Abstract
The gastric epithelium is sustained by a population of stem cells that replenish the various mature epithelial lineages throughout adulthood. Regulation of stem and progenitor cell proliferation occurs via basic developmental signaling pathways, including the Notch pathway, which recently was described to promote gastric stem cell proliferation in both mice and human beings. Current cancer theory proposes that adult stem cells that maintain gastrointestinal tissues accumulate mutations that promote cancerous growth, and that basic signaling pathways, such as Notch, which stimulate stem cell proliferation, can promote tumorigenesis. Accordingly, constitutive Notch activation leads to unchecked cellular proliferation and gastric tumors in genetic mouse models. Furthermore, there is emerging evidence suggesting that the Notch pathway may be activated in some human gastric cancers, supporting a potential role for Notch in gastric tumorigenesis. In this review, we first summarize the current understanding of gastric stem cells defined by genetic mouse studies, followed by discussion of the literature regarding Notch pathway regulation of gastric stem cell function in the mouse and human beings. Notch action to maintain gastric epithelial cell homeostasis and the cellular consequences of dysregulated signaling to promote tumorigenesis are discussed, including studies associating Notch activation with human gastric cancer. Finally, we compare and contrast Notch function in the stomach with other gastrointestinal tissues, including the intestine, to highlight the sensitivity of the stomach to Notch-induced tumors.
Keywords: Stomach, Homeostasis, Gastric Stem Cells, Gastric Cancer
Abbreviations used in this paper: ADAM10, a disintegrin and metalloproteinase 10; GC, gastric cancer; GI, gastrointestinal; GSI, γ-secretase inhibitor; NICD, Notch receptor intracellular domain; TX, tamoxifen
Summary.
The Notch signaling pathway has emerged as a critical regulator of gastric stem cell homeostasis in both mouse and human stomachs. Notch promotes gastric stem/progenitor cell proliferation via activation of the NOTCH1 and NOTCH2 receptors. Accordingly, constitutive Notch activation activates gastric stem cells, resulting in gland fission, tissue expansion, and tumor formation.
Gastric cancer (GC) is a significant human health problem. Worldwide, it is the fifth most common cancer and the third leading cause of cancer-related deaths.1 Despite a recent decrease in incidence in the United States, GC remains a serious burden, with the National Cancer Institute estimating 26,000 new cases and 11,000 deaths in 2016. There are few effective treatment options for GC and only 30% of patients will survive for 5 years or more. The recent genetic profiling of almost 300 human gastric adenocarcinomas in The Cancer Genome Atlas identified 4 molecular GC subgroups: Epstein–Barr virus positive, microsatellite instability, genome stable, and chromosomal instability.2 Each subtype was associated with a distinct molecular signature, providing valuable molecular clues that can help to uncover drivers of tumorigenesis and identify potential therapeutic targets.
A complementary approach to molecular profiling of human gastric cancers is to decipher the key signaling pathways that promote gastric stem/progenitor cell proliferation during homeostasis. Basic developmental signaling pathways that regulate stem cell homeostasis can be key drivers of tumorigenesis in gastrointestinal (GI) tissues. For example, Wnt pathway signaling is required to maintain intestinal stem cells and Wnt-activating mutations are observed in more than 80% of colorectal cancers, primarily via mutation of the Wnt negative regulator APC.3 Current theory is that Wnt-activating mutations accumulate in intestinal stem cells to initiate and maintain tumors.4 A similar unifying tumor-driving pathway required to maintain gastric stem cells has not been identified in stomach, perhaps because of the variability between GC subtypes as well as a comparatively rudimentary understanding of gastric stem cells. The identification of gastric stem cells in mouse models has set the stage to define the niche factors regulating these cells and thereby potential pathways driving gastric cancer cell proliferation.
In this review we summarize studies that have shown a key role for the Notch signaling pathway to maintain gastric stem cells. Notch function in both the corpus and antral regions of the stomach are presented from analysis of mouse models, and mouse and human gastric organoids. The studies have shown that Notch functions to promote stem/progenitor cell proliferation in the stomach. Accordingly, constitutive Notch activation in gastric stem cells was shown to induce gastric tumors in mouse.5, 6 Moreover, there is emerging evidence of Notch pathway activation in some human gastric cancers.7, 8, 9, 10, 11, 12 Hence, the Notch pathway may promote both normal and cancer cell proliferation in the stomach and thus it might provide potential therapeutic targets for treatment of GC.
