Abstract
The integration of lipoprotein-related or apolipoprotein-targeted nanoparticles as pharmaceutical carriers opens new therapeutic and diagnostic avenues in nanomedicine. The concept is to exploit the intrinsic characteristics of lipoprotein particles as being the natural transporter of apolar lipids and fat in human circulation. Discrete lipoprotein assemblies and lipoprotein-based biomimetics offer a versatile nanoparticle platform that can be manipulated and tuned for specific medical applications. This article reviews the possibilities for constructing drug loaded, reconstituted or artificial lipoprotein particles. The advantages and limitations of lipoprotein-based delivery systems are critically evaluated and potential future challenges, especially concerning targeting specificity, concepts for lipoprotein rerouting and design of innovative lipoprotein mimetic particles using apolipoprotein sequences as targeting moieties are discussed. Finally, the review highlights potential medical applications for lipoprotein-based nanoparticle systems in the fields of cardiovascular research, cancer therapy, gene delivery and brain targeting focusing on representative examples from literature.
Keywords: Apolipoproteins, artificial lipoproteins, high density lipoproteins, lipoprotein related nanoparticles, low density lipoproteins, recombinant lipoproteins, reconstituted lipoproteins, synthetic lipoproteins
INTRODUCTION
In the search for new and improved therapeutics, the field of nanomedicine that deals with functionalized nanoparticles for molecular imaging and therapy is rapidly emerging. Nanoparticles offer new opportunities to transfer active substances directly to the diseased area in the body. If appropriately designed, the characteristics of the nanoparticles can easily be tuned to specific needs, especially by surface coatings and functionalization. Within the last two decades, a variety of nanoparticles have been designed for targeted delivery of drugs or contrast agents. Many of these nanoassemblies were constructed for cancer treatment by taking advantage of the leaky vasculature of tumors. However, apart from tumor targeting, ever increasing efforts are devoted to gene delivery and the early recognition and diagnostics of atherosclerotic plaques with the goal to impede cardiovascular events (for a review see ref. [1]). Some more recent developments concentrate on the combination of both therapy and diagnostics within one single nanoparticle. Along these lines, many nanostructured particles have been developed, some of which are on the market, while others are currently being applied in clinical trials [2, 3]. Among them, liposomes formed as single lipid bilayers and polymers are the most extensively explored delivery systems. The inherent problems of nanoparticles, however, are poor biocompatibility and low stability in vivo, since most artificial nanoassemblies become rapidly cleared by the mononuclear phagocyte system. In contrast to synthetic particles, lipoproteins are naturally occurring nanoparticles evading recognition by the body´s immune system, which makes them excellent candidates with highly attractive properties for exploitation as molecular transporters. The endogenous nature of lipoproteins, however, hampers the large-scale applicability of native lipoprotein particles. This fact has prompted researchers to focus on the development of reconstructed lipoprotein-mimetic particles, for instance by synthesizing recombinant lipoproteins from commercially available lipids and apolipoprotein molecules [4]. Recent approaches to develop synthetic lipoprotein-like nanoparticles assembled from lipid microemulsions and apolipoprotein-related peptide sequences or functional synthetic peptides are equally promising. Alternatively, apolipoprotein molecules can be considered as targeting moieties for non-lipidic nanoparticles. This review illustrates key research directions and highlights recent advances in the construction of artificial lipoproteins and, finally, outlines the potential of lipoprotein biomimetics for emerging nanomedical applications.
Lipoproteins as Endogenously Circulating Nanoparticles
Lipoproteins are supramolecular complexes of protein and lipids which transport lipophilic compounds in the blood’s aqueous environment. As naturally occurring nanoassemblies, lipoprotein particles are not immediately cleared by the mononuclear phagocyte system of the liver and spleen and remain in circulation for a longer period of time. During their lifetime lipoproteins are responsible for intercellular lipid transport to supply tissues and cells with cholesterol and fat, in the course of which lipoproteins are recognized by specific cellular receptors.
Structurally, lipoproteins are globular core-shell particles organized into two major compartments: an apolar oily core, comprised primarily of cholesteryl esters (CE) and triglycerides (TG) and an amphiphilic outer shell composed of a phospholipid monolayer embedding cholesterol and specific proteins, named apolipoproteins. Lipoproteins can be categorized into four major classes. Distinguished by their buoyant density, size, chemical composition and electrophoretic mobility, they are named chylomicrons, very low density lipoproteins (VLDL), low density lipoproteins (LDL) and high density lipoproteins (HDL). Fig. (1) gives an overview of the major lipoprotein classes.
Fig. (1).
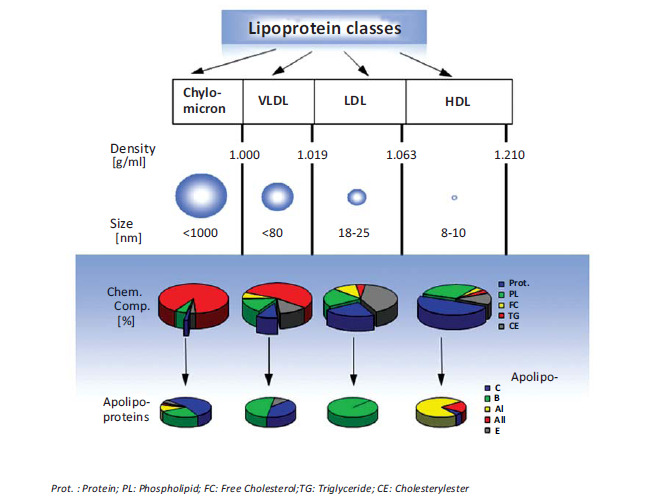
Major lipoprotein classes. Physical characteristics, average chemical composition and apolipoprotein content of different lipoprotein species.
Chylomicrons are the least dense and largest of the lipoproteins with a diameter in the micrometer range. Once in the blood stream, chylomicrons undergo a maturation process and due to successive hydrolysis of triglycerides, the size of chylomicrons decreases forming so-called chylomicron remnants, which are rapidly removed from the circulation by the liver. The next dense class of lipoproteins is the VLDL particle, which measures up to 80 nm in diameter and is rich in triglycerides, which become hydrolyzed by lipoprotein lipases. During the lipolytic removal of triglycerides, the particle size of VLDL decreases while the particle density increases. With time the particles are converted from triglyceride-rich VLDL to cholesteryl ester-rich LDL particles, which are much smaller than VLDL with diameters between 18 and 25 nm [5]. LDL particles are the primary transporters of cholesterol to peripheral tissues and in contrast to the other lipoprotein species, LDL particles possess just one single copy of an apolipoprotein called apolipoprotein B-100 (apo-B100). Apo-B100 is a large, non-exchangeable, amphipathic single-chain glycoprotein which plays a central role in particle stabilization as well as in cellular recognition and receptor mediated endocytosis of LDL. Once internalized by cells, LDL particles are transported into the lysosome for degradation. In humans, the liver and the andrenal glands have the highest expression level of LDL-receptors (LDLR), but LDL-receptors are also expressed in various other tissues including kidney, intestine and, most important for drug delivery, in numerous solid tumors. The LDL-receptor, also named the B/E-receptor, apart from apo-B100 also recognizes apolipoprotein E (apo-E) containing lipoproteins. Apo-E is an exchangeable apolipoprotein associated with different lipoprotein classes; apo-E synthesized in macrophages is involved in the transport of excess cholesterol from the peripheral tissues to the liver for disposal in the bile, a process named reverse cholesterol transport [6, 7]. HDL particles are the smallest and densest lipoprotein entities with diameters in the range of 7 to 15 nm. HDL particles are rich in protein and harbor different amounts of exchangeable apolipoproteins, of which apolipoprotein A-I (apo-AI) is the most abundant. Mature discoidal HDL, for instance, contains two apo-AI molecules wrapped around a lipid bilayer in a double belt-like manner [8], while the most probable structure for apo-AI on cholesterol-rich spherical HDL particles is a trefoil-like arrangement [9]. In any case, amphipathic alpha-helix bundles are a common structural feature of apolipoproteins such as apo-A and apo-E [10-12]. Studies on C-terminal truncated apo-AI by X-ray crystallography as well as the NMR structure of full-length modified apo-E have confirmed this feature [13, 14]. For a very recent review on the structure of apo-AI the reader is referred to ref. [15]. Physiologically, HDL plays a significant role in reverse cholesterol transport, in which apo A-I binds to ATP-binding cassette transporter A1 (ABCA1) in the extra-hepatic tissues for the efflux of cholesterol from the tissue to HDL. Further remodeling occurs through ATP-binding cassette transporter G1 (ABCG1). Apo A-I activates lecithin cholesterol acyltransferase (LCAT) so that the cholesterol is esterified, moves to the center of the lipoprotein converting nascent discoidal HDL to spherical HDL. HDL returns to the liver and binds to scavenger receptor class B type 1 (SR-B1) via apo A-I and downloads the cholesterol in the liver, which is targeted to bile and elimination from the body [16, 17]. For a better understanding, Fig. (2) shows a simplified scheme illustrating the pathways of lipoprotein metabolism and the role of specific lipoprotein receptors (A), and their involvement in reverse cholesterol transport (B).
Fig. (2).
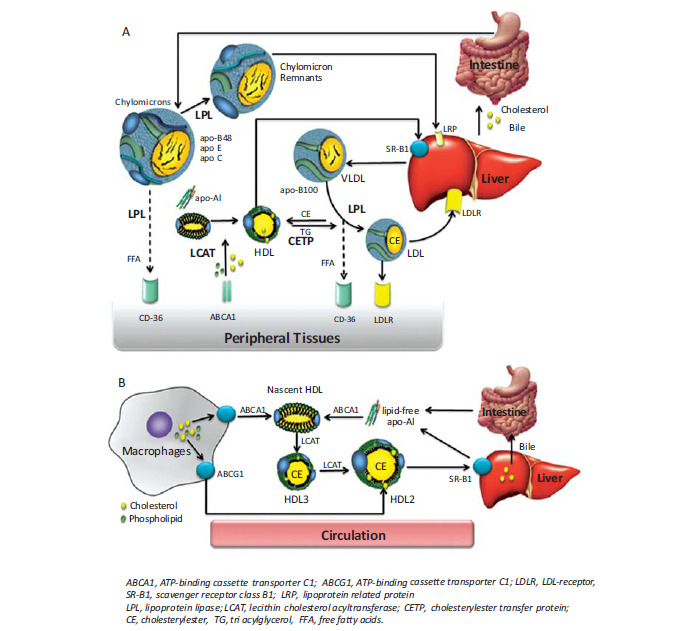
Schematic diagram of lipoprotein metabolism (A) and an overview of reverse cholesterol transport (B).
Having a deep understanding of the principles of natural lipoprotein metabolism is vital for the development of artificial targeted lipoproteins. Another crucial aspect in the development of lipoprotein related therapeutic formulations is their stability, especially during processing and drug loading. Equally important, is the resistance of the formulation to potentially deleterious biochemical modifications as oxidation or structural remodeling. In particular, physicochemical characteristics like size, surface curvature or chemical composition are the key determinants of particle structure and stability [18, 19]. Accordingly, lipoprotein stability and shelf life need to be considered as essential parameter in any kind of manipulation and manufacturing processes.
Adaptation of Lipoproteins to Design a Versatile Nanoparticle Platform for Drug Delivery and Imaging
As most studies concerning therapeutic and diagnostic applications of lipoproteins deal with nanoassemblies related to LDL and HDL, our review will focus on these two lipoprotein classes. Based on the literature review, terms like modified, reconstituted/recombinant, artificial/synthetic or lipoprotein-related nanoparticles can be identified, and are strongly dependent on how the lipoprotein particles are treated or produced. More precisely, native lipoproteins can be modified by loading drugs immediately after isolation and purification from human plasma. Alternatively, native lipoprotein particles can be separated first to be reconstituted from individual lipids and protein moieties later on. This procedure works quite well for HDL particles, while for LDL the reconstitution process is much more complex taking into account the huge size and amphipathic nature of apo-B100. These particles are termed reconstituted or recombinant. More recent studies report on the use of artificially reconstituted lipoprotein particles. Here, commercially available
synthetic lipids are used instead of natural lipid extracts, whereas synthetic lipoprotein particles are typically assembled from lipid microemulsions and peptide sequences resembling apolipoprotein domains. This strategy has met with much success using apo-AI mimetic peptides [20], while only a limited number of studies have been reported using peptide sequences resembling the receptor binding domain of apo-B100. The last category named lipoprotein-related nanoparticles primarily includes lipoprotein-mimetics, which consist of non lipidic nanoparticles targeted by apolipoproteins.
