Abstract
Lipodystrophy is a common complication in HIV-infected patients taking highly active antiretroviral therapy. Its early diagnosis is crucial for timely modification of antiretroviral therapy. We hypothesize that mitochondrial DNA in plasma may be a potential marker of LD in HIV-infected individuals. In this study, we compared plasma mitochondrial DNA levels in HIV-infected individuals and non-HIV-infected individuals to investigate its potential diagnostic value.
Total plasma DNA was extracted from 67 HIV-infected patients at baseline and 12, 24 and 30 months after initiating antiretroviral therapy. Real-time quantitative PCR was used to determine the mitochondrial DNA levels in plasma. Lipodystrophy was defined by the physician-assessed presence of lipoatrophy or lipohypertrophy in one or more body regions.
The mitochondrial DNA levels in plasma were significantly higher at baseline in HIV-infected individuals than in non-HIV-infected individuals (p<0.05). At month 30, 33 out of 67 patients (49.2%) showed at least one sign of lipodystrophy. The mean plasma mitochondrial DNA levels in lipodystrophy patients were significantly higher compared to those without lipodystrophy at month 24 (p<0.001). The receiver operating curve analysis demonstrated that using plasma mitochondrial DNA level (with cut-off value >5.09 log10 copies/ml) as a molecular marker allowed identification of patients with lipodystrophy with a sensitivity of 64.2% and a specificity of 73.0%.
Our data suggest that mitochondrial DNA levels may help to guide therapy selection with regards to HIV lipodystrophy risk.
Keywords: Biomarker, highly active antiretroviral therapy (HAART), human immunodeficiency virus (HIV), lipodystrophy, mitochondrial DNA (mtDNA), plasma
INTRODUCTION
Lipodystrophy (LD) is a common complication in HIV-infected patients taking highly active antiretroviral therapy (HAART), with an estimated prevalence between 11% and 83% [1,2]. It is characterized by peripheral fat loss in the limbs, cheeks and/or buttocks (lipoatrophy) and by accumulation of fat in the abdomen, neck and/or breasts (lipohypertrophy). A number of studies have reported association of nucleoside reverse transcriptase inhibitors (NRTIs), particularly thymidine analogues (zidovudine and stavudine), with lipoatrophy [3,4] while protease inhibitors (PIs) are associated with lipohypertrophy [5,6]. The underlying mechanisms for LD are thought to be due to mitochondrial toxicity [7,8]. Increased apoptosis of adipocytes has been described in NRTIs-induced LD [9,10]. Furthermore, massive cell lysis during adipocyte tissue apoptosis results in mitochondrial DNA (mtDNA) release into plasma. LD resulting in body dysmorphism may lower the quality of life leading to stigma and discrimination among HAART recipients [11]. This may cause poor compliance to treatment, jeopardizing the long-term success of HAART [12]. Improved diagnostic techniques of pre-clinical LD may facilitate earlier HAART modification by the physician.
Plasma mtDNA has recently received increasing attention and has been studied in several acute and chronic disorders. Studies show that plasma mtDNA may act as a potential biomarker of aging, systemic inflammation, and tumors [13-17]. This may be due to cells releasing mitochondrial structures, particularly mtDNA, into the circulation. Since fat-cell damage is the main cause for the development of LD in HAART-treated patients, we hypothesize that plasma mtDNA level may also be a marker of LD in HIV-infected individuals.
Data expressed as mean ±standard deviation or percentages. NRTIs, nucleoside reverse-transcriptase inhibitors; d4T, stavudine; 3TC, lamivudine; AZT, zidovudine.
In this study, we compared plasma mtDNA levels in HIV-infected individuals and non-HIV-infected individuals and investigated its potential role in diagnosing pre-clinical LD.