The Notch Signaling Pathway
The Notch pathway regulates key cellular processes such as proliferation and differentiation through communication between adjacent cells.13 Notch components are expressed broadly to regulate many homeostatic processes in adult tissues, including the GI tract.14 The Notch signaling pathway, present in all metazoans, is highly conserved. In mammals, there are 4 Notch receptors (NOTCH1–4) and 5 Notch ligands (Delta-like 1, 3, 4, and Jagged 1 and 2), all of which are single-pass transmembrane proteins.15 The signal-sending cells express Notch ligand and the signal-receiving cells express Notch receptor. Engagement of ligand and receptor initiates proteolytic cleavage events at the cell membrane to release an intracellular signaling fragment of the Notch receptor: Notch receptor intracellular domain (NICD). In the intestine, the initial Notch receptor cleavage is performed by the membrane-localized sheddase a disintegrin and metalloproteinase 10 (ADAM10) to cleave the receptor ectodomain.16 ADAM10 function in the stomach has not yet been tested. After this receptor cleavage event by ADAM10, the NICD signaling fragment is released via intramembrane cleavage by the γ-secretase complex. Subsequently, NICD translocates to the nucleus and assembles a transcription activation complex with the DNA binding protein RBP-Jκ (also known as CSL) and coactivators, including MAML, to induce the transcription of target genes such as those in the hairy and enhancer of split (Hes)-related gene family. Direct gene targets vary with tissue context, with data suggesting that Hes1 and Olfm4 may be direct Notch target genes in stomach and intestine.5, 17
As a consequence of the requirement for interactions between juxtaposed cells, Notch signaling communicates short-range signals. Furthermore, the signal is short-lived, with receptor destruction an integral aspect of the signaling process, and rapid degradation of NICD resulting from its PEST domain.15 Thus, Notch signaling is well suited as a niche pathway to regulate stem cell behavior in GI tissues.
Mouse Gastric Stem Cells
The adult glandular stomach contains 2 regions: the corpus, whose primary function is the luminal secretion of acid and digestive enzymes, and the more distal antrum, which secretes the hormone gastrin. Distinct pools of actively cycling stem cells in each region fuel epithelial cell turnover throughout life. These active stem cells generate proliferating progenitors that differentiate into the various mature epithelial cell lineages of the stomach.14 In the corpus, adult stem cells thought to be located in the midregion of each gland generate progeny that migrate bidirectionally to form the differentiated cell types, including short-lived surface mucous cells, and longer-lived acid-secreting parietal cells, endocrine cells, and zymogenic lineage cells. In contrast, antral stem cells are located at the gland base and generate surface mucous cells, endocrine cells, including gastrin-producing G cells, and deep mucous cells. In general, cellular turnover is more rapid in the antrum than in the corpus, with a time frame of several days vs several weeks or months (reviewed by Mills and Shivdasani18).
Long-term lineage tracing in genetic mouse models has been the gold standard approach for identifying stem cells in the GI tract. With this approach, genetic markers have been shown to define gastric stem cells that generate all of the differentiated epithelial cell lineages, although discovery in the stomach has lagged well behind parallel studies in the intestine. After the discovery of LGR5 as a marker for intestinal stem cells,19 stem cells in the gastric antrum also were shown to express LGR5 by observation of lineage traces in Lgr5-EGFP-ires-CreERT2 mice more than 20 months after Cre activation with tamoxifen (TX)20 (Table 1). In addition, single Lgr5-GFP+ antral cells isolated from this mouse strain were capable of initiating organoids with the potential to differentiate into mature gastric epithelial cell types, further supporting the conclusion that LGR5 marks an active antral stem cell. Interestingly, Lgr5-GFP cells isolated from stomach or intestine are both capable of forming long-lived organoid lines, although they each retain regional memory to form gastric or intestinal cell types despite growth under similar culture conditions.20, 21 This finding suggests that GI tract stem cells are epigenetically marked to follow prescribed region-specific differentiation programs to generate mature epithelial cells.
Table 1.
Genetic Mouse Strains Expressed in Adult Gastric Stem Cells
| Strain name | Active stem cell? |
Induced stem cell? |
Differentiated cells? | Reference | ||
|---|---|---|---|---|---|---|
| Antrum | Corpus | Antrum | Corpus | |||
| Lgr5-EGFP-ires-CreERT2 | Yes | No | - | - | No | Barker et al20 |
| CCK2R-CreERT | Yes | No | - | - | Yes (corpus) | Hayakawa et al25 |
| Sox2-CreER | Yes | Yes | - | - | Yes | Arnold et al23 |
| Lrig1-CreERT2 | Yes | Yes | - | - | Yes | Powell et al24 and Samuelson, unpublished data |
| eR1-CreERT2 | Yes | Yes | - | - | Yes | Matsuo et al26 |
| 12.4KVil-Cre | No | No | Yes | No | No | Qiao et al28 |
| Troy-eGFP-ires-CreERT2 | No | Noa | No | Yesa | Yes | Stange et al29 |
| Mist1-CreERT2 | No | No,a yesb |
No | Yesa,b | Yes | Hayakawa et al30 and Stange et al29 |
NOTE. Mouse Cre drivers expressed in gastric stem cells are categorized based on their contribution to normal homeostasis (active stem cell) or in response to injury (induced stem cell). Some Cre drivers also are expressed in gastric differentiated cell types.