LDL RELATED PARTICLES FOR DRUG DELIVERY AND IMAGING
Reconstitution Processes, Drug Loading and Modification Procedures for LDL
For medical purposes, the biophysical and structural features of lipoproteins and their biological activity must be preserved. In general, there are several options for creating drug or contrast-agent-loaded and functionalized LDL particles, which are schematically depicted in Fig. (3). With the structural architecture of lipoproteins in mind, there are three principal ways by which LDL can be transformed into a medical nanocarrier: Incorporation of drugs in the oily inner lipid core (i), intercalation or association of active substances to the phospholipid surface monolayer (ii), or covalent binding of ligands to the protein moiety (iii). All such loading routes have been extensively studied and some have turned out to be more beneficial for certain medical applications than others.
Fig. (3).
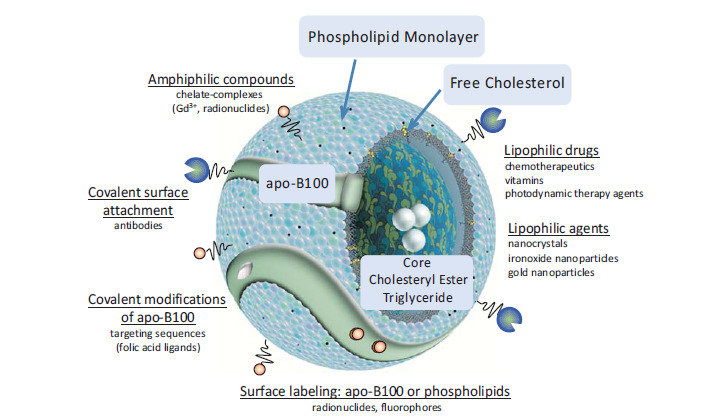
LDL particles can be modified to act as natural endogenous nanoparticles for targeted drug delivery or multifunctional molecular imaging. Representative ways to modify LDL are schematically depicted. Modified with permission from [40] © 2012 Prassl and Laggner, licensee InTech.
Drug Loading in the Inner Lipid Core
Hydrophobic drugs, including chemotherapeutics, antibiotics and vitamins are ideal candidates for incorporation in the lipophilic inner core of LDL. Hydophilic drugs have to be chemically modified before
incorporation to make them more lipophilic, for instance, by covalent coupling of fatty acid derivatives or cholesterol to the drug molecules [21]. Drug incorporation can be accomplished by different techniques including lyophilisation, solvent evaporation and reconstitution procedures [22, 23]. Most of the applied methods are rather harsh involving delipidation and reconstitution of the core using apolar solvents or surfactants, which could easily diminish particle integrity and receptor affinity [21]. Krieger et al. performed detailed comprehensive studies on the core loading reconstitution method, such that this procedure can be performed reproducibly while maintaining the structural and functional integrity of the lipoprotein particles [24-26]. However, to be used as an effective delivery system in vivo, a high payload of drug molecules is needed and the drug-loaded particles have to be stable at physiological conditions before systemic administration. Hence, to achieve reliable remote drug/contrast agent loading into the lipid core of plasma-derived lipoprotein particles, standardized procedures have to be developed and each investigator must conduct similar analyses for the respective diagnostic or therapeutic agent of interest in order to ensure that the integrity of the modified lipoprotein particle is preserved. Another shortcoming of this approach is the need to isolate LDL from human plasma. Thereby, substantial inter-donor variations and heterogeneity of LDL preparations must be considered. The big advantage of core-loading, however, is that drugs are protected from degradation and cytotoxic chemotherapeutics can be transported within lipoprotein particles, thus reducing systemic side effects and toxicity.
Another frequently studied approach makes use of lipid microemulsions containing the drug and detergent-solubilized or solvent-extracted apo-B100 [27-29]. Typically, the protein-free emulsion particles are assembled from natural or synthetic phospholipids, primarily phosphatidylcholines, and cholesterol oleate and/or triglycerides (triolein) [30, 31]. The limitation here is that apo-B100 is difficult to separate from plasma-LDL and due to its amphipathic nature, enormous size and structural sensitivity, it is even more difficult to restore apoB-100 to its native form on the surface of microemulsion particles. More recent studies have shown the feasibility of synthesizing artificial LDL particles using lipid microemulsions in combination with synthetic peptides, whereas the peptide sequence corresponds to the amino acid sequence of the LDL receptor binding domain [32-34]. These LDL mimetic particles were actively taken up by cells via LDL receptor mediated endocytosis [34]. To extend the protocol, Nikanjam et al. [35, 36] have used the same peptide sequence but additionally linked to an 18 amino acid amphiphatic alpha helix that avidly binds to the surface of the lipid emulsion. With fluorescence microscopy, the authors have been able to show in cell culture experiments that these bifunctional peptide-engineered synthetic LDL particles are as efficiently taken up via the LDL receptor pathway as native LDL. As the particle size of synthetic LDL was in between HDL and LDL the authors called their LDL, nano LDL.
Intercalation of Ligands in the Phospholipid Monolayer
Apart from loading drugs in the lipophilic core of LDL, amphiphilic substances (drugs or marker molecules) or fatty acid modified chelator complexes can be easily incorporated into the phospholipid surface monolayer of lipoproteins [37, 38]. Originally, this was done for numerous structural studies on lipoproteins and it is certainly the most convenient way to gain signal-emitting lipoprotein species for fluorescence microscopy and diagnostic imaging. The commercially available fluorescent DiI-LDL from Molecular Probes® (Life Technologies) is an excellent example of this.
Protein-Associated Surface Modifications of LDL
Finally, the surface of LDL can be modified by protein labeling. This is achieved by covalent attachment of molecules to the lysine and cysteine amino acid residues of apo-B100. Such molecules include fluorophores, radionuclides or metal ions for molecular imaging. Here it is important to note that this strategy is limited to relatively small modifications to the amino acid residues of apo-B100, otherwise the receptor recognition and the function of the lipoprotein will become compromised.
Alternatively, targeting sequences (e.g. folic acid) can be coupled to apo-B100, for instance with the purpose of rerouting LDL to alternate receptors, which in the case of folate, are more specifically expressed in tumor cells [39].
Irrespective of the way in which the LDL has been modified, the resulting LDL-drug complex has to be stable and the drug must remain incorporated, coupled or tightly associated to the LDL particle while transported in blood. During drug transport to cells, the LDL particle should protect its cargo from degradation and, if appropriate, reduce its cytotoxicity. Once LDL has entered the targeted cells efficient amounts of drugs have to be released to exert enhanced pharmacological effects, for instance to kill the tumor cells. Accordingly, the efficiency of the delivery system depends on many different parameters like the absolute amount of drug encapsulated and transported, the activity of cell surface receptors, the rate of internalization and finally the intracellular metabolic fate of the particle. LDL particles typically become trapped in lysosomes for cellular degradation and drug release. However, many drugs must escape from lysosomes into the cytosol to become active. To meet this challenge, an attractive solution is provided using light triggered release through photochemical internalization. Through this novel technique, endolysosomal membrane disruption is caused upon activation of photosensitizers, which allows a spatiotemporal control of cytosolic drug release [41].
As most studies are concerned with therapy and imaging of cancer and atherosclerotic plaques, or gene delivery, the next sections will provide a brief overview of the literature and describe some recent achievements in these fields.
LDL as Drug Delivery System in Cancer Therapy
The feasibility of exploiting the lipophilic carrier properties of lipoproteins for the purpose of drug delivery was recognized early [25, 42]. Lipoprotein-mediated delivery systems seemed to be particularly attractive for the transport of cytotoxic drugs, as it was realized that certain tumors have a higher level of LDL receptor activity than normal cells [43, 44]. Among them are malignancies as diverse as breast cancer, ovarian carcinomas, lung cancer, brain cancer, melanoma or leukemic cells (summarized in an early review by Firestone et al. [45]). The pronounced up-regulation of LDL receptors on tumor cells can be explained by a high demand of cholesterol necessary for cell growth and mechanisms directly linked to cell proliferation and de novo membrane synthesis [46]. However, the targeting specificity of LDL is limited as the LDL receptor is ubiquitously expressed throughout the body, most prominent in the liver and in adrenals. Consequently, it is of central concern to enhance the targeting selectivity to malignant tissues. One possibility for approaching this goal is to down-regulate fat metabolism in the liver e.g. by administration of steroids or bile salts [47]. An accumulation of bile salts in hepatocytes suppresses bile salt production and allows intracellular cholesterol pools to increase. Since the cell has ample cholesterol levels it then down regulates membrane LDL-receptor expression [48]. As a result, the circulation time of LDL particles in the bloodstream is enhanced to make more time available for the binding of drug loaded LDL to cell surface receptors on cancer tissues. When recognized by the LDL receptor, LDL particles become internalized to be delivered to the lysosome for the degradation and release of active drugs, while the LDL receptor is readily recycled back to the cell surface. Another strategy to reduce liver uptake of LDL is to reroute LDL from the classical LDL receptor pathway to an alternative receptor pathway with the goal of addressing other cell targets. One viable way is to redirect LDL to scavenger receptors on cells of the monocyte/macrophage system, which are not down regulated by cholesterol accumulation. Lipoprotein rerouting can be achieved by modifying the LDL particle, for instance by acetylation, methylation, derivatization of lysine residues on apo-B100 or by oxidative modifications of LDL [49]. Such chemically modified LDL can be further loaded with drugs or contrast agents to be selectively taken up by different classes of scavenger receptors on macrophages, including lectin-like ox-LDL receptor (LOX1), scavenger receptor class A (SR-A) or SR-B1. Apart from receptor-mediated uptake, some unspecific passive diffusion of LDL in the interstitial of solid tumors is known to occur due to the enhanced permeability of tumor microvasculature with gaps up to 400 nm in size [50].
LDL Particles for Gene Therapy
It was shown years ago that oligonucleotides, especially cholesterol modified oligonucleotides, can be intercalated into the phospholipid layer of LDL forming stable oligonucleotide LDL complexes [51]. More recent studies deal with the incorporation of small interfering RNA (siRNA) into lipoproteins [52, 53]. As such, siRNAs hold a great therapeutic potential to silence the expression of disease-related genes, making the development of proper delivery systems an important issue for pharmaceutical implementations. Still, the in vivo delivery of the highly negatively charged siRNA remains a challenge, as most nonviral cationic delivery systems utilized so far have faced the problem of toxicity. In this vein, it is an excellent strategy to explore biocompatible lipoprotein nanoparticles for the transport of siRNAs, and indeed, the first very promising results have been obtained for chemically modified siRNA. Cholesterol-conjugated siRNA (chol-siRNA) molecules were efficiently incorporated into LDL particles, while maintaining the structural integrity of LDL [53]. However, despite selective uptake of LDL-chol-siRNA nanoparticles into cells via the LDL receptor pathway, silencing efficiency was limited due to the entrapment of LDL-chol-siRNA in lysosomal compartments. To overcome this problem, the authors used photochemical internalization to induce specific cytosolic release. By this means the LDL-chol-siRNA mediated gene knockdown in HepG2 cells (cultured hepatoma cell line) was significantly enhanced [41]. Apart from cholesterol, other hydrophobic molecules like fatty acids or bile acids have been conjugated to siRNA. These lipophilic-siRNAs conjugates were shown to efficiently bind to lipoproteins and revealed enhanced gene silencing activity in vivo as compared to non-lipoprotein-associated lipophilic-siRNA [52]. Furthermore, the authors have compared the delivery routes of LDL to HDL. While LDL-mediated-siRNA transfection primarily occurs in the liver, HDL-bound siRNAs are transported to various tissues, including adrenal, ovary, kidney and liver, where they are selectively taken up by SR-B1 [52]. Although the preliminary results obtained for lipophilic derivatives of siRNA bound to lipoproteins are highly encouraging, optimization procedures and further tests have to be established to allow a wider range of applications for siRNAs to be implemented. For the future it is conceivable that the classical lipoprotein pathway can be circumvented by rerouting siRNA-loaded lipoprotein particles to alternative receptors to obtain a more versatile platform for the treatment of very different kinds of diseases. Some representative examples for LDL particles as drug delivery system are given in Table 1.
Table 1.
