Abstract
The bone morphogenetic proteins, (BMP)s are regulatory peptides that have significant effects on the growth and differentiation of gastrointestinal tissues. In addition, the BMPs have been shown to exert anti-inflammatory actions in the gut and to negatively regulate the growth of gastric neoplasms. The role of BMP signaling in the regulation of gastric metaplasia, dysplasia and neoplasia has been poorly characterized. Transgenic expression in the mouse stomach of the BMP inhibitor noggin leads to decreased parietal cell number, increased epithelial cell proliferation, and to the emergence of SPEM. Moreover, expression of noggin increases Helicobacter-induced inflammation and epithelial cell proliferation, accelerates the development of dysplasia, and it increases the expression of signal transducer and activator of transcription 3 (STAT3) and of activation-induced cytidine deaminase (AID). These findings provide new clues for a better understanding of the pathophysiological mechanisms that regulate gastric inflammation and the development of both dysplastic and neoplastic lesions of the stomach.
Keywords: Cellular Differentiation, Cellular Proliferation, Gastric Inflammation, Cytokines, Chemokines
Abbreviations used in this paper: BMP, bone morphogenetic protein; BMPR-I, BMP type I receptor; EGFR, epidermal growth factor receptor; ERK, extracellular signal-related kinase; IL, interleukin; SPEM, spasmolytic polypeptide expressing metaplasia; TFF2, trefoil factor family 2; TGF, transforming growth factor; TNF, tumor necrosis factor
Summary.
The bone morphogenetic proteins exert important regulatory actions on the homeostasis of the gastric epithelium. Loss of bone morphogenetic protein signaling leads to metaplasia and dysplasia and to enhancement of helicobacter-induced gastric inflammation.
The bone morphogenetic proteins (BMPs) belong to the transforming growth factor-β (TGF-β) superfamily of regulatory peptides. The BMPs have been shown to play a broad array of biological actions on various cell types such as monocytes, epithelial cells, mesenchymal cells, and neurons.1 BMP-2, BMP-4, and BMP-7 are among the best-studied members of the BMP family of regulatory peptides. The physiological importance of these BMPs has been underscored by the observation that mouse embryos homozygous for either the BMP-4 or BMP-2 null alleles exhibit embryonic lethality. Similarly, BMP-7 knockout mice die shortly after birth with defects in the morphogenesis of kidneys and eyes.1 BMP-2, BMP-4, and BMP-7 appear to be significantly expressed in gastrointestinal tissues, where they have been shown to play an important role in the regulation of gastrointestinal growth and differentiation.2, 3, 4, 5, 6, 7, 8, 9, 10, 11
Bone Morphogenetic Protein Signaling
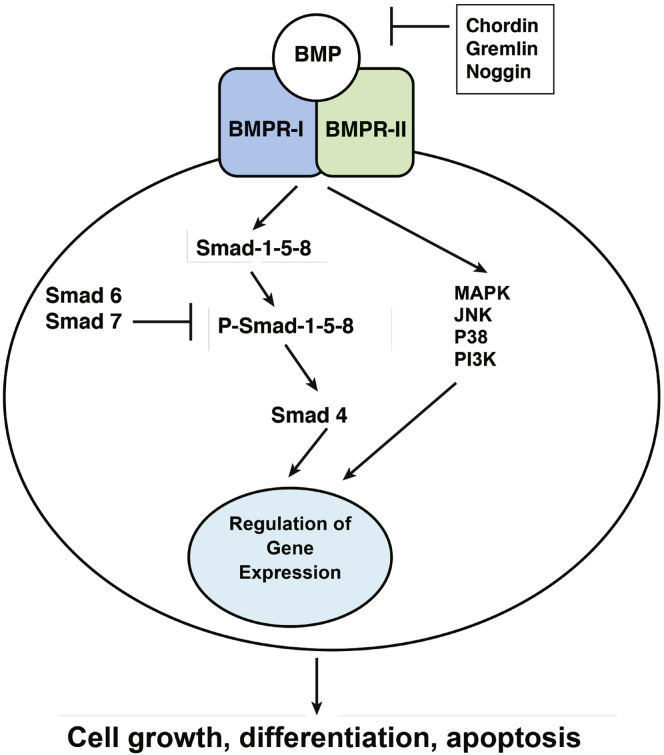
The BMPs activate several complex signal transduction pathways to exert their biological actions.1, 12, 13, 14, 15 In particular, binding of the BMPs to the BMP type I receptor (BMPR-I) leads to the dimerization of BMPR-I with the BMP type II receptor, a molecule that has serine/threonine kinase activity. This event triggers the phosphorylation of both BMPR-I and the regulatory proteins Smad 1, 5, and 8, which are known as R-Smads, which mediate the intracellular actions of the BMPs. On phosphorylation, Smad 1, 5, and 8 associate with Smad 4 in a heterodimeric complex that translocates to the nucleus where it activates the transcription of BMP-regulated genes1, 12 (Figure 1). In contrast to the BMPs, TGF-β peptides signal to the nucleus through the phosphorylation and activation of a different set of regulatory Smad proteins, Smad 2 and 3, which bind Smad 4 to induce gene transcription. The complexity of this system is underscored by the observation that the inhibitory proteins Smad 6 and Smad 7 block the effects of BMP-activated signaling, thus generating a negative feedback loop that controls the level of activation of BMP-mediated signal transduction. Smad 6 and Smad 7 are therefore classified as inhibitory Smads. Although Smad 7 inhibits both TGF-β and BMP-mediated signals, Smad 6 is a relatively specific inhibitor of BMP signaling because it only weakly affects the actions of TGF-β.16 The actions of the BMPs can be blocked by inhibitory molecules such as noggin, gremlin, and chordin, which are expressed in tissues to modulate the level of BMP signaling.14 Of these, noggin, a secreted polypeptide present in several mammalian tissues, has been shown to bind to and inhibit the actions of extracellular BMP-2, BMP-4, and to a lesser extent BMP-7.3, 6, 17, 18, 19 In addition to Smad-dependent signaling, the BMPs have also been shown to activate in some biological systems the extracellular signal-related kinases (ERKs), PI3 kinase, the P38 kinases, and the C-jun N-terminal kinases.14
Figure 1.