MATERIALS AND METHODS
Study population - 67 HIV-infected individuals were included in this study. HIV-infected individuals who received medical care between April 2009 and October 2013 at the Guangzhou Eighth People’s Hospital were enrolled. Only treatment-naïve HIV-infected individuals were included. Patients with opportunistic infections or co-infection with hepatitis B or C were excluded from the study. All patients received an initial regimen of lamivudine (3TC) plus zidovudine (AZT) or stavudine (d4T) with efavirenz (EFV), nelfinavir or lopinavir/ritonavir (LPV/r). LD was defined as subcutaneous fat wasting (lipoatrophy) and/or fat accumulation in the abdomen, neck, or back (lipohypertrophy), reported by the patient and confirmed by the physician examination. Non-HIV-infected controls (n=23) were a group of local volunteers who were seronegative for HIV and had no history of chronic illness or intravenous drug use. All individuals gave written informed consent. This study was approved by the local ethics committee.
Blood sampling and assessment of plasma mtDNA - The blood samples were processed and the DNA was extracted according to our aforementioned protocol [17]. Plasma DNA was measured with cytochrome C oxidase II (Cox II) genes and the GAPDH by real-time quantitative polymerase chain reaction (qPCR) assay, the primer sequences have been described previously [18]. Amplification of mitochondrial products was performed separately in optical 96-well plates (Applied Biosystems). All samples were run in triplicate. Absolute mtDNA copy numbers were calculated using serial dilutions of plasmids with known copy numbers of mtDNA. qPCR was carried out in 20 μl of total reaction volume containing 4 μl H2O, 10μlTaqMan® Universal PCR Master Mix (Applied Biosystems, Branchburg, New Jersey, USA), 1 μl of each of 10 μM primers and 0.5 μl of a 10 μM FAM-labeled probe (both probes from Life Technologies, Guangzhou, China). For each reaction, 3.5 μl of DNA was added. qPCR was performed using the ABI 7500 fast Sequence Detection System (Applied Biosystems, Branchburg, New Jersey, USA) under the following conditions: 10 minutes at 95°C, 2 minutes at 50°C followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C.
Plasma HIV-RNA was measured by quantitative PCR assay (CobasAmpliPrep/CobasTaqman 96; sensitivity of 40 copies/ml; Roche Molecular Systems). CD4 cell count was determined using FACSCanto flow cytometer and CellQuest software (BD Biosciences, San Jose, CA).
Statistical analysis - Comparisons between groups were made using the Mann–Whitney U-test. Multiple comparisons were performed using Kruskal-Wallis test. The diagnostic suitability of plasma mtDNA copy number for identification of patients with LD was determined by the Receiver operating characteristic (ROC) curve analysis. Quantitative data are presented as means ± standard deviations (SD). p<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software (version16.0; SPSS, Chicago, IL).
PATIENTS’ BASIC CHARACTERISTICS
The characteristics of HIV-infected individuals are depicted in Table 1. There was no significant variation in age and gender of non HIV-infected patients (age 34.64± 8.83, female 45.5%, p-value 0.07, 0.86 respectively) compared to HIV-infected individuals group. HAART regimen consisting of two NRTIs in combination with one nonnucleoside reverse transcriptase inhibitors (NNRTIs) or protease inhibitor (PI) was initiated in all HIV-positive patients. They all had AZT and 3TC as their initial NRTIs regimen. The mean duration of d4T treatment was 12.8 months (SD, 10.5). At month 30 visit, 13 (19.4%) patients never experienced exposure to d4T, 14 (20.9%) patients were exposed to d4T less than 12 months, 31 (46.3%) patients had exposure of 12 to 24 months, and 9 (13.4%) patients more than 24 months. Patients taking a d4T-based regimen switched to AZT or TDF (tenofovir) based regimen.
Table 1.
Baseline characteristics of HIV-infected individuals.
| Characteristic | Value |
|---|---|
| Age (years) | 38.21±7.90 |
| Female [n (%)] | 29 (43.3) |
|
Transmission [n (%)] Sexual Blood Intravenous drug use Others/unknown |
50 (74.6) 3 (4.5) 13 (19.4) 1 (1.5) |
| HIVRNA(log10 copies/ml) | 4.65±0.84 |
| CD4+ count (cells/μl) | 98.85±72.60 |
| Lactate level (mmol/l) | 1.17±0.37 |
|
NRTIs [n (%)] d4T + 3TC AZT+ 3TC |
50 (74.6) 17 (25.4) |
MTDNA ASSESSMENT IN PLASMA
mtDNA level was measured in all patients at baseline, month 12, month 24, and month 30 of treatment. Data are reported in Fig. (1). In HIV-infected individuals, the mean plasma mtDNA levels were 5.51, 4.79, 5.00, and 5.10 for baseline, month 12, 24, and 30, respectively. In non-HIV-infected individuals, the mean mtDNA level was 5.03. The plasma mtDNA levels were significantly higher in HIV-infected individuals pre-HAART compared to non-HIV-infected individuals, at months 12, 24, and 30 post-HAART (p = 0.008, p < 0.001, p < 0.001, and p = 0.002 respectively; Fig. 1).
