Abstract
Gastric cancer (GC) remains the third most common cause of cancer death worldwide, with limited therapeutic strategies available. With the advent of next-generation sequencing and new preclinical model technologies, our understanding of its pathogenesis and molecular alterations continues to be revolutionized. Recently, the genomic landscape of GC has been delineated. Molecular characterization and novel therapeutic targets of each molecular subtype have been identified. At the same time, patient-derived tumor xenografts and organoids now comprise effective tools for genetic evolution studies, biomarker identification, drug screening, and preclinical evaluation of personalized medicine strategies for GC patients. These advances are making it feasible to integrate clinical, genome-based and phenotype-based diagnostic and therapeutic methods and apply them to individual GC patients in the era of precision medicine.
Keywords: Gastric Cancer, Cancer Genomics, Molecular Classification, Preclinical Models
Abbreviations used in this paper: CIMP, CpG island methylator phenotype; CIN, chromosomally unstable/chromosomal instability; EBV, Epstein-Barr virus; GAPPS, gastric adenocarcinoma and proximal polyposis of the stomach; GC, gastric cancer; GTPase, guanosine triphosphatase; HDGC, hereditary diffuse gastric cancer; hPSC, human pluripotent stem cell; lncRNA, long noncoding RNA; LOH, loss of heterozygosity; miRNA, microRNA; MSI, microsatellite unstable/instability; MSI-H, high microsatellite instability; MSS/EMT, microsatellite stable with epithelial-to-mesenchymal transition features; NGS, next-generation sequencing; PDX, patient-derived tumor xenografts; TCGA, The Cancer Genome Atlas; TGF, transforming growth factor
Summary.
The following pages provide a summary of current knowledge regarding the genomics of gastric cancer (GC), with a particular emphasis on how new genomic knowledge informs precision medicine and personalized therapies.
Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer death in developed countries, with 984,000 new cases and 841,000 deaths occurring globally in 2013.1 The incidence and mortality of GC are declining, in part because of improved Helicobacter pylori eradication and cancer screening. However, adenocarcinoma of the gastric cardia is increasing in North America and Europe,2, 3 and the incidence of non-cardia GC among whites aged 25–39 years has increased 1.67-fold in the United States during the past 2 decades.4 Moreover, most GC cases are diagnosed at advanced stages, with consequent poor outcome; treatment is mostly restricted to cytotoxic chemotherapy. Thus, there is an urgent need to improve our understanding of the pathogenesis of GC and to identify more effective, less toxic therapeutic strategies. GC is multifactorial, with complex host genetic and environmental factors contributing to its development. GC is also highly heterogeneous; it is customarily divided into 2 main histologic subtypes, intestinal and diffuse, which are based on the Lauren classification.5 However, the use of anti–human epidermal growth factor receptor-2 monoclonal antibody, trastuzumab, and anti–vascular endothelial growth factor receptor-2 monoclonal antibody, ramucirumab, has shifted the previous histopathologic paradigm to incorporate new genetic and molecular features.6, 7 Recently, remarkable advances in next-generation sequencing (NGS) technologies have defined the genomic landscape of GC8, 9, 10; studies of microRNAs (miRNAs) and long noncoding RNAs (lncRNAs)11, 12 as well as novel preclinical models (such as patient-derived tumor xenografts [PDX] and patient-derived organoids) have largely filled the gap between cancer genetics and phenotype.13, 14, 15, 16, 17 These advances have made it possible to integrate traditional, genome-based and phenotype-based diagnostic and therapeutic methods with application to individual GC patients in the era of precision medicine.
