Abstract
Background & Aims
Hepatocellular carcinoma (HCC) occurs more frequently and aggressively in men than in women. Although sex hormones are believed to play a critical role in this disparity, the possible contribution of other factors largely is unknown. We aimed to investigate the role of serotonin on its contribution of sex discrepancy during HCC.
Methods
By using an inducible zebrafish HCC model through hepatocyte-specific transgenic krasV12 expression, differential rates of HCC in male and female fish were characterized by both pharmaceutical and genetic interventions. The findings were validated further in human liver disease samples.
Results
Accelerated HCC progression was observed in krasV12-expressing male zebrafish and male fish liver tumors were found to have higher hepatic stellate cell (HSC) density and activation. Serotonin, which is essential for HSC survival and activation, similarly were found to be synthesized and accumulated more robustly in males than in females. Serotonin-activated HSCs could promote HCC carcinogenesis and concurrently increase serotonin synthesis via transforming growth factor (Tgf)b1 expression, hence contributing to sex disparity in HCC. Analysis of liver disease patient samples showed similar male predominant serotonin accumulation and Tgfb1 expression.
Conclusions
In both zebrafish HCC models and human liver disease samples, a predominant serotonin synthesis and accumulation in males resulted in higher HSC density and activation as well as Tgfb1 expression, thus accelerating HCC carcinogenesis in males.
Keywords: Liver Cancer, TGFB1, Kras, Zebrafish
Abbreviations used in this paper: α-SMA, α-smooth muscle actin; cDNA, complementary DNA; dox, doxycycline; EGFP, enhanced green fluorescence protein; Gfap, glial fibrillary acidic protein; HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; Htr2b, 5-hydoxytryptamine receptor 2B; IF, immunofluorescence; IHC, immunohistochemistry; PCR, polymerase chain reaction; P-Tph1, phosphorylated tryptophan hydroxylase 1; TGF, transforming growth factor; Tph1, tryptophan hydroxylase 1; WT, wild type
Graphical Abstract
See editorial on page 301.
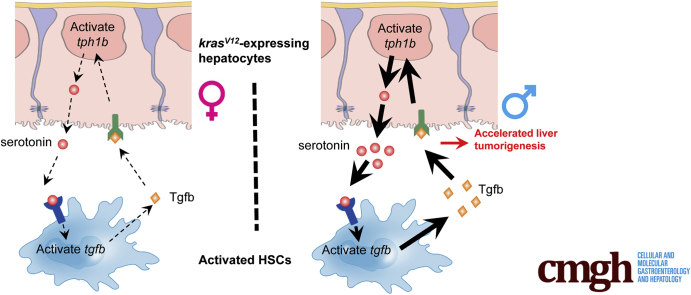
Summary.
A higher serotonin synthesis and accumulation in male zebrafish resulted in increased activation of hepatic stellate cells and transforming growth factor B1 expression and this has contributed to the accelerated progression of hepatocellular carcinoma in male zebrafish with activated kras oncogene expression.
Hepatocellular carcinoma (HCC) is notably more prevalent in men than in women, with a male-to-female ratio of 4.5:1.1 The sex disparity appears to be mediated by a sex hormone–regulated mechanism. After menopause, women showed increased occurrence of HCC, which could be reduced with estrogen treatment.2, 3 In a carcinogen-induced mouse HCC model, administration of estrogen to male mice inhibited HCC development.4 It is interesting to note that effects elicited by sex hormones are associated commonly with tumor microenvironment-mediated inflammatory response. For example, estrogen inhibits interleukin 6 expression in Kupffer cells (liver resident macrophages) and confers resistance to HCC in female mice.5 Androgen signaling polarizes tumor-associated macrophages to a protumor gene expression profile during early hepatocarcinogenesis.6 Interestingly, numerous HCC studies have shown a more robust and active HCC tumor microenvironment in males than in females. For example, infiltrating tumor-associated macrophage density has been found to be higher in males than in females in a mouse HCC model.7 In human HCC patients, men have considerably higher numbers of intratumoral infiltrated CD66b+ neutrophils and CD8+ T cells; both are indicators of poor disease prognosis.8
Hepatic stellate cells (HSCs), the main fibrinogenic cell type in the liver, primarily are responsible for the production of extracellular matrix materials.9, 10, 11 Recent studies have shown a tumor-promoting role of HSCs.12 Tumor cell–secreted signals promote HSCs to transdifferentiate into highly proliferating myofibroblast-like cells, also known as activated HSCs.13 Activated HSCs can induce phenotypic changes in cancer cells, primarily by secretion of protumor growth factors and cytokines such as hepatocyte growth factor and transforming growth factor (TGF)β1.14 An increased HSC-specific gene expression signature in HCC patients indicates poor prognosis.15 Notably, HSCs are found to be of higher density in male than in female HCC patients.16 Because only activated HSCs rapidly proliferate, a higher HSC density in male HCC patients implies that a sex-dependent mechanism could contribute to activation of HSCs.13
Our laboratory previously generated several inducible HCC models by transgenic expression of an oncogene in hepatocytes in zebrafish.17, 18, 19, 20, 21 The inducible nature of these zebrafish HCC models allows the oncogene to be activated at a given and controlled timing in both sexes, providing an excellent platform to study sex disparity in HCC initiation and progression. In this study, we attempted to investigate the mechanism of the sex disparity observed in HCC patients. In our krasV12-expressing HCC zebrafish model, male fish showed accelerated carcinogenesis as compared with females.17 Interestingly, we found an increased level of serotonin production in male krasV12-expressing livers as compared with female counterparts and showed that serotonin was necessary for maintaining HSC survival and activation during HCC carcinogenesis. The activated HSCs in turn promoted HCC progression and at the same time increased serotonin synthesis in hepatocytes via Tgfb1, hence maintaining and promoting the sex disparity observed in krasV12-expressing zebrafish. The findings in zebrafish were confirmed by our analyses of human liver disease patient samples, suggesting that serotonin is involved in the sex disparity of human HCC.
Materials and Methods
Zebrafish Husbandry
Zebrafish were maintained in compliance with Institutional Animal Care and Use Committee guidelines from the National University of Singapore. Five transgenic lines, Tg(fabp10:rtTA2s-M2; TRE2:EGFP-krasG12V) (gz32Tg) in a tetracycline-controlled transcription activation (Tet-On) system for inducible hepatocyte-specific expression of oncogenic krasG12V,17 Tg(hand2:EGFP) (pd24Tg) with EGFP-labeled HSCs under the hand2 promoter,22 Tg(tp1:EGFP) (um14Tg) with EGFP-labeled cholangiocytes under a notch-responsive element,23 Tg(lyz:DsRed2) (nz50Tg) with DsRed-labeled neutrophils under the lysozyme C (lyz) promoter,24 Tg(mpeg1:mCherry) (gl22Tg), with mCherry-labeled macrophages under the mpeg1 promoter,25 and Tg(fabp10:DsRed; ela3l:EGFP) (gz15Tg) with DsRed-labeled hepatocytes under the fabp10 promoter26 were used in this study and referred to as kras+, hand2+, lyz+, mpeg+, and fabp10+, respectively, in the present report.
Chemical Treatment
All chemical/reagent treatments were conducted in 3-month-old adult fish for 7 days. The chemicals/reagents used included doxycycline (dox) (D9891; Sigma-Aldrich, St. Louis, MO), serotonin (14927; Sigma), PCPA (4-chloro-DL-phenylalanine) (C6506; Sigma), BW723C86 (B175; Sigma), SB204741 (S0693; Sigma), and SB431542 (1614; Tocris, Minneapolis, MN). Serotonin, PCPA, BW723C86, SB204741, SB431542, and dox were used for adult exposure at 10 μmol/L, 25 μmol/L, 15 μmol/L, 15 μmol/L, 2.5 μmol/L, and 30 μg/mL, respectively. The dosages were selected based on the highest all-survival concentrations in preliminary experiments.