BMP signaling. Binding of BMPs to BMPR-I leads to dimerization of BMPR-I with BMP type II receptor, a molecule that has serine/threonine kinase activity. This event triggers phosphorylation of both BMPR-I and of Smad 1, 5, and 8, proteins known to mediate the intracellular actions of BMPs. On phosphorylation, Smad 1, 5, and 8 associate with Smad 4 in a heterodimeric complex that translocates to the nucleus where it activates the transcription of BMP-regulated genes. BMP signaling is negatively regulated by the inhibitory Smad proteins, Smad 6 and 7, and by secreted BMP inhibitors such as chordin, gremlin, and noggin that bind to BMPs, blocking their ability to activate signal transduction. In addition to Smad-mediated signaling, BMPs can activate in some systems signal transduction cascades that lead to activation of mitogen-activated protein kinase (MAPK), Jun N-terminal kinase (JNK), P38 kinase, and PI3 kinase.
Localization of Bone Morphogenetic Protein-4 and of Cell Receiving Bone Morphogenetic Protein–generated Signals in the Gastric Mucosa
A series of recently published reports from our laboratory have examined the localization of BMP-4–expressing cells in the gastric mucosa in both the presence and absence of inflammation20 in genetically engineered mice that express a β-galactosidase marked allele of the BMP-4 gene (BMP-4βgal/+ mice). X-gal staining of the gastric mucosa of BMP-4βgal/+ mice indicated that BMP-4 is expressed in mesenchymal cells located both under and between the glands. To analyze the pattern of expression of BMP-4 during inflammation, BMP-4βgal/+ mice were infected with Helicobacter felis for 2 months. H felis induced the expression of TNF-α, MIP-2, and interferon-γ mRNAs in these mice, and it led to the development of significant foci of inflammatory infiltrates in the mucosa of the corpus.20 Moreover, staining of corresponding sections with X-gal demonstrated that BMP-4 is expressed in clusters of cells that appear to be localized in the mesenchymal layers of the mucosa that are adjacent to but not in the inflammatory infiltrates.20 Localization of cells receiving BMP-generated signals was determined in experiments with transgenic mice that express β-galactosidase under the control of a BMP-responsive element. In this system X-gal positively stained cells could be detected mostly at the level of the isthmus and neck of the glands but not in the mesenchyme in both the absence and presence of inflammation.20 Similar results were observed when sections of the gastric mucosa of Helicobacter pylori–infected mice were stained with antibodies recognizing phosphorylated and active forms of the BMP-4 signal transducing proteins, Smad1, 5, and 8.20 Thus, studies conducted with 2 different experimental approaches in the presence of 2 types of Helicobacter organisms confirmed the notion that BMP-generated signals specifically target cells located in the epithelium but not in the mesenchyme or in the inflammatory infiltrates.
Immunohistochemical analysis of sections of the fundic mucosa of H felis–infected BMP-4βgal/+ mice demonstrated that BMP-4 expression can be predominantly detected in alpha smooth muscle actin–positive cells. No significant BMP-4 expression could be identified in cells expressing macrophage, B, T, dendritic, and neutrophil markers.20 Thus, myofibroblasts but not immune cells appear to represent the main source of BMP-4 expression in the gastric mucosa.
Bone Morphogenetic Protein Signaling Regulates Gastric Epithelial Homeostasis
The oxyntic mucosa is a complex structure that contains several types of highly specialized cells such as mucus pit, mucus neck, parietal, zymogenic, and endocrine cells. The mechanisms and the factors that regulate the homeostasis of the gastric epithelium have been only partially characterized.21
Studies from our laboratory have shown that incubation of cultured parietal cells with BMP-4 leads to stimulation of H+/K+-adenosine triphosphatase α-subunit gene expression and to enhancement of secretagogue-stimulated gastric acid production,22 suggesting that BMP signaling exerts regulatory effects on the physiological function of the gastric parietal cells.
