyte ferroportin levels. Immunofluorescent localization of ferroportin showed that the protein localized to the basolateral membrane of duodenal enterocytes in both suckling and weaned mice.
Conclusions
These data show that the high iron absorption occurring during suckling is mediated by ferroportin. However, enterocyte ferroportin is hyporesponsive to hepcidin at this time, despite being expressed on the basolateral membrane. Alterations to ferroportin that prevent hepcidin binding during suckling may allow iron absorption to remain high regardless of hepcidin expression levels, reducing the likelihood of iron deficiency during development.
Keywords: Iron Homeostasis, Inflammation, Iron Deficiency
Abbreviations used in this paper: cDNA, complementary DNA; FDB, fluorescence dilution buffer; Hamp1, hepcidin antimicrobial peptide 1; Hprt, hypoxanthine guanine phosphoribosyl transferase; LPS, lipopolysaccharide; mRNA, messenger RNA; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; Saa1, serum amyloid A1; SDS, sodium dodecyl sulfate; Tmprss6, transmembrane serine protease 6
Summary.
The iron exporter ferroportin is essential for the high iron absorption that occurs in suckling mice, as it is in adults. However, unlike adults, iron absorption is hyporesponsive to the inhibitory effect of the iron regulatory peptide hepcidin during suckling.
An adequate supply of iron is crucial for mammalian health, but is particularly important for the rapid growth that occurs during the suckling period immediately after birth.1, 2 Suboptimal iron levels during this time can lead to permanent cognitive and psychomotor impairment that often cannot be reversed by subsequent iron treatment.3 To protect against this, maternal iron transfer to the developing fetus increases toward the end of gestation such that full-term mammals are well endowed with iron.4, 5 In addition, although breast milk has a relatively low iron content, intestinal iron absorption by the suckling intestine is generally far more efficient than in weaned mammals.1 This is evident most clearly in rodents, with numerous studies showing that the percentage of iron absorbed from a test dose decreases from more than 80% during suckling to approximately 10%–20% shortly after weaning.6, 7, 8, 9, 10 The reason for this decrease, however, is unclear because there is no obvious change in iron stores or growth rate,8 making it unlikely that alterations in body iron demand are responsible.
Although the mechanisms underlying the increased dietary iron intake in suckling infants are unclear, the pathways involved in the absorption of iron and its regulation in adults are relatively well defined. In weaned mammals, dietary iron is absorbed predominantly by the enterocytes of the duodenum and upper jejunum, and is transported across the brush-border membrane and into these cells by the iron import protein divalent metal-ion transporter 1.11, 12 If required by the body, the iron then is exported across the basolateral membrane and released into the extracellular fluid by the iron transporter ferroportin.13, 14, 15 The export of iron from enterocytes generally is considered to be the rate-limiting step in iron absorption,16 and it is at this point that the amount of iron entering the body is tightly regulated by the iron regulatory hormone hepcidin.17 This peptide is produced by the liver in response to alterations in body iron demand and secreted into the circulation, where it inhibits the release of iron from various cells, including enterocytes, by binding to ferroportin and triggering its internalization and degradation.18 Thus, hepcidin is a negative regulator of iron absorption. Various stimuli affect the production of hepcidin. Those associated with an increase in iron demand, for example, iron deficiency19 and increased red blood cell production,20 inhibit hepcidin synthesis, whereas stimuli that reduce iron absorption, such as iron loading19 and inflammation,20 increase hepcidin expression.
Despite extensive data showing the importance of the hepcidin/ferroportin axis in maintaining iron homeostasis in adults, the role of this regulatory pathway during suckling is unclear. A study performed in human infants has shown that iron supplementation has no effect on dietary iron absorption at 6 months of age, while reducing absorption in 9-month-old infants.21 Another study showed that iron supplements provided at 4–6 months of age increased hemoglobin concentration regardless of the initial iron status of the infant, whereas supplements at 9 months of age did not affect hemoglobin levels in iron-replete children.22 These studies suggested that the normal regulatory mechanisms controlling dietary iron absorption are not active in the immature intestine. Further evidence for this lack of regulation comes from studies in rats. We and others have shown that iron absorption in suckling rats is hyporesponsive to stimuli that normally would decrease iron absorption, such as iron loading and inflammation, despite increasing hepatic hepcidin expression.10, 23 We also were unable to detect ferroportin protein in suckling rat enterocytes despite high intestinal absorption,10 suggesting that ferroportin-independent pathways predominate at this time. In contrast, a study using ferroportin knockout mice showed that pups lacking intestinal ferroportin were paler that wild-type littermates late in the suckling period,24 although detailed absorption studies were not performed, leaving the role of ferroportin unclear.
To understand mechanisms of iron absorption at this critical stage of life, there is a need to define the roles of ferroportin and hepcidin in suckling mammals. To investigate this, we used mouse models to extend our previous studies in rats. In the current study we show that, not only is the ferroportin protein detectable in suckling mouse enterocytes, it is essential for the high iron absorption that occurs at this time. However, ferroportin, and therefore iron absorption, remain hyporesponsive to the inhibitory effect of circulating hepcidin.
Materials and Methods
Animals
C57BL/6 mice were obtained from the Animal Resources Centre (Perth, Australia) as pregnant dams or as lactating dams with pups. Mice lacking ferroportin specifically in enterocytes were produced by crossing ferroportin floxed mice24 with vil-Cre-ERT2 mice25 (both on a C57BL/6 background), which express tamoxifen-inducible Cre recombinase under the expression of the villin promoter. Cre recombinase expression was induced in these mice by injecting tamoxifen (75 μg/g body weight) subcutaneously at 7 days of age. Ferroportin floxed littermates lacking Cre recombinase also were injected with tamoxifen and used to obtain control samples. Homozygous transmembrane serine protease 6 (Tmprss6) knockout mice26 on a C57BL/6 background were used as a model of constitutively high hepcidin production. Both wild-type and heterozygous littermates were used as controls because we observed no phenotypic differences between these genotypes. In all experiments, mice were allowed unlimited access to a standard pellet diet (120 mg Fe/kg; Norco Stockfeeds, South Lismore, Australia) and water unless otherwise described. Results from male and female mice were pooled for all studies because gender differences were minor at the ages examined and had no effect on key comparisons or on the data interpretation. All pups were weaned at 21 days of age. In some studies, pups were weaned onto an iron-deficient diet based on AIN93G (1 mg Fe/kg,27 SF01-017; Specialty Feeds, Glen Forrest, Australia). Control animals for these studies were weaned onto a control diet (68 mg Fe/kg, AIN93G; Specialty Feeds). Some mice were injected intraperitoneally with 1 mg/kg lipopolysaccharide (LPS) (from Escherichia coli 0111:B4; Sigma-Aldrich, Sydney, Australia) to induce an acute phase response because this is known to stimulate hepcidin production. LPS-treated mice were killed 4 hours after injection. In all studies, suckling mice were euthanized at 15 days of age and weaned animals at 25–28 days of age. Before euthanasia, mice used for tissue collection were anesthetized (200 mg/kg ketamine, 10 mg/kg xylazine) and blood was withdrawn by cardiac puncture and allowed to clot for serum iron determination as described later. After euthanasia, liver tissue was snap frozen in liquid nitrogen for gene expression analysis. Intestinal enterocytes were isolated as described later and snap frozen in liquid nitrogen for RNA and protein expression studies. In some studies, duodenal tissue was formalin-fixed and paraffin-embedded for use in immunofluorescence analysis. All experimental procedures were approved by the QIMR Berghofer Medical Research Institute Animal Ethics Committee.