Fig. (1).
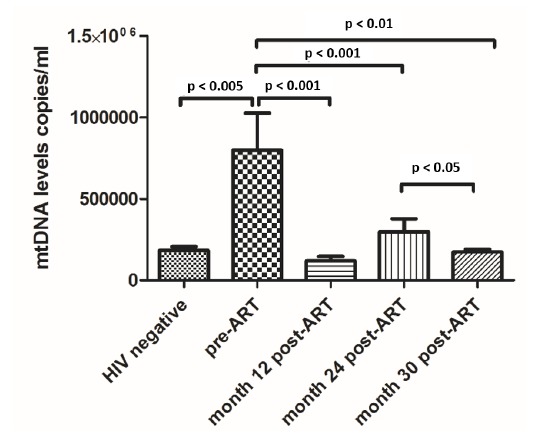
Comparison of plasma mtDNA levels in non-HIV-infected individuals, HIV-infected individuals’ pre-ART, month 12, month 24 and month 30 post-ART. ART: antiretroviral therapy.
To study the association of plasma mtDNA levels with duration of d4T exposure, we compared mtDNA levels of HIV-infected individuals at baseline, with HAART duration of less than 12 months, between 12 months and 24 months, and more than 24 months. There was no significant difference in plasma mtDNA levels between these groups (5.09, 5.09, 5.17, 5.22 log10 copies/ml, respectively).
COMPARED PLASMA mtDNA IN PATIENTS WITH OR WITHOUT CLINICAL LD
At month 30 of initiating HAART, 33 out of 67 patients (49.2%) showed at least one sign of LD. The characteristics of HIV-infected individuals with and without LD are presented in Table 2. In patients with LD, the mean plasma mtDNA (log10copies/ml) was 5.54 at baseline, 4.86 at month 12, 5.14 at month 24 and 5.22 at 30 months post-HAART. In patients without LD, it was 5.48, 4.71, 4.81, and 4.90 respectively (Fig. 2). The plasma mtDNA levels in patients with LD were significantly higher than those without LD at month 24 and at month 30 post-HAART (both p<0.001). However, similar trends were observed at baseline (p = 0.74) and at month 12 (p = 0.21) (Fig. 2).
Table 2.
The characteristics of HIV-infected individuals with and without LD.
| With LD (n=33) | Without LD (n=34) | p-Value | |
|---|---|---|---|
| Age (years) | 37.5±8.2 | 38.5±8.3 | 087 |
| Female [n (%)] | 14 (42.4%) | 13 (38.2%) | 0.86 |
| BMI (kg/m2) | 20.3±2.7 | 21.1±2.6 | 0.26 |
| CD4+ count (cells/μl) at baseline | 111±70.1 | 92±69.0 | 0.28 |
| CD4+ count (cells/μL) at month 24 | 374±254.5 | 424±184.0 | 0.47 |
| HIV RNA(log10 copies/ml) at baseline | 4.5±0.8 | 4.8±0.9 | 0.31 |
| NRTI exposure(months) Stavudine* Zidovudine Tenofovir |
14.1±5.4 6.4±6.1 3.1±5.6 |
9.5±9.8 12.0±10.7 2.3±5.3 |
0.02 0.06 0.44 |
| Triglycerides | 2.5±2.4 | 2.1±1.6 | 0.59 |
| Cholesterol | 4.6±1.0 | 4.5±0.9 | 0.64 |
Data expressed as mean (SD) or n (percentage). *Statistically significant.