Several reports have shown that the parietal cells play an important role in the process of differentiation and development of other cell lineages in the gastric mucosa. Indeed, studies have shown that loss of mature parietal cells leads to a block in the differentiation program of the zymogenic lineages and to the development of different types of mucosal cell remodeling.23, 24, 25, 26, 27 It has been proposed that the cause of these events might reside in the observation that the parietal cells are a major site for the production of regulatory factors and morphogens in the gastric epithelium4, 28, 29 and that loss of these peptides could contribute to the development of metaplasia. On the basis of this hypothesis it has been suggested that BMP signaling could regulate the homeostasis of the gastric epithelium through its ability to control the biological functions of the parietal cells. However, a recent report has demonstrated that induction of selective parietal cell apoptosis by means of diphtheria toxin is not sufficient to induce aberrations in gastric epithelial homeostasis30 and that additional mechanisms are likely to be involved in the pathophysiology of these events. It is conceivable that in addition to the parietal cells, BMP-activated signals could target other cell lineages. It is clear that additional studies are necessary to define more precisely the role of BMP signaling in the regulation of parietal cell biology.
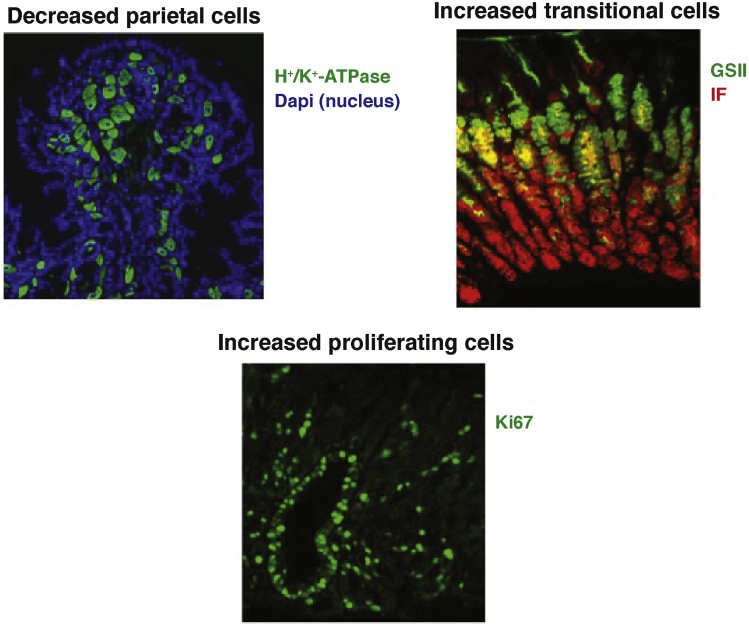
We and others have demonstrated in a series of in vivo investigations that inhibition of BMP signaling in the gastric mucosa causes profound aberrations in the normal mechanisms that control the proliferation, maturation, and differentiation of several lineages of gastric epithelial cells, underscoring the importance of BMP signaling in the regulation of gastric epithelial homeostasis.31, 32 In particular, we showed that transgenic expression in the mouse stomach of the BMP inhibitor noggin leads to decreased parietal cell number, increased epithelial cell proliferation, and to expansion of cells that express markers of both mucus neck and zymogenic cell differentiation31 (Figure 2).
Figure 2.
Cellular changes in noggin transgenic (TG) mice. Inhibition of BMP signaling in gastric mucosa achieved by transgenic expression of the BMP inhibitor noggin leads to decreased parietal cell number, increased epithelial cell proliferation, and to expansion of cells that express markers of both mucus neck and zymogenic cell differentiation.
(Reprinted with permission from Shinohara M, et al. Gastroenterology 2010;139:2050–2060.)
Another significant phenotypic feature of the noggin transgenic mice is the development of spasmolytic polypeptide expressing metaplasia (SPEM), a type of metaplasia that is characterized by the aberrant expression of trefoil factor family 2 (TFF2) and of mucins that bind the lectin GSII at the base of fundic glands. One interesting theory is that SPEM could evolve from transdifferentiation of chief cells.33, 34, 35, 36, 37 Accordingly, it is possible that inhibition of BMP signaling might lead to profound changes in the normal mechanisms that regulate zymogenic cell differentiation, leading to the aberrant expression of TFF2 at the base of the fundic glands.