Occasional slowly expanding lineage stripes appear to originate from differentiated chief cells, with accelerated expansion after loss of active stem/progenitor cells (Stange et al29).
Lineage stripes appear to originate from the isthmus region from slowly cycling cells, with accelerated expansion after loss of active stem/progenitor cells (Hayakawa et al30).
Unfortunately, a specific marker for the active stem cell in the adult corpus region of the stomach has not yet been described. In contrast to the antrum, Lgr5-EGFP-ires-CreERT2 does not mark active corpus stem cells; however, it does mark progenitors in the immature neonatal stomach that form adult corpus stem cells.20 Thus, the corpus stands apart from more distal regions of the GI tract in regard to expression of Lgr5. Recently, it was shown that the Wnt signaling pathway drives gastric corpus patterning during mouse stomach development, and that Wnt activation in cultured human pluripotent stem cells induces differentiation into corpus epithelial cell types,22 an important finding that will further our understanding of pathway regulation of adult gastric corpus stem cells.
In addition to specific stem cell markers, mouse genetic alleles have been described that are expressed in differentiated gastric cell types as well as in active stem cells. Although not specific to stem cells, these Cre drivers still can be useful tools to manipulate stem cell function because they have been observed to generate long-term lineage stripes after Cre activation (Table 1). These strains include Sox2-CreER,23 Lrig1-CreERT2,24 CCK2R-CreERT,25 and eR1-CreERT226 (Table 1). The Sox2 allele is particularly useful because it is expressed in both corpus and antral stem cells, but it is not expressed in the intestine. This is hugely advantageous for studying pathway regulation of gastric stem cells because it will allow genetic manipulation of the stomach without affecting the intestine, where changes to stem cell function can limit animal viability. One consideration is that most of the genetic mouse strains that have been identified to mark gastric stem cells have not been studied extensively, so the general utility of an individual strain is uncertain.
Cellular plasticity is a hallmark of GI tissues, where cellular reprogramming has been suggested to allow committed progenitors or even mature cells to serve as reserve stem cells when active stem cells are compromised.27 The existence of dedicated quiescent, or reserve, stem cell populations in the stomach has not been established definitively. However, certain populations of nonproliferating gastric epithelial cells have been shown to expand during tissue injury and show active stem cell lineage tracing activity. These include rare quiescent antral stem cells that express a Villin-Cre driver,28 and a small population of differentiated corpus chief cells expressing Troy-29 and Mist1-Cre drivers29, 30 (Table 1). One of these studies reported that Mist1-CreERT2 also is expressed in rare quiescent stem cells in the isthmus of corpus glands that can serve as the cell-of-origin for gastric cancer in mouse models.30 Furthermore, recent studies have reported that CCK2R-CreERT–expressing antral stem cells expand in response to gastrin,25 and that LGR5+ antral stem cells can expand in response to Helicobacter infection.31
One major challenge for stem cell studies in the stomach is the extensive gastric cell toxicity observed in genetic models using TX-regulated Cre drivers. TX targets the parietal cells, inducing rapid cell death, with increased corpus progenitor cell proliferation and metaplasia following cell death. These cellular changes are transient and normal cellular homeostasis returns within 3 weeks.32 Parietal cells are well known to be lynchpins in the stomach, required for corpus epithelial cell homeostasis and maintenance of the zymogenic cells in the gland base.27 Almost all of the genetic alleles that have been described to manipulate gastric stem cells in the mouse are TX-regulated Cre drivers (Table 1). The TX-induced parietal cell damage is dose-dependent,32 and in many instances the observed lineage traces reported for many of the gastric Cre drivers used doses that would be expected to induce parietal cell damage. Thus, there is likely to be an injury component to the response that needs to be considered. To avoid parietal cell injury, new genetic tools are needed that do not rely on acute TX treatment to manipulate genes in gastric stem cells.