Isolation of Intestinal Enterocytes
A section of the proximal small intestine immediately distal to the pylorus (2 cm for suckling mice and 3 cm for postweaned animals) was excised from euthanized mice and the intestine was cut longitudinally and rinsed with ice-cold phosphate-buffered saline (PBS). The tissue then was transferred to a 10-mL tube containing 4 mL PBS plus 1.5 mmol/L EDTA, 500 μmol/L phenylmethylsulfonyl fluoride, and cOmplete Protease Inhibitor Cocktail (Roche Diagnostics Australia, Castle Hill, Australia), and mixed by inversion for 30 minutes at 4°C. Residual tissue was removed and the isolated enterocytes were washed with ice-cold PBS before being snap frozen in liquid nitrogen.
Measurement of Dietary Iron Absorption
Dietary iron absorption was determined with 59Fe using 2 methods. For both methods, mice were fasted for 4 hours before dosing. The first method has been described previously.28 Fasted mice were gavaged with 10 mmol/L HCl containing 200 μmol/L ferrous sulfate and 3 μCi 59FeCl3 (PerkinElmer, Glen Waverley, Australia). The total gavage volume was 50 μL for pups and 100 μL for postweaned animals. The amount of radioactivity in each animal was determined immediately after dosing using a Ram DA Counter with a PM-11 tube (Rotem Industries, Arava, Israel). The mice then were allowed unrestricted access to food or returned to their mothers. After 5 days, the amount of radioactivity remaining in each mouse was determined as described earlier and the proportion of 59Fe absorbed from the test dose was calculated by dividing the radioactivity in the mouse after 5 days by the initial radioactivity for that animal. For the second method, fasted mice were gavaged with 59Fe as described earlier. After 90 minutes, the mice were anesthetized (200 mg/kg ketamine, 10 mg/kg xylazine), euthanized by cervical dislocation, and the entire gastrointestinal tract from the esophagus to the distal colon was removed. The amount of radioactivity in the carcass and in the carcass plus the gastrointestinal tract was measured as described earlier. During the 90-minute time frame of the experiment, no 59Fe was detected in the feces or bedding, indicating that the entire test dose was contained within the animal. The proportion of 59Fe from the test dose transferred to the body was calculated by dividing the radioactivity in the carcass alone by the radioactivity in the carcass plus the gastrointestinal tract.
Determination of Serum Iron Levels
Serum iron levels were determined using the Iron/TIBC Reagent Kit (Pointe Scientific, Canton, MI). The volume of each reagent used was reduced 20-fold from that recommended by the manufacturer to allow the assay to be performed in a microtiter plate and to use a much smaller volume of serum.
RNA Extraction and Gene Expression Analysis
RNA was extracted from liver samples using TRIzol reagent (ThermoFisher Scientific, Scoresby, Australia) as per the manufacturer’s instructions. RNA quality was confirmed by formaldehyde gel electrophoresis and UV spectroscopy, and 500 ng of RNA from each sample was used to synthesize complementary DNA (cDNA) using Superscript III Reverse Transcriptase (ThermoFisher Scientific) and an oligo deoxythymidine primer as per the manufacturer’s instructions. A standard curve also was established using a 5-fold dilution series of RNA ranging from 3.2 to 2000 ng per reaction along with minus Superscript III and minus template negative controls. The 20-μL cDNA samples then were diluted to 500 μL with 10 mmol/L Tris–HCl pH 7.5 and stored at -20°C. Gene expression analysis using real-time polymerase chain reaction (PCR) was performed by adding 3 μL of each cDNA sample (or standard or negative control) to a PCR reaction mix to produce a final volume of 10 μL containing 1× LightCycler 480 SYBR Green I Master (Roche Diagnostics Australia) and 500 nmol/L of the appropriate forward and reverse primers. PCR products for each primer set were sequenced to ensure that the correct product was amplified. Each PCR reaction was performed in triplicate in 384-well plates on a LightCycler 480 real-time PCR machine (Roche Diagnostics Australia) using the following cycling conditions: 95°C for 10 minutes, and 45 cycles of 95°C for 10 seconds, 62°C for 10 seconds, and 72°C for 10 seconds. The PCR cycle was followed by melt curve analysis (65°C for 1 minute followed by an increase to 97°C with a ramp rate of 0.11°C/s and 5 acquisitions per degree) to ensure a single PCR product. The gene expression in each sample then was determined by comparing its Ct with the standard curve. The PCR was not accepted if the efficiency of the standard curve was not between 1.8 and 2.2. Gene expression data were normalized to the expression of the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (Hprt). The PCR primers used were as follows: Hamp1 forward: AGAGCTGCAGCCTTTGCAC; Hamp1 reverse: ACACTGGGAATTGTTACAGCATT; Hprt forward: GGACTGATTATGGACAGGA; Hprt reverse: GAGGGCCACAATGTGATG; serum amyloid A1 (Saa1) forward: AGAGGACATGAGGACACCAT; Saa1 reverse: CAGGAGGTCTGTAGTAATTGG.
Measurement of Serum Hepcidin1 Concentration
Serum hepcidin1 concentration was determined using the Hepcidin Murine-Complete Enzyme-Linked Immunosorbent Assay Kit (Intrinsic LifeSciences, La Jolla, CA) as per the manufacturer’s instructions.
Analysis of Ferroportin Protein Expression by Western Blot
Protein was extracted from isolated enterocytes by lysis in 10 volumes of triple-detergent buffer (50 mmol/L Tris-HCl [pH 8.0], 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, and 0.5% sodium deoxycholate) containing fresh cOmplete Protease Inhibitor Cocktail (Roche Diagnostics Australia). The solution was incubated on ice for 30 minutes before being centrifuged at 16,000g for 5 minutes at 4°C. The supernatant was collected and the protein concentration was determined using the bicinchoninic acid assay. For Western blot, 50 μg of total protein was mixed with SDS loading buffer (final concentration, 50 mmol/L Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 0.1% bromophenol blue, and 5% β-mercaptoethanol) and incubated at room temperature for 5 minutes before being run on a 10% SDS polyacrylamide gel. After electrophoresis, the protein was transferred to an Immobilon-FL polyvinylidene difluoride transfer membrane (Merck Millipore, Bayswater, Australia). The membrane then was incubated in Odyssey Blocking Buffer (LI-COR Biotechnology, Lincoln, NE) for 1 hour at room temperature. All incubations were performed with constant gentle agitation. The blocking step was followed by incubation of the membrane with Odyssey Blocking Buffer (LI-COR Biotechnology) plus 0.1% Tween-20 containing the antiferroportin antibody (rabbit anti-mouse MTP1 IgG, cat. no. MTP11-A, 1:2500; Alpha Diagnostics, San Antonio, TX) and the anti–β-actin antibody (anti–β-actin antibody [AC-15], cat. no. ab6276, 1:10,000; Abcam, Melbourne, Australia) overnight at 4°C. The membrane then was washed with Tris-buffered saline containing 0.1% Tween-20 (3 × 10 min, at room temperature), incubated with the secondary antibodies (IRDye 800CW goat anti-rabbit IgG [H + L], cat. no. 926-32211, IRDye 680RD goat anti-mouse IgG [H + L], cat. no. 926-68070, 1:10,000; LI-COR Biotechnology) diluted in Odyssey Blocking Buffer (LI-COR Biotechnology) plus 0.1% Tween-20 for 1 hour at room temperature in the dark, before being washed again in Tris-buffered saline containing 0.1% Tween-20 (3 × 10 min, at room temperature). The membrane then was dried overnight and scanned using the Odyssey CLx Infrared Imaging System (LI-COR Biotechnology).
