Abstract
The cellular origin of digestive cancers has been a long-standing question in the cancer field. Mouse models have identified long-lived stem cells in most organ systems, including the luminal gastrointestinal tract, and numerous studies have pointed to tissue resident stem cells as the main cellular origin of cancer. During gastric carcinogenesis, chronic inflammation induces genetic and epigenetic alterations in long-lived stem cells, along with expansion of stem cell niches, eventually leading to invasive cancer. The gastric corpus and antrum have distinct stem cells and stem cell niches, suggesting differential regulation of cancer initiation at the 2 sites. In this short review, we discuss recent experimental models and human studies, which provide important insights into the pathogenesis of gastric cancer.
Keywords: Gastric Cancer, Stem Cell, Stem Cell Niche, Lgr5, Mist1, CCK2R
Abbreviations used in this paper: CCK2R, cholecystokinin receptor 2; IM, intestinal metaplasia; SPEM, spasmolytic polypeptide-expressing metaplasia
Summary.
In this perspective, we briefly summarize recent advances in our understanding of gastric stem cells and their link to gastric cancer. Although the discussion is focused largely on mouse experimental models and the gastric mucosa, we correlate experimental observations with human studies as well as findings from other cancer fields, most of which have pointed to stem cells as the origin of cancer. We also comment on the role of alterations in the stem cell niche as fundamental to the development of gastric cancer. Although other gastric cell types may possess some degree of cellular plasticity and could contribute to cancer development, accumulating evidence indicates that stem cells are the major cellular origin of most cancers, including gastric cancer.
Considerable data exist regarding gastric preneoplasia and early histopathologic changes that can progress to gastric cancer. Gastric cancer was linked strongly to chronic inflammation of the stomach, later recognized as being caused by Helicobacter pylori infection. However, gastric cancer takes many years to develop. Pathologic studies by Correa1 have shown that intestinal-type gastric cancer develops through a series of histopathologic stages that include chronic gastritis, atrophic gastritis, intestinal metaplasia (IM), dysplasia, and cancer. However, although there has been intense interest in the past in altered cellular differentiation or metaplasia, it is highly likely that gastric cancer, similar to other cancers, arises from aberrant stem cells through a process of field cancerization, the clonal expansion from multipotent stem cells that possess gene mutations,2, 3, 4, 5, 6, 7, 8 and thus a deep understanding of gastric carcinogenesis will require detailed elucidation of the role of gastric stem cells.
In organs such as the luminal gastrointestinal tract, which turn over rapidly and continuously, multipotent stem cells reside at the top of the self-renewal hierarchy and govern organ homeostasis.9, 10, 11, 12 Gastrointestinal stem cells give rise to committed progenitor cells of the epithelial lineages, including proliferative transit-amplifying cells, which then give rise to fully differentiated epithelial lineages that are mostly nonproliferative. Because cancers arise only after the acquisition of multiple mutagenic events, long-lived cells would be the only cells capable of serving as reservoirs of mutated stem cells.4, 13, 14 Stem cells appear to be the ideal cellular targets for the accumulation of genetic alterations, given their fundamental properties of longevity and self-renewal.2, 15 Mutations have been shown to be accumulated in stem cells,16 whereas mutations that occur in differentiated, postmitotic epithelial cells would not be propagated to progeny, and in general differentiated cells would not survive for the many decades required to achieve the mutational threshold for malignant transformation.14 In addition, studies in mice have suggested that mutations that occur in gastrointestinal stem cells more often lead to cancer,10, 14, 17, 18, 19 although cancer can arise when mutations are targeted to some long-lived differentiated cells, such as tuft cells.20 Indeed, although there has been some debate as to whether the primary determinant of cancer risk at a particular organ site is the actual number of stem cells vs other extrinsic factors/environmental risk, there has been little debate regarding the stem cell origins of cancer.21, 22 Short-lived progenitors are able to interconvert into stem-like cells after tissue damage,10, 23, 24 but it remains unclear whether such interconverted cells can develop into cancer. In organs such as pancreas and liver that do not frequently divide in the normal state, cancers may arise from more differentiated acinar and hepatocyte compartments, but recent evidence has suggested the existence within these compartments of specific facultative reserve stem-like cells, which preferentially may contribute to regeneration and give rise to cancer.25, 26, 27, 28, 29, 30 In any case, although it may be possible for non–stem cells to contribute to gastric regeneration and cancer, chronic inflammation associated with H pylori infection typically induces a regenerative and reparative response orchestrated by tissue resident stem cells,31 which are activated and expand as part of an injury response, predisposing to the acquisition of genetic and epigenetic alterations (Figure 1).