Analysis of the noggin transgenic mice also led to the observation that inhibition of BMP signaling in the gastric mucosa causes induction of ERK activation, hypergastrinemia, and stimulation of epidermal growth factor receptor (EGFR) ligands gene expression, events that are likely to contribute to the hyperproliferative state of the stomach of these mice.31 These observations are in agreement with previous reports conducted both in vivo and in vitro that have indicated that BMP-4 inhibits EGF-stimulated ERK activation in isolated parietal cells22 and that prolonged overexpression of growth factors in the stomach of mice alters the normal architecture of the gastric mucosa, leading to increased ERK activation, loss of parietal cells, and foveolar hyperplasia.38, 39, 40 The crucial role of gastrin in the expression of the proliferative changes of the noggin transgenic mice was further supported by the observation that crossing of noggin transgenic mice to gastrin knockout mice leads to inhibition of cell proliferation.41
Bone Morphogenetic Protein Signaling Regulates Gastric Inflammation
Several reports have suggested that the BMPs might be involved in the regulation of the inflammatory response. In support of this hypothesis, the gastric mucosa of patients infected with H pylori exhibits increased expression of both BMP-2 and BMP-4,42 indicating that these peptides might play important regulatory actions during the process of Helicobacter-induced gastric inflammation. Moreover, BMP-7 appears to decrease the degree of inflammation seen during ischemic acute renal failure in rats.43 Similarly, BMP-7 ameliorates the severity of colonic inflammation, and it accelerates the healing of colitis in rats exposed to trinitrobenzenesulfonic acid, a well-established inducer of experimental colitis in rodents.44, 45 The anti-inflammatory effect of BMP signaling was further substantiated by the observation that deletion of Bmpr1a in the colonic mucosa leads to enhancement of Dextran Sulfate Sodium-induced colonic injury and inflammation.45 Finally, transgenic expression of BMP-4 in the skin of mice treated with both the carcinogen N-methyl-N′-nitrosoguanidine and the tumor promoter 12-O-tetradecanoylphorbol-13-acetate leads to a marked decrease in the degree of cellular hyperproliferation and inflammation induced by these agents.46 The significance of these findings has been further confirmed by the observation that patients with inflammatory bowel disease can exhibit alterations in the TGF-β signaling cascade and that these abnormalities can render patients unable to mount an effective anti-inflammatory response in the gastrointestinal tract.47 Indeed, recent studies have shown that administration of mongersen, an oral antisense oligonucleotide that targets the TGF-β signaling inhibitor Smad 7, to the ileal and colonic mucosa of patients with Crohn’s disease leads to restoration of the anti-inflammatory effects of TGF-β and to improvement of disease activity.48 Taken together, these observations suggest that members of the TGF-β/BMP family of regulatory peptides might represent novel and important regulators of gastrointestinal inflammation.
Chronic inflammation of the gastric mucosa has been recognized as an important causative factor for the development of dysplasia and neoplasia.49 The mechanisms involved in the pathogenesis of gastric neoplasms in the context of chronic inflammation have been only partially characterized. One current hypothesis is that chronic inflammatory stimuli such as infection with Helicobacter organisms can cause aberrations in the normal biological functions of gastric stem/progenitor cells, leading to the development of metaplastic and dysplastic changes of the gastric mucosa and ultimately to neoplasias.50 Indeed, both intestinal metaplasia and SPEM have been associated with the development of inflammation-induced gastric neoplasms.51, 52 Studies have also indicated that the development of gastric inflammation is mediated by the release of a broad array of cytokines and chemokines such as interleukin (IL) 6, IL1β, TNF-α, interferon-γ, and IL8.53, 54, 55, 56, 57, 58, 59, 60, 61 Several complex signal transduction pathways regulate the expression and the biological actions of cytokines and chemokines. Activation of the IκB kinases, the mitogen-activated protein kinases, and of the STAT proteins STAT1 and STAT3 in particular has been linked to the induction of gastric inflammation and to the development, in some instances, of dysregulated gastric mucosal cell growth and transformation.62, 63, 64, 65, 66
The role of BMP signaling in gastric carcinogenesis has been substantiated by the observations that BMP-2 inhibits the growth of gastric cancer cells and that epigenetic silencing of the BMP-2 gene through methylation can be detected in gastric cancers.11, 67 Moreover, conditional inactivation of Bmpr1a leads to increased cellular proliferation and to the development of tumors in mice at the level of gastric epithelial transition zones68 and in the antrum.69 In support of these observations, recent reports have demonstrated that some sets of human gastric neoplasms exhibit decreased expression of component of the BMP signal transduction pathway,70 confirming the notion that the BMPs can exert significant inhibitory effects on the growth of gastric tumors.