Immunofluorescent Localization of Ferroportin Protein
Tissue sections were stained for ferroportin protein using the following protocol. Paraffin sections of formalin-fixed duodenal tissue were dewaxed and rehydrated using a Leica Autostainer XL (Leica Microsystems, North Ryde, Australia). Slides then were transferred to 10 mmol/L citrate buffer plus 0.05% Tween-20, pH 6.0, and maintained at 95°C–99°C for 20 minutes to facilitate antigen retrieval. Slides were allowed to cool in antigen retrieval buffer before being washed with PBS (3 × 5 min, at room temperature). The slides then were incubated in freshly prepared 50 mmol/L glycine/50 mmol/L ammonium chloride (15 min, at room temperature) to remove endogenous fluorescence. Blocking was achieved by incubating the slides in 10% fluorescence dilution buffer (FDB) (PBS containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, 5% goat serum, 5% fetal bovine serum, and 2% bovine serum albumin, for 1 hour, at room temperature). The primary antibody (rabbit antiferroportin antibody,28 1:500) in 1.5% FDB in PBS was added to each slide and the sections were incubated overnight at 4°C in a humidified chamber. The slides were washed with PBS (3 × 5 min, at room temperature) before the secondary antibody (goat anti-rabbit IgG [H + L] secondary antibody, Alexa Fluor 594 conjugate, 1:500; ThermoFisher Scientific) in 1.5% FDB in PBS was added to each slide and the sections were incubated for 30 minutes at room temperature in a humidified chamber. The slides were washed with PBS (3 × 5 min, at room temperature) before the nuclei were stained using 4’,6-diamidine-2’-phenylindole dihydrochloride (1:10,000, 5 min, at room temperature; Roche Diagnostics, Australia) in PBS. After PBS washes (3 × 5 min, at room temperature), the slides were mounted using ProLong Gold Antifade Mountant (ThermoFisher Scientific) and visualized using a LSM 780-NLO confocal microscope (Zeiss, North Ryde, Australia).
Statistics
All values are expressed as means ± SEM. The equality of the variances was determined using the Levene test. Statistical differences between means were calculated by analysis of variance followed by either the Tukey post hoc test for samples with equal variances or the Games–Howell post hoc test for samples with unequal variances using IBM SPSS Statistics software (IBM Australia, St Leonards, Australia). A P value of less than .05 was considered significant.
Results
Intestinal Iron Absorption Is Increased During Suckling in Mice and Correlates With Reduced Hepcidin Expression
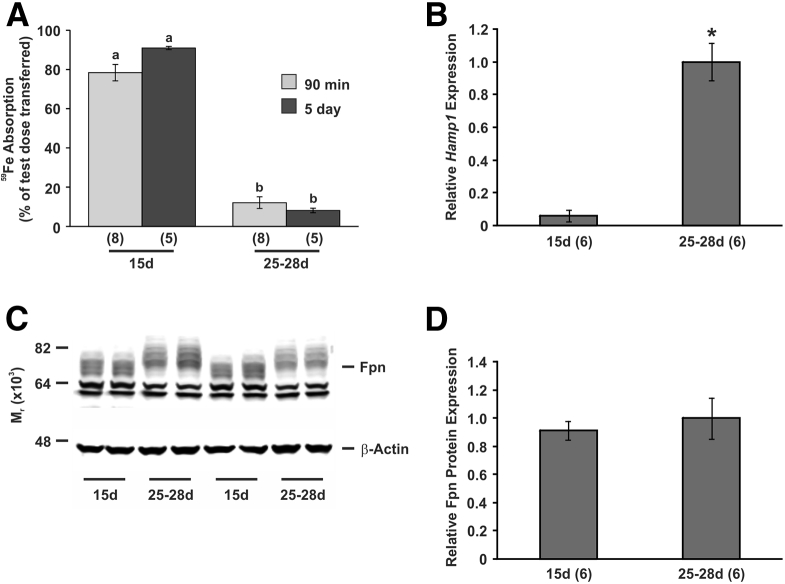
Previous studies in human beings and rats have suggested that the well-characterized hepcidin/ferroportin axis that regulates dietary iron absorption in adults may not be functional in suckling mammals.10, 21, 22, 23 To study this further, we have characterized iron absorption in suckling mice to ensure that the mouse is a suitable model to study this phenomenon. Similar to rats, we found that intestinal iron absorption was very high in suckling mice, and decreased rapidly to normal adult levels shortly after weaning (91% vs 8% absorption for suckling and weaned mice, respectively), when measured using our standard 5-day absorption assay (Figure 1A). However, there is evidence suggesting that the turnover of enterocytes is much slower in suckling mammals,29 potentially increasing the functional lifespan of the enterocytes. This would make it difficult to obtain definitive information on the mechanism underlying the enhanced iron absorption of suckling because either an increased rate of iron absorption or a slower release of iron from enterocytes over a longer period of time could lead to the same effect. However, by studying absorption over both 90 minutes and 5 days we could discriminate between these 2 possibilities. We found no significant difference in the amount of iron absorbed using either time period (Figure 1A), which indicates that most of the iron was absorbed within the first 90 minutes. Consequently, 90-minute absorption measurements were used for subsequent experiments unless otherwise indicated.
Figure 1.
Intestinal iron absorption and gene and protein expression in suckling and weaned mice. (A) The absorption of a test dose of 59Fe was determined in 15-day-old and 25- to 28-day-old C57BL/6J mice using both the 90 minute assay (90 min) and the 5-day assay (5 day) as described in the Materials and Methods section and the proportion of the test dose absorbed is shown. Tissues were taken from separate cohorts of mice at the same ages and the expression of (B) hepatic Hamp1 mRNA and (C and D) ferroportin was determined in duodenal enterocytes by quantitative PCR and Western blot, respectively. Gene expression levels were calculated relative to the housekeeping gene Hprt and are expressed as a proportion of 25- to 28-day-old levels. Ferroportin protein expression levels were calculated relative to β-actin and are expressed as a proportion of 25- to 28-day-old levels. (C) A representative Western blot is shown. The number of mice analyzed in each group is indicated in parentheses under each bar. The data represent means ± SEM. (A) Bars with the same letter are not statistically different from each other. (B and D) *P < .001. 15d, 15 days old; Fpn, ferroportin; 25-28d, 25-28 days old.