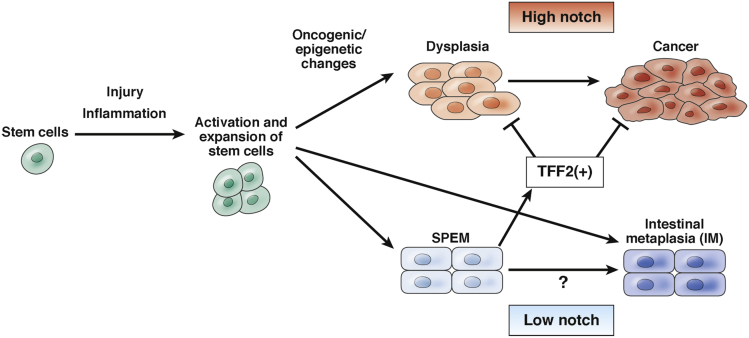
Figure 1.
Model of stem cell–derived gastric carcinogenesis. During gastric carcinogenesis, long-lived stem cells and their niche are activated and expanded in response to tissue injury and inflammation. Activated stem cells give rise to metaplasia and dysplasia after accumulation of genetic and epigenetic changes. During Barrett’s esophagus development, dysplasia can progress to cancer with high Notch expression, while metaplasia appears to be postmitotic and a distinct lineage with low Notch expression.46 Given that Barrett’s esophagus may originate from gastric cardia glands, this may be the case in gastric metaplasia/dysplasia development. Indeed, notch signaling has been shown to increase proliferation and decrease differentiation, and thus clearly regulate mucous cell phenotypes in the stomach.99, 100, 101, 102 Aberrant notch activation leads to hyperplasia and dysplasia both in the corpus and antrum.68, 101, 102 SPEM cells express TFF2, which inhibits cancer progression.54, 55
Nevertheless, exactly how cancer is initiated remains unclear. Traditionally, cancer initiation was thought to commence after mutation in an oncogene or tumor-suppressor gene, such as APC in the colon, and this may be true in a subset of colon cancers.32 However, in gastric cancer, The Cancer Genome Atlas (TCGA) and other studies have shown 4 distinct molecular subtypes, but have not defined a clear dominant mutational pathway.33, 34 The different molecular subtypes could in theory represent different cellular origins, as has been postulated for colorectal cancer and other cancers,35, 36 but this notion has not yet been supported experimentally. TP53 is the most mutated gene found in approximately half of all gastric cancer patients, and a TP53 mutation sometimes is observed even in early lesions.33, 34, 37, 38 However, the role of TP53 mutations in cancer initiation has not been fully elucidated. Furthermore, more than 10% of gastric cancer cases have very few gene mutations, suggesting that genetic events may not be required for cancer initiation and progression.33, 34, 37 It is possible that in many tumors, epigenetic changes induced by chronic Helicobacter infection39, 40 or other factors41 may play more important roles in triggering cancer initiation. There is strong evidence from analysis of human resection specimens that these mutations are established within stem cells in the gastric glands, and that clonal alterations can spread by means of gland fission.5, 6, 7, 8
Mutations present in gastric dysplasia or cancer also can be found in adjacent IM, suggesting a shared clonal origin.5, 6, 7, 8 Fifty years ago, researchers assumed that gastric dysplasia and cancer arose directly from IM cells. However, it now has become evident that IM is not highly proliferative and likely represents terminally differentiated, postmitotic cells42 (Figure 1). Indeed, in Barrett’s esophagus, which likely originates from migrated