A series of recently published studies from our laboratory in noggin transgenic mice tested the hypothesis that the BMPs inhibit gastric inflammation and that loss of this signaling mechanism leads to metaplastic and dysplastic changes of the gastric mucosa.20
Indeed, microscopic analysis of hematoxylin-eosin–stained sections of the fundic mucosa of the noggin transgenic mice but not of wild-type control mice revealed the presence of foci of mild to moderate inflammatory infiltrates.20 Moreover, measurement by quantitative reverse transcriptase polymerase chain reaction of TNF-α, interferon-γ, MIP-2, and IL1β mRNAs demonstrated that inhibition of BMP signaling causes a significant increase in the expression of these inflammatory molecules.20
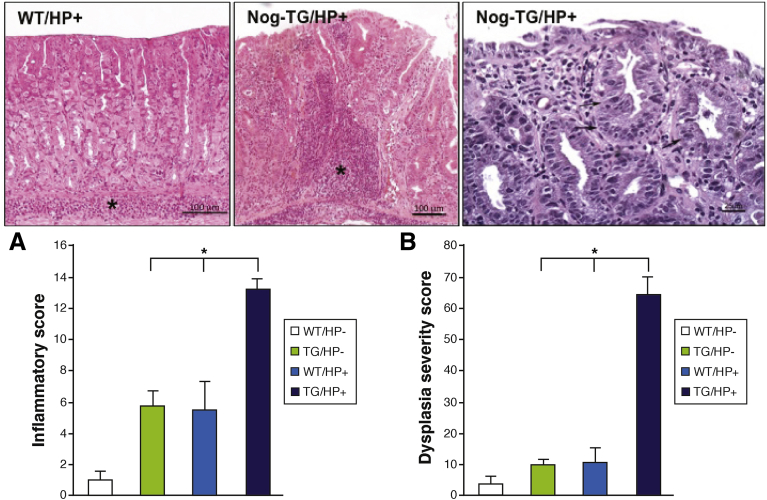
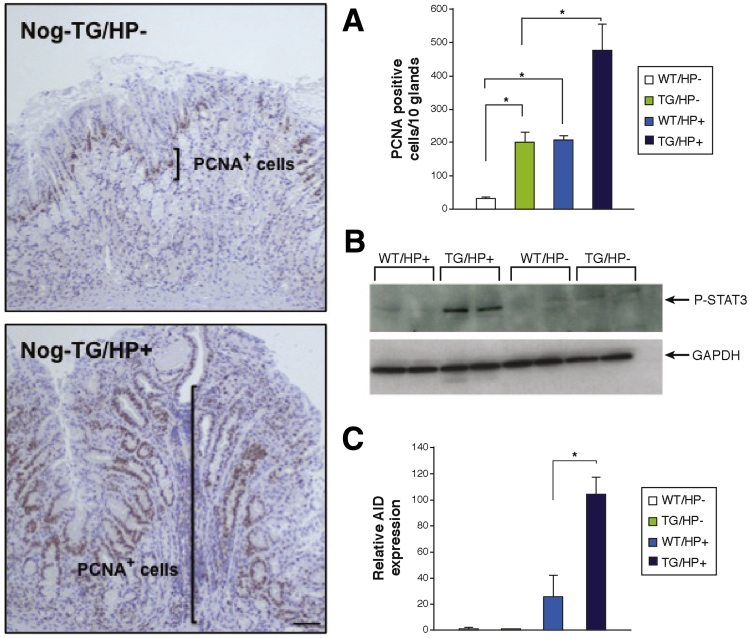
Challenge of 3-month-old noggin transgenic mice with the SS1 strain of H pylori, which is known to induce an inflammatory response in the gastric mucosa of mice,71, 72, 73, 74, 75 led to a significant increase in the severity of the inflammatory infiltrates and to the development of areas of dysplastic mucosa when compared with non-transgenic/noninfected, non-transgenic/H pylori–infected, and transgenic/noninfected, age-matched littermates20 (Figure 3). In agreement with these observations, the gastric mucosa of H pylori–infected noggin transgenic mice exhibited enhanced production of cytokines, induction of cell proliferation and of STAT3 phosphorylation, and increased expression of activation-induced cytidine deaminase,20 a molecule that has been shown to mediate some of the pro-oncogenic actions of H pylori in the stomach76 (Figure 4). Thus, inhibition of BMP signaling in the gastric epithelium leads to the development of a pro-oncogenic environment characterized by increased cell proliferation, dysplasia, and by enhanced expression of STAT3 and activation-induced cytidine deaminase. The observation that these changes were associated with enhanced expression of TFF220 confirms the notion that inhibition of BMP signaling and H pylori–induced inflammation can contribute to the emergence of SPEM, and that this type of metaplasia is associated with the development of dysplasia.
Figure 3.
Enhanced inflammation and accelerated dysplasia in gastric epithelium of Helicobacter-infected noggin transgenic (TG) mice. Representative hematoxylin-eosin–stained gastric paraffin sections of the corpus of H pylori (HP)-infected wild-type (WT) and Nog-TG mice. Asterisks mark inflammatory infiltrates. The magnified image shows dysplastic changes in Helicobacter-infected Nog-TG mice. Arrows point to areas of dysplastic epithelium. Graph bars represent inflammatory (A) and dysplasia severity (B) scores calculated in both WT and TG mice in the presence and absence of H pylori (HP). Values are shown as means ± standard error of the mean, n = 4. *P < .05. For histologic scoring each field was scored separately for the presence or absence of neutrophilic and mononuclear cell infiltration, gastritis, and epithelial metaplasia/dysplasia. The score was expressed as a percent affected fields.20 Severity of the changes was graded by using a scale from 1 (mild) to 3 (severe).20
Figure 4.