AHL, asymptomatic hyperlactatemia; BMI, body mass index; HAART, antiretroviral treatment; NRTI, nucleoside reverse transcriptase inhibitor.
Fig. (2).
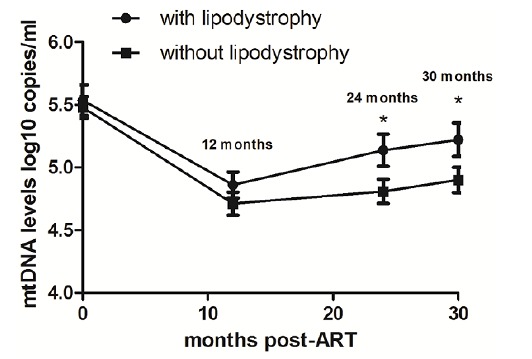
The different values of plasma mtDNA levels (pre-ART, month 12, month 24, and month 30) in patients with and without lipodystrophy.
EVALUATION OF PLASMA mtDNA LEVEL AS A DIAGNOSTIC MARKER FOR EARLY IDENTI-FICATION OF PATIENTS WITH LD
To test whether quantification of plasma mtDNA copy number allows identification of patients with LD at month 24 post-HAART, we conducted a parametric ROC curve analysis, which correlates true and false positive rates (sensitivity and 1-specificity). As shown in Fig. (3), when the optimal cut-off value of plasma mtDNA was defined at > 5.09 log10 copies/ml by the ROC analysis, the area under the curve (AUC) was 0.74 (95% confidence interval: 0.61–0.87). Sensitivity and specificity of plasma mtDNA for diagnosis of patients with LD was 64.2% and 73.0%, respectively, suggesting that the level of plasma mtDNA may serve as a useful biomarker to identify patients with LD.
Fig. (3).
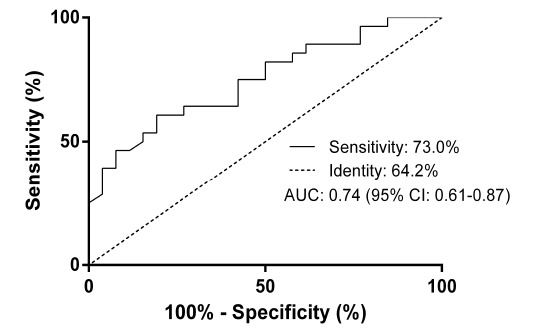
Receiver operating characteristic (ROC) curve of plasma mtDNA for discrimination between patients with lipodystrophy from patients without lipodystrophy. Sensitivity of the individual biomarkers is plotted on the y-axis, whereas 1-specificity or the false positive rates plot on the x-axis. The data are shown with 95%CI. AUC, area under curve; CI, confidence interval.
CONCLUSION
In this study, we present measured plasma mtDNA levels in HIV-infected individuals at baseline and during 30 months of HAART treatment. Our study evaluates the role of plasma mtDNA levels in identification of pre-clinical LD. Our results show that the plasma mtDNA levels in patients with LD were significantly higher than those without LD 30 months after HAART initiation. Similar results are seen at month 24.
Our data are consistent with previous studies demonstrating that plasma mtDNA levels are elevated in HIV-infected individuals prior to initiation of antiretroviral therapy [19]. Plasma mtDNA levels are also significantly increased in patients with tissue injury and various tumors [15,20,21]. The mechanism that cells use to release free circulating DNA into the blood is still not elucidated. An increase in plasma DNA concentration may be due to either increased liberation from cells or decreased clearance efficiency [22,23]. Our observation of a significant decrease of plasma mtDNA levels after 12 months of HAART initiation indicates that the HIV virus may disturb the equilibrium between mtDNA production and clearance.
Studies showed that about 50% of patients had at least one physical abnormality characteristic of LD after 12–18 months of HAART treatment [24,25]. However, it is difficult to predict pre-clinical LD and the need for changing medication [26,27]. In this study, clinical investigations showed a higher frequency of LD at month 30 of HAART. At month 24 of HAART therapy, patients who subsequently developed LD at month 30 have shown higher levels of mtDNA compared to non-LD subjects. This suggests that plasma mtDNA levels may serve as a diagnostic biomarker for LD in HIV-infected individuals taking HAART.