gastric cardia glands,38, 43, 44, 45, 46 goblet cell differentiation is associated with reduced Notch signaling and reduced proliferation,46, 47 and clinically a high goblet cell count is associated with a reduced risk of cancer.48, 49 Greater focus has been given recently to another form of metaplasia called spasmolytic polypeptide-expressing metaplasia (SPEM), which appears more proliferative and shows a stronger association with gastric cancer.50, 51, 52 SPEM usually is located closer to the stem cell zone, likely precedes IM, and probably represents a precursor of IM.42 However, evidence again suggests that SPEM does not give rise directly to dysplasia and cancer,42, 53 but rather represents a fairly stable differentiated lesion that may in fact inhibit tumor progression54, 55 (Figure 1). The cardinal marker for SPEM, the peptide Trefoil factor 2 (TFF2), is anti-inflammatory and tumor suppressive, and knockout of the Tff2 gene leads to more rapid gastric cancer progression.54, 55 At a population level, SPEM and IM almost certainly are risk factors for gastric cancer,38, 50, 52 given their association with H pylori infection and chronic atrophic gastritis, but this is not sufficient to assume that they are direct cellular precursors of cancer.38 Indeed, the presence of SPEM and IM likely are risk factors for cancer because they reflect early changes to the pool of undifferentiated gastric stem cells that are the origins of cancer. Nevertheless, in our opinion, metaplasia is a distinct lineage from cancer, and greater attention should be given to the undifferentiated stem cells that are the direct source of cancer (Figure 1).
Gastric antral stem cells were the first epithelial stem cells characterized in the stomach. Earlier studies have suggested that stem cells reside at the isthmus in the antral glands, a region above the base where the glands narrow, with clear evidence of bidirectional migration from the stem cell zone.56 Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) is a reported antral stem cell marker, and similar to the findings in the intestine, Lgr5 was expressed at the base of the glands.12 Although the location of Lgr5+ antral cells was not entirely consistent with the location of stem cells from earlier studies, Lgr5+ cells did overlap somewhat with the antral proliferative zone in the lower isthmus, and lineage-tracing studies with Lgr5-CreERT mice consistently showed tracing events of the antral glands. Recently, more rapidly cycling, long-lived stem cells were identified that express the gastrin receptor cholecystokinin receptor 2 (CCK2R).57 These Lgr5-negative CCK2R+ antral stem cells are highly proliferative, divide on average once a day, and often are present as single cells near the +4 position (Figure 2). Several other stem cell markers such as Sox2 or eR1 have been reported,58, 59 but the possible overlap between these markers needs to be elucidated further.
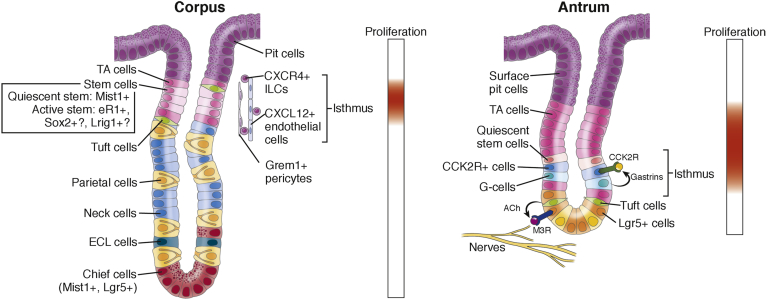
Figure 2.