Notch Regulation of Gastric Epithelial Cell Proliferation
Notch recently was established as a crucial pathway regulating gastric stem cell proliferation and differentiation. In the fetal mouse stomach, Notch pathway components Notch1 and Jag2, and the Notch target gene Hes1, have been mapped to the nascent gastric epithelium.6 Analysis of Hes1 mutant mice showed that the Notch pathway regulates cellular differentiation in the developing stomach, with excessive endocrine cell differentiation observed in perinatal mice.33 In the adult stomach, the consequences of Notch pathway inhibition and constitutive pathway activation also have been established from analysis of mouse models. Notch inhibition by γ-secretase inhibitor (GSI) treatment in adult mice showed reduced progenitor cell proliferation in both corpus6, 34 and antrum.5, 6, 35 Importantly, GSI treatment also reduced the number of proliferating Lgr5-GFP antral stem cells.5 Furthermore, single fluorescence-activated cell sorter–isolated Lgr5-GFP stem cells derived from GSI-treated mice initiated fewer organoids than stem cells derived from control mice, suggesting that Notch is required to support antral stem cell function.5 In contrast, constitutive Notch activation in LGR5+ antral stem cells using genetic overexpression of NICD induced stem and progenitor cell proliferation.5 Similarly, NICD expression in immature parietal cells in fetal mice and in adult corpus stem cells induced gastric epithelial cell proliferation.6, 34 Furthermore, gastric organoids derived from Notch-activated adult corpus and antral stem cells grow faster, consistent with Notch pathway induction of stem and progenitor cell proliferation.5, 34 Overall, these studies suggest that Notch promotes epithelial cell proliferation and is required to maintain stem cells in both corpus and antrum.
Studies in both mice and human beings showed that Notch regulation of gastric antral and corpus progenitor cell proliferation occurs via NOTCH1 and NOTCH2 receptor signaling. Gene expression analysis has shown that NOTCH1 and NOTCH2 are the predominant Notch receptors expressed in the mouse and human stomach.34, 35 Moreover, a Notch1-CreERT mouse strain induced full antral lineage stripes, indicating that active NOTCH1-receptor signaling occurs in antral stem cells.5, 35 Inhibitory antibodies that target NOTCH1 or NOTCH2 significantly reduced stem and progenitor cell proliferation in mouse stomach and decreased growth of corpus and antral organoids, suggesting that both receptors promote gastric epithelial cell proliferation in vivo and in vitro.34, 35 Furthermore, inhibition of NOTCH1 and NOTCH2 in organoids derived from human gastric glands from both corpus and antrum also led to reduced organoid growth, suggesting that the Notch pathway is crucial to support human gastric stem cell function.34, 35 Importantly, the human and mouse studies concur, validating the mouse as a reliable model to probe pathway regulation of human gastric stem cells.
Notch Regulation of Gastric Epithelial Cell Differentiation
In addition to regulation of proliferation, the Notch pathway regulates epithelial cell differentiation in the antral stomach. Notch signaling appears to act in a global manner, generally promoting progenitor cell proliferation and inhibiting differentiation. Analysis of mice after Notch inhibition via GSI treatment or antibody-mediated blockade of both NOTCH1 and NOTCH2 increased expression of all antral epithelial cell lineage markers, including surface mucous, deep mucous, and endocrine cells.5, 35 Accordingly, there was increased expression of lineage-directing transcription factors FoxQ1 (surface mucous),36 Spdef (deep mucous),37 and Neurog3 (endocrine)38 in the Notch-inhibited antral stomach.5, 35 Similarly, expression of differentiated markers was increased in antral organoids treated with GSI or NOTCH1- and NOTCH2-receptor antibodies. In addition, Notch inhibition led to epithelial cell remodeling, with increased expression of secretory proteins normally associated with other regions of the GI tract, including intestine.35
In contrast to the general increase in antral epithelial cell differentiation observed after Notch inhibition, constitutive Notch activation via NICD expression in LGR5+ antral stem cells reduced differentiation, including decreased expression of transcription factors and markers for all epithelial cell lineages.5 Furthermore, antral organoids expressing NICD showed decreased expression of transcription factors and markers for endocrine and deep mucous cells. Together, the inhibition and activation data suggest that Notch inhibits overall differentiation in the antral stomach.5, 35 This is in contrast to the intestine, where Notch functions to regulate cell fate choice, directing progenitor cells to adopt an absorptive rather than secretory cell fate via regulation of the transcription factor ATOH1.39, 40, 41
Although Notch signaling appears to suppress differentiation in the antrum, the role of Notch to regulate differentiation in the gastric corpus is less clear. Cellular turnover in the stomach varies regionally, with renewal of all antral epithelial cell lineages occurring within several weeks, and cellular renewal in the corpus occurring between several days (surface mucous) to several months (parietal, chief) (reviewed by Mills and Shivdasani18). Because Notch inhibition with GSI or NOTCH1- and NOTCH2-receptor antibodies results in rapid animal mortality,34 study of Notch regulation of complete corpus epithelial cellular renewal has not been feasible. However, it has been shown that although differentiation of surface mucous cells in the antrum is Notch-dependent,5, 35 this same lineage in the gastric corpus does not appear to be affected by Notch inhibition,34 suggesting that corpus surface mucous cells are Notch-independent. Therefore, key molecular differences in corpus vs antral stem cell differentiation regulated by Notch signaling will be an important future area of research.