Etiologic Factors in Gastric Carcinogenesis
Environmental Factors
Among clinical risk factors for GC, which include smoking, high-salt diet, high intake of meats, and bile reflux, infection with H pylori is a leading factor, especially in distal GC.18, 19, 20, 21 On the basis of improved estimates from prospective studies, 89% of new non-cardia GC cases are attributable to H pylori worldwide.22 H pylori–mediated gastric carcinogenesis involves several mechanisms: cytotoxin-associated gene A, vacuolating cytotoxin A–induced chronic inflammation, oxidative damage, genomic instability, and epigenetic changes in gastric epithelial cells.18, 23, 24, 25, 26 Interestingly, an inverse relation between H pylori infection and the risk of proximal GC has been observed in Western countries.27
Epstein-Barr virus (EBV) occurs in 2%–20% of GC, with a worldwide average of 10%.28 In EBV-associated GC, latent membrane protein 2A activates DNA methyltransferase 1 by inducing phosphorylation of STAT3, thereby causing CpG island hypermethylation of the PTEN promoter.29 Specific EBV transcripts, including latent genes and viral miRNAs, also have oncogenic properties such as increased cell proliferation and motility, impairment of apoptosis, and increased chemoresistance.30
Host Factors
Hereditary cancer syndromes linked to 1%–3% of GC consist of 3 principal syndromes: hereditary diffuse gastric cancer (HDGC), gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), and familial intestinal GC.31 Germline mutations in CDH1, CTNNA1, and other tumor suppressor genes, including BRCA2, STK11, and SDHB, have been identified in HDGC.32 CDH1 mutations are prognostic genetic markers in HDGC. GAPPS is characterized by autosomal dominant transmission of fundic gland polyposis restricted to the proximal stomach, without evidence of colorectal or duodenal polyposis or other hereditary gastrointestinal cancer syndromes.33 GC is also increased in other heritable syndromes, such as Li-Fraumeni syndrome with germline mutation of TP53, Peutz-Jeghers syndrome with frameshift mutation in STK11, hereditary nonpolyposis colorectal cancer with germline DNA mismatch repair gene mutation, and familial adenomatous polyposis with germline APC mutation.31, 34
From Histologic to Molecular Classification
GC has long been categorized by using histomorphologic classification systems. According to the Lauren classification, GCs are divided into 2 main subtypes, intestinal and diffuse.5 However, these histologic classifications are not sufficient to reflect the molecular and genetic characteristics of GC or to develop personalized treatment strategies in the era of precision medicine.
Recently, advances in genomic technology and high-throughput analysis have helped reveal the molecular genetic landscape of GC (Figure 1). Several molecular classification systems have been proposed, and distinct molecular subtypes have been identified.8, 9, 10, 35, 36, 37 In 2014, a landmark study by The Cancer Genome Atlas (TCGA) proposed 4 subtypes: (1) EBV-positive (8.8%), (2) microsatellite unstable/instability (MSI, 21.7%), (3) genomically stable (19.7%), and (4) chromosomally unstable/chromosomal instability (CIN, 49.8%).8 Most EBV-positive tumors occurred in male patients and in the gastric fundus or body, displaying extreme DNA hypermethylation and amplification of JAK2 and PD-L1/2, with 80% harboring non-silent PIK3CA mutations. All EBV-positive GCs displayed CDKN2A promoter hypermethylation, while lacking the MLH1 hypermethylation characteristic of the MSI-associated CpG island methylator phenotype (CIMP).8, 38 Strong interleukin-12–mediated signaling signatures suggested a robust immune cell presence in this subtype. In contrast, MSI-subtype tumors tended to occur in female patients, diagnosed at advanced ages, and characterized by elevated mutation rates, including mutations of genes encoding targetable oncogenic signaling proteins. The genomically stable subtype lacked numerous molecular alterations, correlated well with the Lauren diffuse histologic variant, but harbored mutations of RHOA or fusions involving RHO-family guanosine triphosphatase (GTPase)–activating proteins. The active GTP-bound form of RHOA activates STAT3 to promote tumorigenesis.39 Finally, CIN subtype tumors were frequent at the gastroesophageal junction/cardia, correlated well with the Lauren intestinal histologic variant, showed marked aneuploidy, and harbored focal amplifications of receptor tyrosine kinases (RTKs), in addition to recurrent TP53 mutations and RTK-RAS activation.8
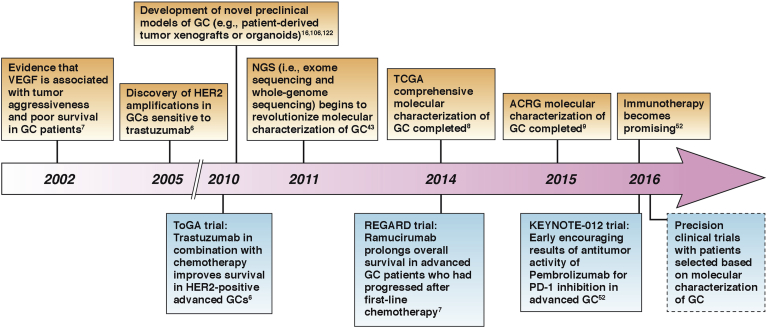
Figure 1.