Photography and Image Analysis
At each time point of chemical treatment experiments, more than 10 adult fish in each group were used for imaging analyses. These zebrafish were anesthetized in 0.08% tricaine (E10521; Sigma) and immobilized in 3% methylcellulose (M0521; Sigma) before imaging. Each zebrafish was photographed individually with an Olympus microscope (Olympus, Tokyo, Japan).
Isolation of Hepatocytes, Neutrophils, Macrophages, Cholangiocytes, and HSCs by Fluorescence-Activated Cell Sorting
Fabp10+, lyz+, mpeg+, tp1+, and hand2+ transgenic zebrafish in wild-type background were used for fluorescence-activated cell sorting isolation of hepatocytes, neutrophils, macrophages, cholangiocytes, and HSCs, respectively, using a cell sorter (643245; BD Biosciences, Singapore). Ten adult livers were pooled and dissociated into single cells in the presence of 0.05% trypsin (T1426; Sigma) using a 40-μm mesh (352340; Fisher Scientific, Pittsburgh, PA) as previously described.27 Hepatocytes (fabp10+), cholangiocytes (tp1+), and HSCs (hand2+) were isolated based on EGFP expression, neutrophils (lyz+) were isolated based on DsRed expression, and macrophages (mpeg+) were isolated based on mCherry expression.
RNA Extraction, Complementary DNA Amplification, and Reverse-Transcription Quantitative Polymerase Chain Reaction
Total RNA was extracted using the RNeasy mini kit (74104; Qiagen, Singapore). A total of 5 ng RNA was used as a template to synthesize and amplify complementary DNA (cDNA) using the QuantiTect Whole Transcriptome Kit (207043; Qiagen). Amplified cDNA was performed for real-time quantitative polymerase chain reaction (PCR) with LightCycler 480 SYBR Green I Master (04707516001; Roche, Singapore). Targeted cDNAs were amplified for 40 cycles (95°C, 20 s; 65°C, 15 s; and 72°C, 30 s). The sequences of PCR primers used were as follows: β-actin: CTCTGGGTCACCGCTTCTTT (forward) and CAGATGCTCACGAAACCCCT (reverse); tph1b: CTAAGAGCATACGGGGCTGG (forward) and GGACGCTGGATTGTCTTTGC (reverse); htr2b: CGCAGGATGTCCACCATAGG (forward) and TGCACCTTTCACAGAGCACA (reverse); and tgfb1: AGCAGAATTGCGTCTTCGGA (forward) and TCAAATGAGAGCCAGCGGTT (reverse).
Histologic and Cytologic Analyses
Ten adult livers were fixed in 4% paraformaldehyde in phosphate-buffered saline (P6748; Sigma), embedded in paraffin, and sectioned at 5-μm thickness using a microtome, followed by H&E, PicroSirus Red, Gomori’s trichome, immunohistochemistry (IHC), or immunofluorescence (IF) stainings. H&E (H-3404; Vector Laboratories, Burlingame, CA, USA), Picrosirius Red (24901B; Polyscience, Inc, Warrington, PA), and Gomori’s trichome (87021; Thermo Fisher Scientific, Singapore) stainings were conducted according to the manufacturers’ protocols. For IHC and IF stainings, the primary antibodies were derived mostly from rabbits, including anti-proliferating cell nuclear antigen (PCNA) (FL-261; Santa Cruz Biotechnology, Dallas, TX), anti–caspase 3 (C92-065; BD Biosciences, Singapore), antiserotonin (C5545; Sigma), anti-TgfB1 (04-953; EMD Millipore, Billerica, MA), anti-PTph (SC135716; Santa Cruz), anti–α-smooth muscle actin (α-Sma) (ab15734; Abcam, Singapore), anti-PSmad2 (3101; Cell Signaling Technology, Danvers, MA) and anti–collagen I (ab23730; Abcam), except for anti-glial fibrillary acidic protein (Gfap) (154474; Abcam) from the mouse. Anti-rabbit or anti-mouse secondary antibodies were purchased from Thermo Fisher Scientific. At least 8 fish from each treatment group were examined and 1 high-power field was selected randomly from each fish liver and IF signals were counted manually for quantitative analyses.
Human Patient Samples
Paraffin-embedded human liver disease progression tissue microarray slides were purchased from Biomax, Inc (Derwood, MD) (LV8011a). Patients were classified into 4 groups: normal (n = 5), inflammation (n = 7), cirrhosis (n = 16), and HCC (n = 30). Histopathology of all patients was diagnosed by a pathologist based on the information provided from Biomax, Inc (https://www.biomax.us/tissue-arrays/Liver/LV8011a). Patient samples slides were subjected to H&E staining and IHC staining for serotonin and TGFβ1, respectively.