Applications of LDL-related particles as drug delivery systems.
| Lipoprotein | Drug | Application | Indication | Reference |
|---|---|---|---|---|
| LDL | doxorubicin derivative | in vitro/in vivo | cancer | [54] |
| m-LDL | cytotoxic compound 25 | in vitro | cancer | [28] |
| LDL | daunomycin | in vitro | cancer/lung | [55] |
| LDL | mitoclomine derivative | in vitro/in vivo | cancer/leukemia | [47] |
| LDL | daunorubicin derivatives | in vitro | cancer | [56] |
| LDL | ellipticin derivatives | in vitro/in vivo | cancer/melanoma | [46] |
| LDL | elliptinium oleate | in vitro/in vivo | cancer/melanoma | [57] |
| LDL | vincristine | in vivo (human) | cancer / ovarian | [58] |
| LDL | AD32/WB4291 | in vitro | acute myeloblastic leukemia | [29] |
| ac-LDL | ketoconazole | in vitro | infection | [59] |
| mod-LDL | thymidine | in vitro | macrophage associated diseases | [49] |
| r-LDL | dioleylfloxuridine /methotrexate | in vitro / in vivo (rat) | cancer | [60] |
| r-LDL | nucleoside | in vitro | HIV | [61] |
| r-LDL | tetra-t-butyl silicon phthalocyanine | in vitro | cancer / liver cells (photodynamic therapy) |
[62] |
| r-LDL | silicon naphtythalocyanine bisoleate | in vitro /in vivo (mice) | cancer / liver (photodynamic therapy) |
[23] |
| r-LDL | bacteriochlorin e6 bisoleate | in vivo | cancer / liver (photodynamic therapy) |
[63] |
| LDL | hypericin | in vitro | photodynamic therapy | [64] |
| dextran-LDL | hypericin | in vitro | cancer (photodynamic therapy) | [65] |
| syn-LDL | paclitaxel oleate | in vitro | cancer / glioblastoma | [35] |
| syn-LDL | in vitro | leukemic cell lines; CML; stem cell | [66] | |
| LDL | Chol-siRNA | in vitro / in vivo | gene silencing | [52] |
| LDL | Chol-siRNA | in vitro | gene silencing | [53] |
| galactosylated LDL | fluoresceinated ovalbumin | in vitro | antigen / Kupffer cells | [67] |
r-LDL: Reconstituted LDL; m-LDL: Model LDL; ac-LDL: Acetylated LDL; mod-LDL: modified LDL; syn-LDL: synthetic LDL; CML: Chronic Myeloid Leukemia.
Lipoproteins Loaded with Contrast Agents for Non-Invasive Medical Imaging
Non-invasive medical imaging requires the accumulation of contrast agents at the target site of disease. So far different non-invasive imaging techniques have been investigated for the detection, visualization and diagnosis of cancer and atherosclerosis. Apart from nuclear imaging by positron emission tomography (PET) or single-photon emission computed tomography (SPECT) the use of radioactive tracer molecules, optical imaging techniques like near infrared spectroscopy (NIR) or magnetic resonance imaging (MRI) is gaining importance. Each of the above-mentioned techniques has their own benefits and drawbacks [68, 69]. Both PET and SPECT have excellent sensitivity in the picomolar range but require the use of radioisotopes and have low spatial resolution. In comparison, magnetic resonance imaging (MRI) has much higher spatial resolution but lower sensitivity and thus needs higher concentrations of contrast agents to be accumulated in the diseased region [70]. Typical MRI contrast agents are iron-oxide nanoparticles or gadolinium chelates. Thereby, paramagnetic gadolinium ions (Gd3+) become chelated, mostly by diethylenetriaminepentaacetic acid (DTPA), in order to abolish the toxicity of free gadolinium. The Gd-chelates can be covalently coupled to phospholipid molecules to be incorporated into the
phospholipid monolayer of lipoproteins. In contrast to gadolinium complexes, ironoxide nanoparticles, whose size typically lies in the range between 3 and 200 nm, have to be embedded in the lipophilic core, where only the smallest ironoxide nanoparticles are appropriate for encapsulation. One example in which this procedure has proven effective is described Jung et al. who replaced the lipid core of rHDL by 7nm iron oxide nanoparticles [71]. Some recent studies point to the potential to create an inorganic nanocrystalline core within synthetic lipoprotein particles [72]. In this context, a novel labeling procedure for LDL with diagnostically active nanocrystals was described by Allijn et al. [73]. The method relies on the encapsulation of surface coated nanocrystals in micelles with subsequent translocation in the core of LDL.
LDL for Medical Imaging of Cancer
Initial reports on lipoprotein particles applied as signal emitting agents for tumor imaging date back to the early 1980´s. Initially, LDL particles were labeled with radionuclides as gallium (68Ga), technetium(99mTc) or indium (111In) [74-79]. The radioactive labels were either conjugated to apo-B100 or complexed by amphiphilic chelator molecules being intercalated in the surface phospholipid monolayer. Originally, the signal-emitting LDL particles were used for basic research studies as markers to determine LDL-receptor activity and LDL metabolism rather than for diagnostic purposes [80]. Similarly, various fluorescence dyes have been attached or incorporated into LDL for in vitro imaging. More recently, NIR fluorescent probes, such as carbocyanine derivatives conjugated to a lipid anchor, have been incorporated for optical imaging of tumor models. Using these conjugated LDL particles, a sufficient accumulation in tumor xenografts has been achieved [81, 82].
More recently, gadolinium agents were attached to LDL for improved tumor imaging using MRI. For this approach, gadolinium ions are caught in a chelator complex, very similar to the well-established protocols available for radionuclide complexation [37, 83]. In contrast to PET imaging, where few radiolabels are sufficient for obtaining high signal intensities, a high payload of contrast agents on LDL is required to overcome the low inherent sensitivity of MRI. Corbin et al have managed to load up to about 500 Gd3+ ions per LDL particle by intercalation of the amphiphilic DTPA-bis(stearylamide) complex into LDL before gadolinium loading [37]. The paramagnetic LDL nanoparticles behaved very similarly in vitro as native LDL being efficiently taken up by tissues that overexpress LDL receptors. In vivo, the particles exhibited a strong MR signal in a mouse model with human hepatoblastoma (HepG2) xenografts 24 hours after administration. Crich et al. investigated less bulky lipophilic chelator complexes for the immobilization of gadolinium to achieve higher relaxivities in MRI and to guarantee a higher thermodynamic stability of the complex [83]. The latter is important considering the known renal toxicity related to free gadolinium. The authors report on a selective accumulation of the novel Gd-chelated LDL particles for enhanced in vivo visualization of subcutaneous tumors in a mouse model. While most studies with LDL nanoparticles have been conducted in vitro or in animal models, there is one clinical study examining autologous LDL as a delivery vehicle for boron neutron capture therapy in human malignant glioma [84].
Rerouting Strategies for LDL-Mediated Cancer Diagnosis
As only a limited number of tumors overexpress the classical LDL-receptor, which specifically recognizes apo-B100 and apo-E, it would be advantageous to redirect LDL particles to alternative surface receptors more frequently and more specifically expressed on tumor cells. To achieve this task, tumor-specific ligands are conjugated to surface-exposed lysine residues of apo-B100. Indeed, by covalently linking folic acid via NHS-ester conjugation to native LDL, the folic-acid targeted particles were avidly taken up by folate receptor overexpressing KB-cells but not by LDL-receptor overexpressing cells [39]. The proof of concept that rerouting of LDL works in vivo was attained by IR fluorescence imaging after incorporation of a NIR dye into the phospholipid monolayer of LDL. In this manner, tumor accumulation, tissue distribution as well as clearance rates could be monitored non-invasively in living animals over a time period of 24 hours [38]. Although numerous reactions will compete with the targeted uptake of rerouted LDL particles by cancer cells, these preliminary studies show promise that ligands other than folic acid could be coupled to LDL to increase the affinity for cell-specific targeted uptake. The same authors also suggest using either larger VLDL particles for targeting tumor neovascularization or smaller HDL particles for targeting solid tumors by traversing the vascular endothelium by extravasation [38].
LDL for Imaging of Atherosclerotic Plaques
Non-oncologic applications of lipoproteins in imaging are focusing on the diagnosis of atherosclerotic plaques, especially the urgent need for early detection and differentiation between stable and vulnerable plaques have driven research in the field of imaging of atherosclerosis.
Apart from their physiological role in lipid transport, lipoproteins may be involved in the progression of atherosclerosis [85]. Atherosclerosis is a chronic inflammatory disorder of the blood vessels and a prevalent cause of cardiovascular disease and is a leading cause of morbidity and mortality worldwide [86]. Endogenous modifications of LDL, primarily by oxidation, enzymatic degradation or lipolysis are the initiating factors in early atherosclerosis. Thereby, LDL particles accumulate in the intima of the arterial wall where apo-B100 binds to proteoglycans of the extracellular matrix through ionic interactions. As a consequence, LDL particles are retained in the subendothelium, where they are prone to further oxidative modifications, aggregation and fusion. Such modified particles are rapidly taken up by macrophages to form foam cells. This event is a key step in the progression of atherosclerosis which implies the accumulation of LDL particles in plaque regions [87]. Mainly for that reason many researchers have explored the intrinsic targeting properties of LDL to atherosclerotic plaques for the early diagnosis and detection of atherosclerotic lesions by different non-invasive imaging modalities. Especially oxidatively modified LDLs (oxLDL) are known to play an important role in the course of plaque formation. Despite the fact that oxidation of LDL is primarily associated with lipid peroxidation and generation of reactive oxygen species, it has become evident that modifications and fragmentations of apo-B100 are responsible for altered receptor affinity. The modifications primarily involve surface-exposed lysine residues [88]. Besides modifications of lysine residues, apo-B100 was successfully derivatized by reductive lactosamination leading to an enhanced uptake of lactosylated LDL by galactose-specific receptors in the liver [89, 90]. In this context, lactosylated LDLs efficaciously loaded with cholesterol-conjugated oligodeoxynucleotides revealed a substantially increased uptake of oligonucleotides by Kupffer cells in vivo [91].
So far a broad arsenal of studies in the literature report on the use of radiolabeled LDL and modifications thereof for imaging of atherosclerosis (for comprehensive reviews see refs. [92, 93]). A variety of different radionuclides, including 111In, 99mTc, 68Ga, 18F, were coupled to LDL as a tracer for the detection of atherosclerotic lesions. The efficient uptake of radiolabeled LDL in atherosclerotic plaques was demonstrated in numerous studies and as lipoprotein contrast agents preferentially accumulate in macrophages they were used to determine the content and location of macrophages within the plaque using an established mouse model for atherosclerosis (apo E-/- mouse) [94].
For MRI imaging of atheromas, only a limited number of reports applying LDL are available. In one study, LDL particles were enriched with hydrophobic paramagnetic manganese-mesoporphyrin as an MRI-active contrast agent. The complex showed a strong MRI signal after incubation with foam cells [95]. In another study, a new Gd-chelate (GdDO3A-monoamide chelate with a long alkenyl anchor) was synthesized and incorporated in the phospholipid monolayer of native LDL. In vivo studies in the apo E-/- mouse model showed significant retention of the paramagnetic LDL complexes in atherosclerotic plaques and a clear signal enhancement in MR [96]. Lowell et al. incorporated an oleic acid conjugated DO3A-chelate complex in native LDL followed by a subsequent coordination reaction with Gd3+ [97]. The post chelating with Gd3+ citrate solution resulted in an extremely high payload of about 200 Gd3+ ions per LDL particle. The Gd3+ loaded LDL was extremely stable and revealed biophysical characteristics similar to native LDL. At 48 hours post injection in apo E-/- mice, the authors found a pronounced signal enhancement in the in vivo MR images of the atheroma in the mouse arteries indicating a high accumulation of Gd3+- LDL in the plaque regions. The authors propose that the Gd-modified LDL particles are most probably taken up by macrophages inside the plaque. Interestingly, the authors point out the possibility of converting autologous LDL isolated from one patient into MR-active LDL to be returned to the blood of the same patient. This extracorporeal approach was suggested as an essential step towards new strategies in personalized medicine to achieve selective detection of atherosclerotic plaques [98].
To summarize, non-invasive visualization of cardiovascular events by exploiting the targeting potential of multimodal nanoparticles with special focus on modified lipoproteins is becoming increasingly important, especially for the early assessment of individual risk profiles of vascular dysfunction and heart failure.
HDL RELATED PARTICLES FOR DRUG DELIVERY AND IMAGING
In principle, HDL particles can be modified in the same way as described for LDL particles. However, compared to LDL it is much easier and more convenient to reassemble HDL particles from individual lipids and protein than to modify native HDL isolated from human plasma [99]. This is due to the fact that HDL contains water-soluble, small apolipoproteins, most importantly the aforementioned apo-AI, instead of the huge amphipathic apo-B100 molecule. Even more interesting is the fact that the association of amphipathic α-helical peptides with lipid nanoparticles is sufficient to mimic the metabolic properties of native HDL. As such, the use of artificial HDL as a transport vehicle becomes more and more attractive for therapeutic applications or as flexible nanoparticle template for diagnostics. More recent papers even point to the possibility of using HDL-like nanoparticles for combined therapy and diagnostics (theranostics). In the field of cancer research, lipoprotein-inspired nanoparticles for theranostic strategies by loading and delivering cancer therapeutic and diagnostic agents are most promising [100]. The small size of HDL (less than 20 nm) allows them to maneuver deeply into tumors and HDL can be targeted either to their endogenous receptors, when those are implicated in cancer, or to other cancer-specific receptors. A recent review by Liu et. al. outlines the main mechanisms predicted to improve the function and targeting specificity of HDL in vivo in more detail [101].