Notch and Gastric Tumorigenesis
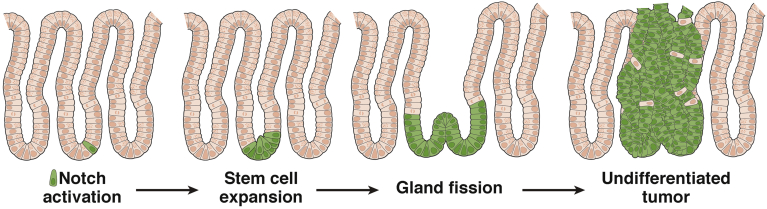
Notch regulation of gastric stem cell function in the adult mouse antrum has been more attainable than in the mouse corpus owing to the identification of LGR5 as an antral stem cell marker and the ability to track and manipulate these cells using the Lgr5-GFP-CreERT2 mouse model.20 Thus, in the mouse antrum, Notch is thought to regulate gastric stem cell number, as suggested by regulation of organoid establishment activity, and by observation of tissue expansion via increased proliferation, gland fission, and enhanced conversion of adult antral glands to monoclonality after Notch activation.5 Gland fission is a tissue growth phenomenon that normally occurs in the postnatal stomach to increase organ size with the growth of the organism.42 Fission is thought to occur in response to increased numbers of stem cells to spatially accommodate the increased cellular production,43 and results in a single glandular unit bifurcating, or splitting, into 2 individual glands. Gland fission during homeostatic conditions in the adult stomach is rare. The extensive gland fission that occurs as a result of constitutive NICD expression leads to tissue expansion and polyp formation within 6 months, showing that Notch activation in antral stem cells is tumorigenic (Figure 1). The NICD-induced tumors are highly proliferative, largely composed of undifferentiated cells that express progenitor cell markers, although interestingly not LGR5.5 This also has been shown in the mouse corpus, where constitutive NICD expression in parietal progenitor cells and in adult SOX2+ stem/progenitor cells induces hyperproliferative glands.6, 34
Figure 1.
Notch activation in antral stem cells induces tumorigenesis. In the mouse, NICD activation in an LGR5+ stem cell (green) at the gland base results in increased stem cell proliferation and number followed by antral gland fission. This leads to overall tissue expansion and eventual tumorigenesis. Such tumors are hyperproliferative and undifferentiated, expressing stem and progenitor cell markers.
The human metaplastic stomach contains clonal patches of epithelial cells, a process termed field cancerization.44, 45 Whether this process is regulated by Notch signaling in the human stomach has not been shown, but our evidence from the mouse stomach suggests that unchecked signaling in pathways that regulate proliferation may give progenitor cells a selective advantage, leading to clonal expansion.5 In addition, in the human stomach it has been shown that a clonal mutational field can acquire additional new mutations, suggesting that gastric cancer may arise from a genetically advantageous proliferative subclone within the founder clonal field.44
Human Gastric Cancer Stem Cells
Little is known about human gastric stem cells. Putative markers of gastric stem cells have been proposed based on their expression in cancer-initiating cells (also called cancer stem cells). The proliferative capacity and self-renewal property of stem cells makes them susceptible to tumor-initiating mutations, and therefore the transformation of normal stem cells into cancer stem cells remains a likely theory for tumorigenesis (reviewed by Vries et al4). Markers of such cancer stem cells in the human stomach include CXCR4,46 and the cell surface markers CD133, also known as Prominin-1,47, 48, 49 and CD44,50 in particular CD44v651 and CD44v9.52 In addition, expression of SOX2 is altered in gastric cancer, although it is unclear whether SOX2 is oncogenic53, 54 or has tumor-suppressing capabilities.55, 56, 57 Whether these markers also are expressed in normal human gastric stem cells is an important future question that will advance our understanding of human gastric epithelial cell biology.