Statistical Analysis
Statistical significance between the 2 groups was evaluated by a 2-tailed unpaired Student t test using inStat version 5.0 for Windows (GraphPad, San Diego, CA). Statistical data are presented as means ± SDs.
Results
More Aggressive HCC Progression in Male KrasV12-Expressing Livers
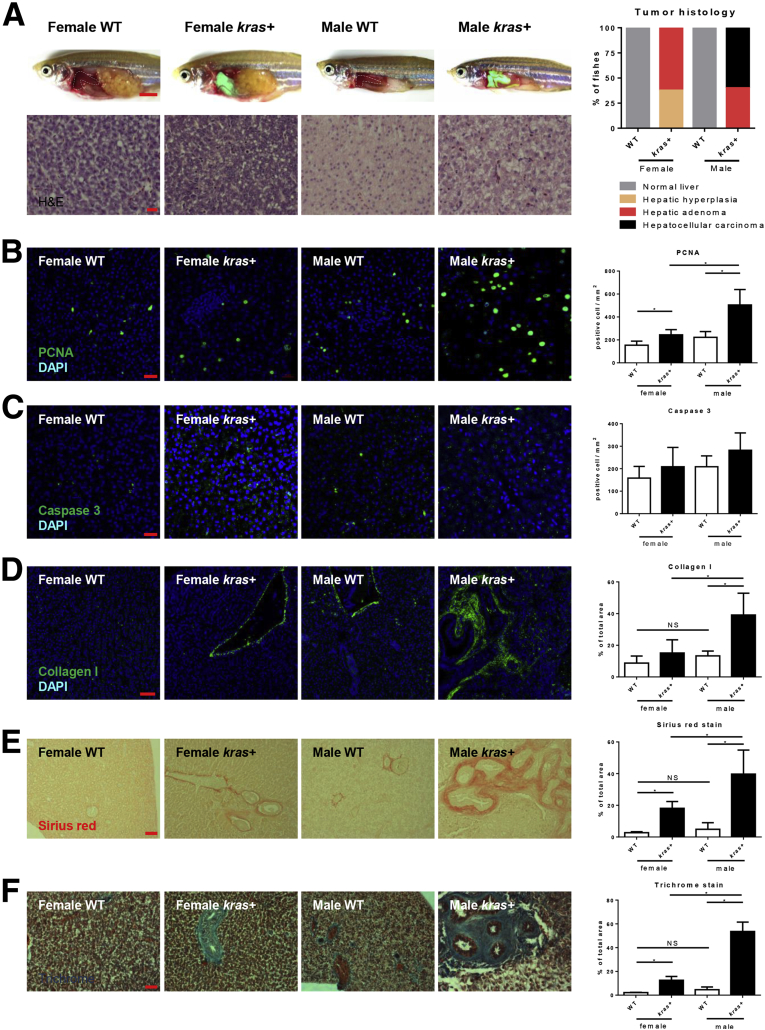
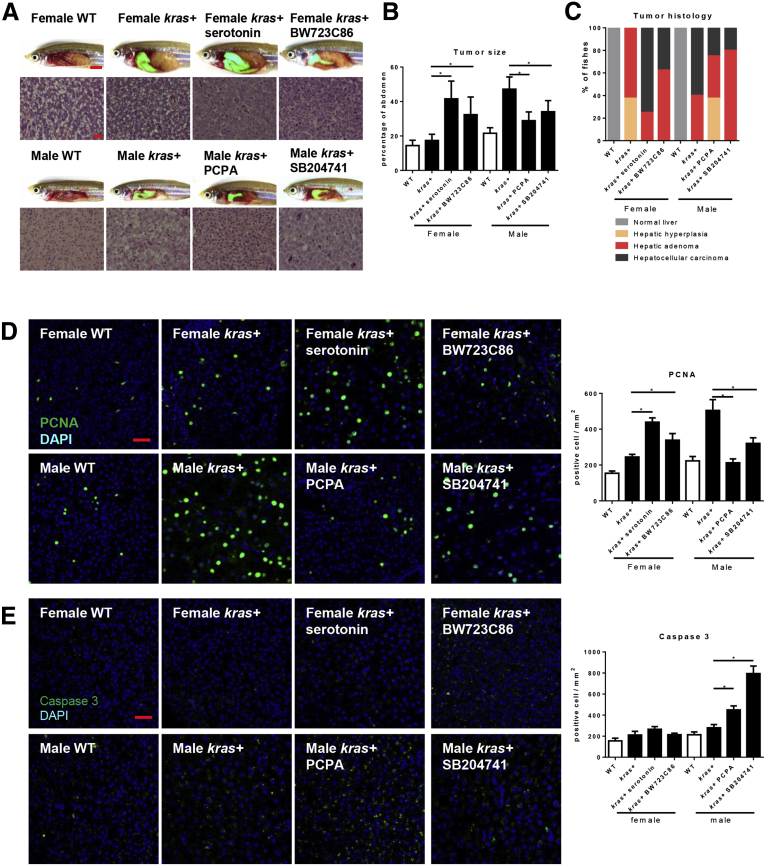
In human patients, men develop more aggressive HCC with larger tumors and a lower survival rate than women.28, 29, 30 To examine if our krasV12-expressing HCC zebrafish model recapitulated the sex disparity, male and female adult kras+ zebrafish were exposed to dox for 7 days. Initial gross morphologic examination of these kras+ zebrafish showed significantly enlarged livers in kras+ males than in kras+ females after 7 days of dox induction (Figure 1A). Histologically, krasV12-expressing livers in males showed more advanced HCC phenotype than that in females. In males, krasV12-expressing hepatocytes were densely situated with lost organization of the typical 2-cell hepatic plates; these are typical features of carcinoma. In contrast, female krasV12-expressing livers were either hyperplastic or adenoma; these livers largely maintained the 2-cell plate but had a more prominent nucleoli and vacuolated cytoplasm (Figure 1A). As summarized in Figure 1A, 60% of female krasV12-expressing livers showed adenoma histology and the remaining 40% had hepatic hyperplasia; in contrast, 100% of the male krasV12-expressing livers showed carcinoma or adenoma histology.
Figure 1.
Sex disparity in krasV12-induced HCC progression. Three-month-old adult zebrafish were treated with 30 μg/mL dox for 7 days and examined by various assays as described in the text. (A) Gross morphology and histology of kras+ and WT (control) male and female zebrafish after dox exposure. Male krasV12-expressing liver (green for green fluorescent protein expression) were enlarged significantly as compared with female krasV12-expressing liver and also with WT male and female livers (dotted line enclosed). Bottom left: H&E staining of the liver sections of dox-treated kras+ and WT (control) male and female zebrafish. Right: Quantification of tumor histology observed in the H&E-stained liver sections of dox-treated kras+ male and female zebrafish is shown (n = 10 each group). (B–D) IF staining of (B) PCNA, (C) caspase 3, and (D) collagen I in liver sections of dox-treated kras+ and WT male and female zebrafish. Quantifications of stained cells are shown on the right (n > 8 in each group). (E) Picrosirius Red staining of the liver sections of dox-treated kras+ and WT male and female zebrafish. Quantification of fibrotic liver tissue area in Picrosirius Red–stained liver sections is shown on the right (n > 10 in each group). (F) Gomori’s trichrome staining of the liver sections of dox-treated kras+ and WT male and female zebrafish. Quantification of fibrotic liver tissue area in Gomori’s trichrome–stained liver sections is shown on the right (n > 8 in each group). *P < .05. Scale bars: (A) 3 mm (top row), 20 μm (bottom row), (E–F) 500 μm, (D) 50 μm, and (B and C) 20 μm. DAPI, 4′,6-diamidino-2-phenylindole.
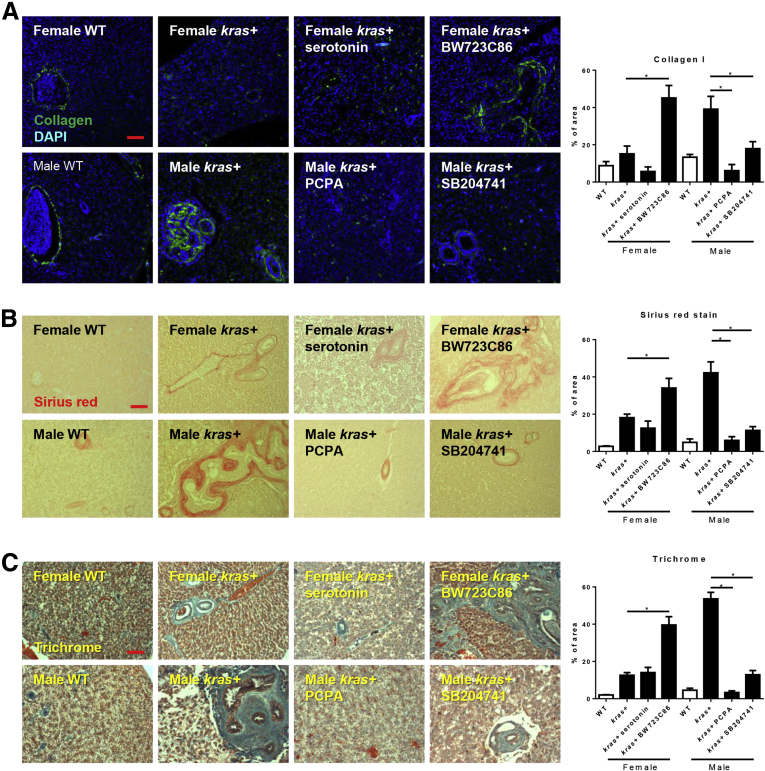
To further investigate the mechanism behind this sex disparity, the cell proliferation, apoptosis, and extent of hepatic fibrosis were examined. As expected, krasV12-expressing hepatocytes had higher rates of cell proliferation and apoptosis than their wild-type (WT) siblings (Figure 1B and C). When comparing the sexes, male krasV12-expressing hepatocytes had a significantly higher numbers of proliferating but not apoptotic cells than female krasV12-expressing hepatocytes. The extent of liver fibrosis could be a marker for early HCC.31 By IF staining, male krasV12-expressing livers showed a significantly higher expression of collagen I than WT siblings and also female krasV12-expressing livers, reflecting its more advanced liver disease status (Figure 1D). Picrosirius Red and Gomori’s trichrome staining, both of which stained for fibrotic tissues, showed consistent results with collagen I staining (Figure 1E and F).