Reconstituted (Recombinant) HDL – rHDL
Depending on which lipids are used for the reconstitution of HDL, the nanoparticles formed are either lipoprotein nanodiscs or spherical nanoparticles. Traditionally, HDL particles are reassembled from apo-AI and phospholipids to form nascent discoidal HDL particles. In the course of this procedure, commercially-available bilayer-forming phospholipids, mainly dimyristoylphosphatidylcholine (DMPC) and dimytristoylphosphatidylglycerol (DMPG), sodium cholate and apo-AI are used for the self-assembling process. The particles are formed by detergent-removal and are recovered by gel chromatography or density gradient ultracentrifugation [99]. To convert the discoidal HDL nanoparticles into spherical HDL particles, they are incubated with LDL and the enzyme lecithin-cholesterol acyltransferase (LCAT) to induce lipid exchange. Alternatively, spherical HDL can be prepared by sonication from lipid suspensions containing phospholipids and CE. With the addition of apo-AI, the protein associates to the lipid suspension to form spherical HDL [102]. Recently, Kim et al. presented an innovative and very elegant production technique for rHDL using microfluidics. In a single step procedure, multifunctional rHDL particles could be conveniently synthesized by mixing apo-AI with lipids and imaging/therapeutic agents. By manipulating the mixing speed and the lipid to protein ratio, the physicochemical characteristics (e.g. size, morphology) of the HDL-derived nanomaterials could be tuned. Experimentally, fluorescence dyes and the hydrophobic drug simvastatin were incorporated to form discoidal rHDL particles for drug delivery. Likewise, inorganic gold, ironoxide and quantum dot nanocrystals codispersed with lipids in organic solution were incorporated as nanocrystalline core of spherical rHDL nanoparticles for computer tomography (CT), MRI and fluorescence microscopy, respectively [103]. A scheme for the microfluidic synthesis procedure is provided in Fig. (4).
Fig. (4).
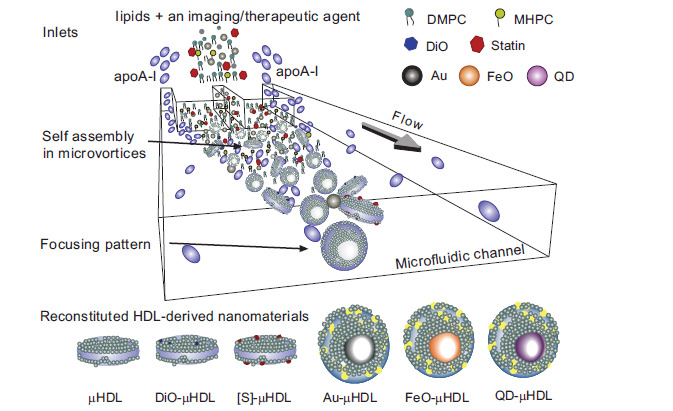
Microfluidic reconstitution of HDL nanoparticles. A schematic depiction of a microfluidic platform that allows for a single-step and large scale production of HDL particles. Reconstituted discoidal HDL (µHDL); DiO-µHDL for fluorescence; [S]-µHDL statin loaded rHDL as therapeutics; Au-µHDL, FeO-µHDL and QD-µHDL spherical rHDL with nanocrystalline core of gold, ironoxide and quantum dots for CT imaging, MRI and fluorescence microscopy, respectively. Reprinted with permission from [103]; copyright 2013 American Chemical Society.
rHDL-Based Therapy
Numerous studies deal with the intrinsic anti-atherogenic and cardioprotective properties of HDL and with the use of rHDL as a therapeutic agent [104]. Specifically, repeated treatment with rHDL has been shown to reduce inflammatory and atherogenic processes in different animal studies. The preclinical studies have been further extended to human clinical trials revealing that rHDL is well tolerated by patients. It was found that intravenous injections of rHDL significantly raise the levels of HDL in plasma of humans. This is considered positive as it is expected that increased levels of HDL lead to a reduction of atheroma volume and plaque remodeling. Since the therapeutic options of using rHDL are not in the focus of the current review, we would like to refer to two excellent reviews by Murphy et al. [105] and Damiano et al. [106] who point out that the administration of rHDL is a promising strategy for treating atherosclerosis and other inflammatory related diseases.
rHDL as Drug Delivery Systems
To come back to the potential of rHDL as a targeted delivery system, it is important to note that similar to LDL, HDL is excellently suited for the delivery of lipophilic and poorly water soluble drugs, which account for almost 40% of all drugs. The drugs can be easily accommodated within the phospholipid bilayer of rHDL-nanodiscs. Accordingly, hydrophobic drugs like all-trans retinoic acid, a retinoid hormone, amphotericin B, an antifungal substance or curcumin have been formulated in rHDL-nanodiscs [107-110]. The latter formulation, for example, demonstrated less toxicity in cell culture compared to the conventional formulation and was shown to be highly effective in animal models [108]. In another example, rHDL was examined as a delivery system for nosiheptide, a lipophilic peptide with anti-hepatitis B viral activity. Complexed with rHDL, the drug was efficiently transported to the liver after intravenous injection in rats [111]. Similar results were obtained by the same group [112] using palmitate-modified acyclovir complexed in rHDL as antiviral therapeutics targeting the liver. In other studies, rHDL was employed as a carrier for anticancer drugs [113, 114]. When the lipophilic anticancer drug paclitaxel was loaded into spherical rHDL particles, a much better tolerance was achieved in mice compared to the free drug, while the drug was taken up primarily by SR-B1 expressed on cancer cells [114, 115]. Equally good results were obtained for rHDL loaded with doxorubicin. The particles showed high absorption to SR-B1 positive hepatocytes in vitro and when applied in vivo tumor growth was more effectively reduced than by liposomal doxorubicin [116]. In another example, HDL was pre-incubated with cholesterol-modified siRNA and the HDL-bound cholesterol-siRNA was injected into Styrian hamster [52]. In this study, HDL cholesterol uptake and the expression of SR-B1 correlated well with the tissues that were identified to take up the cholesterol-siRNA. The data also indicated a significant enhancement of gene silencing efficiency for HDL-bound siRNA as compared to equal amounts of unbound siRNA [52] indicating that HDL is capable to transport and deliver functional siRNA in vivo targeting SR-B1 expressing cells. Only recently was it reported that HDL mimicking peptide-phospholipid scaffolds can be employed to achieve selective, efficient and direct cytosolic delivery of cholesterol modified siRNA [117]. It is assumed that the cholesterol-siRNA molecules stick to the surface of the rHDL particle as shown by an increase of the hydrodynamic radius of the nanoparticle and an increased negative surface charge upon loading with anionic cholesterol-siRNA. While apo-AI functionalized cholesterol-siRNA-containing particles were taken up by SR-B1, analogous particles functionalized with apo-E were internalized by the LDL receptor [118]. Shahzad et al. produced another kind of spherical rHDL particle that contains an siRNA-oligolysine core surrounded by a lipid layer and apo-AI [119]. These particles were selectively taken up by tumor xenografts which overexpress SR-B1; almost no uptake was observed in normal tissues. To date, only limited data are available for the delivery of nucleic acids using lipoprotein-related nanoparticles, however, recent studies provide strong evidence that rHDL nanoparticles could serve as efficient non-viral vectors for gene delivery. Detailed background information on the current challenges of nucleic acid therapy and a closer description of the strategy why and how to use HDL as transporter for nucleic acids is provided in recent reviews [106, 120]. Table 2 summarizes some representative examples of studies developing HDL-related particles for drug delivery.
Table 2.
Applications of HDL-related particles as drug delivery systems.
| Lipoprotein | Drug | Application | Indication | Reference |
|---|---|---|---|---|
| rHDL (nanodisc) | curcumin | in vitro | anti-inflammatory, anti-proliferative cancer | [109, 110] |
| rHDL (nanodisc) | retinoic acid | cancer | [107] | |
| rHDL (nanodisc) | amphotericin B | in vitro/in vivo | anti-fungal | [108] |
| rHDL (spherical) | nosiheptide | in vivo | anti-viral | [111] |
| rHDL (spherical) | fluorouracil (5-FU), 5-iododeoxyuridine, doxorubicin (Dox), vindesine |
in vitro | cancer | [113] |
| rHDL (synthetic, spherical) | paxlitaxel | in vitro/ in vivo | cancer | [114] |
| rHDL (synthetic, spherical) | paxlitaxel | in vivo | cancer | [115] |
| rHDL (spherical) | doxorubicin | in vitro/in vivo | cancer | [116] |
| HDL (native) | chol-siRNA | in vivo | gene delivery | [52] |
| HDL (mimic) | chol-siRNA | in vitro | gene delivery | [117] |
| HDL (mimic) | chol-siRNA | in vivo | gene delivery / tumor growth inhibition | [121] |
| rHDL (spherical) | oligolysine-siRNA | in vivo | cancer | [119] |
| HDL (functional mimic, spherical) |
gold nanoparticles | in vitro | cholesterol binding /cholesterol efflux | [122, 123] |
| HDL (synthetic, spherical) |
gold nanocrystalline core | in vivo | cancer, lymphoma | [124] |
| HDL (synthetic, spherical) |
gold nanocrystalline core, chol-DNA | in vitro | gene delivery | [125] |
| rHDL (folic acid targeted) | fluorescent | in vitro | cancer | [126] |
r-HD: Reconstituted HDL; chol: cholesterol
rHDL for Medical Imaging
Modified rHDL has been frequently studied as a contrast agent for atherosclerotic imaging. As already mentioned, the main reasons to perform vascular imaging are to achieve an early recognition of atherosclerotic lesions and to be able to differentiate between stable and vulnerable plaques. The latter are prone to rupture causing stroke or myocardial infarction and are identifiable by a thin fibrous cap, strong neovascularization and a higher amount of monocyte-derived macrophages, especially in the area of the plaque shoulder [127, 128]. Along this line, HDLs have been demonstrated to target macrophages and due to their small size can penetrate the plaque, whereas macrophage infiltration is positively correlated with lesion progression, plaque size and intimal thickness [129]. Based on these considerations, HDL particles seem to be excellently suited for the recognition of atherosclerotic plaques through targeting of macrophages. Shaish et al. were the first to show an accumulation of
radiolabeled HDL in atherosclerotic lesions of the aortic arch in apo E-/- mice [130]. Based on these very promising results, Cormode and colleagues have continued to adopt HDL for contrast-enhanced MRI [131, 132]. To achieve MR signaling, HDL was reconstituted from a single phospholipid species, cholesteryl esters and apo-AI by established techniques. But in addition to standard lipids a chelate carrying phospholipid species (e.g. 1,2 di-myristoyl -sn-glycero-3-phosphatidyl-ethanolamine-diethylenetriamine pentaacetic acid (DMPE-DTPA)) that provides the possibility to complex gadolinium ions, has been included. In addition, the authors have incorporated amphiphilic fluorescent dyes like Rhodamine B or Cy5.5-labeled phosphatidylethanolamine in the phospholipid layer. With dual-labeled rHDL, multimodal imaging with MRI and optical techniques is performed in vivo as well as ex vivo after injection in apo E-/- mice. The particles were shown to accumulate in the macrophage-rich domains of aortic plaques [133]. Similar results were obtained by the same group producing discoidal rHDL particles instead of spherical ones [134]. Later on, the PE-DTPA lipid was substituted by an AAZTA-(1,4-bis(hydroxycarbonylmethyl)-6-[bis(hydroxycarbonyl-methyl)]amino-6-methylperhydro-1,4-diazepine)-lipid. Such modified particles revealed a higher relaxivity than the Gd-DTPA containing rHDL, making them even more efficacious contrast agents [135]. For tumor imaging, a novel chemically stable bacteriochlorophyll analog coupled to two unsaturated hydrocarbon chains was synthesized for NIR fluorescence imaging. The dye was incorporated into rHDL particles during reconstitution by sonication of phospholipids and cholesterol oleate before the addition of apo-AI [136]. These particles were shown successful in targeting cancer cells that express SR-B1.