The hepatic expression of the hepcidin antimicrobial peptide 1 (Hamp1) gene, which encodes the functional hepcidin peptide in mice, was very low in suckling mice compared with recently weaned animals (the value in suckling mice was 6% of the weaned mouse value) (Figure 1B). Again, this is similar to our previous expression studies in rats and is not surprising given the inhibitory effect of hepcidin on iron absorption. However, unlike our previous data, we were able to detect ferroportin protein in duodenal cell isolates from suckling mice (Figure 1C and D). Duodenal ferroportin protein levels were similar in suckling and weaned animals, despite the increased iron absorption that was seen at the younger time point. In addition, the normally broad ferroportin band found in adult intestinal cells was slightly smaller in protein samples extracted from 15-day-old mouse enterocytes. These results indicate that the mouse is an appropriate model for examining the molecular basis of intestinal iron absorption and its regulation during suckling, although the failure to show increased ferroportin protein expression in the gut during suckling suggests that ferroportin-independent mechanisms may mediate iron transfer to the body at this time.
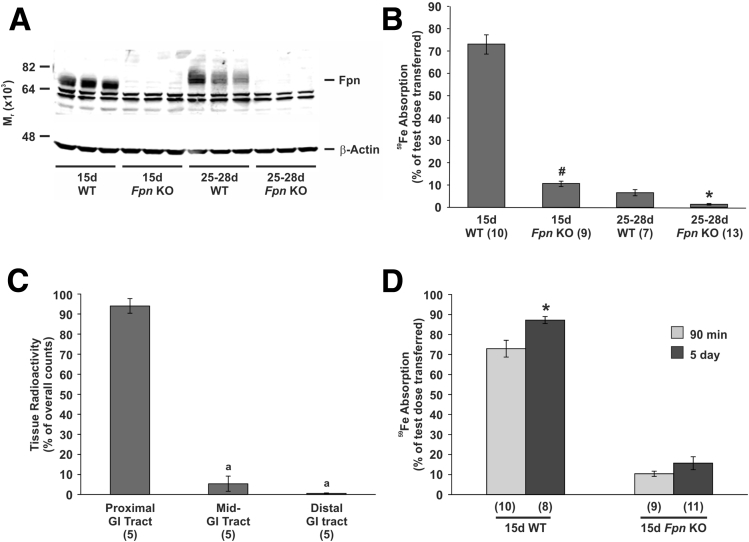
Intestinal Iron Absorption in Suckling Mice Is Dependent on the Iron Export Protein Ferroportin
To determine the extent of ferroportin involvement in dietary iron absorption during suckling, we examined the absorption of radioactive iron in both suckling and weaned animals lacking ferroportin specifically in intestinal enterocytes. Western blot analysis showed no major band corresponding to ferroportin in enterocytes isolated from knockout mice at either age, confirming both the success of the Cre induction and the specificity of the ferroportin antibody (Figure 2A). Loss of enterocyte ferroportin caused a significant decrease in 59Fe absorption from 72.9% to 10.4% of a test dose in suckling mice and from 6.4% to 1.3% of a test dose for weaned animals when measured using the 90-minute absorption assay (Figure 2B). Interestingly, the absorption of 59Fe in wild-type, weaned mice (6.4%) was lower than that of 15-day-old ferroportin knockout mice (10.4%), implying that, although the majority of iron is absorbed via a ferroportin-dependent pathway during suckling, other mechanisms may be responsible for approximately 14% of iron transfer to the body at this time. We have published data previously in rats that indicated that ferroportin in the distal alimentary canal may play a role in iron absorption during suckling.9 Because the villin promoter, which drives Cre recombinase in our ferroportin knockout mice, is expressed at lower levels in the distal alimentary canal,30 it is possible that some ferroportin activity may remain in the ileum and colon, and may be responsible for the residual iron absorption in suckling ferroportin knockout mice. To investigate this, we examined the progression of 59Fe along the gastrointestinal tract in suckling ferroportin knockout mice over the 90-minute absorption assay. We found that, of the 59Fe remaining in the gastrointestinal tract, approximately 94% had not progressed past the first half of the small intestine, with almost no counts detected in the colon (Figure 2C). In addition, a comparison of iron absorption measured using the 90-minute assay (Figure 2B) and the 5-day assay showed no significant difference in 15-day-old ferroportin knockout mice (Figure 2D). These results show that iron absorption in suckling ferroportin knockout mice is largely complete after 90 minutes when most of the test dose is still in the upper gastrointestinal tract, and that no further absorption occurs as the 59Fe moves into the more distal regions, implying that the distal gastrointestinal tract is not responsible for the residual iron absorption seen in suckling ferroportin knockout mice. These results also suggest that the increased absorptive capacity in the distal small intestine and colon9 is not responsible for the high iron absorption that occurs in suckling wild-type mice, because most of the iron is absorbed within 90 minutes and before it reaches the distal gastrointestinal tract.
Figure 2.
Intestinal iron absorption in suckling and weaned mice with intestine-specific ferroportin deletion. Tissues were taken from 15-day-old and 25- to 28-day-old mice specifically lacking ferroportin in intestinal enterocytes or in wild-type littermate controls, and the level of ferroportin protein in duodenal enterocytes was determined by Western blot. (A) A representative blot is shown. (B) The absorption of a test dose of 59Fe was determined in duplicate groups of mice at the same ages using the 90-minute assay and the proportion of the test dose absorbed is shown. (A and B) Statistical significance was calculated by comparing knockout mice with their age-matched littermates. (C) The distribution of 59Fe along the gastrointestinal tract 90 minutes after dosing was determined in ferroportin knockout mice. The data are presented as a proportion of the total counts from the entire gastrointestinal tract. (B) The 90-minute absorption data were compared with (D) 5-day absorptions in 15-day-old ferroportin knockout mice and littermate controls. Statistical significance was calculated by comparing the 2 techniques in each strain. The number of mice analyzed in each group is indicated in parentheses under each bar. The data represent means ± SEM. (B and D) *P < .05; #P < .001. (C) Bars with the same letter are not statistically different from each other. Distal GI tract, the cecum and colon; 15d Fpn KO, 15-day-old intestinal-specific ferroportin knockout mice; 15d WT, 15-day-old wild-type littermates; Fpn, ferroportin; GI, gastrointestinal; mid-GI tract, the distal half of the small intestine; proximal GI tract, the stomach and the proximal half of the small intestine; 25-28d Fpn KO, 25- to 28-day-old intestinal-specific ferroportin knockout mice; 25-28d WT, 25- to 28-day-old wild-type littermates.