Accelerated Carcinogenesis in Male KrasV12-Expressing Livers Correlated With Increased HSC Density and Serotonin Synthesis
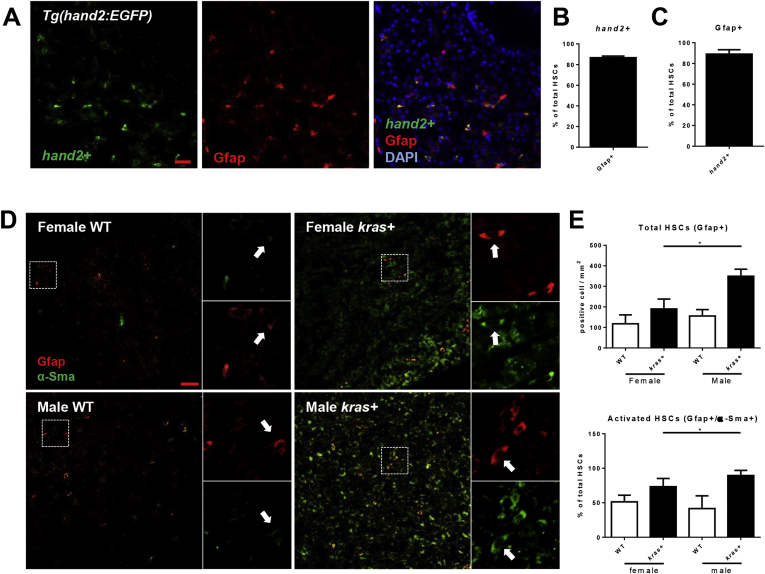
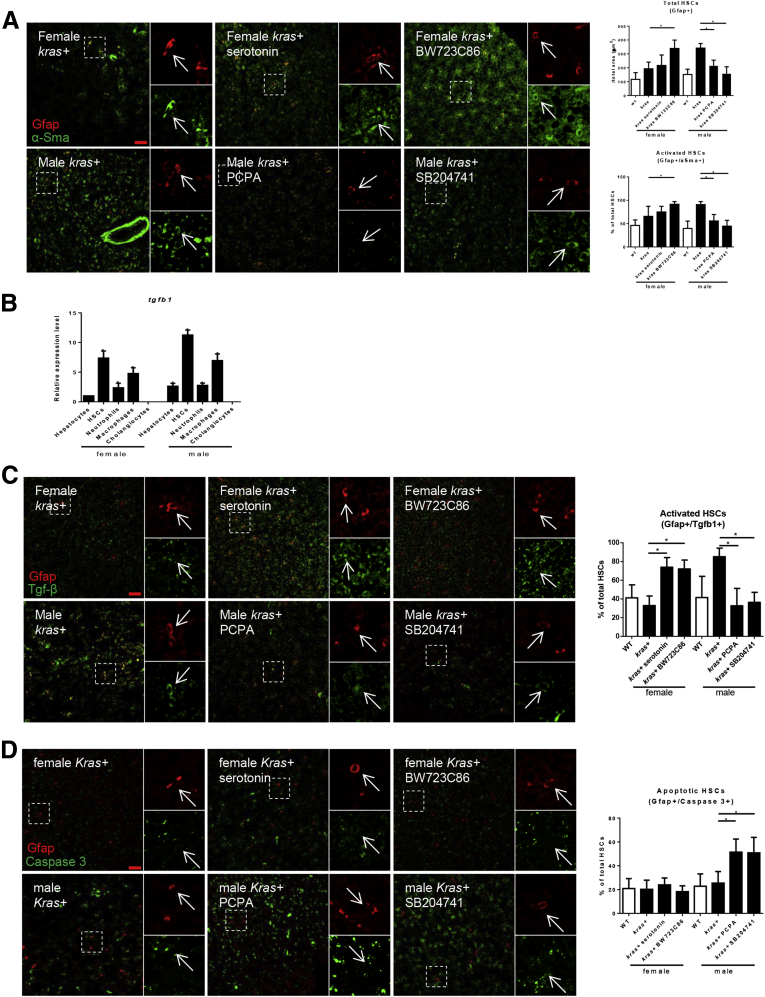
In human HCC, male HCC patients have a higher HSC density than female patients and a high HSC density signals a poor prognosis.15, 16 Hence, HSC density and activation were investigated in our kras+ HCC zebrafish model. Gfap+ cells in the liver demarcate both activated and quiescent HSCs.32 IF staining of Gfap on HSC reporter transgenic zebrafish, hand2+, showed nearly 90% hand2+ cells were positive for Gfap staining in the liver; similarly, 89% of Gfap+ cells were hand2+ cells (Figure 2A–C). Thus, there was high overlap between the 2 molecular markers for HSCs.22 α-Sma is a marker for activated HSC33; hence, Gfap+/α-Sma+ cells in the liver were used to indicate activated HSCs (Figure 2D). With krasV12 expression, both total HSC density and the percentage of activated HSCs were significantly higher in male krasV12-expressing livers than female (Figure 2E), indicating sex disparity in HSC activation in krasV12-expressing livers.
Figure 2.
Sex disparate increase of HSCs and activated HSCs after krasV12induction. (A) Overlap of hand2+ and Gfap expression. Gfap expression was examined in liver sections of hand2+ transgenic fish with 4′,6-diamidino-2-phenylindole (DAPI) staining for nuclei (blue) 8 days after fertilization. (B) Percentage of Gfap+ HSCs as marked by hand2:gfp expression in liver sections (n = 20). (C) Percentage of hand2+ HSCs as marked by Gfap+ expression in liver sections (n = 20). (D) IF co-staining of GFAP (red, general HSCs) and α-Sma (green, activated HSCs) in liver sections of adult zebrafish. Three-month-old kras+ and WT, male and female zebrafish were treated with 30 μg/mL dox for 7 days. Total HSCs, activated HSCs, and serotonin were examined. White boxes indicate enlarged regions shown on the right of each photograph. Arrows indicate Gfap+ cells. (E) Quantification of HSC density (Gfap+, top) and percentages of activated HSCs (Gfap+/α-Sma+, bottom) in liver sections (n > 8 in each group). *P < .05. Scale bar: 20 μm.
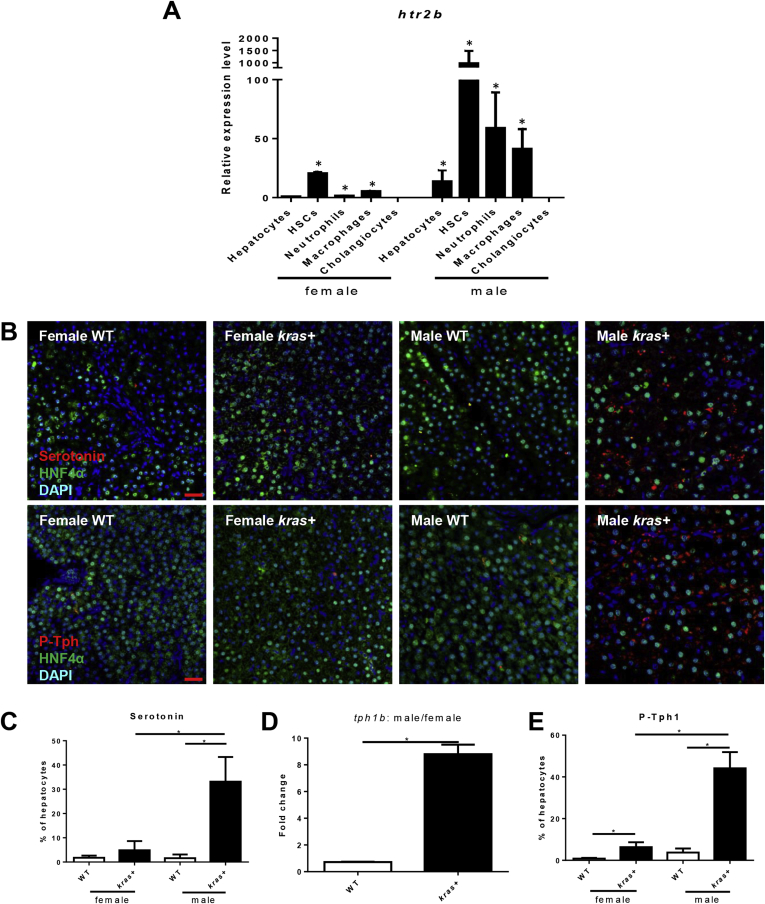
Serotonin has been shown to play an activating role on HSCs via 5-hydoxytryptamine receptor 2B (Htr2b) in a mouse liver regeneration model.34 To investigate if htr2b played a role in sex disparity in the kras+ HCC model in zebrafish, reverse-transcription quantitative PCR was performed in fluorescence-activated cell sorting–isolated hepatocytes, neutrophils, macrophages, HSCs and cholangiocytes. Htr2b was found to be expressed the most strongly in both male and female HSCs, with marginal expression in neutrophils and macrophages, but almost no expression was found in hepatocytes and cholangiocytes of both sexes (Figure 3A). Overall, the levels of htr2b expression was higher in males than in females in all cell types examined.
Figure 3.