Rerouting Strategies for HDL
A highly attractive alternative to address a broader range of tumors is to modulate the in vivo performance of HDL particles by rerouting them to biomarkers other than their natural receptors [126, 137, 138]. This can be achieved by surface functionalization of rHDL particles with targeting sequences. Apart from prior identification of specific targeting ligands, it is equally important to establish a proper conjugation chemistry to preserve the endogenous structure of HDL. A successful example of rHDL being rerouted is demonstrated by the conjugation of folate to the surface of fluorescent-labeled rHDL particles with the aim of targeting folic acid receptors, which are overexpressed on ovarian cancer cell lines [126]. In another example, Chen et al. have coupled an αvβ3-integrin specific RGD-peptide to rHDL to target angiogenic endothelial cells [139]. The peptide functionalized rHDL particles were loaded with signal-emitting molecules to enable in vivo multimodal MR and NIR imaging simultaneously. The authors found a preferential uptake of the RGD-peptide functionalized particles in tumor endothelial cells. For the first time, molecular imaging of angiogenesis through rerouting of targeted rHDL was achieved.
Synthetic HDL
Over the last decade, the research groups around Cormode, Mulder, Fayad and colleagues have further refined the method for lipoprotein-inspired imaging of atherosclerosis by producing fully synthetic HDL-particles containing a spherical nanocrystalline core. The inner lipophilic core is surrounded by a monolayer of phospholipids with apo-AI molecules associated to the surface. The nanocrystalline core can be composed of either Au-nanoparticles for CT imaging, quantum dots for optical imaging or ironoxide-nanoparticles for MRI [72, 140]. Additionally, fluorescent dyes or phospholipid anchored Gd-chelates can be incorporated into the outer phospholipid monolayer of the synthetic HDLs to construct multimodal signal-emitting particles for molecular imaging. The synthetic HDL particles are spherical and highly homogeneous with a diameter and a lipid to protein ratio similar to naturally occurring HDL. As seen in in vivo experiments in the apo E-/- mouse model for atherosclerosis, the nanocrystalline HDL particles are readily taken up in the vessel walls and macrophages of atherosclerotic plaques [72]. Only recently, the biomimetic synthetic HDL particles containing a gold nanocrystalline core have proven effective in the treatment of lymphoma by inhibiting B-cell lymphoma growth through binding to SR-B1, cholesterol starvation and selective induction of apoptosis [124]. In an alternative approach, McMahon et al. explored synthetic HDL with a gold nanocrystalline core for gene delivery [125]. In this study, gold nanoparticles (AuNP) were mixed with apo-AI in an aqueous solution prior to assembly with lipids. Subsequently, cholesterol-modified DNA was absorbed to form hybrid chol-DNA-HDL AuNPs (see Fig. 5). The conjugate was internalized by human cells and efficient cellular nuclear acid delivery was achieved. Overall for the first time, this study combines lipid-based nucleic acid transfection strategies with HDL biomimicry for cell-specific targeting.
Fig. (5).
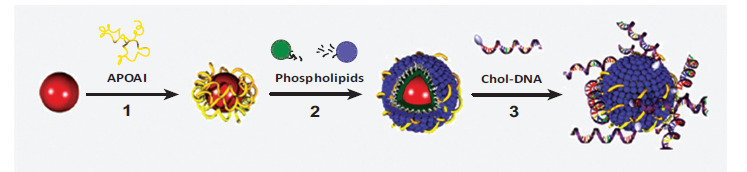
Biomimetic HDL particles for nucleic acid delivery. (1) An aqueous solution of gold nanoparticles (AuNPs) is mixed with apo-AI. (2) A mixture of phospholipids is then added to the surface of the AuNPs to form biomimetic HDL AuNPs. HDL AuNPS are purified by centrifugation and are resuspended in water. (3) Chol-DNA is added to purified HDL AuNPs and the chol-DNA-HDL-AuNP conjugates are purified by centrifugation and resuspended in buffer. Reprinted with permission from [125]; copyright 2011 American Chemical Society.
Nanodiscoidal HDL with Peptide Mimetics
The recent efforts to develop synthetic HDLs have been expanded to include peptide sequences instead of apolipoprotein molecules to mimic natural HDL particles. These peptides possess amphipathic helix motifs similar to those present in apo-A or apo-E [10, 12], but do not necessarily show sequence homology to apolipoproteins. The only requirement is that the peptide sequences show a high binding affinity to lipids. To this end, various lipid-peptide complexes are synthesized with different lipid compositions as well as lipid to peptide ratios [141]. In one promising study, a synthetic HDL nanodisc carrying the apo-AI mimetic peptide termed 37pA was functionalized with Gd-bearing agents and rhodamine for combined MRI and fluorescence imaging, respectively. A comparison between apo E-/- mice and wild type mice after in vivo administration of peptide functionalized HDL nanodiscs revealed a significant enhancement of the MRI signal in the aortic plaques of the apo E-/- mouse and no significant signal in aortas of wild type mice [142]. Ex vivo fluorescence microscopy confirmed that the HDL nanodiscs preferentially accumulate in the macrophages in atherosclerotic lesions. The same group showed that the targeting potential can be further enhanced using an apo-E derived lipopeptide, termed P2fA2 [143]. The apo-E based peptide sequence is a cationic arginine-rich tandem dimer, which shows remarkable membrane penetrating properties. The peptide was conjugated with two fatty acid chains to create a cationic lipopeptide to be more easily incorporated into the phospholipid layer. For further details on the versatility of synthetic HDL for molecular imaging, the reader is referred to several excellent reviews performed by the same group [72, 93, 144].
Non-Lipid-Based Apolipoprotein-Targeted Nano-particles for Drug Delivery
Next to other lipoprotein nanoparticles described before, non-lipidic nanoparticles coated with apolipoproteins are being developed as drug carriers. Among them are so-called proticles, which are based on protamine and oligonucleotides. Protamine is a relatively small polycationic peptide with a molecular weight of approximately 4000 Da. In the sperm of salmon, protamine condenses DNA and delivers it to the nucleus of the egg after fertilization. This property of protamine has been exploited to condense plasmid DNA into compact DNA structures. In combination with smaller antisense oligonucleotides, proticles increased the oligunucleotide stability against nuclease activity, and improved cellular uptake of the antisense molecules [145]. Proticles have several properties that make them potential candidates for drug delivery: i) The production is simple and rapid and is based on self-assembly of charged macromolecules [145]. ii) Proticles display extremely low (cyto) toxicity in vitro as compared to other nanomaterials [146]. Consequently, proticles coated with apolipoproteins were investigated as targeted carriers to improve uptake efficacy across the blood brain barrier (BBB). Kratzer et al. [147] have evaluated the effects of apoA-I coated proticles on the functional properties of primary porcine brain capillary endothelial cells (BCEC) and characterized the uptake and transcytosis of proticles in this cell culture model. Transcytosis of 125I-labeled proticles across polarized BCEC cultures occurred in a time- and concentration-dependent manner. ApoA-I coating enhanced proticle delivery to astrocytes in this in vitro model of the BBB almost twofold. The blocking of SR-B1 reduced transcytosis of apoA-I-coated proticles to levels observed for uncoated proticles. Therefore the authors concluded that apoA-I coating of proticles could be a feasible targeting technology to improve drug delivery across the BBB [147].
CONCLUSION AND PERSPECTIVE
Lipoproteins are flexible nanostructures for the delivery of both therapeutics and imaging agents alike. While the lipoprotein platform is still in its infancy, first groundbreaking results in enhanced multimodal imaging, treatment of tumors, gene therapy and visualization of atherosclerotic plaques show promise that further endeavors to implement theranostics will be successful, especially with respect to personalized medicine. More recent approaches focus on the exploration of sophisticated lipoprotein-mimetic nanomaterials as a biocompatible platform for medical applications. In the near future it is likely that the development of functionalized lipoprotein mimetics with specific targeting properties will prove to be successful. However, despite promising options provided by pre-clinical studies, only a limited number of clinical studies are currently available. This fact directly reflects the challenges of the future, namely to create standardized protocols for widespread use. In parallel, new technologies have to be established for the reproducible and controlled manufacturing of synthetic lipoprotein particles with high yield and homogeneity. Given the versatility and physical tunability of composition, size and morphology of artificial lipoprotein particles, this will be one of the major tasks in the more distant future. Moreover, toxicological and immunological data are required before human trials can be conducted, assuring that the products are safe with a comparably low toxicity profile. Given these requirements, it is reasonable to expect that the pharmaceutical industry will pursue the translation of the lipoprotein platform to clinics and commercialization.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge support by the European Union (“NanoAthero” project FP7-NMP-2012-LARGE-6-309820) and the Austrian Science Fonds (FWF Project No. I 1109-N28).
LIST OF ABBREVIATIONS
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- Apo-A1
Apolipoprotein A-1
- Apo-B100
Apolipoprotein B-100
- Apo-E
Apolipoprotein E
- BBB
Blood Brain Barrier
- BCEC
Brain capillary endothelial cells
- CE
Cholesteryl esters
- CETP
Cholesteryl ester transfer protein
- CHOL
Cholesterol
- DTPA
Diethylenetriaminepentaacetic acid
- HDL
High density lipoprotein
- LCAT
Lecithin cholesterol acyltransferase
- LDL
Low density lipoprotein
- LDLR
Low density lipoprotein receptor
- LOX1
Lectin-like ox-LDL receptor
- MRI
Magnetic resonance imaging
- NIR
Near infrared spectroscopy
- oxLDL
Oxidatively modified low density lipoprotein
- PET
Positron emission tomography
- rHDL
Reconstituted/recombinant HDL
- siRNA
Small interfering RNA
- SPECT
Single-photon emission computed tomography
- SR-A
Scavenger receptor class A
- SR-B1
Scavenger receptor class B type 1
- TG
Triglycerides, triacylglycerols
- VLDL
Very low density lipoprotein
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Lobatto M.E., Fuster V., Fayad Z.A., Mulder W.J. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011;10(11):835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan R., Gaspar R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011;8(6):2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 3.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Rensen P.C., de Vrueh R.L., Kuiper J., Bijsterbosch M.K., Biessen E.A., van Berkel T.J. Recombinant lipoproteins: lipoprotein-like lipid particles for drug targeting. Adv. Drug Deliv. Rev. 2001;47(2-3):251–276. doi: 10.1016/s0169-409x(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 5.Prassl R., Laggner P. Molecular structure of low density lipoprotein: Current status and future challenges. Eur. Biophys. J. 2009;38(2):145–158. doi: 10.1007/s00249-008-0368-y. [DOI] [PubMed] [Google Scholar]
- 6.Mahley R.W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 7.Fielding C.J., Fielding P.E. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995;36(2):211–228. [PubMed] [Google Scholar]
- 8.Segrest J.P., Jones M.K., Klon A.E., Sheldahl C.J., Hellinger M., DeLoof H., Harvey S.C. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J. Biol. Chem. 1999;274(45):31755–31758. doi: 10.1074/jbc.274.45.31755. [DOI] [PubMed] [Google Scholar]