Timeline of selected major developments in GC (above arrow) and related clinical trials (below arrow) in recent years.
In 2015, the Asian Cancer Research Group proposed a new classification system associated with distinct genomic alterations, disease progression, and prognosis across multiple GC cohorts.9 On the basis of whole-genome sequencing, gene expression profiling, genome-wide copy number microarrays, and targeted gene sequencing, 4 molecular subtypes were identified: (1) MSI, (2) microsatellite stable with epithelial-to-mesenchymal transition features (MSS/EMT), (3) MSS/TP53 mutant (MSS/TP53+), and (4) MSS/TP53 wild-type (MSS/TP53–).9 One strong point of this study was the availability of long-term follow-up data, enabling association of this molecular classification with clinical outcome. The postoperative surveillance program for recurrence in the Asian Cancer Research Group study consisted of performing follow-up exams every 6 months until 5 years after the date of surgery.9 MSI tumors were hypermutated, intestinal, usually antral, and diagnosed at clinical stage I/II. MSI tumors had the best prognosis; their recurrence rate after surgical resection of primary GC was the lowest among all 4 subtypes (22%). MSS/TP53+ tumors were linked to EBV infection and had the next best prognosis, followed by MSS/TP53- tumors. MSS/EMT tumors occurred at a younger age, mostly diagnosed at clinical stage III/IV, and were the Lauren diffuse histologic type. The MSS/EMT subtype had the worst prognosis and the highest recurrence rate (63%), with recurrences located mostly in the peritoneal cavity.9
Continuing to refine molecular classification, regular mutated (2.4 mutations/megabase; range, 0–8.3) and hypermutated (20.5 mutations/megabase; range, 9.6–200.2) GC types were identified in another recent study.10 The regular mutated type was further subclassified into 2 subgroups, C1 and C2. The first subgroup, C1, was enriched in mutations of TP53, XIRP2, and APC and was associated with a significantly better prognostic outcome, whereas C2 was overrepresented by mutations in ARID1A, CDH1, PIK3CA, ERBB2, and RHOA. Furthermore, consistent with the Asian Cancer Research Group study, this research team observed that CDH1 mutations were associated with a worse outcome in diffuse-type GC, independent of disease stage.10 Because ARID1A is frequently mutated in both EBV and MSI subtypes, its mutation alone is not likely to constitute an alternative GC pathway.
Molecular Genetic Profiling of Gastric Cancer
Somatic Mutations in Gastric Cancer
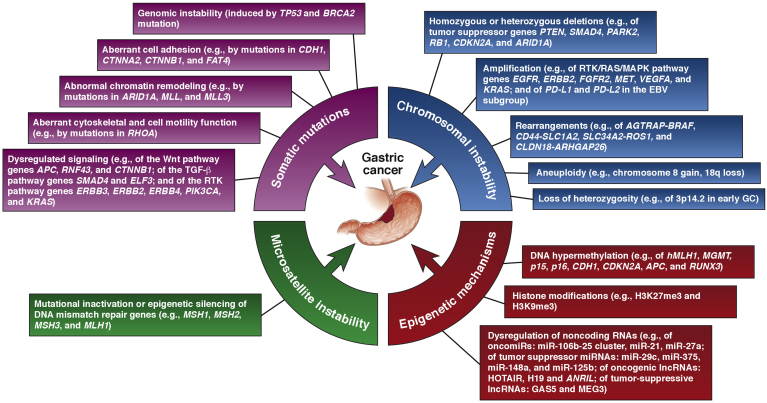
The mutational landscape of GC has been deciphered by using large-scale analyses of data from genomic, expressional, and mutational profiling studies, especially potential clinically relevant driver mutations (Figure 2). Approximately 16.4% of GC cases exhibit hyperdense mutation frequencies.10
Figure 2.
Genetic and epigenetic characteristics of GCs.