Intestinal Iron Absorption in Suckling Mice Is Affected Minimally by Increased Hamp1 Expression
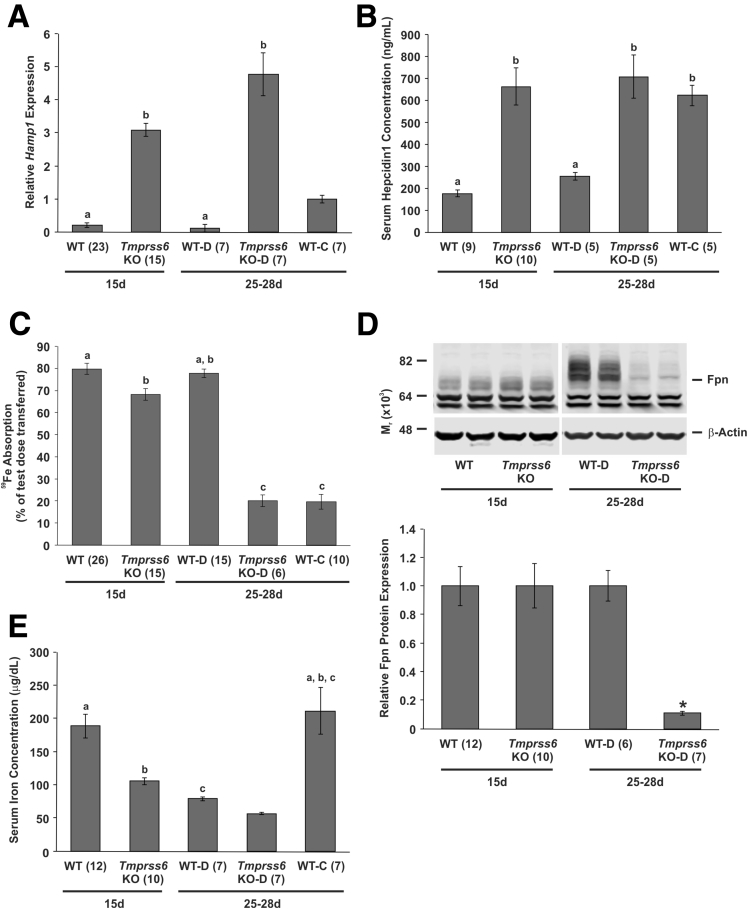
Having established that ferroportin is essential for the high iron absorption that occurs during suckling, we examined whether absorption at this time was regulated by hepcidin as it is in adults because our previous data in rats suggested that this was not the case.10 For these studies, we chose to use the well-characterized Tmprss6 knockout mouse. This mouse strain has constitutively high hepatic hepcidin expression owing to a lack of functional Tmprss6 protein.26 In this study, the 25- to 28-day-old Tmprss6 knockout and littermate control mice were weaned onto an iron-deficient diet at 21 days of age. This ensured that the weaned animals had a similar level of iron absorption and Hamp1 expression to the suckling mice. A separate group of littermates was weaned onto a control diet with the same basal composition but with a normal iron level.
Expression of hepatic Hamp1 messenger RNA (mRNA) was increased significantly in Tmprss6 knockout mice at both 15 and 25–28 days of age compared with littermate controls, despite the weaned Tmprss6 knockout mice being maintained on an iron-deficient diet (Figure 3A). In fact, the level of Hamp1 expression in 25- to 28-day-old Tmprss6 knockout mice on the deficient diet was higher than that seen in littermates weaned onto a control diet. Wild-type littermates weaned onto the iron-deficient diet had Hamp1 mRNA levels similar to 15-day-old wild-type littermates. However, when serum hepcidin1 concentration was examined, there was no significant difference between wild-type littermates weaned onto a control diet and Tmprss6 knockout mice at either age despite higher Hamp1 message levels in the later groups (Figure 3B). Both Tmprss6 knockout groups were consuming low-iron diets (breast milk and iron-deficient diet), suggesting that this somehow inhibits Hamp1 message translation or hepcidin1 processing and secretion. It also implies that intestinal iron absorption should be similar in these 3 groups of mice. When intestinal iron absorption was examined, however, absorption was significantly lower in Tmprss6 knockout mice weaned onto a deficient diet and littermates weaned onto a control diet than in 15-day-old knockouts (Figure 3C). This hyporesponsiveness to hepcidin during suckling also was reflected in the analysis of duodenal ferroportin protein, with no change seen in 15-day-old Tmprss6 knockout mice compared with 15-day-old wild-type mice (Figure 3D). In contrast, when both Tmprss6 knockout and wild-type mice were weaned onto iron-deficient diets, intestinal ferroportin expression was 11% of wild-type levels in the knockout animals. Interestingly, serum iron levels decreased in Tmprss6 knockout mice at both 15 and 25–28 days of age (Figure 3E), suggesting that, although intestinal absorption is hyporesponsive to hepcidin during suckling, the release of iron from other body tissues is regulated by hepcidin as it is in adults.
Figure 3.
Intestinal iron absorption, gene and protein expression, and serum iron levels in suckling and weaned Tmprss6 knockout mice. Tissues were taken from 15-day-old Tmprss6 knockout mice or wild-type littermate controls, or from 25- to 28-day-old Tmprss6 knockout mice or wild-type littermate controls that had been weaned onto an iron-deficient diet at 21 days of age. Tissues also were taken from 25- to 28-day-old wild-type littermate controls weaned onto a control diet. (A) Relative hepatic Hamp1 mRNA expression is shown and (B) serum hepcidin1 concentration is shown. (C) The absorption of a test dose of 59Fe was measured in separate cohorts of similarly treated mice and the proportion of the test dose absorbed is shown. (D) The level of ferroportin protein in isolated duodenal enterocytes and (E) serum iron concentration also were determined. Gene expression levels were calculated relative to the housekeeping gene Hprt and are expressed as a proportion of the values of 25- to 28-day-old wild-type littermates on the control diet. Ferroportin protein expression levels were calculated relative to β-actin and are expressed as a proportion of wild-type littermates at each time point. (D) A representative blot is shown. The number of mice analyzed in each group is indicated in parentheses under each bar. The data represent means ± SEM. (A, B, C, and E) Bars with the same letter are not statistically different from each other. (D) *P < .005. 15d, 15 days old; Fpn, ferroportin; Tmprss6 KO, Tmprss6 knockout mice; Tmprss6 KO-D, Tmprss6 knockout mice on an iron deficient diet; 25-28d, 25–28 days old; WT, wild-type littermates; WT-C, wild-type littermates on a control diet; WT-D, wild-type littermates on an iron-deficient diet.
Ferroportin Protein Localization in the Intestine Is Unchanged in Suckling and Weaned Mice
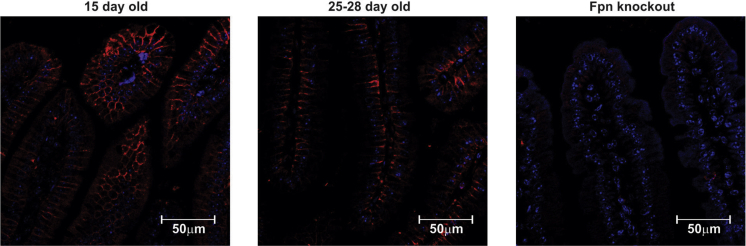
It is possible that changes in the localization of enterocyte ferroportin during suckling, such that the iron export protein is inaccessible to circulating hepcidin, may explain the lack of regulation seen at this time. Therefore, we examined the localization of ferroportin protein in duodenal sections using immunofluorescence microscopy. We saw no obvious difference in localization, with ferroportin detected on the basolateral surface of enterocytes in both suckling and weaned mice (Figure 4). The specificity of the antibody for immunofluorescence was confirmed by the lack of specific staining in duodenal sections taken from ferroportin knockout mice. Although these results do not rule out minor changes in ferroportin localization, they suggest that major changes in the localization of ferroportin are unlikely to be responsible for the lack of response to hepcidin in the suckling intestine.