- 9.Silva R.A., Huang R., Morris J., Fang J., Gracheva E.O., Ren G., Kontush A., Jerome W.G., Rye K.A., Davidson W.S. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. USA. 2008;105(34):12176–12181. doi: 10.1073/pnas.0803626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borhani D.W., Rogers D.P., Engler J.A., Brouillette C.G. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc. Natl. Acad. Sci. USA. 1997;94(23):12291–12296. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisgraber K.H., Newhouse Y.M., Mcpherson A. Crystallization and preliminary-x-ray analysis of human plasma apolipoprotein c-i. J. Mol. Biol. 1994;236(1):382–384. doi: 10.1006/jmbi.1994.1146. [DOI] [PubMed] [Google Scholar]
- 12.Wilson C., Wardell M.R., Weisgraber K.H., Mahley R.W., Agard D.A. Three dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252:1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- 13.Mei X., Atkinson D. Crystal structure of C-terminal truncated apolipoprotein A-I reveals the assembly of high density lipoprotein (HDL) by dimerization. J. Biol. Chem. 2011;286(44):38570–38582. doi: 10.1074/jbc.M111.260422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Chen J., Wang J. A complete backbone spectral assignment of lipid-free human apolipoprotein E (apoE). Biomol. NMR Assign. 2008;2(2):207–210. doi: 10.1007/s12104-008-9122-8. [DOI] [PubMed] [Google Scholar]
- 15.Mei X., Atkinson D. Lipid-free apolipoprotein a-i structure: insights into HDL formation and atherosclerosis development. Arch. Med. Res. doi: 10.1016/j.arcmed.2015.05.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niesor E.J. Will lipidation of ApoA1 through interaction with ABCA1 at the intestinal level affect the protective functions of HDL? Biology (Basel) 2015;4(1):17–38. doi: 10.3390/biology4010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinecke J.W. A new era for quantifying HDL and cardiovascular risk? Nat. Med. 2012;18(9):1346–1347. doi: 10.1038/nm.2930. [DOI] [PubMed] [Google Scholar]
- 18.Shen B.W., Scanu A.M., Kézdy F. Structure of human serum lipoproteins inferred from compositional analysis. Proc. Natl. Acad. Sci. USA. 1977;74:837–841. doi: 10.1073/pnas.74.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajima S., Yokoyama S., Yamamoto A. Effect of lipid particle size on association of apolipoproteins with lipid. J. Biol. Chem. 1983;258(16):10073–10082. [PubMed] [Google Scholar]
- 20.Zhang Z.H., Chen J., Ding L.L., Jin H.L., Lovell J.F., Corbin I.R., Cao W.G., Lo P.C., Yang M., Tsao M.S., Luo Q.M., Zheng G. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small. 2010;6(3):430–437. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 21.Kader A., Davis P.J., Kara M., Liu H. Drug targeting using low density lipoprotein (LDL): physicochemical factors affecting drug loading into LDL particles. J. Control. Release. 1998;55(2-3):231–243. doi: 10.1016/s0168-3659(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 22.Hammel M., Laggner P., Prassl R. Structural characterisation of nucleoside loaded low density lipoprotein as a main criterion for the applicability as drug delivery system. Chem. Phys. Lipids. 2003;123(2):193–207. doi: 10.1016/s0009-3084(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 23.Song L.P., Li H., Sunar U., Chen J., Corbin I., Yodh A.G., Zheng G. Naphthalocyanine-reconstituted LDL nanoparticles for in vivo cancer imaging and treatment. Int. J. Nanomedicine. 2007;2(4):767–774. [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger M., Brown M.S., Faust J.R., Goldstein J.L. Replacement of endogenous cholesteryl esters of low density lipoprotein with exogenous cholesteryl linoleate. J. Biol. Chem. 1978;253(12):4093–4101. [PubMed] [Google Scholar]
- 25.Krieger M., Smith L.C., Anderson R.G., Goldstein J.L., Kao Y.J., Pownall H.J., Gotto A.M., Jr, Brown M.S. Reconstituted low density lipoprotein: a vehicle for the delivery of hydrophobic fluorescent probes to cells. J. Supramol. Struct. 1979;10:467–478. doi: 10.1002/jss.400100409. [DOI] [PubMed] [Google Scholar]
- 26.Krieger M. Reconstitution of the hydrophobic core of low-density lipoprotein. Methods Enzymol. 1986;128:608–613. doi: 10.1016/0076-6879(86)28094-5. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg B., Suominen L. Preparation of biologically active analogs of serum low density lipoprotein. J. Lipid Res. 1984;25(6):550–558. [PubMed] [Google Scholar]
- 28.Lundberg B. Preparation of drug-low density lipoprotein complexes for delivery of antitumoral drugs via the low density lipoprotein pathway. Cancer Res. 1987;47(15):4105–4108. [PubMed] [Google Scholar]
- 29.Masquelier M., Lundberg B., Peterson C., Vitols S. Cytotoxic effect of a lipophilic alkylating agent after incorporation into low density lipoprotein or emulsions: studies in human leukemic cells. Leuk. Res. 2006;30(2):136–144. doi: 10.1016/j.leukres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Ginsburg G.S., Small D.M., Atkinson D. Microemulsion of phospholipids and cholestrol esters. Protein- free models of low-density lipoprotein. J. Biol. Chem. 1982;257:8216–8227. [PubMed] [Google Scholar]
- 31.Hirata R.D., Hirata M.H., Mesquita C.H., Cesar T.B., Maranhao R.C. Effects of apolipoprotein B-100 on the metabolism of a lipid microemulsion model in rats. Biochim. Biophys. Acta. 1999;1437(1):53–62. doi: 10.1016/s1388-1981(98)00004-3. [DOI] [PubMed] [Google Scholar]
- 32.Owens M.D., Baillie G., Halbert G.W. Physicochemical properties of microemulsion analogues of low density lipoprotein containing amphiphatic apoprotein B receptor sequences. Int. J. Pharm. 2001;228(1-2):109–117. doi: 10.1016/s0378-5173(01)00818-3. [DOI] [PubMed] [Google Scholar]
- 33.Baillie G., Owens M.D., Halbert G.W. A synthetic low density lipoprotein particle capable of supporting U937 proliferation in vitro. J. Lipid Res. 2002;43(1):69–73. [PubMed] [Google Scholar]
- 34.Hayavi S., Baillie G., Owens M.D., Halbert G.W. Receptor dependent cellular uptake of synthetic low density lipoprotein by mammalian cells in serum-free tissue culture. J. Pharm. Pharmacol. 2006;58(10):1337–1342. doi: 10.1211/jpp.58.10.0006. [DOI] [PubMed] [Google Scholar]
- 35.Nikanjam M., Gibbs A.R., Hunt C.A., Budinger T.F., Forte T.M. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J. Control. Release. 2007;124(3):163–171. doi: 10.1016/j.jconrel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Nikanjam M., Blakely E.A., Bjornstad K.A., Shu X., Budinger T.F., Forte T.M. Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme. Int. J. Pharm. 2007;328(1):86–94. doi: 10.1016/j.ijpharm.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Corbin I.R., Li H., Chen J., Lund-Katz S., Zhou R., Glickson J.D., Zheng G. Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia. 2006;8(6):488–498. doi: 10.1593/neo.05835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Corbin I.R., Li H., Cao W., Glickson J.D., Zheng G. Ligand conjugated low-density lipoprotein nanoparticles for enhanced optical cancer imaging in vivo. J. Am. Chem. Soc. 2007;129(18):5798–5799. doi: 10.1021/ja069336k. [DOI] [PubMed] [Google Scholar]
- 39.Zheng G., Chen J., Li H., Glickson J.D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc. Natl. Acad. Sci. USA. 2005;102(49):17757–17762. doi: 10.1073/pnas.0508677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prassl R., Laggner P. Lipoprotein Structure and Dynamics:Low density lipoprotein viewed as a highly dynamic and flexible nanoparticle. In: Frank S., Kostner G.M., editors. Lipoproteins role in health and diseases. InTech; 2012. pp. 4–20. [Google Scholar]
- 41.Jin H., Lovell J.F., Chen J., Ng K., Cao W., Ding L., Zhang Z., Zheng G. Cytosolic delivery of LDL nanoparticle cargo using photochemical internalization. Photochem. Photobiol. Sci. 2011;10(5):810–816. doi: 10.1039/c0pp00350f. [DOI] [PubMed] [Google Scholar]
- 42.Gal D., Ohashi M., MacDonald P.C., Buchsbaum H.J., Simpson E.R. Low-density lipoprotein as a potential vehicle for chemotherapeutic agents and radionucleotides in the management of gynecologic neoplasms. Am. J. Obstet. Gynecol. 1981;139(8):877–885. doi: 10.1016/0002-9378(81)90952-2. [DOI] [PubMed] [Google Scholar]
- 43.Ho Y.K., Smith R.G., Brown M.S., Goldstein J.L. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood. 1978;52(6):1099–1114. [PubMed] [Google Scholar]
- 44.Vitols S., Peterson C., Larsson O., Holm P., Aberg B. Elevated uptake of low density lipoproteins by human lung cancer tissue in vivo. Cancer Res. 1992;52(22):6244–6247. [PubMed] [Google Scholar]
- 45.Firestone R.A. Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells. Bioconjug. Chem. 1994;5(2):105–113. doi: 10.1021/bc00026a002. [DOI] [PubMed] [Google Scholar]
- 46.Favre G. Targeting of tumor cells by low density lipoproteins: principle and use of ellipticin derivatives. C. R. Seances Soc. Biol. Fil. 1992;186(1-2):73–87. [PubMed] [Google Scholar]
- 47.Vitols S., Soderberg-Reid K., Masquelier M., Sjostrom B., Peterson C. Low density lipoprotein for delivery of a water-insoluble alkylating agent to malignant cells. In vitro and in vivo studies of a drug- lipoprotein complex. Br. J. Cancer. 1990;62(5):724–729. doi: 10.1038/bjc.1990.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hynds S.A., Welsh J., Stewart J.M., Jack A., Soukop M., McArdle C.S., Calman K.C., Packard C.J., Shepherd J. Low-density lipoprotein metabolism in mice with soft tissue tumours. Biochim. Biophys. Acta. 1984;795(3):589–595. doi: 10.1016/0005-2760(84)90189-9. [DOI] [PubMed] [Google Scholar]
- 49.Mankertz J., Nundel M., von Baeyer H., Riedel E. Low density lipoproteins as drug carriers in the therapy of macrophage- associated diseases. Biochem. Biophys. Res. Commun. 1997;240(1):112–115. doi: 10.1006/bbrc.1997.7625. [DOI] [PubMed] [Google Scholar]
- 50.Danquah M.K., Zhang X.A., Mahato R.I. Extravasation of polymeric nanomedicines across tumor vasculature. Adv. Drug Deliv. Rev. 2011;63(8):623–639. doi: 10.1016/j.addr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 51.de Smidt P.C., Le Doan T., de Falco S., van Berkel T.J. Association of antisense oligonucleotides with lipoproteins prolongs the plasma half-life and modifies the tissue distribution. Nucleic Acids Res. 1991;19(17):4695–4700. doi: 10.1093/nar/19.17.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfrum C., Shi S., Jayaprakash K.N., Jayaraman M., Wang G., Pandey R.K., Rajeev K.G., Nakayama T., Charrise K., Ndungo E.M., Zimmermann T., Koteliansky V., Manoharan M., Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007;25(10):1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 53.Jin H., Lovell J.F., Chen J., Lin Q., Ding L., Ng K.K., Pandey R.K., Manoharan M., Zhang Z., Zheng G. Mechanistic insights into LDL nanoparticle-mediated siRNA delivery. Bioconjug. Chem. 2012;23(1):33–41. doi: 10.1021/bc200233n. [DOI] [PubMed] [Google Scholar]
- 54.Masquelier M., Vitols S., Peterson C. Low-density lipoprotein as a carrier of antitumoral drugs: in vivo fate of drug-human low-density lipoprotein complexes in mice. Cancer Res. 1986;46(8):3842–3847. [PubMed] [Google Scholar]
- 55.Kerr D.J., Hynds S.A., Shepherd J., Packard C.J., Kaye S.B. Comparative cellular uptake and cytotoxicity of a complex of daunomycin-low density lipoprotein in human squamous lung tumour cell monolayers. Biochem. Pharmacol. 1988;37(20):3981–3986. doi: 10.1016/0006-2952(88)90083-4. [DOI] [PubMed] [Google Scholar]
- 56.Masquelier M., Vitols S., Palsson M., Mars U., Larsson B.S., Peterson C.O. Low density lipoprotein as a carrier of cytostatics in cancer chemotherapy: study of stability of drug-carrier complexes in blood. J. Drug Target. 2000;8(3):155–164. doi: 10.3109/10611860008996861. [DOI] [PubMed] [Google Scholar]
- 57.Samadi-Baboli M., Favre G., Canal P., Soula G. Low density lipoprotein for cytotoxic drug targeting: improved activity of elliptinium derivative against B16 melanoma in mice. Br. J. Cancer. 1993;68(2):319–326. doi: 10.1038/bjc.1993.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filipowska D., Filipowski T., Morelowska B., Kazanowska W., Laudanski T., Lapinjoki S., Akerlund M., Breeze A. Treatment of cancer patients with a low-density-lipoprotein delivery vehicle containing a cytotoxic drug. Cancer Chemother. Pharmacol. 1992;29(5):396–400. doi: 10.1007/BF00686010. [DOI] [PubMed] [Google Scholar]