Gastric corpus and antrum stem cells and their niche. Stem cells are thought to reside within proliferative isthmus (upper part of the corpus glands and lower part of the antral glands). In the corpus glands, the isthmus is located between abundant transit-amplifying (TA) cells. There is a slow-cycling stem cell that expresses Mist1 and a rapidly cycling stem cell that may express eR1, Sox2, or Lrig1, and they can differentiate into various cell types such as pit, parietal, neck, enterochromaffin-like (ECL), and tuft cells. C-X-C motif chemokine ligand 12 (CXCL12)+ endothelial cells, C-X-C motif chemokine receptor type 4 (CXCR4)+ innate lymphoid cells (ILCs), and Grem1+ pericytes appear to compose the corpus stem cell niche. Chief cells at the base express Mist1 and Lgr5, but they normally are postmitotic and do not divide. In the antrum, several rapidly cycling stem cells are reported just below the major TA cell zone, including Lgr5+ cells at the base and CCK2R+ cells at +4. Lgr5+ cells can be activated by acetylcholine (ACh)-producing nerves and tuft cells through muscarinic acetylcholine receptor subtype 3 (M3R). Gastrins secreted from antral G cells can regulate CCK2R+ stem cell function in a paracrine manner. Slow-cycling stem cells in the antrum have not been identified yet.
In animal models, both Lgr5+ stem cells and CCK2R+ stem cells are able to interconvert with each other, and can give rise to cancer.12, 60, 61 Importantly, the expression of CCK2R in antral +4 stem cells potentially explains the gastrin-mediated effects on cancer progression in mouse models.62 In mice, increased levels of amidated gastrin promote proximal corpus carcinogenesis but inhibit antral cancer development, whereas knockout of gastrin promotes antral cancer development.41, 63, 64, 65 In the antrum, amidated gastrin suppresses CCK2R+ stem cell activity57; on the other hand, CCK2R-expressing stem cells in the cardia and progenitors in the corpus are activated by amidated gastrin45 (Figure 2). Therefore, CCK2R modulates proximal and distal gastric stem cells in distinct ways, and thus cancer risk.62 In recent decades, there has been a dramatic epidemiologic shift in the location of gastric cancer, with a decrease in antral cancer and an increase in proximal corpus/cardia cancer.38 These trends in theory could be related in part to increased levels of amidated gastrins, which might be facilitated by the frequent use of acid-suppressing drugs.
In contrast to the antral glands, the corpus glands are functionally and morphologically distinct, with very different stem cell populations (Figure 2). Earlier studies that included electron microscopy suggested the presence of undifferentiated, granule-free stem cells within the corpus isthmus with bidirectional migration of progenitor and daughter cells.66 Lrig1 and Sox2 may be expressed in a subset of corpus stem or progenitor cells,59, 67 but to date these markers have not been linked directly to the granule-free stem cell. We recently found that Mist1, a marker of gastric chief cells, is expressed significantly within the corpus granule-free stem cell population.68 Although Mist1 protein expression is difficult to confirm in these isthmus stem cells, endogenous Mist1 messenger RNA readily is detectable. Mist1+ isthmus cells are single, relatively slow-cycling stem cells, dividing on average every 5 days, and can serve as a cellular origin of gastric corpus cancer. Given that Mist1 is expressed abundantly in 9–10 mature chief cells per gland, but present in only 1–2 Mist1+ isthmus stem cells per gland, Mist1 is clearly not a specific stem cell marker. However, most stem cell markers are not specific for stem cells and label a heterogeneous cell population, and classically purification and characterization of stem cell populations have been achieved by flow cytometry using 4–5 markers, rather than relying on a single marker.
Importantly, lineage tracing in Mist1-CreERT mice clearly indicates that glands are traced from rare Mist1+ isthmus stem cells, not basal Mist1+ chief cells. This can be shown by ablating chief cells using Lgr5-DTR-GFP mice, which express diphtheria toxin receptor (DTR) and green fluorescent protein (GFP) in Lgr5+ cells,69 and by ablating isthmus stem cells using treatment with 5-fluorouracil.70 Lineage tracing in Mist1-CreERT mice is not reduced by chief cell ablation, but is inhibited by isthmus cell ablation.68 It is perhaps not widely appreciated that although Lgr5-CreERT recombines in only a small subset of chief cells, Lgr5 (and GFP) are expressed broadly at the base of oxyntic glands as shown in Lgr5-DTR-GFP mice,70 (Figure 2). Therefore, ablation of Lgr5+ chief cells eliminated Cre-mediated recombination of chief cells by Mist1-CreERT, but did not reduce oxyntic gland tracing.