Htr2b expression and serotonin production after krasV12induction. (A) Expression of htr2b in hepatocytes, HSCs, neutrophils, macrophages, and cholangiocytes. These cells were isolated by fluorescence-activated cell sorting based on DsRed, GFP, DsRed, mCherry, and GFP expression, respectively from fabp10+, hand2+, lyz+, mpeg+, and tp1+ transgenic zebrafish. Total RNA was extracted and htr2b expression was determined by reverse-transcription quantitative PCR. Relative expression levels are shown with the value from female hepatocytes set as 1. (B) IF co-staining of Hnf4a/Serotonin (top) and Hnf4a/P-Tph1 in liver sections. Three-month-old kras+ and WT, male and female zebrafish were treated with 30 μg/mL dox for 7 days. Serotonin and P-Tph level were examined. (C) Quantification of serotonin-positive hepatocytes in these zebrafish (n > 8 in each group). (D) Reverse-transcription quantitative PCR determination of tph1b expression in hepatocytes isolated by fluorescence-activated cell sorting from dox-treated kras+ and fabp10+ (kras- control) male and female fish. Fold change is shown between males and females in WT and kras+ fish. (E) Quantification of percentage of P-Tph1–positive hepatocytes in these zebrafish (n > 8 in each group). *P < .05. Scale bar: 20 μm. DAPI, 4′,6-diamidino-2-phenylindole.
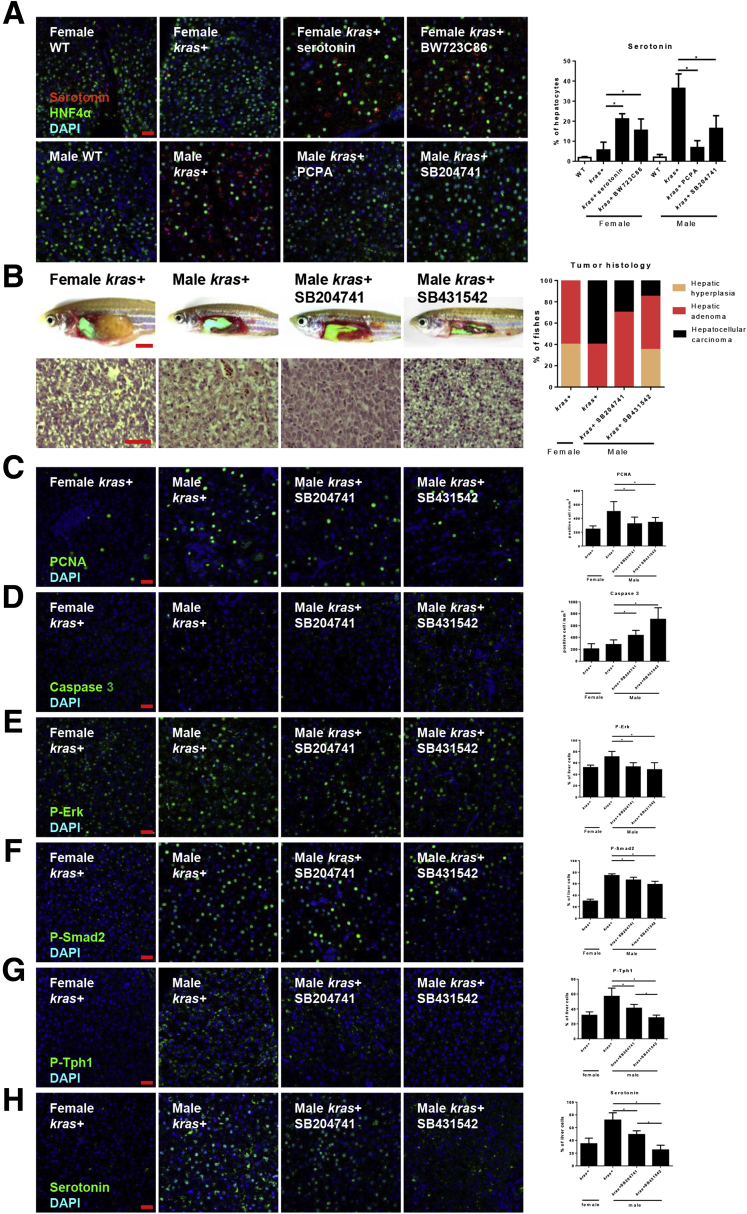
To account for the increased HSC density and activation, serotonin level was examined in male and female krasV12-expressing livers. Interestingly, IF staining of serotonin indicated a more significantly increase in krasV12-expressing hepatocytes in males than in females (Figure 3B and C). To investigate the sex difference in serotonin level, expression of tryptophan hydroxylase 1b (Tph1b), the rate-limiting enzyme for serotonin biosynthesis, was examined. Male krasV12-expressing hepatocytes showed an 8-fold increase in tph1b messenger RNA expression (Figure 3D). Tph1 can undergo phosphorylation to enhance protein stability.35 IF staining of phosphorylated Tph1 (P-Tph1) in liver sections showed a higher expression of the phosphorylated enzyme in male krasV12-expressing hepatocytes than female krasV12-expressing hepatocytes (Figure 3B and E), suggesting that the sex disparity in serotonin levels was owing to the higher expression and phosphorylation of Tph1b in males than in females.
Serotonin Promotes KrasV12-Induced Carcinogenesis in Zebrafish via Activation of the Htr2b Receptor in HSCs
To examine if serotonin or Htr2b-receptor activation contributed significantly to the differential rates of HCC between sexes, serotonin and the Htr2b-receptor agonist BW723C86 were used to detect kras+ female zebrafish and the Tph1 inhibitor PCPA and the Htr2b-receptor antagonist SB204741 were used for kras+ male zebrafish for 7 days. Gross morphology of both serotonin- and BW723C86-treated kras+ females showed significantly enlarged livers as compared with WT control females. In contrast, kras+ male zebrafish showed a significant deterrence of liver enlargement with exposure to PCPA or SB204741 (Figure 4A and B). Histologically, exposure to serotonin or activation of the Htr2b receptor significantly accelerated the rate of carcinogenesis because an increased portion of female krasV12-expressing livers showed a loss of 2-cell hepatic plates. However, decreased percentages of fish with HCC histology were observed in male krasV12-expressing livers after exposure to PCPA or SB204741 (Figure 4C). As summarized in Figure 4C, 75% and 38% of female krasV12-expressing livers showed HCC histology when exposed to serotonin and BW723C86, respectively. In contrast, 25% and 20% of male krasV12-expressing livers showed milder histology when exposed to PCPA and SB204741, respectively.
Figure 4.
Effects of serotonin level and HSC activation on krasV12-induced carcinogenesis. Three-month-old adult zebrafish were treated with dox with or without serotonin, BW23C86, PCPA, and SB204741 for 7 days. (A) Gross morphology and H&E staining of liver sections. (B) Quantification of tumor size as a percentage of total abdominal area. (C) Quantification of tumor histology observed in the H&E-stained liver sections of these zebrafish is shown on the right (n > 8 in each group). (D and E) IF staining and quantification of (D) PCNA+ and (E) caspase 3+ cells in liver sections of these zebrafish (n > 8). *P < .05. Scale bars: (A) 3 mm for gross observation images and 20 μm for histology images, and (D and E) 20 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Cytologic analysis of cell proliferation was consistent with morphologic and histologic changes. After 7 days of dox exposure, male krasV12-expressing livers had a significantly higher level of proliferating cells than females. Exposure of serotonin or BW723C86 to kras+ females significantly increased the level of proliferating cells. Conversely, exposure of SB204741 and PCPA significantly reduced the number of proliferating cells (Figure 4D). In contrast, exposure to PCPA or SB204741 to kras+ males increased apoptotic liver cells significantly (Figure 4E). IF staining of collagen I, Picrosirius Red, or Gomori’s trichrome staining consistently indicated an increased collagen deposition in the extracellular matrix when comparing male and female krasV12-expressing livers (Figure 5). Exposure of kras+ female fish to BW723C86 increased collagen deposition as compared with kras+ female controls. In contrast, exposure to either SB204741 or PCPA decreased collagen deposition as compared with male kras+ control (Figure 5). Hence, either serotonin or the activation of the Htr2b receptor could accelerate carcinogenesis significantly in our krasV12-expressing model.