- 59.Nicolas J.M., Pirson P., Leclef B., Trouet A. Acetylated low-density lipoprotein as a vehicle for antiinfectious drugs: preparation and antileishmanial activity of Ac-LDL containing ketoconazole-oleate. Ann. Trop. Med. Parasitol. 1990;84(4):325–336. doi: 10.1080/00034983.1990.11812476. [DOI] [PubMed] [Google Scholar]
- 60.de Smidt P.C., van Berkel T.J. LDL-mediated drug targeting. Crit. Rev. Ther. Drug Carrier Syst. 1990;7(2):99–120. [PubMed] [Google Scholar]
- 61.Schultis H.W., von Baeyer H., Neitzel H., Riedel E. Preparation of nucleoside-LDL-conjugates for the study of cell- selective internalization: stability characteristics and receptor affinity. Eur. J. Clin. Chem. Clin. Biochem. 1991;29(10):665–674. doi: 10.1515/cclm.1991.29.10.665. [DOI] [PubMed] [Google Scholar]
- 62.Li J.J., Guo Y.L., Yang Y.J. Enhancing anti-inflammatory cytokine IL-10 may be beneficial for acute coronary syndrome. Med. Hypotheses. 2005;65(1):103–106. doi: 10.1016/j.mehy.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 63.Marotta D.E., Cao W., Wileyto E.P., Li H., Corbin I., Rickter E., Glickson J.D., Chance B., Zheng G., Busch T.M. Evaluation of bacteriochlorophyll-reconstituted low-density lipoprotein nanoparticles for photodynamic therapy efficacy in vivo. Nanomedicine (Lond.) 2011;6(3):475–487. doi: 10.2217/nnm.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee P., Adhikary R., Halder M., Petrich J.W., Miskovsky P. Accumulation and interaction of hypericin in low-density lipoprotein--a photophysical study. Photochem. Photobiol. 2008;84(3):706–712. doi: 10.1111/j.1751-1097.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- 65.Huntosova V., Buzova D., Petrovajova D., Kasak P., Nadova Z., Jancura D., Sureau F., Miskovsky P. Development of a new LDL-based transport system for hydrophobic/amphiphilic drug delivery to cancer cells. Int. J. Pharm. 2012;436(1-2):463–471. doi: 10.1016/j.ijpharm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Zhou P., Hatziieremia S., Elliott M.A., Scobie L., Crossan C., Michie A.M., Holyoake T.L., Halbert G.W., Jorgensen H.G. Uptake of synthetic Low Density Lipoprotein by leukemic stem cells--a potential stem cell targeted drug delivery strategy. J. Control. Release. 2010;148(3):380–387. doi: 10.1016/j.jconrel.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Wu F., Wuensch S.A., Azadniv M., Ebrahimkhani M.R., Crispe I.N. Galactosylated LDL nanoparticles: a novel targeting delivery system to deliver antigen to macrophages and enhance antigen specific T cell responses. Mol. Pharm. 2009;6(5):1506–1517. doi: 10.1021/mp900081y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choudhury R.P., Fisher E.A. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 2009;29(7):983–991. doi: 10.1161/ATVBAHA.108.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rudd J.H., Hyafil F., Fayad Z.A. Inflammation imaging in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29(7):1009–1016. doi: 10.1161/ATVBAHA.108.165563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanwar R.K., Chaudhary R., Tsuzuki T., Kanwar J.R. Emerging engineered magnetic nanoparticulate probes for molecular MRI of atherosclerosis: how far have we come? Nanomedicine (Lond.) 2012;7(6):899–916. doi: 10.2217/nnm.12.57. [DOI] [PubMed] [Google Scholar]
- 71.Jung C., Kaul M.G., Bruns O.T., Ducic T., Freund B., Heine M., Reimer R., Meents A., Salmen S.C., Weller H., Nielsen P., Adam G., Heeren J., Ittrich H. Intraperitoneal injection improves the uptake of nanoparticle-labeled high-density lipoprotein to atherosclerotic plaques compared with intravenous injection: a multimodal imaging study in ApoE knockout mice. Circ Cardiovasc Imaging. 2014;7(2):303–311. doi: 10.1161/CIRCIMAGING.113.000607. [DOI] [PubMed] [Google Scholar]
- 72.Cormode D.P., Skajaa T., van Schooneveld M.M., Koole R., Jarzyna P., Lobatto M.E., Calcagno C., Barazza A., Gordon R.E., Zanzonico P., Fisher E.A., Fayad Z.A., Mulder W.J. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8(11):3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allijn I.E., Leong W., Tang J., Gianella A., Mieszawska A.J., Fay F., Ma G., Russell S., Callo C.B., Gordon R.E., Korkmaz E., Post J.A., Zhao Y., Gerritsen H.C., Thran A., Proksa R., Daerr H., Storm G., Fuster V., Fisher E.A., Fayad Z.A., Mulder W.J., Cormode D.P. Gold nanocrystal labeling allows low-density lipoprotein imaging from the subcellular to macroscopic level. ACS Nano. 2013;7(11):9761–9770. doi: 10.1021/nn403258w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sinzinger H., Bergmann H., Kaliman J., Angelberger P. Imaging of human atherosclerotic lesions using 123I-low-density lipoprotein. Eur. J. Nucl. Med. 1986;12(5-6):291–292. doi: 10.1007/BF00251990. [DOI] [PubMed] [Google Scholar]
- 75.Rosen J.M., Butler S.P., Meinken G.E., Wang T.S., Ramakrishnan R., Srivastava S.C., Alderson P.O., Ginsberg H.N. Indium-111-labeled LDL: a potential agent for imaging atherosclerotic disease and lipoprotein biodistribution. J. Nucl. Med. 1990;31(3):343–350. [PubMed] [Google Scholar]
- 76.Moerlein S.M., Daugherty A., Sobel B.E., Welch M.J. Metabolic imaging with gallium-68- and indium-111-labeled low-density lipoprotein. J. Nucl. Med. 1991;32(2):300–307. [PubMed] [Google Scholar]
- 77.Ponty E., Favre G., Benaniba R., Boneu A., Lucot H., Carton M., Soula G. Biodistribution study of 99mTc-labeled LDL in B16-melanoma- bearing mice. Visualization of a preferential uptake by the tumor. Int. J. Cancer. 1993;54(3):411–417. doi: 10.1002/ijc.2910540311. [DOI] [PubMed] [Google Scholar]
- 78.Jasanada F., Urizzi P., Souchard J.P., Le Gaillard F., Favre G., Nepveu F. Indium-111 labeling of low density lipoproteins with the DTPA- bis(stearylamide): evaluation as a potential radiopharmaceutical for tumor localization. Bioconjug. Chem. 1996;7(1):72–81. doi: 10.1021/bc950073l. [DOI] [PubMed] [Google Scholar]
- 79.Urizzi P., Souchard J.P., Palevody C., Ratovo G., Hollande E., Nepveu F. Internalization of indium-labeled LDL through a lipid chelating anchor in human pancreatic-cancer cells as a potential radiopharmaceutical for tumor localization. Int. J. Cancer. 1997;70(3):315–322. doi: 10.1002/(sici)1097-0215(19970127)70:3<315::aid-ijc12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 80.Lees A.M., Lees R.S. 99mTechnetium-labeled low density lipoprotein: receptor recognition and intracellular sequestration of radiolabel. J. Lipid Res. 1991;32(1):1–8. [PubMed] [Google Scholar]
- 81.Li H., Zhang Z.H., Blessington D., Nelson D.S., Zhou R., Lund-Katz S., Chance B., Glickson J.D., Zheng G. Carbocyanine labeled LDL for optical Imaging of tumors. Acad. Radiol. 2004;11(6):669–677. doi: 10.1016/j.acra.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Zheng G., Li H., Yang K., Blessington D., Licha K., Lund-Katz S., Chance B., Glickson J.D. Tricarbocyanine cholesteryl laurates labeled LDL: new near infrared fluorescent probes (NIRFs) for monitoring tumors and gene therapy of familial hypercholesterolemia. Bioorg. Med. Chem. Lett. 2002;12(11):1485–1488. doi: 10.1016/s0960-894x(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 83.Crich S.G., Lanzardo S., Alberti D., Belfiore S., Ciampa A., Giovenzana G.B., Lovazzano C., Pagliarin R., Aime S. Magnetic resonance imaging detection of tumor cells by targeting low-density lipoprotein receptors with Gd-loaded low-density lipoprotein particles. Neoplasia. 2007;9(12):1046–1056. doi: 10.1593/neo.07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leppala J., Kallio M., Nikula T., Nikkinen P., Liewendahl K., Jaaskelainen J., Savolainen S., Gylling H., Hiltunen J., Callaway J., Kahl S., Farkkila M. Accumulation of Tc-99M-low-density lipoprotein in human-malignant glioma. Br. J. Cancer. 1995;71(2):383–387. doi: 10.1038/bjc.1995.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lusis A.J. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 87.Williams K.J., Tabas I. Lipoprotein retention--and clues for atheroma regression. Arterioscler. Thromb. Vasc. Biol. 2005;25(8):1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 88.Lund-Katz S., Laplaud P.M., Phillips M.C., Chapman M.J. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry. 1998;37(37):12867–12874. doi: 10.1021/bi980828m. [DOI] [PubMed] [Google Scholar]
- 89.Bijsterbosch M.K., Ziere G.J., van Berkel T.J. Lactosylated low density lipoprotein: a potential carrier for the site-specific delivery of drugs to Kupffer cells. Mol. Pharmacol. 1989;36(3):484–489. [PubMed] [Google Scholar]
- 90.Bijsterbosch M.K., van Berkel T.J. Uptake of lactosylated low-density lipoprotein by galactose-specific receptors in rat liver. Biochem. J. 1990;270(1):233–239. doi: 10.1042/bj2700233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bijsterbosch M.K., Manoharan M., Dorland R., Waarlo I.H., Biessen E.A., van Berkel T.J. Delivery of cholesteryl-conjugated phosphorothioate oligodeoxynucleotides to Kupffer cells by lactosylated low-density lipoprotein. Biochem. Pharmacol. 2001;62(5):627–633. doi: 10.1016/s0006-2952(01)00705-5. [DOI] [PubMed] [Google Scholar]
- 92.Frias J.C., Lipinski M.J., Lipinski S.E., Albelda M.T. Modified lipoproteins as contrast agents for imaging of atherosclerosis. Contrast Media Mol. Imaging. 2007;2(1):16–23. doi: 10.1002/cmmi.124. [DOI] [PubMed] [Google Scholar]
- 93.Cormode D.P., Skajaa T., Fayad Z.A., Mulder W.J. Nanotechnology in medical imaging: probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2009;29(7):992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lipinski M.J., Amirbekian V., Frias J.C., Aguinaldo J.G., Mani V., Briley-Saebo K.C., Fuster V., Fallon J.T., Fisher E.A., Fayad Z.A. MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn. Reson. Med. 2006;56(3):601–610. doi: 10.1002/mrm.20995. [DOI] [PubMed] [Google Scholar]
- 95.Mitsumori L.M., Ricks J.L., Rosenfeld M.E., Schmiedl U.P., Yuan C. Development of a lipoprotein based molecular imaging MR contrast agent for the noninvasive detection of early atherosclerotic disease. Int. J. Cardiovasc. Imaging. 2004;20(6):561–567. doi: 10.1007/s10554-004-7020-4. [DOI] [PubMed] [Google Scholar]
- 96.Yamakoshi Y., Qiao H., Lowell A.N., Woods M., Paulose B., Nakao Y., Zhang H., Liu T., Lund-Katz S., Zhou R. LDL-based nanoparticles for contrast enhanced MRI of atheroplaques in mouse models. Chem. Commun. (Camb.) 2011;47(31):8835–8837. doi: 10.1039/c1cc10924c. [DOI] [PubMed] [Google Scholar]
- 97.Lowell A.N., Qiao H., Liu T., Ishikawa T., Zhang H., Oriana S., Wang M., Ricciotti E., FitzGerald G.A., Zhou R., Yamakoshi Y. Functionalized low-density lipoprotein nanoparticles for in vivo enhancement of atherosclerosis on magnetic resonance images. Bioconjug. Chem. 2012;23(11):2313–2319. doi: 10.1021/bc300561e. [DOI] [PubMed] [Google Scholar]
- 98.Chen R., Yu H., Jia Z.Y., Yao Q.L., Teng G.J. Efficient nano iron particle-labeling and noninvasive MR imaging of mouse bone marrow-derived endothelial progenitor cells. Int. J. Nanomedicine. 2011;6:511–519. doi: 10.2147/IJN.S16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jonas A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- 100.Ng K.K., Lovell J.F., Zheng G. Lipoprotein-Inspired Nanoparticles for Cancer Theranostics. Acc. Chem. Res. 2011;44(10):1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X., Suo R., Xiong S.L., Zhang Q.H., Yi G.H. HDL drug carriers for targeted therapy. Clin. Chim. Acta. 2013;415:94–100. doi: 10.1016/j.cca.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 102.Pittman R.C., Glass C.K., Atkinson D., Small D.M. Synthetic high density lipoprotein particles. Application to studies of the apoprotein specificity for selective uptake of cholesterol esters. J. Biol. Chem. 1987;262(6):2435–2442. [PubMed] [Google Scholar]
- 103.Kim Y., Fay F., Cormode D.P., Sanchez-Gaytan B.L., Tang J., Hennessy E.J., Ma M., Moore K., Farokhzad O.C., Fisher E.A., Mulder W.J., Langer R., Fayad Z.A. Single step reconstitution of multifunctional high-density lipoprotein-derived nanomaterials using microfluidics. ACS Nano. 2013;7(11):9975–9983. doi: 10.1021/nn4039063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sirtori C.R., Calabresi L., Franceschini G. Recombinant apolipoproteins for the treatment of vascular diseases. Atherosclerosis. 1999;142(1):29–40. doi: 10.1016/s0021-9150(98)00247-0. [DOI] [PubMed] [Google Scholar]