Recent studies have suggested a tissue stem cell model that comprises both rapidly cycling (active) and relatively slow-cycling (quiescent) stem cells, which can interconvert.71 Although Lgr5+ stem cells are reported to divide symmetrically and expand clonally through the neutral-drift model,72, 73 our discovery of Mist1+ relatively quiescent gastric stem cells seems more consistent with the classic paradigm of rare master stem cells that maintain the glands.74, 75, 76 Earlier label-retaining studies in the intestine suggested a model of solitary +4-type stem cells that divide asymmetrically in the normal state, with symmetric division (leading to crypt fission) occurring only when there is a need to expand the stem cell population, such as in regenerative and carcinogenic states.75, 76 Recent studies have suggested that such +4-type stem cells are present within the intestinal crypts, and may express several markers.77, 78, 79 In the gastric antrum, CCK2R+ cells are rapidly cycling stem cells, and thus there also may exist an additional adjacent, relatively slow-cycling antral stem cell. Similarly, in the corpus, it is probably the case that in addition to the slow-cycling Mist1+ isthmus stem cell, there also are rapidly cycling, non-Mist1 stem cells within the corpus glands (Figure 2). Recently, eR1+ cells have been described in the gastric isthmus, and these might represent active corpus stem cell,58 although eR1 does not mark a solitary cell. Further characterization of the heterogeneous isthmus stem cell population is required.
Mist1+ corpus stem cells can serve as an origin of gastric cancer, including both intestinal and diffuse types. Diffuse-type gastric cancer was generated by knockout of the Cdh1 gene in Mist1+ isthmus cells, in combination with Helicobacter species infection, whereas intestinal-type cancers were generated by knockout of Apc along with Kras mutation in Mist1+ cells.68 Induction of a mutant Kras gene in Mist1+ corpus stem cells leads to the rapid development of SPEM, and the finding of SPEM in Mist1-CreERT;LoxP-Stop-LoxP (LSL)-Kras mice was confirmed by another group.80
The stem cell niche is critical for the maintenance and control of tissue stem cells, maintaining their quiescence and promoting self-renewal, but stem cell niches can be altered and expanded after injury and inflammation.68, 81, 82, 83, 84 An activated niche can facilitate the development of cancer derived from stem cells, and we and others have defined important cellular components of the gastric stem cell niche, including myofibroblasts, nerves, endothelial cells, innate lymphoid cells, pericytes, tuft cells, and hormone-producing cells20, 45, 57, 68, 85, 86, 87, 88 (Figure 2). Targeting these niche cells may be potentially useful in the inhibition of stem cell expansion and cancer development.
Gastric cancer develops after many years of chronic H pylori infection, in a chronically inflamed stomach characterized by atrophic glands and expanded stroma. Thus, alterations in specified niches precede the activation of gastric stem cells and the initiation of cancer. Although a number of mouse models of gastric cancer now have been described (please see Hayakawa et al89 for a more complete summary), the Helicobacter infection model and the interleukin 1β overexpression mouse model90 have been used commonly to model inflammation-dependent gastric carcinogenesis. Given that Rag2-/- immunodeficient mice have minimal Helicobacter-associated pathology,91, 92 one could argue that H pylori induces cancer indirectly through recruitment of immune cells and induction of high levels of proinflammatory cytokines and oxidative stress.93, 94, 95 In particular, activation and recruitment of immature CD11b+Gr1+ myeloid cells,90 along with cancer-associated fibroblasts,87 are early events associated with malignant progression in the stomach.