Increased cell proliferation and expression of pro-oncogenic molecules in Helicobacter-infected noggin transgenic (TG) mice. Gastric paraffin sections from noninfected and H pylori (HP)-infected Nog-TG mice were stained with anti-PCNA antibodies. Size bar, 50 μm. Graph bars represent number of PCNA-positive nuclei detected in WT and TG mice in the presence and absence of H pylori (HP). Values are shown as means ± standard error of the mean, n = 4. *P < .05 (A). Phosphorylation and activation of STAT3 in wild-type (WT) and TG mice in the presence and absence of H pylori were studied by Western blots using an anti-phospho-STAT3 antibody (B). Activation-induced cytidine deaminase (AID) mRNA signals in WT mice were compared with those detected in TG mice in the presence and absence of HP by using quantitative reverse transcription polymerase chain reaction and displayed as fold-increase over the WT negative controls. Values are shown as means ± standard error of the mean, n = 4. *P < .05 (C).
In vitro studies conducted in primary cultures of canine parietal and mucus cells also demonstrated that BMP-4, BMP-2, and BMP-7 can significantly inhibit both basal and TNF-α–stimulated IL8 gene expression in these cells, supporting the notion that BMPs exert direct inhibitory effects on the activation of inflammatory mechanisms in gastric epithelial cells.20 The observation that BMP-4 also attenuates TNF-α–stimulated IL8 gene expression and release in AGS human gastric epithelial cells underscored the relevance of this signaling mechanism in human pathophysiology.20
Conclusions
BMP signaling appears to target the gastric epithelium where it exerts inhibitory effects on the expression of proinflammatory mediators. Loss of this signaling mechanism appears to cause the development of metaplastic and dysplastic changes of the gastric mucosa (Table 1). These findings underscore the importance of BMP signaling in the regulation of gastric inflammation and epithelial homeostasis. Future investigations focused on the elucidation of the specific targets of this signaling mechanism and the role of BMPs in the regulation of gastric stem/progenitor cells in the stomach will provide new clues for a better understanding of the pathophysiological mechanisms that lead to the development of both dysplastic and neoplastic lesions in the stomach. Therefore, possible manipulations of the BMP signal transduction pathway might offer future, novel opportunities for the treatment of gastric inflammation and carcinogenesis.
Table 1.
Mouse Models of Inhibition of BMP Signaling in the Stomach
| Mouse model | Phenotype |
|---|---|
|
H/K-noggin mouse31 Expression of noggin in the corpus by means of the H+/K+-ATPase α-subunit gene promoter |
SPEM Inflammation (+) Decreased parietal cells Increased number of transitional cells Hypergastrinemia Increased cell proliferation Increased growth factor expression Induction of ERK activation |
| H felis/H pylori–infected H/K-noggin mouse20 | SPEM Inflammation (+++) Dysplasia STAT3 activation Induction of activation-induced cytidine deaminase expression |
| H/K-noggin mouse crossed to gastrin/KO mouse41 | SPEM Altered parietal and zymogenic cell differentiation Diminished cell proliferation in the absence of gastrin |
|
Foxa3-Cre;bmpr1aflox/flox mouse32 Cre-mediated deletion of bmpr1a in the foregut endoderm by using the Foxa3 promoter |
SPEM Decreased parietal cells Increased number of endocrine cells |
|
Mx1-Cre; bmpr1aflox/flox mouse68 Cre-mediated deletion of bmpr1a in the gastric epithelium by means of a type I interferon-inducible promoter |
Epithelial hyperplasia in proximal fundic region Development of tumors in squamocolumnar and gastrointestinal transition zones |
|
CAGG-Cre;bmpr1aflox/flox mouse69 Cre-mediated global deletion of bmpr1a by using a modified chicken β-actin promoter |
Formation of antral polyps |
Footnotes
Conflicts of interest The author discloses no conflicts.
Funding Supported by NIDDK grants RO1DK083373, by the University of Michigan Gastrointestinal Peptide Research Center (grant P30-DK-34933), and by the Funderburg Award in Gastric Biology Related to Cancer.