Notch and Gastric Cancer
Studies in mice have shown that NICD expression in parietal progenitor cells,6 adult SOX2+ corpus stem cells,34 and LGR5+ antral stem cells5 lead to hyperplastic polyp development in corpus or antrum. In human beings, Notch pathway dysregulation, particularly via up-regulation of ligands and/or receptors, has been observed in human gastric cancer. Importantly, correlations between high expression of NOTCH1, NOTCH2, and Jagged 1 have been associated with gastric cancer morbidity.7, 8, 12 NOTCH1 activation in human gastric cancer has been associated with DLL1 promoter methylation,11 suggesting that epigenetic dysregulation of Notch signaling also may contribute to gastric disease. Functional studies in human gastric cancer cell lines show that pharmacologic Notch inhibition can reduce growth and invasion in lines that show increased NOTCH1 expression.10 Notch manipulation also can inhibit gastric tumor growth via regulation of PTEN expression, where Notch inhibition with small interfering RNA against NOTCH1 or GSI treatment leads to activation of nuclear PTEN expression, growth inhibition, and apoptosis of gastric cancer cells.9 Furthermore, Notch activation via expression of NICD in the SC-M1 human gastric adenocarcinoma cell line promoted colony formation and tumor growth in mouse xenograft models through activation of cyclooxygenase-2 expression,12 suggesting that Notch may initiate gastric tumorigenesis via cross-talk with other signaling pathways. Finally, the Notch pathway has been shown to increase expression of CD4458 and CD133,59 putative markers of gastric cancer stem cells. Taken together, current data suggest significant Notch pathway involvement in the regulation of gastric cancer cell growth, proliferation, and metastasis, highlighting Notch as a key pathway to explore further for the development of targeted therapies for gastric cancer treatment.
Notch Regulation of Gastric Versus Intestinal Stem Cells
Gastric and intestinal stem cells are both regulated by Notch and Wnt, commonly using the same pathway components, such as LGR5,19, 20 and NOTCH1 and NOTCH2.35, 60, 61, 62, 63, 64 However, these stem cell populations intrinsically are primed to perform functions specific to each tissue program. Thus, pathway regulation likely depends on specific molecular cues from the gastric or intestinal stem cell niche as it relates to overall tissue function. Interestingly, stem cells in stomach and intestine are differentially sensitive to Notch pathway activation in the context of tumorigenesis. Although constitutive expression of NICD in LGR5+ stem cells induces antral tumors with 100% penetrance, intestinal or colonic tumors are not observed.5 These findings suggest that Notch tone in the stomach and intestine differ, with gastric stem cells more sensitive to Notch-activating mutations than intestinal stem cells. Surprisingly, NICD-expressing intestinal stem cells appear to be lost, suggesting that they are at a competitive disadvantage to normal intestinal stem cells. These findings in mice would predict that Notch might play a larger role in the development of human gastric cancers than in human colon cancer.
Both gastric and intestinal stem cells are affected similarly by Wnt pathway manipulation. Wnt activation via genetic deletion of Apc in mouse gastric and intestinal stem cells leads to epithelial cell hyperproliferation and tumorigenesis in both intestine65, 66 and stomach.20, 56 Patients with the Wnt activating genetic disorder familial adenomatous polyposis develop hundreds to thousands of intestinal adenomas that progress to colorectal cancer via accumulation of additional tumorigenic mutations (reviewed by Fearon67). Interestingly, almost all familial adenomatous polyposis patients also develop gastric polyps, typically fundic gland polyps, although they largely are benign and the risk for gastric adenocarcinoma development is low compared with the risk for colorectal cancer.68 This finding suggests that, in contrast to enhanced gastric stem cell sensitivity to Notch activation, the intestine may be more sensitive to Wnt activation than the stomach. Indeed, although adenomas are observed in both tissues after Apc deletion in LGR5 cells in mouse genetic models, the intestinal tumor burden is greater, limiting the duration of study owing to animal morbidity.20 Future studies will be necessary to determine how Notch and Wnt intersect to support the gastric and intestinal stem cell niche during normal tissue homeostasis and with diseases such as cancer.
Summary and Future Directions/Questions
Notch functions to balance cellular proliferation and differentiation in the stomach, with Notch inhibition leading to reduced stem and progenitor cell proliferation and increased differentiation, although Notch activation results in increased stem and progenitor cell proliferation and reduced differentiation. Furthermore, genetic overexpression of NICD induces tissue expansion via gland fission and gastric tumors. Thus, Notch appears to be a central signaling pathway that promotes gastric stem cell proliferation and expansion. Furthermore, Notch pathway activation also has been reported in human gastric cancer, suggesting that this pathway may prove to be a useful therapeutic target for treatment of some human gastric cancers.
Several important questions remain unanswered that undoubtedly will further our understanding of Notch pathway function in mouse and human gastric stem cells. These questions include the following:
-
•
How is the Notch niche defined? What niche cells present ligand to gastric stem cells?
-
•
What are the key Notch ligands in the stomach?
-
•
How does Notch regulate gastric stem cell self-renewal and proliferation?