Although H pylori can have direct effects on gastric epithelial cells, questions remain as to whether H pylori interacts directly with gastric stem cells to promote neoplasia. Given that the relative abundance of H pylori in the stomach markedly decreases with the development of atrophy and metaplasia, any direct effects of H pylori more likely occur during early preneoplasia rather than as a late event in carcinogenesis. Indeed, Lgr5+ antral stem cells can be activated by H pylori colonization at early histopathologic stages.31 An additional role for H pylori may be to induce gastric dysbiosis or bacterial overgrowth at later stages. Chronic infection with H pylori and subsequent gastric atrophy lead to an increased gastric pH level, resulting in expansion of the microflora of the stomachs.96, 97, 98 This bacterial overgrowth, which can sustain a high level of chronic inflammation and oxidative stress, is associated significantly with late-stage progression to gastric cancer because the development of dysplasia in monoassociated H pylori–infected Ins-Gas mice was delayed significantly by housing the mice in otherwise germ-free conditions.97, 98
Finally, relatively novel contributors to the evolving cancer niche are neurons and their close cousins, tuft cells (Figure 2). Gastric stem cells are supported strongly by cholinergic signaling emanating in part from the vagus nerve acting through the muscarinic-3 receptor, and thus vagotomy markedly suppresses gastric cancer development in a variety of mouse models.85 More recently, we have shown that both Dclk1+ tuft cells and nerves are important sources of acetylcholine within the gastric mucosa, and the cholinergic stimulation induces nerve growth factor expression, leading to an expansion of enteric nerves in the stomach, thus promoting gastric carcinogenesis.86 Blockade of nerve growth factor signaling inhibits gastric tumor development, thus establishing the acetylcholine–nerve growth factor axis as a key component of the gastric cancer stem cell niche.86 Comprehensive understanding of gastric stem cells and their niche is indeed a key to open up a new road for cancer therapy, and thus still needs to be intensely elucidated further.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding The authors are supported by the National Institute of Health grant U54CA126513, R01CA093405, R01CA120979, R01DK052778, R35CA210088 (T.C.W.), T32OD010978, P30ES002109, and P01CA28842 (J.G.F.), the Clyde Wu Family Foundation (T.C.W.), the Project for Cancer Research And Therapeutic Evolution (P-CREATE) from the Japan Agency of Medical Research and Development, AMED, the Grant-in-Aid for Research Activity Start-up from Japan Society for the Promotion of Science, the Kobayashi Foundation for Cancer Research, the Mochida Memorial Foundation for Medical and Pharmacological Research, and the Tokyo Society of Medical Sciences (Y.H.).
References
- 1.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 2.Rossi D.J., Jamieson C.H., Weissman I.L. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Zeuner A., Todaro M., Stassi G. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell. 2014;15:692–705. doi: 10.1016/j.stem.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Greaves M., Maley C.C. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham T.A., McDonald S.A., Wright N.A. Field cancerization in the GI tract. Future Oncol. 2011;7:981–993. doi: 10.2217/fon.11.70. [DOI] [PubMed] [Google Scholar]
- 6.Cross W., Graham T.A., Wright N.A. New paradigms in clonal evolution: punctuated equilibrium in cancer. J Pathol. 2016;240:126–136. doi: 10.1002/path.4757. [DOI] [PubMed] [Google Scholar]
- 7.McDonald S.A., Greaves L.C., Gutierrez-Gonzalez L. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez-Gonzalez L., Graham T.A., Rodriguez-Justo M. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology. 2011;140:1251–1260. doi: 10.1053/j.gastro.2010.12.051. e1–6. [DOI] [PubMed] [Google Scholar]
- 9.Donati G., Watt F.M. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell. 2015;16:465–476. doi: 10.1016/j.stem.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 11.Barker N., van Es J.H., Kuipers J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 12.Barker N., Huch M., Kujala P. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Oh J., Lee Y.D., Wagers A.J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker N., Ridgway R.A., van Es J.H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 15.Reya T., Morrison S.J., Clarke M.F. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 16.Blokzijl F., de Ligt J., Jager M. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538:260–264. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanpain C. Tracing the cellular origin of cancer. Nat Cell Biol. 2013;15:126–134. doi: 10.1038/ncb2657. [DOI] [PubMed] [Google Scholar]