Figure 5.
Effects of serotonin level and HSC activation on krasV12-induced liver fibrosis. Three-month-old adult zebrafish were treated with dox with or without serotonin, BW23C86, PCPA, and SB204741 for 7 days. (A) IF staining of collagen I antibody and quantification of fibrotic liver tissue area in collagen I antibody–stained liver sections (n > 8 in each group). (B) Staining and quantification of fibrotic liver tissue area in Picrosirius Red–stained liver sections (n > 8 in each group). (C) Staining and quantification of fibrotic liver tissue area in Gomori’s trichrome–stained liver sections (n > 8 in each group). *P < .05. Scale bars: (A) 50 μm, and (B and C) 500 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Serotonin-Activated HSCs Promote Carcinogenesis and Serotonin Synthesis via Tgfb1
It has been reported previously that serotonin is capable of activating HSCs via the Htr2b receptor.34 To investigate if serotonin was responsible for the sex disparity in HSC density through controlling the Htr2b downstream signaling, IF co-staining of Gfap and α-Sma was performed on both female and male krasV12-expressing livers. As shown in Figure 6A, activation of Htr2b signaling by BW723C86 but not serotonin significantly increased both the total HSC density and percentage of activated HSCs whereas inhibition of serotonin synthesis by PCPA or inhibition of Htr2b signaling by SB204741 decreased both the density and percentage of activated HSCs, as compared with female or male krasV12-expressing livers, respectively (Figure 6A). Thus, serotonin regulates HSCs through Htr2b, which could increase both the proliferation and activation of HSCs.
Figure 6.
Manipulation of serotonin level and HSC density affects HSC activation and apoptosis. (A) IF staining and quantification of HSC density (Gfap+) in liver sections (n > 8 in each group). Three-month-old adult zebrafish were treated with dox with or without serotonin, BW23C86, PCPA, and SB204741 for 7 days. Percentages of activated HSCs (Gfap+/α-Sma+) in the liver sections (n > 8 in each group). (B) Expression of tgfb1 in hepatocytes, HSCs, neutrophils, macrophages, and cholangiocytes. These cells were isolated by fluorescence-activated cell sorting based on DsRed, GFP, DsRed, mCherry, and GFP expression, respectively, from fabp10+, hand2+, lyz+, mpeg+, and tp1+ transgenic zebrafish. Total RNAs were extracted and htr2b expression was determined by reverse-transcription quantitative PCR. Relative expression levels are with the values with female hepatocytes set as 1. (C and D) IF staining and quantification of percentages of (C) TgfB1+ HSCs (Gfap+/Tgfb1+) and (D) apoptotic HSCs in the liver sections as described in panel A. n > 8 in each group. *P < .05. Scale bar: 20 μm.
It has been reported that Htr2b activation is required for both HSC survival and tgfb1a expression.34 To further elucidate the molecular outcome of Htr2b activation, expression of tgfb1a was examined in various cell types in the liver, including hepatocytes, HSCs, neutrophils, macrophages, and cholangiocytes. As shown in Figure 6B, HSCs had the highest level of tgfb1a expression in both male and female adult zebrafish (Figure 6B). Treatments with serotonin or BW723C86 significantly increased the percentage of Tgfb1-expressing HSCs in female krasV12-expressing livers whereas treatments with PCPA or SB204741 significantly decreased HSCs of male krasV12-expressing livers (Figure 6C). IF co-staining of Gfap with caspase-3 or Tgfb1 also was performed to show apoptotic HSCs (Gfap+/Cas3+). In contrast, the percentage of apoptotic HSCs remained unchanged in female krasV12-expressing livers exposed to serotonin or BW723C86, but there was a significant increase in apoptotic HSCs in male krasV12-expressing livers exposed to PCPA or SB204741 (Figure 6D). In contrast, therefore, serotonin activation of Htr2b signaling in HSCs is necessary for HSC survival as well as increased Tgfb1 expression.
Curiously, IF staining of serotonin indicated that BW723C8 activation of Htr2b in female krasV12-expressing hepatocytes increased serotonin significantly, suggesting increased Tgfb1 or HSCs activation could promote serotonin synthesis (Figure 7A). To confirm the role of HSCs or Tgfb1 in serotonin synthesis, 3-month-old adult kras+ male zebrafish were exposed to either SB204741 or SB431542 (Tgfβ receptor 2 inhibitor) for 7 days. Inhibition of HSCs or Tgfβ signaling relaxed the HCC histology in male krasV12-expressing livers (Figure 7B). Cytologic analysis of proliferating cells showed a significant decrease with both SB204741 and SB431541 exposure (Figure 7C). Tgfb1 is known to induce both cell senescence and apoptosis.36, 37 β-galactosidase staining for senescent cells showed no difference between 3-month-old adult kras+ female and kras+ male zebrafish after exposure to SB204741 or SB431542 (data not shown), whereas IF staining of caspase-3 showed significantly higher apoptotic cells with either SB204741 or SB431542 exposure to kras+ males (Figure 7D). Notably, both SB204741- and SB431542-treated zebrafish showed decreased Tgfb1 expression and corresponding down-regulation of phosphorylated Erk (P-Erk) and phosphorylated Smad2 (P-Smad2) signaling (Figure 7E and F), suggesting that inhibition of Htr2b-mediated HSC activation or inhibition of Tgfbr2 decreased both Tgfb1 expression and its downstream signaling. Interestingly, inhibition of both HSC activation and Tgfβ1 signaling showed an inhibition of Tph1 phosphorylation and hence the serotonin production (Figure 7G and H), suggesting a potential role of HSC and their secreted Tgfb1 in regulating Tph1 activity.
Figure 7.
Tgfb1 promotes serotonin synthesis and krasV12-induced carcinogenesis. Three-month-old adult zebrafish were treated for 7 days with 30 μg/mL dox with or without 2 μmol/L serotonin, BW23C86, PCPA, and SB204741. (A) IF staining of serotonin in liver section of these zebrafish. These slides also were co-stained for hepatocytes (HNF4a) and nuclei (4′,6-diamidino-2-phenylindole [DAPI]). Right: Quantification of serotonin-positive liver cells as percentages of hepatocytes (n = 10). Three-month-old Male krasV12-expressing zebrafish were treated with dox with or without SB204741 and SB431542, respectively. (B) Gross morphology and H&E staining of liver sections of these zebrafish. Quantification of tumor histology observed in the H&E-stained liver sections of these zebrafish (n > 8 in each group). (C–H) IF staining and quantification of (C) PCNA+, (D) caspase 3+, (E) P-Erk+, (F) P-Smad2, (G) P-Tph+, and (H) serotonin+ cells in liver sections (n > 8 in each group). *P < .05. Scale bars: (B) 3 mm (toprow) and 50 μm (bottom row), and (A, C–H) 20 μm.