-
•
How does Notch cooperate with Wnt and other signaling pathways to both maintain homeostasis and promote tumorigenesis?
-
•
What are the molecular markers for human gastric stem cells?
-
•
Can Notch pathway inhibition be effective for gastric cancer treatment?
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Institutes of Health F32-DK093340, Michigan Institute for Clinical Health Research Postdoctoral Translational Scholars Program National Institutes of Health Clinical and Translational Science Award UL1TR000433, National Institutes of Health T32-HD007505, and an American Association for Cancer Research-Debbie's Dream Foundation Career Development Award (E.S.D.); and by research support through National Institutes of Health P01-DK062041, National Institutes of Health 5R01-DK096972, National Cancer Institute P50-CA130810, and the American Gastroenterological Association Research Foundation (L.C.S.).
References
- 1.Torre L.A., Bray F., Siegel R.L. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huels D.J., Sansom O.J. Stem vs non-stem cell origin of colorectal cancer. Br J Cancer. 2015;113:1–5. doi: 10.1038/bjc.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vries R.G., Huch M., Clevers H. Stem cells and cancer of the stomach and intestine. Mol Oncol. 2010;4:373–384. doi: 10.1016/j.molonc.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demitrack E.S., Gifford G.B., Keeley T.M. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–2536. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim T.H., Shivdasani R.A. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med. 2011;208:677–688. doi: 10.1084/jem.20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer L., Takacs A., Slotta-Huspenina J. Clinical significance of NOTCH1 and NOTCH2 expression in gastric carcinomas: an immunohistochemical study. Front Oncol. 2015;5:94. doi: 10.3389/fonc.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu K.W., Hsieh R.H., Huang K.H. Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression. Carcinogenesis. 2012;33:1459–1467. doi: 10.1093/carcin/bgs165. [DOI] [PubMed] [Google Scholar]
- 9.Kim S.J., Lee H.W., Baek J.H. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene. 2016;35:251–260. doi: 10.1038/onc.2015.80. [DOI] [PubMed] [Google Scholar]
- 10.Li L.C., Peng Y., Liu Y.M. Gastric cancer cell growth and epithelial-mesenchymal transition are inhibited by gamma-secretase inhibitor DAPT. Oncol Lett. 2014;7:2160–2164. doi: 10.3892/ol.2014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piazzi G., Fini L., Selgrad M. Epigenetic regulation of delta-like1 controls Notch1 activation in gastric cancer. Oncotarget. 2011;2:1291–1301. doi: 10.18632/oncotarget.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh T.S., Wu C.W., Hsu K.W. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69:5039–5048. doi: 10.1158/0008-5472.CAN-08-4021. [DOI] [PubMed] [Google Scholar]
- 13.Bray S.J. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 14.Demitrack E.S., Samuelson L.C. Notch regulation of gastrointestinal stem cells. J Physiol. 2016;594:4791–4803. doi: 10.1113/JP271667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopan R., Ilagan M.X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai Y.H., VanDussen K.L., Sawey E.T. ADAM10 regulates Notch function in intestinal stem cells of mice. Gastroenterology. 2014;147:822–834 e13. doi: 10.1053/j.gastro.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanDussen K.L., Carulli A.J., Keeley T.M. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills J.C., Shivdasani R.A. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker N., van Es J.H., Kuipers J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 20.Barker N., Huch M., Kujala P. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Sato T., Vries R.G., Snippert H.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 22.McCracken K.W., Aihara E., Martin B. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold K., Sarkar A., Yram M.A. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell A.E., Wang Y., Li Y. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayakawa Y., Jin G., Wang H. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–553. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuo J., Kimura S., Yamamura A. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology. 2017;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Mills J.C., Sansom O.J. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao X.T., Ziel J.W., McKimpson W. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stange D.E., Koo B.K., Huch M. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayakawa Y., Ariyama H., Stancikova J. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigal M., Rothenberg M.E., Logan C.Y. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148:1392–1404 e21. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 32.Huh W.J., Khurana S.S., Geahlen J.H. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24 e7. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen J., Pedersen E.E., Galante P. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 34.Demitrack E.S., Gifford G.B., Keeley T.M. NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am J Physiol Gastrointest Liver Physiol. 2017;312:G133–G144. doi: 10.1152/ajpgi.00325.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gifford G.B., Demitrack E.S., Keeley T.M. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut. 2016 doi: 10.1136/gutjnl-2015-310811. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verzi M.P., Khan A.H., Ito S. Transcription factor foxq1 controls mucin gene expression and granule content in mouse stomach surface mucous cells. Gastroenterology. 