- 18.White A.C., Lowry W.E. Refining the role for adult stem cells as cancer cells of origin. Trends Cell Biol. 2015;25:11–20. doi: 10.1016/j.tcb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visvader J.E. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 20.Westphalen C.B., Asfaha S., Hayakawa Y. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasetti C., Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S., Powers S., Zhu W. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Es J.H., Sato T., van de Wetering M. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tetteh P.W., Basak O., Farin H.F. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Bird T.G., Forbes S.J. Two fresh streams to fill the liver's hepatocyte pool. Cell Stem Cell. 2015;17:377–378. doi: 10.1016/j.stem.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Wollny D., Zhao S., Everlien I. Single-cell analysis uncovers clonal acinar cell heterogeneity in the adult pancreas. Dev Cell. 2016;39:289–301. doi: 10.1016/j.devcel.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Wang B., Zhao L., Fish M. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Font-Burgada J., Shalapour S., Ramaswamy S. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westphalen C.B., Takemoto Y., Tanaka T. Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell. 2016;18:441–455. doi: 10.1016/j.stem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huch M., Dorrell C., Boj S.F. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigal M., Rothenberg M.E., Logan C.Y. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148:1392–1404 e21. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 32.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cristescu R., Lee J., Nebozhyn M. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 35.Polak P., Karlic R., Koren A. Cell-of-origin chromatin organization shapes the mutational landscape of cancer. Nature. 2015;518:360–364. doi: 10.1038/nature14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Sousa E.M.F., Wang X., Jansen M. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 37.Wang K., Yuen S.T., Xu J. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa Y., Sethi N., Sepulveda A.R. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16:305–318. doi: 10.1038/nrc.2016.24. [DOI] [PubMed] [Google Scholar]
- 39.Hattori N., Ushijima T. Epigenetic impact of infection on carcinogenesis: mechanisms and applications. Genome Med. 2016;8:10. doi: 10.1186/s13073-016-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maekita T., Nakazawa K., Mihara M. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 41.Tomita H., Takaishi S., Menheniott T.R. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140:879–891. doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldenring J.R., Nam K.T., Wang T.C. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. 2210 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald S.A., Lavery D., Wright N.A. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol. 2015;12:50–60. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 44.Jansen M., Wright N.A. Distal esophageal adenocarcinoma and gastric adenocarcinoma: time for a shared research agenda. Adv Exp Med Biol. 2016;908:1–8. doi: 10.1007/978-3-319-41388-4_1. [DOI] [PubMed] [Google Scholar]
- 45.Lee Y., Urbanska A.M., Hayakawa Y. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett's-like esophagus. Oncotarget. 2017;8:203–214. doi: 10.18632/oncotarget.10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quante M., Bhagat G., Abrams J.A. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menke V., van Es J.H., de Lau W. Conversion of metaplastic Barrett's epithelium into post-mitotic goblet cells by gamma-secretase inhibition. Dis Model Mech. 2010;3:104–110. doi: 10.1242/dmm.003012. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava A., Golden K.L., Sanchez C.A. High goblet cell count is inversely associated with ploidy abnormalities and risk of adenocarcinoma in Barrett's esophagus. PLoS One. 2015;10:e0133403. doi: 10.1371/journal.pone.0133403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavery D.L., Martinez P., Gay L.J. Evolution of oesophageal adenocarcinoma from metaplastic columnar epithelium without goblet cells in Barrett's oesophagus. Gut. 2016;65:907–913. doi: 10.1136/gutjnl-2015-310748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halldorsdottir A.M., Sigurdardottrir M., Jonasson J.G. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 51.Wang T.C., Goldenring J.R., Dangler C. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt P.H., Lee J.R., Joshi V. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 53.Goldenring J.R., Ray G.S., Coffey R.J. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 54.Farrell J.J., Taupin D., Koh T.J. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox J.G., Rogers A.B., Whary M.T. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2 -/- C57BL6 x Sv129 Helicobacter pylori-infected mice. Am J Pathol. 2007;171:1520–1528. doi: 10.2353/ajpath.2007.070249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee E.R., Leblond C.P. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–224. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- 57.Hayakawa Y., Jin G., Wang H. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–553. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuo J., Kimura S., Yamamura A. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology. 2017;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Arnold K., Sarkar A., Yram M.A. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X.B., Yang G., Zhu L. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res. 2016;26:838–849. doi: 10.1038/cr.