Figure 4.
Immunofluorescence localization of ferroportin protein in the duodenum of suckling and weaned mice. Duodenal tissue was taken from 15-day-old and 25- to 28-day-old C57BL/6J mice and formalin-fixed sections were stained for ferroportin protein and imaged by confocal microscopy. Ferroportin protein is shown in red and nuclei is shown in blue (4’,6-diamidine-2’-phenylindole dihydrochloride). Intestinal-specific ferroportin knockout mice were used as a negative control to ensure antibody specificity. At least 6 sections were stained from each age group and representative images are shown. Fpn, ferroportin.
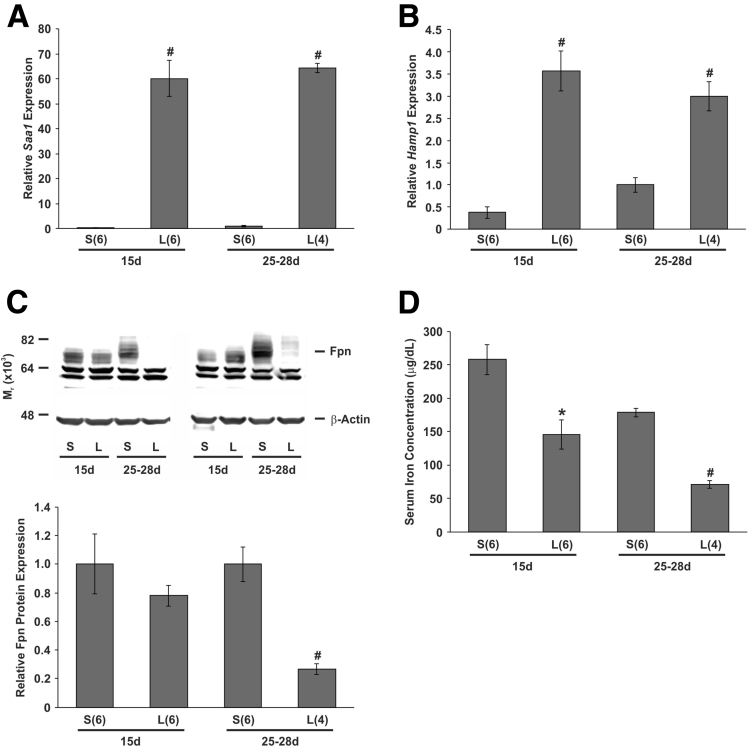
Duodenal Ferroportin Expression Is Unaffected in Suckling Mice Treated With LPS Despite Increased Hamp1 Expression
Stimulation of hepcidin production in adult mice occurs during iron loading19 and inflammation.20 Dietary iron loading is unlikely to occur in suckling mammals, however, infections are common at this age. Therefore, we investigated the effect of acute inflammation on intestinal ferroportin expression by injecting LPS into both suckling and weaned mice. After the injection of LPS, the hepatic inflammatory marker Saa1 was increased at least 60-fold in both suckling and weaned mice (Figure 5A). Likewise, hepatic Hamp1 expression also was increased in response to LPS treatment, with mRNA levels reaching a similar level in mice of both ages (Figure 5B). We were unable to examine intestinal iron absorption directly because LPS treatment reduces gastric motility,31 delaying the passage of iron into the duodenum and causing much of the 59Fe delivered to remain in the stomach for the 90-minute assay (data not shown). However, analysis of duodenal ferroportin levels showed that LPS treatment reduced the iron export protein to approximately 27% of saline-injected controls in weaned mice, whereas no significant change was seen in suckling animals (Figure 5C). This suggests that there would be little, if any, change in the absorption of iron from the intestinal lumen in response to LPS treatment in suckling mice. Despite this, a decrease in serum iron was observed in suckling mice treated with LPS (Figure 5D), similar to the decrease noted in Tmprss6 knockout mice (Figure 3D), again suggesting that the inability of hepcidin to down-regulate ferroportin is confined to enterocytes.
Figure 5.
Gene and protein expression and serum iron levels in suckling and weaned mice treated with LPS. Fifteen-day-old and 25- to 28-day-old C57BL/6J mice were injected intraperitoneally with either 1 mg/kg LPS in sterile saline or sterile saline alone. Four hours after injection, the mice were euthanized and tissues were taken. Relative hepatic (A) Saa1 and (B) Hamp1 mRNA expression, the (C) level of ferroportin protein in isolated duodenal enterocytes, and the (D) serum iron concentration were determined. Gene expression levels were calculated relative to the housekeeping gene Hprt and are expressed as a proportion of the 25- to 28-day-old saline-injected group. Ferroportin protein expression levels were calculated relative to β-actin and are expressed as a proportion of saline-injected controls at each time point. (C) A representative blot is shown. The number of mice analyzed in each group is indicated in parentheses under each bar. The data represent means ± SEM. 15d, 15 days old; Fpn, ferroportin; L, LPS-injected mice; 25-28d, 25–28 days old; S, saline-injected mice. *P < .05; #P < .01.
Discussion
Iron is crucial for infant mammals, particularly during central nervous system development, in which iron deficiency can have lifelong consequences.3 Adequate iron levels are maintained, in part, by the high dietary iron absorption that occurs during suckling.1, 6, 7, 8, 9, 10 Previous studies showing that stimuli known to decrease iron absorption in adults were unable to do so before weaning10, 21, 22, 23 have questioned the role of the well-characterized hepcidin/ferroportin axis17 during suckling. We have addressed this concern in the present study, and have confirmed that ferroportin mediates the majority of iron absorption that occurs in suckling mice, as it does in adults. Surprisingly, we saw no change in ferroportin protein levels between preweaned and postweaned mice despite an 11-fold decrease in absorption over this time. Although the reason for this is unclear, this result indicates that factors other than ferroportin protein levels can affect the efficiency of ferroportin-mediated iron transfer to the body.
Although our results clearly indicate that ferroportin is essential for the majority of iron absorption during suckling, 15-day-old ferroportin knockout mice still were able to absorb 10% of a test dose of iron. It is possible that residual ferroportin activity in the duodenum and upper jejunum may be responsible for iron absorption in these mice. Close examination of the Western blots in Figure 2A shows faint bands of the correct size in ferroportin knockout mice. However, these bands are far fainter than those seen in wild-type mice, making it unlikely that they are responsible for the 10% absorption seen in knockout animals, and are more likely to represent nonspecific background bands. Another possible explanation involves the suggestion by Ezekiel6 in 1967 that the increased pinocytotic capacity of the immature intestine might be responsible for the high iron absorption in suckling mammals. Although largely discounted as a major iron absorption pathway,32 it remains possible that pinocytosis may play a minor role in dietary iron uptake during suckling.