- 105.Murphy A.J., Chin-Dusting J., Sviridov D. Reconstituted HDL: a therapy for atherosclerosis and beyond. Clin. Lipidol. 2009;4(6):731–739. [Google Scholar]
- 106.Damiano M.G., Mutharasan R.K., Tripathy S., McMahon K.M., Thaxton C.S. Templated high density lipoprotein nanoparticles as potential therapies and for molecular delivery. Adv. Drug Deliv. Rev. 2013;65(5):649–662. doi: 10.1016/j.addr.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 107.Singh A.T., Evens A.M., Anderson R.J., Beckstead J.A., Sankar N., Sassano A., Bhalla S., Yang S., Platanias L.C., Forte T.M., Ryan R.O., Gordon L.I. All trans retinoic acid nanodisks enhance retinoic acid receptor mediated apoptosis and cell cycle arrest in mantle cell lymphoma. Br. J. Haematol. 2010;150(2):158–169. doi: 10.1111/j.1365-2141.2010.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oda M.N., Hargreaves P.L., Beckstead J.A., Redmond K.A., Van Antwerpen R., Ryan R.O. Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin B. J. Lipid Res. 2006;47(2):260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 109.Ghosh M., Singh A.T., Xu W., Sulchek T., Gordon L.I., Ryan R.O. Curcumin nanodisks: formulation and characterization. Nanomedicine (Lond.) 2011;7(2):162–167. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh A.T., Ghosh M., Forte T.M., Ryan R.O., Gordon L.I. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk. Lymphoma. 2011;52(8):1537–1543. doi: 10.3109/10428194.2011.584253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feng M.Q., Cai Q.S., Shi X.L., Huang H., Zhou P., Guo X. Recombinant high-density lipoprotein complex as a targeting system of nosiheptide to liver cells. J. Drug Target. 2008;16(6):502–508. doi: 10.1080/10611860802200938 . [DOI] [PubMed] [Google Scholar]
- 112.Feng M., Cai Q., Huang H., Zhou P. Liver targeting and anti-HBV activity of reconstituted HDL-acyclovir palmitate complex. Eur. J. Pharm. Biopharm. 2008;68(3):688–693. doi: 10.1016/j.ejpb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 113.Kader A., Pater A. Loading anticancer drugs into HDL as well as LDL has little effect on properties of complexes and enhances cytotoxicity to human carcinoma cells. J. Control. Release. 2002;80(1-3):29–44. doi: 10.1016/s0168-3659(01)00536-3. [DOI] [PubMed] [Google Scholar]
- 114.McConathy W.J., Nair M.P., Paranjape S., Mooberry L., Lacko A.G. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anticancer Drugs. 2008;19(2):183–188. doi: 10.1097/CAD.0b013e3282f1da86. [DOI] [PubMed] [Google Scholar]
- 115.Mooberry L.K., Nair M., Paranjape S., McConathy W.J., Lacko A.G. Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. J. Drug Target. 2010;18(1):53–58. doi: 10.3109/10611860903156419. [DOI] [PubMed] [Google Scholar]
- 116.Yuan Y., Wang W., Wang B., Zhu H., Zhang B., Feng M. Delivery of hydrophilic drug doxorubicin hydrochloride-targeted liver using apoAI as carrier. J. Drug Target. 2013;21(4):367–374. doi: 10.3109/1061186X.2012.757769. [DOI] [PubMed] [Google Scholar]
- 117.Yang M., Jin H., Chen J., Ding L., Ng K.K., Lin Q., Lovell J.F., Zhang Z., Zheng G. Efficient cytosolic delivery of siRNA using HDL-mimicking nanoparticles. Small. 2011;7(5):568–573. doi: 10.1002/smll.201001589. [DOI] [PubMed] [Google Scholar]
- 118.Nakayama T., Butler J.S., Sehgal A., Severgnini M., Racie T., Sharman J., Ding F., Morskaya S.S., Brodsky J., Tchangov L., Kosovrasti V., Meys M., Nechev L., Wang G., Peng C.G., Fang Y., Maier M., Rajeev K.G., Li R., Hettinger J., Barros S., Clausen V., Zhang X., Wang Q., Hutabarat R., Dokholyan N.V., Wolfrum C., Manoharan M., Kotelianski V., Stoffel M., Sah D.W. Harnessing a physiologic mechanism for siRNA delivery with mimetic lipoprotein particles. Mol. Ther. 2012;20(8):1582–1589. doi: 10.1038/mt.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shahzad M.M., Mangala L.S., Han H.D., Lu C., Bottsford-Miller J., Nishimura M., Mora E.M., Lee J.W., Stone R.L., Pecot C.V., Thanapprapasr D., Roh J.W., Gaur P., Nair M.P., Park Y.Y., Sabnis N., Deavers M.T., Lee J.S., Ellis L.M., Lopez-Berestein G., McConathy W.J., Prokai L., Lacko A.G., Sood A.K. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13(4):309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McMahon K.M., Thaxton C.S. High-density lipoproteins for the systemic delivery of short interfering RNA. Expert Opin. Drug Deliv. 2014;11(2):231–247. doi: 10.1517/17425247.2014.866089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin Q.Y., Chen J., Jin H.L., Ng K.K., Yang M., Cao W.G., Ding L.L., Zhang Z.H., Zheng G. Efficient systemic delivery of siRNA by using high-density lipoprotein-mimicking peptide lipid nanoparticles. Nanomedicine (Lond.) 2012;7(12):1813–1825. doi: 10.2217/nnm.12.73. [DOI] [PubMed] [Google Scholar]
- 122.Luthi A.J., Zhang H., Kim D., Giljohann D.A., Mirkin C.A., Thaxton C.S. Tailoring of biomimetic high-density lipoprotein nanostructures changes cholesterol binding and efflux. ACS Nano. 2012;6(1):276–285. doi: 10.1021/nn2035457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luthi A.J., Lyssenko N.N., Quach D., McMahon K.M., Millar J.S., Vickers K.C., Rader D.J., Phillips M.C., Mirkin C.A., Thaxton C.S. Robust passive and active efflux of cellular cholesterol to a designer functional mimic of high density lipoprotein. J. Lipid Res. 2015;56(5):972–985. doi: 10.1194/jlr.M054635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang S., Damiano M.G., Zhang H., Tripathy S., Luthi A.J., Rink J.S., Ugolkov A.V., Singh A.T., Dave S.S., Gordon L.I., Thaxton C.S. Biomimetic, synthetic HDL nanostructures for lymphoma. Proc. Natl. Acad. Sci. USA. 2013;110(7):2511–2516. doi: 10.1073/pnas.1213657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McMahon K.M., Mutharasan R.K., Tripathy S., Veliceasa D., Bobeica M., Shumaker D.K., Luthi A.J., Helfand B.T., Ardehali H., Mirkin C., Volpert A.O., Thaxton C. S. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011;11(3):1208–1214. doi: 10.1021/nl1041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Corbin I.R. Ligand-coupled lipoprotein for ovarian cancer-specific drug delivery. Methods Mol. Biol. 2013;1049:467–480. doi: 10.1007/978-1-62703-547-7_35. [DOI] [PubMed] [Google Scholar]
- 127.Mangge H., Almer G., Truschnig-Wilders M., Schmidt A., Gasser R., Fuchs D. Inflammation, adiponectin, obesity and cardiovascular risk. Curr. Med. Chem. 2010;17(36):4511–4520. doi: 10.2174/092986710794183006. [DOI] [PubMed] [Google Scholar]
- 128.Gao T., He X., Yu W., Zhang Z., Wang Y. Atherosclerotic plaque pathohistology and classification with high-resolution MRI. Neurol. Res. 2011;33(3):325–330. doi: 10.1179/016164110X12767786356318. [DOI] [PubMed] [Google Scholar]
- 129.Naresh N.K., Xu Y., Klibanov A.L., Vandsburger M.H., Meyer C.H., Leor J., Kramer C.M., French B.A., Epstein F.H. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology. 2012;264(2):428–435. doi: 10.1148/radiol.12111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shaish A., Keren G., Chouraqui P., Levkovitz H., Harats D. Imaging of aortic atherosclerotic lesions by (125)I-LDL, (125)I-oxidized-LDL, (125)I-HDL and (125)I-BSA. Pathobiology. 2001;69(4):225–229. doi: 10.1159/000055947. [DOI] [PubMed] [Google Scholar]
- 131.Cormode D.P., Frias J.C., Ma Y., Chen W., Skajaa T., Briley-Saebo K., Barazza A., Williams K.J., Mulder W.J., Fayad Z.A., Fisher E.A. HDL as a contrast agent for medical imaging. Clin. Lipidol. 2009;4(4):493–500. doi: 10.2217/clp.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skajaa T., Cormode D.P., Falk E., Mulder W.J., Fisher E.A., Fayad Z.A. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30(2):169–176. doi: 10.1161/ATVBAHA.108.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frias J.C., Williams K.J., Fisher E.A., Fayad Z.A. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 2004;126(50):16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 134.Frias J.C., Ma Y., Williams K.J., Fayad Z.A., Fisher E.A. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6(10):2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- 135.Briley-Saebo K.C., Geninatti-Crich S., Cormode D.P., Barazza A., Mulder W.J., Chen W., Giovenzana G.B., Fisher E.A., Aime S., Fayad Z.A. High-relaxivity gadolinium-modified high-density lipoproteins as magnetic resonance imaging contrast agents. J. Phys. Chem. B. 2009;113(18):6283–6289. doi: 10.1021/jp8108286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cao W., Ng K.K., Corbin I., Zhang Z., Ding L., Chen J., Zheng G. Synthesis and evaluation of a stable bacteriochlorophyll-analog and its incorporation into high-density lipoprotein nanoparticles for tumor imaging. Bioconjug. Chem. 2009;20(11):2023–2031. doi: 10.1021/bc900404y. [DOI] [PubMed] [Google Scholar]
- 137.Corbin I.R., Chen J., Cao W., Li H., Lund-Katz S., Zheng G. Enhanced cancer-targeted delivery using engineered high-density lipoprotein-based nanocarriers. J. Biomed. Nanotechnol. 2007;3(4):367–376. [Google Scholar]
- 138.Corbin I.R., Ng K.K., Ding L., Jurisicova A., Zheng G. Near-infrared fluorescent imaging of metastatic ovarian cancer using folate receptor-targeted high-density lipoprotein nanocarriers. Nanomedicine (Lond.) 2013;8(6):875–890. doi: 10.2217/nnm.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen W., Jarzyna P.A., van Tilborg G.A., Nguyen V.A., Cormode D.P., Klink A., Griffioen A.W., Randolph G.J., Fisher E.A., Mulder W.J., Fayad Z.A. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. 2010;24(6):1689–1699. doi: 10.1096/fj.09-139865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Skajaa T., Cormode D.P., Jarzyna P.A., Delshad A., Blachford C., Barazza A., Fisher E.A., Gordon R.E., Fayad Z.A., Mulder W.J. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials. 2011;32(1):206–213. doi: 10.1016/j.biomaterials.2010.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mishra V.K., Anantharamaiah G.M., Segrest J.P., Palgunachari M.N., Chaddha M., Sham S.W., Krishna N.R. Association of a model class A (apolipoprotein) amphipathic alpha helical peptide with lipid: high resolution NMR studies of peptide lipid discoidal complexes. J. Biol. Chem. 2006;281(10):6511–6519. doi: 10.1074/jbc.M511475200. [DOI] [PubMed] [Google Scholar]
- 142.Cormode D.P., Briley-Saebo K.C., Mulder W.J., Aguinaldo J.G., Barazza A., Ma Y., Fisher E.A., Fayad Z.A. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4(9):1437–1444. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]
- 143.Chen W., Vucic E., Leupold E., Mulder W.J., Cormode D.P., Briley-Saebo K.C., Barazza A., Fisher E.A., Dathe M., Fayad Z.A. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast. Media Mol. 2008;3(6):233–242. doi: 10.1002/cmmi.257. [DOI] [PubMed] [Google Scholar]
- 144.Skajaa T., Cormode D.P., Falk E., Mulder W.J., Fisher E.A., Fayad Z.A. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30(2):169–176. doi: 10.1161/ATVBAHA.108.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Junghans M., Kreuter J., Zimmer A. Antisense delivery using protamine-oligonucleotide particles. Nucleic Acids Res. 2000;28(10):E45. doi: 10.1093/nar/28.10.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weyermann J., Lochmann D., Zimmer A. Comparison of antisense oligonucleotide drug delivery systems. J. Control. Release. 2004;100(3):411–423. doi: 10.1016/j.jconrel.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 147.Kratzer I., Wernig K., Panzenboeck U., Bernhart E., Reicher H., Wronski R., Windisch M., Hammer A., Malle E., Zimmer A., Sattler W. Apolipoprotein A-I coating of protamine-oligonucleotide nanoparticles increases particle uptake and transcytosis in an in vitro model of the blood-brain barrier. J. Control. Release. 2007;117(3):301–311. doi: 10.1016/j.jconrel.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