Sex Differences in Serotonin and TGFB1 Level in Human Liver Disease Patients
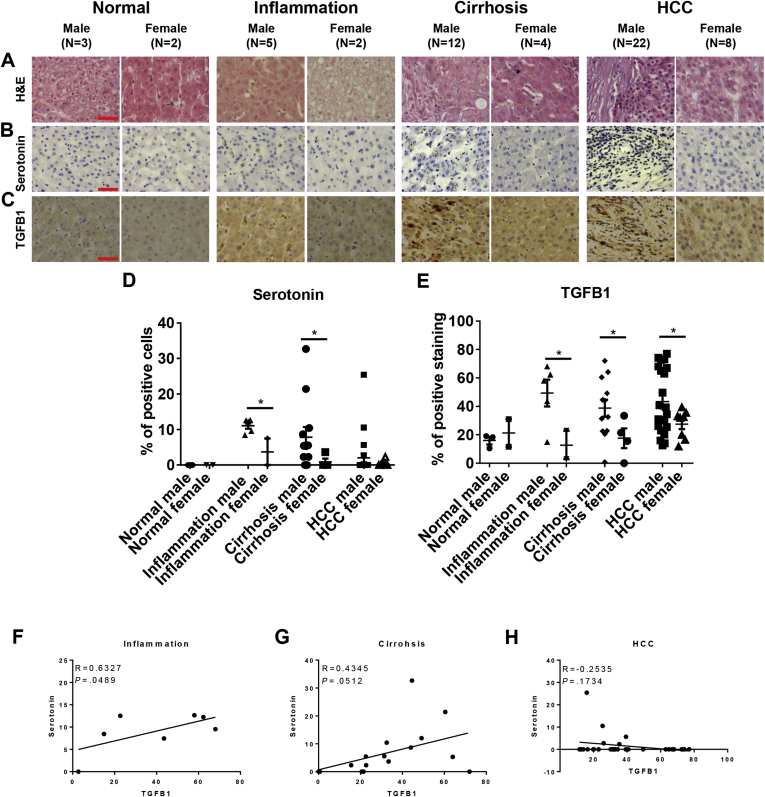
In human liver diseases, serum serotonin levels have been shown to increase significantly in patients with cirrhosis and HCC.38 To observe if the sex disparate serotonin levels observed in our krasV12 HCC zebrafish model would be reflected in human patients, a panel of liver disease samples, including normal, liver inflammation, cirrhosis, and HCC, were analyzed for levels of serotonin and TGFB1. Each sample then was stained for H&E, serotonin, and TGFB1, respectively (Figure 8A–C). Notably, serotonin levels in inflammation and cirrhosis samples were significantly higher in males than in females (Figure 8D). This sex disparity was less evident in HCC samples but still was very significant. Similarly, male-biased TGFB1 expression also was evident in inflammation, cirrhosis, and HCC samples (Figure 8E). Serotonin and TGFB1 protein showed a positive and significant correlation in both inflammation and cirrhosis patients, suggesting the molecular mechanism of serotonin-induced TGFB1 expression potentially translated in this cohort of patients. However, the correlation between the 2 molecules was abrogated in HCC patients, suggesting other confounding factors might contribute to TGFB1 expression in HCC patients (Figure 8F-H).
Figure 8.
Sex difference in serotonin and TGFB1 levels in human liver disease samples. A panel of liver disease samples from human patients was examined for histology by H&E staining and for serotonin and TGFB1 levels by antibody staining. These samples were categorized into normal, inflammation, cirrhosis, and HCC for both males and females. (A) H&E staining of human liver disease samples. (B and C) IHC staining of antibody against (B) serotonin and (C) TGFB1. (D and E) Quantification of the percentages of (D) serotonin or (E) TGFB1-positive liver cells in inflammation, cirrhosis, and HCC patients. (F–H) Quantification of correlation between serotonin with TGFB1 in (F) inflammation, (G) cirrhosis, and (H) HCC patients. *P < .05. Scale bar: 20 μm.
Discussion
Higher Expression of Serotonin in Males Contributed to Sex Disparity via Htr2b Activation of HSCs
In our investigation of human liver disease samples, serotonin levels were increased significantly in inflammation and cirrhosis, and marginally in HCC samples as compared with normal liver control samples. This is consistent with a previous observation that serotonin was a more sensitive serum marker for cirrhosis and HCC than α-fetoprotein.32 The level of serotonin in HCC was significantly lower than in inflammation and cirrhosis samples, suggesting that the contribution of serotonin to liver diseases is possibly more important in early phases of the progression to HCC. Notably, in the cohort of human patients we analyzed, an apparent sex-dependent accumulation was observed in both inflammation and cirrhosis samples because serotonin was generally not detected in female patients of both stages. This is in line with the prevailing knowledge that men have a significantly higher rate of serotonin synthesis than women.39 Our zebrafish studies recapitulated the sex disparity of serotonin accumulation, which likely can be attributed to the difference in the expression and phosphorylation of rate-limiting enzyme Tph1a. A previously conducted study suggested androgen to be responsible for the activation of Tph1 in macaques, thus providing a link to the sex difference in serotonin.40 The sex differential production of serotonin and HSC density and activation also similarly were observed in other oncogene-induced (xmrk and Myc) zebrafish HCC models generated in our laboratory18, 19; thus, the involvement of serotonin and HSCs in the sex disparity of HCC is not oncogene-specific (our unpublished observations).
Serotonin can act on a variety of serotonin receptors. We showed that Htr2b was highly expressed in HSCs, consistent with a previously reported mouse study.34 The sex disparity in serotonin synthesis resulted in a higher rate of Htr2b-mediated activation of HSCs in males than in females, and led to both a higher density of total HSCs and a higher percentage of activated HSCs, in line with a human HCC patient study in which male patients had a higher density of HSCs than female patients.16 In this study, increased Htr2b activation in HSCs resulted in overexpression of Tgfb1. Both inactivation of HSCs and depletion of TgfB1 signaling significantly relaxed the rate of carcinogenesis in male krasV12-expressing livers, consistent with the current notion that TGFB1 acts as a protumor cytokine in human HCC.41
HSCs Maintain Sex Disparity in HCC via a Serotonin/Tgfβ1 Loop
In our zebrafish study, serotonin level was found to be dependent on Htr2b activation. A role of TGFB1 in the regulation of TPH1 expression and phosphorylation via ERK and SMAD pathways has been shown in human cells.42, 43 Depletion of HSCs via Htr2b antagonist and inhibition of Tgfβ signaling in krasV12-expressing livers of male zebrafish inhibited P-Tph1 and serotonin expression, suggesting that the role of activated HSCs on serotonin synthesis is mediated by Tgfβ signaling. Hence, the robust activation of HSCs via Htr2b in male krasV12-expressing livers would establish a positive feedback loop, ensuring constant and high levels of serotonin synthesis, thus accelerating carcinogenesis via the multifunctional cytokine, Tgfb1. Although this molecular mechanism similarly would exist in female krasV12-expressing livers, the lower initial P-Tph level would result in the initiation of a less-robust feedback loop. This is at least in part consistent with our analyses on human liver disease samples. TGFB1 showed a similar sex-biased expression in inflammation, cirrhosis, and HCC patients. However, significant correlation between serotonin and TGFB1 was observed only in inflammation and cirrhosis samples but not in HCC patients, possibly because of many other factors affecting TGFB1 expression during carcinogenesis.
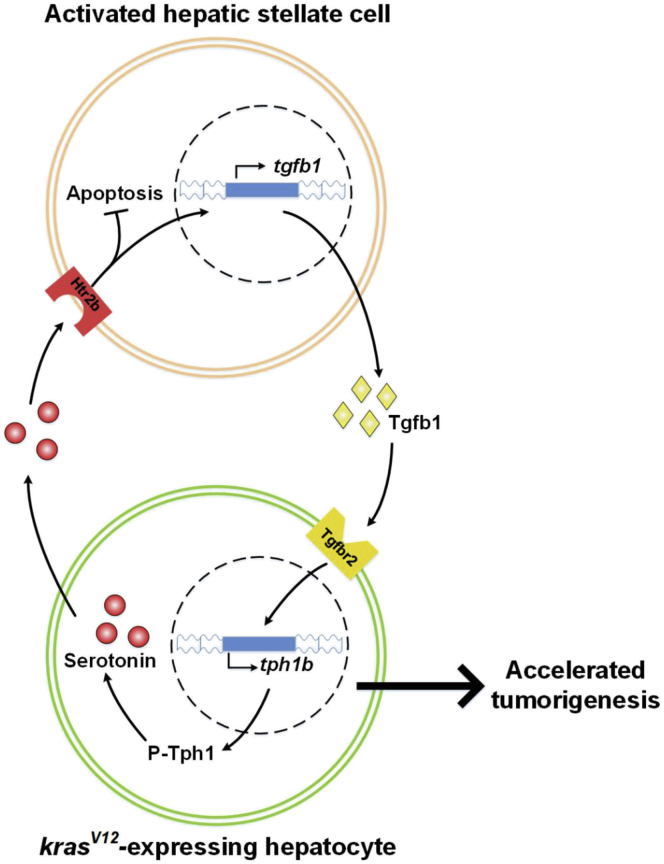
In summary, as shown in Figure 9, data from both zebrafish models and human liver disease samples showed the differential accumulation of serotonin during liver disease progression between sexes, which could be attributed to the disparity of P-Tph level in the 2 sexes of kras+ zebrafish. We further showed the novel role of serotonin in initiating and promoting a sex-disparate tumor microenvironment via Htr2b activation of HSCs. Htr2b-activated HSCs expressed Tgfb1, which not only promoted HCC progression but also increased serotonin synthesis. Hence, HSCs not only promote liver tumor progression but also play a vital role in maintaining the sex disparity observed in HCC patients.