References
- 1.Kawabata M., Imamura T., Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 2.Hardwick J.C., Van Den Brink G.R., Bleuming S.A. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111–121. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 3.Madison B.B., Braunstein K., Kuizon E. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 4.van den Brink G.R., Hardwick J.C., Tytgat G.N. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;21:317–328. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X.P., Woodford-Richens K., Lehtonen R. Germline mutations in BMPR1A/ALK3 cause a subset of cases of juvenile polyposis syndrome and of Cowden and Bannayan-Riley-Ruvalcaba syndromes. Am J Hum Genet. 2001;69:704–711. doi: 10.1086/323703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haramis A.P., Begthel H., van den Born M. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 7.He X.C., Zhang J., Tong W.G. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 8.Howe J.R., Sayed M.G., Ahmed A.F. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet. 2004;41:484–491. doi: 10.1136/jmg.2004.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaestner K.H., Silberg D.G., Traber P.G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11:1583–1595. doi: 10.1101/gad.11.12.1583. [DOI] [PubMed] [Google Scholar]
- 10.Beck S.E., Jung B.H., Fiorino A. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol. 2006;291:G135–G145. doi: 10.1152/ajpgi.00482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen X.Z., Miyake S., Akiyama Y. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004;316:100–106. doi: 10.1016/j.bbrc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Wrana J.L. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 13.Massagué J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 14.Bragdon B., Moseychuk O., Saldanha S. Bone morphogenetic proteins: a critical review. Cell Signaling. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Brazil D.P., Church R.H., Surae S. BMP signaling: agony and antagony in the family. Trends Cell Biol. 2015;25:249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Goto K., Kamiya Y., Imamura T. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J Biol Chem. 2007;282:20603–20611. doi: 10.1074/jbc.M702100200. [DOI] [PubMed] [Google Scholar]
- 17.Wu X.B., Li Y., Schneider A. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest. 2003;112:924–934. doi: 10.1172/JCI15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunet L.J., McMahon J.A., McMahon A.P. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1350. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman L.B., De Jesús-Escobar J.M., Harland R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 20.Takabayashi H., Shinohara M., Mao M. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology. 2014;147:396–406. doi: 10.1053/j.gastro.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willet S.G., Mills J.C. Stomach organ and cell lineage differentiation: from embryogenesis to adult homeostasis. Cell Mol Gastroenterol Hepatol. 2016;2:546–559. doi: 10.1016/j.jcmgh.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitsche H., Ramamoorthy S., Sareban M. Functional role of bone morphogenetic protein-4 in isolated canine parietal cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G607–G614. doi: 10.1152/ajpgi.00194.2006. [DOI] [PubMed] [Google Scholar]
- 23.Loopez-Diaz L., Hinkle K.L., Jain R.N. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol. 2006;290:G970–G979. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- 24.Li Q., Karam S.M., Gordon J.I. Simian virus 40 T antigen-induced amplification of pre-parietal cells in transgenic mice: effects on other gastric epithelial cell lineages and evidence for a p53-independent apoptotic mechanism that operates in a committed progenitor. J Biol Chem. 1995;270:15777–15788. doi: 10.1074/jbc.270.26.15777. [DOI] [PubMed] [Google Scholar]
- 25.Li Q., Karam S.M., Gordon J.I. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–3676. [PubMed] [Google Scholar]
- 26.Canfield V., West A.B., Goldenring J.R. Genetic ablation of parietal cells in transgenic mice: a new model for analyzing cell lineage relationships in the gastric mucosa. Proc Natl Acad Sci USA. 1996;93:2431–2435. doi: 10.1073/pnas.93.6.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenring J.R., Ray G.S., Coffey R.J. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 28.Beauchamp R.D., Barnard J.A., McCutchen C.M. Localization of transforming growth factor alpha and its receptor in gastric mucosal cells: implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989;84:1017–1023. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain R.N., Samuelson L.C. Differentiation of the gastric mucosa: II—role of gastrin in gastric epithelial cell proliferation and maturation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G762–G765. doi: 10.1152/ajpgi.00172.2006. [DOI] [PubMed] [Google Scholar]
- 30.Burclaff J., Osaki L.H., Liu D. Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology. 2017;152:762–766. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinohara M., Mao M., Keeley T.M. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloum F., Allaire J.M., Gagné-Sansfaçon J. Epithelial BMP signaling is required for proper specification of epithelial cell lineages and gastric endocrine cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1065–G1079. doi: 10.1152/ajpgi.00176.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura S., Baxter T., Yamaguchi H. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Goldenring J.R., Nomura S. Differentiation of the gastric mucosa III: animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–G1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 35.Petersen C.P., Mills J.C., Goldenring J.R. Murine models of gastric corpus preneoplasia. Cell Mol Gastroenterol Hepatol. 2017;3:11–26. doi: 10.1016/j.jcmgh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam K.T., Lee H.-J., Sousa J.F. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quante M., Marrache F., Goldenring J.R. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dempsey P.J., Goldenring J.R., Soroka C.J. Possible role of transforming growth factor alpha in the pathogenesis of Menetrier's disease: supportive evidence form humans and transgenic mice. Gastroenterology. 1992;103:1950–1963. doi: 10.1016/0016-5085(92)91455-d. [DOI] [PubMed] [Google Scholar]