Our study has some limitations. First, we did not follow a HAART-naïve HIV-infected group, so we could not assess the contribution of the virus alone to plasma mtDNA levels over time. This of course would be ethically unacceptable in the era of therapy. Second, HAART regimens were modified for HIV-infected individuals who had side effects or intolerance. This may cause uncertainty in assessing the impact of each regimen on plasma DNA levels. Finally, our sample size was small and inferences should be drawn with caution. Further studies with larger sample size are warranted to evaluate the possibility of using mtDNA levels to predict pre-clinical LD.
In conclusion, plasma mtDNA levels may help earlier identification of pre-clinical LD. Plasma mtDNA could be a valuable addition to assessing patients with LD. The relatively higher specificity suggests that mtDNA levels may help in earlier detection and treatment modification of patient populations at risk for HIV lipodystrophy.
ACKNOWLEDGEMENTS
We thank Carissa Chu for critical reading of the manuscript.
ABBREVIATIONS
- 3TC
Lamivudine
- AUC
Area under the curve
- AZT
Zidovudine
- Cox II
Cytochrome C oxidase
- d4T
Stavudine
- EFV
Efavirenz
- HAART
Highly active antiretroviral therapy
- HIV
Human immunodeficiency virus
- LD
Lipodystrophy
- LPV/r
Lopinavir/ritonavir
- mtDNA
Mitochondrial DNA
- NRTIs
Nucleoside reverse transcriptase inhibitors
- PIs
Protease inhibitors
- qPCR
Quantitative PCR
- ROC
Receiver operating characteristic
- SD
Standard deviation
- TDF
Tenofovir
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Grinspoon S., Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Feleke Y., Fekade D., Mezegebu Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop. Med. J. 2012;50:221–230. [PubMed] [Google Scholar]
- 3.Tebas P., Zhang J., Hafner R., et al. Peripheral and visceral fat changes following a treatment switch to a non-thymidine analogue or a nucleoside-sparing regimen in HIV-infected subjects with peripheral lipoatrophy: results of ACTG A5110. J. Antimicrob. Chemother. 2009;63:998–1005. doi: 10.1093/jac/dkp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyle G.J., Sabin C.A., Cartledge J., et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20:2043–2050. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
- 5.John M., McKinnon E.J., James I.R., et al. Randomized, controlled, 48-week study of switching stavudine and/or protease inhibitors to combivir/abacavir to prevent or reverse lipoatrophy in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2003;33:29–33. doi: 10.1097/00126334-200305010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Estrada V., De Villar N.G., Larrad M.T., Lopez A.G., Fernandez C., Serrano-Rios M. Long-term metabolic consequences of switching from protease inhibitors to efavirenz in therapy for human immunodeficiency virus-infected patients with lipoatrophy. Clin. Infect. Dis. 2002;35:69–76. doi: 10.1086/340863. [DOI] [PubMed] [Google Scholar]
- 7.McComsey G.A., O'Riordan M., Choi J., et al. Mitochondrial function, inflammation, fat and bone in HIV lipoatrophy: randomized study of uridine supplementation or switch to tenofovir. Antivir. Ther. 2012;17:347–353. doi: 10.3851/IMP1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallewa J.E., Wilkins E., Vilar J., et al. HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J. Antimicrob. Chemother. 2008;62:648–660. doi: 10.1093/jac/dkn251. [DOI] [PubMed] [Google Scholar]
- 9.Cherry C.L., Lal L., Thompson K.A., et al. Increased adipocyte apoptosis in lipoatrophy improves within 48 weeks of switching patient therapy from Stavudine to abacavir or zidovudine. J. Acquir. Immune Defic. Syndr. 2005;38:263–267. [PubMed] [Google Scholar]
- 10.McComsey G.A., Walker U.A. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion. 2004;4:111–118. doi: 10.1016/j.mito.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Shenoy A., Ramapuram J.T., Unnikrishan B., et al. Effect of Lipodystrophy on the Quality of Life among People Living with HIV/AIDS (PLHIV) on Highly Active Antiretroviral Therapy. J. Int. Assoc. Provid. AIDS Care. 2014;13:471–475. doi: 10.1177/2325957413488205. [DOI] [PubMed] [Google Scholar]