Our results also indicate that enterocyte ferroportin is hyporesponsive to the degradative effects of circulating hepcidin during suckling. Immunofluorescence studies have shown that this is not caused by changes in protein localization making ferroportin inaccessible to circulating hepcidin (Figure 4). Furthermore, the decrease in serum iron levels in suckling mice after hepcidin stimulation suggests that the hepcidin peptide produced is able to inhibit iron release in other body tissues, implying that the hyporesponsiveness is specific to intestinal enterocytes. A possible explanation is suggested by the Western blot in Figure 1A, which shows that the ferroportin protein is smaller in suckling mice, providing evidence for the age-specific modification of the transporter in intestinal enterocytes. This may represent an alternatively spliced form of ferroportin, although differences in glycosylation also could explain the observation, with either option potentially explaining the hyporesponsiveness to hepcidin. It also is possible that the size difference is not related to ferroportin function and that a more subtle modification is occurring. A recent study suggested that many of the residues involved in hepcidin binding lie in the metal binding cavity of ferroportin, and that the binding of hepcidin prevents ferroportin from undergoing a conformational change that is essential for iron export.33 The post-translational modification of any of these specific hepcidin-binding residues during suckling could render ferroportin unresponsive to hepcidin while retaining its iron export capacity, resulting in unregulated iron absorption regardless of circulating hepcidin levels.34 Any modification of ferroportin also could explain our previous inability to detect the protein in enterocytes isolated from suckling rats because any age-specific modifications also may alter antibody binding in that species.
Regardless of the mechanism involved, if human infants are similarly unable to down-regulate dietary iron absorption, as some studies have suggested,21, 22 these findings have implications for the widespread use of iron supplements. Indeed, there is evidence suggesting that the supplementation of iron-replete infants can negatively affect growth, an effect not seen in iron-deficient infants.35, 36 An inability to down-regulate dietary iron absorption during infancy may contribute to the detrimental effect of iron supplements at this time, suggesting that health policies encouraging the universal use of iron supplements in infants should be reconsidered.
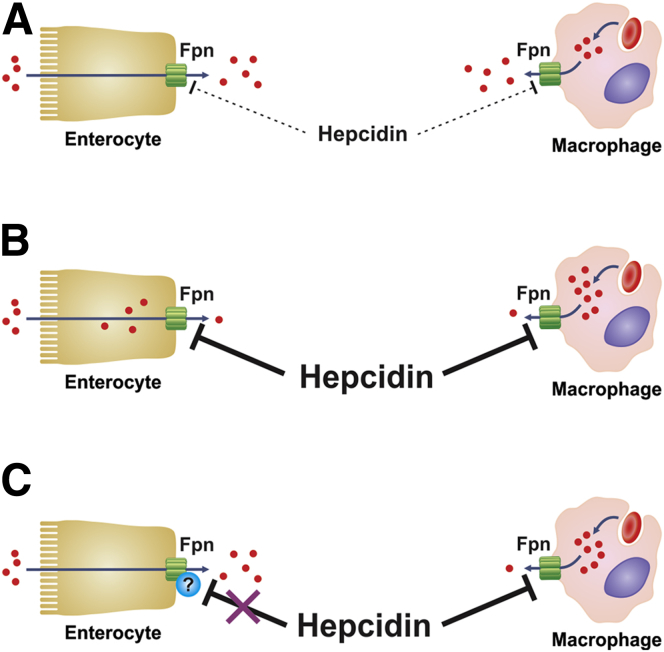
Precisely why mammals have evolved the inability to down-regulate dietary iron absorption during suckling is unclear, however, it is most likely a protective mechanism against iron deficiency, which is particularly common at this time. The altered hepcidin/ferroportin axis appears to specifically protect the intestine against the inhibitory effect of increasing hepcidin levels, which is normally a consequence of iron loading or inflammation.19, 20 Although iron loading is rare in infants, particularly those who are breast fed, infection and inflammation are common. In adults, the increase in hepcidin production during inflammation inhibits iron release from various body cells, including enterocytes.37 The resulting hypoferremia is thought to play an important role in host defense by limiting the supply of iron to invading pathogens.37 Enterocyte-specific modifications that prevent increases in hepcidin from affecting ferroportin activity would be advantageous during infancy because dietary iron absorption would proceed unimpeded, whereas the effect of hepcidin on other tissues would remain, allowing a degree of hypoferremia to occur (Figure 6). Therefore, total body iron stores would not be affected by inflammation and the likelihood of iron deficiency occurring would be reduced. It is interesting to speculate that, if such mechanisms exist in human infants, advice to prolong breast feeding in areas where iron deficiency is common may increase the success of supplementation schemes. Obviously, more research is required to determine if such advice is warranted.
Figure 6.
The effect of hepcidin in suckling mice. (A) In suckling mice and in iron-deficient, weaned animals, circulating hepcidin is very low. This allows ferroportin to persist on the surface of cells such as enterocytes and macrophages where it facilitates iron efflux. (B) In weaned animals, after a stimulus to increase hepcidin production, circulating hepcidin causes the degradation of ferroportin, which reduces the export of iron from all body cells. This includes enterocytes, and this in turn leads to a decrease in dietary iron absorption. (C) Most body cells are affected similarly when a stimulus to increase hepcidin expression occurs during suckling, however, unknown factors render enterocyte ferroportin resistant to hepcidin-induced degradation at this time. This allows dietary iron absorption to proceed unimpeded when hepcidin levels increase, for example, during inflammation. Fpn, ferroportin.
The iron binding protein lactoferrin has been suggested to play a role in iron absorption during suckling.38 This homolog of serum transferrin is the major iron binding protein in human milk38 and a lactoferrin receptor has been isolated from fetal enterocyte brush-border vesicles.39 However, mice born to lactoferrin knockout mothers show no sign of reduced iron uptake during suckling.40 In addition, several studies have suggested that, unlike human milk, the level of lactoferrin in rodent milk is relatively low and that transferrin is the major iron-binding protein present.40, 41 Regardless of which iron-binding protein is present, the current study and other studies from our laboratory9, 10 have shown greatly increased iron absorption in the absence of added lactoferrin or transferrin, implying that they are not necessary for the high iron absorption that occurs during suckling, at least in rodents. Further studies have suggested a role for lactoferrin in the modulation of the intestinal microbiome during suckling,42 rather than the protein playing a direct role in iron absorption.
In conclusion, we have shown that ferroportin is essential for the high dietary iron absorption that occurs in suckling mice, although ferroportin, and therefore iron absorption, is hyporesponsive to the inhibitory effect of hepcidin. We hypothesize that the constitutively high iron absorption during suckling provides an advantage to the developing infant by reducing the chances of iron-deficient growth occurring, particularly in sensitive organs such as the brain. This would be particularly advantageous during periods of infection and inflammation that frequently occur in infants and normally would inhibit iron absorption in adults. If a similar phenomenon occurs in human beings, these findings may have implications for the use of iron supplementation in both iron-deficient and iron-replete infants.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by a project grant from the National Health and Medical Research Council of Australia (G.J.A. and D.M.F.), a Senior Research Fellowship from the National Health and Medical Research Council of Australia (G.J.A.), and an Australian Liver Foundation–Hospitality Industry Career Development Research Fellowship (D.M.F.).