Genomic instability, and thus mutability, endows cells with genetic alterations, which in turn aid tumorigenesis and tumor progression.40 As the guardian of the genome, TP53 plays a central role in maintenance of genome integrity.41 TP53 mutations allow the accumulation of genetic alterations, leading to genomic instability. As the most frequently mutated gene, TP53 mutations occurred in about 50% of GC and 71% of CIN subtype tumors, on the basis of TCGA data.8 BRCA2 is also involved in maintaining genomic integrity. BRCA2 mutations were identified in 8% of the Tianjin (northern Chinese) cohort, validated in the TCGA cohort, and associated with significantly better survival.37
Recurrent somatic mutations in cell adhesion genes (eg, CDH1, CTNNA2, CTNNB1, and FAT4) and chromatin remodeling genes (eg, ARID1A, MLL, and MLL3) have been identified as the most commonly perturbed pathways in GC.8, 9, 10, 42, 43, 44 Among mutated genes in GC exomes, cell adhesion was the most significantly enriched biological process, consistent with a marked tendency of GC toward loss of cell-cell adhesion.42, 43 CDH1 mutations occurred in 11.6% of regular mutated GC and constituted a significant negative prognostic factor in diffuse-type GC, because they were associated with shortened patient survival independent of TNM staging.10 Nineteen percent to 40% of HDGC families have germline CDH1 mutations.32 CTNNA2, encoding a component of cell adhesion complexes, was mutated in 6.4% of MSS-subtype GCs,44 whereas CTNNB1 was mutated in 3.1% of regular mutated GCs.10 Additional CTNNA2 and CTNNB1 functions include regulation of β-catenin signaling during early embryonic development.8, 44 ARID1A encodes a member of the SWI-SNF protein complex, which represses gene activity via chromatin remodeling. An interesting finding was the inverse relationship between ARID1A and TP53; TP53 mutations were uncommon in both MSI and EBV subtypes, which possessed frequent ARID1A mutations (83% and 73%, respectively).43 Moreover, ARID1A mutations were associated with better prognosis in a stage-independent manner. Thus, independent of TP53, mutation of ARID1A, encoding a chromatin-remodeling enzyme, may constitute an alternative GC developmental pathway, with distinct clinical behavior.42, 43
RHOA, belonging to the Rho GTP family, mediates anoikis, focal adhesions, and cell adherens junctions.35, 44 Residues Arg5, Tyr42, Gly17, and Leu57 are RHOA mutational hotspots.8, 35, 44 RHOA mutation, specifically in the diffuse-type or GS subtypes, showed a predilection for the antrum and body, with fewer TP53 mutations.8, 35, 44
Dysregulated signaling, including the canonical Wnt, transforming growth factor (TGF)-β, and RTK pathways, in each of which several mutated genes are involved, has also been identified on the basis of NGS data. APC and RNF43 are associated with truncating or inactivating mutations as negative regulators of the Wnt pathway.8, 44 CTNNB1 encodes β-catenin; its mutation enhances resistance of intranuclear β-catenin to degradation, leading to continuous Wnt signaling.8, 45
Wnt pathway-mutated GCs exhibited longer survival than CDH1-mutated GCs in Chinese patients.37 Mutations of SMAD4 and ELF3, in the TGF-β pathway, were critical drivers in both the MSI and MSS subtypes.44 ERBB3 mutations occurred principally in hypermutated GC, with recurrent mutation sites or COSMIC-reported sites, whereas ERBB2 was significantly mutated in non-hypermutated GC, occurring at known hotspots.8 ERBB4 and its ligand NRG1 were mutated in 10% of GC; ERBB4 mutations occurred in both the receptor and kinase domains, activating both ERBB4 and PI3K-AKT signal transduction.37 PIK3CA was frequently mutated in EBV-positive or MSI subtypes.8, 9 KRAS, an important downstream molecule in RTK signaling, was mutated in MSI subtype tumors.9
Chromosomal Instability