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Syu L.J., Zhao X., Zhang Y. Invasive mouse gastric adenocarcinomas arising from Lgr5+ stem cells are dependent on crosstalk between the Hedgehog/GLI2 and mTOR pathways. Oncotarget. 2016;7:10255–10270. doi: 10.18632/oncotarget.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayakawa Y., Chang W., Jin G. Gastrin and upper GI cancers. Curr Opin Pharmacol. 2016;31:31–37. doi: 10.1016/j.coph.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Wang T.C., Dangler C.A., Chen D. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 64.Zavros Y., Eaton K.A., Kang W. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–2366. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 65.Takaishi S., Tu S., Dubeykovskaya Z.A. Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol. 2009;175:365–375. doi: 10.2353/ajpath.2009.081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 67.Powell A.E., Wang Y., Li Y. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayakawa Y., Ariyama H., Stancikova J. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian H., Biehs B., Warming S. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stange D.E., Koo B.K., Huch M. Differentiated troy(+) chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L., Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snippert H.J., van der Flier L.G., Sato T. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 73.Leushacke M., Ng A., Galle J. Lgr5+ gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep. 2013;5:349–356. doi: 10.1016/j.celrep.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 74.Potten C.S., Kovacs L., Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 75.Potten C.S., Owen G., Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 76.Booth C., Potten C.S. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montgomery R.K., Carlone D.L., Richmond C.A. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sangiorgi E., Capecchi M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takeda N., Jain R., LeBoeuf M.R. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi E., Hendley A.M., Bailey J.M. Expression of activated ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology. 2016;150:918–930. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stzepourginski I., Nigro G., Jacob J.M. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A. 2017;114:E506–E513. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kabiri Z., Greicius G., Madan B. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 83.Miyoshi H., Ajima R., Luo C.T. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lane S.W., Williams D.A., Watt F.M. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao C.M., Hayakawa Y., Kodama Y. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayakawa Y., Sakitani K., Konishi M. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quante M., Tu S.P., Tomita H. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Worthley D.L., Churchill M., Compton J.T. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayakawa Y., Fox J., Gonda T. Mouse models of gastric cancer. Cancers. 2013;5:92–130. doi: 10.3390/cancers5010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tu S., Bhagat G., Cui G. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roth K.A., Kapadia S.B., Martin S.M. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 92.Lee C.W., Rao V.P., Rogers A.B. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2-/- mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun. 2007;75:2699–2707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Q.B., Nakashabendi I.M., Mokhashi M.S. Association of cytotoxin production and neutrophil activation by strains of Helicobacter pylori isolated from patients with peptic ulceration and chronic gastritis. Gut. 1996;38:841–845. doi: 10.1136/gut.38.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davies G.R., Simmonds N.J., Stevens T.R. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naito Y., Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med. 2002;33:323–336. doi: 10.1016/s0891-5849(02)00868-7. [DOI] [PubMed] [Google Scholar]
- 96.Sheh A., Fox J.G. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4:505–531. doi: 10.4161/gmic.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lertpiriyapong K., Whary M.T., Muthupalani S. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014;63:54–63. doi: 10.1136/gutjnl-2013-305178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lofgren J.L., Whary M.T., Ge Z. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–220. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Demitrack E.S., Gifford G.B., Keeley T.M. NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am J Physiol Gastrointest Liver Physiol. 2017;312:G133–G144. doi: 10.1152/ajpgi.00325.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gifford G.B., Demitrack E.S., Keeley T.M. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut. 2016 Mar 1 doi: 10.1136/gutjnl-2015-310811. http://dx.doi.org/10.1136/gutjnl-2015-310811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Demitrack E.S., Gifford G.B., Keeley T.M. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–2536. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim T.H., Shivdasani R.A. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med. 2011;208:677–688. doi: 10.1084/jem.20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]