- 12.Tungsiripat M., McComsey G. Pathogenesis and management of lipoatrophy. Curr. HIV/AIDS Rep. 2008;5:55–63. doi: 10.1007/s11904-008-0010-8. [DOI] [PubMed] [Google Scholar]
- 13.Jylhava J., Nevalainen T., Marttila S., Jylha M., Hervonen A., Hurme M. Characterization of the role of distinct plasma cell-free DNA species in age-associated inflammation and frailty. Aging Cell. 2013;12:388–397. doi: 10.1111/acel.12058. [DOI] [PubMed] [Google Scholar]
- 14.Puskarich M.A., Shapiro N.I., Trzeciak S., Kline J.A., Jones A.E. Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock. 2012;38:337–340. doi: 10.1097/SHK.0b013e318266a169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung C.T., Hsiao S.Y., Tsai T.C., et al. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J. Transl. Med. 2012;10:130. doi: 10.1186/1479-5876-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnalich F., Maldifassi M.C., Ciria E., et al. Plasma levels of mitochondrial and nuclear DNA in patients with massive pulmonary embolism in the emergency department: a prospective cohort study. Crit. Care. 2013;17:R90. doi: 10.1186/cc12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler C., Radpour R., Barekati Z., et al. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Mol. Cancer. 2009;8:105. doi: 10.1186/1476-4598-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K., Sun Y., Liu D., et al. Mitochondrial toxicity studied with the PBMC of children from the Chinese national pediatric highly active antiretroviral therapy cohort. PLoS One. 2013;8:e57223. doi: 10.1371/journal.pone.0057223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cossarizza A., Pinti M., Nasi M., et al. Increased plasma levels of extracellular mitochondrial DNA during HIV infection: a new role for mitochondrial damage-associated molecular patterns during inflammation. Mitochondrion. 2011;11:750–755. doi: 10.1016/j.mito.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Nakahira K, Kyung SY, Rogers A, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. . PLoS Med 2013; 10: e1001577; discussion e1001577. 2013. [DOI] [PMC free article] [PubMed]
- 21.Budnik L.T., Kloth S., Baur X., Preisser A.M., Schwarzenbach H. Circulating mitochondrial DNA as biomarker linking environmental chemical exposure to early preclinical lesions elevation of mtDNA in human serum after exposure to carcinogenic halo-alkane-based pesticides. PLoS One. 2013;8:e64413. doi: 10.1371/journal.pone.0064413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q., Raoof M., Chen Y., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Vaart M., Pretorius P.J., Circulating D.N. Its origin and fluctuation. Ann. N. Y. Acad. Sci. 2008;1137:18–26. doi: 10.1196/annals.1448.022. [DOI] [PubMed] [Google Scholar]
- 24.Miro O., Lopez S., Cardellach F., Casademont J. Mitochondrial studies in HAART-related lipodystrophy: from experimental hypothesis to clinical findings. Antivir. Ther. 2005;10(Suppl. 2):M73–M81. [PubMed] [Google Scholar]
- 25.Feeney E.R., Mallon P.W. Impact of mitochondrial toxicity of HIV-1 antiretroviral drugs on lipodystrophy and metabolic dysregulation. Curr. Pharm. Des. 2010;16:3339–3351. doi: 10.2174/138161210793563482. [DOI] [PubMed] [Google Scholar]
- 26.McComsey G.A., Lo Re V., III, O'Riordan M., et al. Effect of reducing the dose of stavudine on body composition, bone density, and markers of mitochondrial toxicity in HIV-infected subjects: a randomized, controlled study. Clin. Infect. Dis. 2008;46:1290–1296. doi: 10.1086/529384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maagaard A., Kvale D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: a continuum or distinct underlying mechanisms? J. Antimicrob. Chemother. 2009;64:901–909. doi: 10.1093/jac/dkp316. [DOI] [PubMed] [Google Scholar]