Contributor Information
David M. Frazer, Email: david.frazer@qimrberghofer.edu.au.
Gregory J. Anderson, Email: greg.anderson@qimrberghofer.edu.au.
References
- 1.Domellof M. Iron requirements, absorption and metabolism in infancy and childhood. Curr Opin Clin Nutr Metab Care. 2007;10:329–335. doi: 10.1097/MCO.0b013e3280523aaf. [DOI] [PubMed] [Google Scholar]
- 2.Collard K.J. Iron homeostasis in the neonate. Pediatrics. 2009;123:1208–1216. doi: 10.1542/peds.2008-1047. [DOI] [PubMed] [Google Scholar]
- 3.Lozoff B., Georgieff M.K. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Rao R., Georgieff M.K. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddappa A.M., Rao R., Long J.D. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezekiel E. Intestinal iron absorption by neonates and some factors affecting it. J Lab Clin Med. 1967;70:138–149. [PubMed] [Google Scholar]
- 7.Gallagher N.D., Mason R., Foley K.E. Mechanisms of iron absorption and transport in neonatal rat intestine. Gastroenterology. 1973;64:438–444. [PubMed] [Google Scholar]
- 8.Anderson G.J., Walsh M.D., Powell L.W. Intestinal transferrin receptors and iron absorption in the neonatal rat. Br J Haematol. 1991;77:229–236. doi: 10.1111/j.1365-2141.1991.tb07982.x. [DOI] [PubMed] [Google Scholar]
- 9.Frazer D.M., Wilkins S.J., Anderson G.J. Elevated iron absorption in the neonatal rat reflects high expression of iron transport genes in the distal alimentary tract. Am J Physiol Gastrointest Liver Physiol. 2007;293:G525–G531. doi: 10.1152/ajpgi.00579.2006. [DOI] [PubMed] [Google Scholar]
- 10.Darshan D., Wilkins S.J., Frazer D.M. Reduced expression of ferroportin-1 mediates hyporesponsiveness of suckling rats to stimuli that reduce iron absorption. Gastroenterology. 2011;141:300–309. doi: 10.1053/j.gastro.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Fleming M.D., Trenor C.C., 3rd, Su M.A. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 12.Gunshin H., Mackenzie B., Berger U.V. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 13.Donovan A., Brownlie A., Zhou Y. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 14.McKie A.T., Marciani P., Rolfs A. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 15.Abboud S., Haile D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 16.Conrad M.E., Jr., Crosby W.H. Intestinal mucosal mechanisms controlling iron absorption. Blood. 1963;22:406–415. [PubMed] [Google Scholar]
- 17.Nemeth E., Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemeth E., Tuttle M.S., Powelson J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 19.Pigeon C., Ilyin G., Courselaud B. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 20.Nicolas G., Chauvet C., Viatte L. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domellof M., Lonnerdal B., Abrams S.A. Iron absorption in breast-fed infants: effects of age, iron status, iron supplements, and complementary foods. Am J Clin Nutr. 2002;76:198–204. doi: 10.1093/ajcn/76.1.198. [DOI] [PubMed] [Google Scholar]
- 22.Domellof M., Cohen R.J., Dewey K.G. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr. 2001;138:679–687. doi: 10.1067/mpd.2001.112895. [DOI] [PubMed] [Google Scholar]
- 23.Leong W.I., Bowlus C.L., Tallkvist J. Iron supplementation during infancy–effects on expression of iron transporters, iron absorption, and iron utilization in rat pups. Am J Clin Nutr. 2003;78:1203–1211. doi: 10.1093/ajcn/78.6.1203. [DOI] [PubMed] [Google Scholar]
- 24.Donovan A., Lima C.A., Pinkus J.L. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 25.el Marjou F., Janssen K.P., Chang B.H. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 26.Folgueras A.R., de Lara F.M., Pendas A.M. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112:2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 27.Aslam M.F., Frazer D.M., Faria N. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice. FASEB J. 2014;28:3671–3678. doi: 10.1096/fj.14-251520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frazer D.M., Wilkins S.J., Becker E.M. Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. Gastroenterology. 2002;123:835–844. doi: 10.1053/gast.2002.35353. [DOI] [PubMed] [Google Scholar]
- 29.Koldovsky O., Sunshine P., Kretchmer N. Cellular migration of intestinal epithelia in suckling and weaned rats. Nature. 1966;212:1389–1390. doi: 10.1038/2121389a0. [DOI] [PubMed] [Google Scholar]
- 30.Madison B.B., Dunbar L., Qiao X.T. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 31.Collares E.F. Effect of bacterial lipopolysaccharide on gastric emptying of liquids in rats. Braz J Med Biol Res. 1997;30:207–211. doi: 10.1590/s0100-879x1997000200008. [DOI] [PubMed] [Google Scholar]
- 32.Frazer D.M., Darshan D., Anderson G.J. Intestinal iron absorption during suckling in mammals. Biometals. 2011;24:567–574. doi: 10.1007/s10534-011-9429-2. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi R., Kato H.E., Font J. Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat Commun. 2015;6:8545. doi: 10.1038/ncomms9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace D.F., Harris J.M., Subramaniam V.N. Functional analysis and theoretical modeling of ferroportin reveals clustering of mutations according to phenotype. Am J Physiol Cell Physiol. 2010;298:C75–C84. doi: 10.1152/ajpcell.00621.2008. [DOI] [PubMed] [Google Scholar]
- 35.Dewey K.G., Domellof M., Cohen R.J. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr. 2002;132:3249–3255. doi: 10.1093/jn/132.11.3249. [DOI] [PubMed] [Google Scholar]
- 36.Lind T., Seswandhana R., Persson L.A. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr. 2008;97:770–775. doi: 10.1111/j.1651-2227.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 37.Ganz T., Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15:500–510. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brock J.H. Lactoferrin–50 years on. Biochem Cell Biol. 2012;90:245–251. doi: 10.1139/o2012-018. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami H., Lonnerdal B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am J Physiol. 1991;261:G841–G846. doi: 10.1152/ajpgi.1991.261.5.G841. [DOI] [PubMed] [Google Scholar]
- 40.Ward P.P., Mendoza-Meneses M., Cunningham G.A. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol. 2003;23:178–185. doi: 10.1128/MCB.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee E.Y., Barcellos-Hoff M.H., Chen L.H. Transferrin is a major mouse milk protein and is synthesized by mammary epithelial cells. In Vitro Cell Dev Biol. 1987;23:221–226. doi: 10.1007/BF02623583. [DOI] [PubMed] [Google Scholar]
- 42.Lonnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 2009;12:293–297. doi: 10.1097/MCO.0b013e328328d13e. [DOI] [PubMed] [Google Scholar]