- 39.Burdick J.S., Chung E., Tanner G. Treatment of Menetrier's disease with a monoclonal antibody against the epidermal growth factor receptor. N Engl J Med. 2000;343:1697–1701. doi: 10.1056/NEJM200012073432305. [DOI] [PubMed] [Google Scholar]
- 40.Khurana S.S., Riehl T.E., Moore B.D. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todisco A., Mao M., Keeley T.M. Regulation of gastric epithelial cell homeostasis by gastrin and bone morphogenetic protein signaling. Physiol Rep. 2015;3 doi: 10.14814/phy2.12501. pii:e12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleuming S.A., Kodach L.L., Garcia Leon M.J. Altered bone morphogenetic protein signaling in the Helicobacter pylori-infected stomach. J Pathol. 2006;209:190–197. doi: 10.1002/path.1976. [DOI] [PubMed] [Google Scholar]
- 43.Vukicevic S., Basic V., Rogic D. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest. 1998;102:202–214. doi: 10.1172/JCI2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maric I., Kucic N., Turk Wensveen T. BMP signaling in the rats with TNBS induced colitis following BMP7 therapy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1151–G1162. doi: 10.1152/ajpgi.00244.2011. [DOI] [PubMed] [Google Scholar]
- 45.Ji T., Takabayashi H., Mao M. Regulation and function of bone morphogenetic protein signaling in colonic injury and inflammation. Am J Physiol Gastrointest Liver Physiol. 2017;312:G24–G33. doi: 10.1152/ajpgi.00169.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blessing M., Nanney L.B., King L.E. Chemical skin carcinogenesis is prevented in mice by the induced expression of a TGF-beta related transgene. Teratog Carcinog Mutagen. 1995;15:11–21. doi: 10.1002/tcm.1770150103. [DOI] [PubMed] [Google Scholar]
- 47.Monteleone G., Kumberova A., Croft N.M. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:523–526. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monteleone G., Neurath M.F., Ardizzone S. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015;372:1104–1113. doi: 10.1056/NEJMoa1407250. [DOI] [PubMed] [Google Scholar]
- 49.Fox J.G., Wang T.C. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levi E., Sochacki P., Khoury N. Cancer stem cells in Helicobacter pylori infection and aging: implications for gastric carcinogenesis. World J Gastrointest Pathophysiol. 2014;5:366–372. doi: 10.4291/wjgp.v5.i3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halldorsdottir A.M., Sigurdardottir M., Jonasson J.G. Spasmolytic polypeptide expressing metaplasia (SPEM) associated with gastric cancer in island. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 52.Goldenring J.R., Nam K.T., Wang T.C. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crabtree J.E. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig Dis Sci. 1998;43:46S–55S. [PubMed] [Google Scholar]
- 54.Mukaida N., Harada A., Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 55.Peek R.M., Jr. IV Helicobacter pylori strain-specific activation of signal transduction cascades related to gastric inflammation. Am J Physiol Gastrointest Liver Physiol. 2001;280:G525–G530. doi: 10.1152/ajpgi.2001.280.4.G525. [DOI] [PubMed] [Google Scholar]
- 56.Obonyo M., Guiney D.G., Harwood J. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang W., Rathinavelu S., Samuelson L.C. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest. 2005;85:702–715. doi: 10.1038/labinvest.3700260. [DOI] [PubMed] [Google Scholar]
- 58.Smythies L.E., Waites K.B., Lindsey J.R. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 59.Amieva R.M., El-Omar E.M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Hasegawa S., Nishikawa S., Miura T. Tumor necrosis factor-alpha is required for gastritis induced by Helicobacter felis infection in mice. Microb Pathog. 2004;37:119–124. doi: 10.1016/j.micpath.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Yasumoto K., Okamoto S., Mukaida N. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 62.Karin M., Greten F.R. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 63.Cobb M.H., Goldsmith E.J. How MAP kinases are regulated. J Biol Chem. 2005;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 64.Widmann C., Gibson S., Jarpe M.B. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 65.Giraud A.S., Jackson C., Menheniott T.R. Differentiation of the gastric mucosa IV: role of trefoil peptides and IL-6 cytokine family signaling in gastric homeostasis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1–G5. doi: 10.1152/ajpgi.00382.2006. [DOI] [PubMed] [Google Scholar]
- 66.Ernst M., Najdovska M., Grail D. STAT3 and STAT1 mediate IL-11 dependent and inflammation-associated gastric tumourogenesis in gp130receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen X.Z., Akiyama Y., Baylin S.B. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene. 2006;25:2666–2673. doi: 10.1038/sj.onc.1209297. [DOI] [PubMed] [Google Scholar]
- 68.Bleuming S.A., He X.C., Kodach L.L. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–8155. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 69.Huh W.J., Mysorekar I.U., Mills J.C. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am J Physiol Gastrointest Liver Physiol. 2010;299:G368–G380. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kao J.Y., Rathinavelu S., Eaton K.A. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am J Physiol Gastrointest Liver Physiol. 2006;291:G73–G81. doi: 10.1152/ajpgi.00139.2005. [DOI] [PubMed] [Google Scholar]
- 72.Eaton K.A., Benson L.H., Haeger J. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect Immun. 2006;74:4673–4684. doi: 10.1128/IAI.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kao J.Y., Zhang M., Miller M.J. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eaton K.A., Ringler S.R., Danon S.J. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eaton K.A., Danon S.J., Krakowka S. A reproducible scoring system for quantification of histologic lesions of inflammatory disease in mouse gastric epithelium. Comp Med. 2007;57:57–65. [PubMed] [Google Scholar]
- 76.Matsumoto Y., Marusawa H., Kinoshita K. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]