Figure 9.
Schematic summary of serotonin-activated HSCs and Tgfb signal in accelerating liver tumorigenesis in kras+-expressing male transgenic fish.
Acknowledgment
Chuan Yan, Qiqi Yang, and Zhiyuan Gong conceived and designed the experiments; Qiqi Yang and Chuan Yan performed the experiments; Qiqi Yang, Chuan Yan, Chunyue Yin, and Zhiyuan Gong analyzed the data; Chuan Yan, Qiqi Yang, and Zhiyuan Gong wrote the paper; and Chunyue Yin provided material.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by research grants from the National Medical Research Council and the Ministry of Education of Singapore. The sponsors had no role in the study design, or the collection, analysis, or interpretation of data.
References
- 1.Lee C.M., Lu S.N., Changchien C.S. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer. 1999;86:1143–1150. doi: 10.1002/(sici)1097-0142(19991001)86:7<1143::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Mucci L.A., Kuper H.E., Tamimi R. Age at menarche and age at menopause in relation to hepatocellular carcinoma in women. BJOG. 2001;108:291–294. doi: 10.1111/j.1471-0528.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 3.Yu M.W., Chang H.C., Chang S.C. Role of reproductive factors in hepatocellular carcinoma: impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393–1400. doi: 10.1016/j.hep.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Nakatani T., Roy G., Fujimoto N. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn J Cancer Res. 2001;92:249–256. doi: 10.1111/j.1349-7006.2001.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naugler W.E., Sakurai T., Kim S. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 6.Wolf M.J., Adili A., Piotrowitz K. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang W., Lu Y., Xu Y. Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs) J Biol Chem. 2012;287:40140–40149. doi: 10.1074/jbc.M112.348763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y.W., Qiu S.J., Fan J. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Krizhanovsky V., Yon M., Dickins R.A. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bocca C., Novo E., Miglietta A. Angiogenesis and fibrogenesis in chronic liver diseases. Cell Mol Gastroenterol Hepatol. 2015;1:477–488. doi: 10.1016/j.jcmgh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth K.J., Copple B.L. Role of hypoxia-inducible factors in the development of liver fibrosis. Cell Mol Gastroenterol Hepatol. 2015;1:589–597. doi: 10.1016/j.jcmgh.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsova P., Gores G.J. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2015;1:17–27. doi: 10.1016/j.jcmgh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amann T., Bataille F., Spruss T. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100:646–653. doi: 10.1111/j.1349-7006.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang N., Gores G.J., Shah V.H. Hepatic stellate cells: partners in crime for liver metastases? Hepatology. 2011;54:707–713. doi: 10.1002/hep.24384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji J., Eggert T., Budhu A. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015;62:481–495. doi: 10.1002/hep.27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai C.X., Gao Q., Q S.J. Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer. 2009;9:418. doi: 10.1186/1471-2407-9-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew T.W., Liu X.J., Liu L. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene. 2014;33:2717–2727. doi: 10.1038/onc.2013.240. [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Huang X., Zhan H. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J Hepatol. 2012;56:419–425. doi: 10.1016/j.jhep.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Li Z., Zheng W., Wang Z. A transgenic zebrafish liver tumor model with inducible Myc expression reveals conserved Myc signatures with mammalian liver tumors. Dis Model Mech. 2013;6:414–423. doi: 10.1242/dmm.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen A.T., Emelyanov A., Koh C.H. An inducible kras(V12) transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis Model Mech. 2012;5:63–72. doi: 10.1242/dmm.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L., Nguyen A.T., Spitsbergen J.M. Myc-induced liver tumors in transgenic zebrafish can regress in tp53 null mutation. PLoS One. 2015;10:e0117249. doi: 10.1371/journal.pone.0117249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin C., Evason K.J., Maher J.J. The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver. Hepatology. 2012;56:1958–1970. doi: 10.1002/hep.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons M.J., Pisharath H., Yusuff S. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall C., Flores M.V., Storm T. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellett F., Pase L., Hayman J.W. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117:e49–e56. doi: 10.1182/blood-2010-10-314120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korzh S., Pan X., Garcia-Lecea M. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev Biol. 2008;8:84. doi: 10.1186/1471-213X-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manoli M., Driever W. Fluorescence-activated cell sorting (FACS) of fluorescently tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harb Protoc. 2012;2012:8. doi: 10.1101/pdb.prot069633. [DOI] [PubMed] [Google Scholar]
- 28.Dohmen K., Shigematsu H., Irie K. Longer survival in female than male with hepatocellular carcinoma. J Gastroenterol Hepatol. 2003;18:267–272. doi: 10.1046/j.1440-1746.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- 29.Farinati F., Sergio A., Giacomin A. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2009;21:1212–1218. doi: 10.1097/MEG.0b013e32831a86f8. [DOI] [PubMed] [Google Scholar]
- 30.Hefaiedh R., Ennaifer R., Romdhane H. Gender difference in patients with hepatocellular carcinoma. Tunis Med. 2013;91:505–508. [PubMed] [Google Scholar]
- 31.Huang M., Chang A., Choi M. Antagonistic interaction between Wnt and Notch activity modulates the regenerative capacity of a zebrafish fibrotic liver model. Hepatology. 2014;60:1753–1766. doi: 10.1002/hep.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morini S., Carotti S., Carpino G. GFAP expression in the liver as an early marker of stellate cells activation. Ital J Anat Embryol. 2005;110:193–207. [PubMed] [Google Scholar]
- 33.Carpino G., Morini A., Ginanni Corradini S. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37:349–356. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahimkhani M.R., Oakley F., Murphy L.B. Stimulating healthy tissue regeneration by targeting the 5-HT(2)B receptor in chronic liver disease. Nat Med. 2011;17:1668–1673. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Z., Liu T., Chattoraj A. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J Pineal Res. 2008;45:506–514. doi: 10.1111/j.1600-079X.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senturk S., Mumcuoglu M., Gursoy-Yuzugullu O. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- 37.Jang C.W., Chen C.H., Chen C.C. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol. 2002;4:51–58. doi: 10.1038/ncb731. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Razik A., Elhelaly R., Elzehery R. Could serotonin be a potential marker for hepatocellular carcinoma? A prospective single-center observational study. Eur J Gastroenterol Hepatol. 2016;28:599–605. doi: 10.1097/MEG.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 39.Nishizawa S., Benkelfat C., Young S.N. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bethea C.L., Reddy A.P., Robertson N. Effects of aromatase inhibition and androgen activity on serotonin and behavior in male macaques. Behav Neurosci. 2013;127:400–414. doi: 10.1037/a0032016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannelli G., Villa E., Lahn M. Transforming growth factor-beta as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014;74:1890–1894. doi: 10.1158/0008-5472.CAN-14-0243. [DOI] [PubMed] [Google Scholar]
- 42.Imbe H., Senba E., Kimura A. Activation of mitogen-activated protein kinase in descending pain modulatory system. J Signal Transduct. 2011;2011:468061. doi: 10.1155/2011/468061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Estevez M., Estevez A.O., Cowie R.H. The voltage-gated calcium channel UNC-2 is involved in stress-mediated regulation of tryptophan hydroxylase. J Neurochem. 2004;88:102–113. doi: 10.1046/j.1471-4159.2003.02140.x. [DOI] [PubMed] [Google Scholar]