2008;135:591–600. doi: 10.1053/j.gastro.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horst D., Gu X., Bhasin M. Requirement of the epithelium-specific Ets transcription factor Spdef for mucous gland cell function in the gastric antrum. J Biol Chem. 2010;285:35047–35055. doi: 10.1074/jbc.M110.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C.S., Perreault N., Brestelli J.E. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shroyer N.F., Helmrath M.A., Wang V.Y. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 40.VanDussen K.L., Samuelson L.C. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215–223. doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Q., Bermingham N.A., Finegold M.J. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 42.Nomura S., Kaminishi M., Sugiyama K. Clonal analysis of isolated intestinal metaplastic glands of stomach using X linked polymorphism. Gut. 1998;42:663–668. doi: 10.1136/gut.42.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeffler M., Bratke T., Paulus U. Clonality and life cycles of intestinal crypts explained by a state dependent stochastic model of epithelial stem cell organization. J Theor Biol. 1997;186:41–54. doi: 10.1006/jtbi.1996.0340. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez-Gonzalez L., Graham T.A., Rodriguez-Justo M. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology. 2011;140:1251–1260. doi: 10.1053/j.gastro.2010.12.051. e1–6. [DOI] [PubMed] [Google Scholar]
- 45.McDonald S.A., Greaves L.C., Gutierrez-Gonzalez L. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 46.Fujita T., Chiwaki F., Takahashi R.U. Identification and characterization of CXCR4-positive gastric cancer stem cells. PLoS One. 2015;10:e0130808. doi: 10.1371/journal.pone.0130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto K., Aoyagi K., Isobe T. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014;17:97–106. doi: 10.1007/s10120-013-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen L., Chen X.Z., Yang K. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS One. 2013;8:e59154. doi: 10.1371/journal.pone.0059154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao P., Li Y., Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghaffarzadehgan K., Jafarzadeh M., Raziee H.R. Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World J Gastroenterol. 2008;14:6376–6381. doi: 10.3748/wjg.14.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Cunha C.B., Oliveira C., Wen X. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab Invest. 2010;90:1604–1614. doi: 10.1038/labinvest.2010.155. [DOI] [PubMed] [Google Scholar]
- 52.Hirata K., Suzuki H., Imaeda H. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379–386. doi: 10.1038/bjc.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutz K., Mejias-Luque R., Farsakova K. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis. 2014;35:942–950. doi: 10.1093/carcin/bgt410. [DOI] [PubMed] [Google Scholar]
- 54.Matsuoka J., Yashiro M., Sakurai K. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res. 2012;174:130–135. doi: 10.1016/j.jss.2010.11.903. [DOI] [PubMed] [Google Scholar]
- 55.Otsubo T., Akiyama Y., Yanagihara K. SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer. 2008;98:824–831. doi: 10.1038/sj.bjc.6604193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarkar A., Huebner A.J., Sulahian R. Sox2 suppresses gastric tumorigenesis in mice. Cell Rep. 2016;16:1929–1941. doi: 10.1016/j.celrep.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 57.Wang S., Tie J., Wang R. SOX2, a predictor of survival in gastric cancer, inhibits cell proliferation and metastasis by regulating PTEN. Cancer Lett. 2015;358:210–219. doi: 10.1016/j.canlet.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 58.Li L.C., Wang D.L., Wu Y.Z. Gastric tumor-initiating CD44+ cells and epithelial-mesenchymal transition are inhibited by gamma-secretase inhibitor DAPT. Oncol Lett. 2015;10:3293–3299. doi: 10.3892/ol.2015.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konishi H., Asano N., Imatani A. Notch1 directly induced CD133 expression in human diffuse type gastric cancers. Oncotarget. 2016;7:56598–56607. doi: 10.18632/oncotarget.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carulli A.J., Keeley T.M., Demitrack E.S. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402:98–108. doi: 10.1016/j.ydbio.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fre S., Hannezo E., Sale S. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One. 2011;6:e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riccio O., van Gijn M.E., Bezdek A.C. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran I.T., Sandy A.R., Carulli A.J. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y., Cain-Hom C., Choy L. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 65.Barker N., Ridgway R.A., van Es J.H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 66.Powell A.E., Vlacich G., Zhao Z.Y. Inducible loss of one Apc allele in Lrig1-expressing progenitor cells results in multiple distal colonic tumors with features of familial adenomatous polyposis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G16–G23. doi: 10.1152/ajpgi.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fearon E.R. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 68.Arnason T., Liang W.Y., Alfaro E. Morphology and natural history of familial adenomatous polyposis-associated dysplastic fundic gland polyps. Histopathology. 2014;65:353–362. doi: 10.1111/his.12393. [DOI] [PubMed] [Google Scholar]