The dynamic process of CIN constitutes a major event in tumor progression. By involving gains or losses of entire chromosomes or fractions of chromosomes, CIN leads to altered DNA copy number (aneuploidy), amplification, deletion, loss of heterozygosity (LOH), or rearrangement. CIN is common in GC; gastric tumors with a high level of CIN, rather than with TP53 mutation, were more likely to benefit from cisplatin-based neoadjuvant chemotherapy.46 Specific gains or losses of chromosomes are also associated with tumor type and progression. Frequent chromosome 8 gain occurred in MSI subtype, whereas 18q loss prevailed in EBV-positive GC.44 Copy number gains at 8q, 17q, and 20q occurred in intestinal-type GC, whereas gains at 12q and 13q occurred in diffuse-type tumors.47, 48 Gains at 8q23.3 and 8q23.2 were most frequently associated with early GC, whereas gains at 8q24.21 and 8q24.3 were associated with advanced GC.49 Finally, gain of 1q32.3 and loss of 18q22.1 were associated with poor clinical outcome.50
Frequent focal amplifications of EGFR, ERBB2, FGFR2, MET, VEGFA, and KRAS are involved in RTK/RAS/MAPK signaling and were associated with increased tumor cell proliferation, differentiation, apoptosis, adhesion, and migration.8, 9, 51 The utilization of trastuzumab, a monoclonal antibody targeting ERBB2, is a successful example of translational genetic profiling in GC. One international randomized controlled trial (ToGA) demonstrated that human epidermal growth factor 2–positive advanced GC patients exhibited prolonged survival when treated with trastuzumab plus chemotherapy.6 Recurrent amplifications at 9p24.1, the locus containing CD274 and PDCD1LG2, which encode the immunosuppressant proteins PD-L1 and PD-L2, were enriched in the EBV subgroup.8 A recent open-label phase 1b trial (KEYNOTE-012) demonstrated manageable toxicity and promising antitumor activity of anti–PD-1 antibody (pembrolizumab) in advanced GC.52
Additional amplified genes in GC comprise oncogenic transcription factors, including MYC, GATA4, GATA6, and KLF5, and cell-cycle regulators such as CCNE1, CCND1, and CDK6.8, 9, 51, 53
Focal genomic deletions in tumor suppressor genes such as PTEN, SMAD4, PARK2, RB1, CDKN2A, and ARID1A have been identified in GC.8, 9, 51 LOH pattern also differed significantly between early and advanced GCs; the most frequent LOH was at 3p14.2 in early GCs, whereas in advanced GCs the most frequent LOH occurred at 11q24.3-25, 11q23.2-24.1, 11q14.1, and 12p11.21-13.33.49
Although the creation of fusion genes via genomic rearrangement is less reported in solid than in liquid tumors, fusion genes have been identified in GC. In one study, approximately 1%–2% of GCs harbored RAF pathway gene rearrangements wherein exon 8 of BRAF was fused with exon 5 of AGTRAP.54 CD44-SLC1A2 and SLC34A2-ROS1 fusions were also identified in GCs.55, 56 Interchromosomal rearrangements between claudin 18 (CLDN18) and Rho GTPase-activating protein 6 (ARHGAP26) were exclusively identified in the GS subtype in the TCGA study8; this fusion resulted in impaired epithelial integrity and wound healing.57
Microsatellite Instability
MSI is characterized by alterations in length within short repeated DNA sequences (microsatellites), resulting from mutational inactivation or epigenetic silencing of DNA mismatch repair genes (eg, MSH1, MSH2, MSH3, and MLH1).58 These mutations include coding region frameshift mutations caused by MSI, which can drive oncogenesis by inactivating tumor-suppressor genes or disrupting other noncoding regulatory sequences.59, 60 MSI, a major type of genetic instability in cancer, occurs in a substantial portion of GCs.61, 62, 63 On the basis of the frequency of mutations within microsatellite markers, GC can be classified as MSS or MSI, which includes high-frequency (MSI-H) and low-frequency subtypes. In Korean GC-derived cell lines and primary tissues, the number of microsatellite mutations causing insertions or deletions in gene-encoding regions was 4-fold to 6-fold higher in MSI-H than in MSS samples, which was based on genome-wide and transcriptome-wide analyses of mutations associated with MSI.62 MSI-H GC is characterized by older age, mostly female, distal location, and better survival.64 Mismatch repair–deficient cancers may stimulate the immune system, making them susceptible to immune checkpoint blockade therapy. This theory was confirmed in a recent clinical trial in which patients with MSI-positive tumors across 6 cancer types, including 1 case of GC treated with a PD-1 inhibitor (pembrolizumab), had improved outcomes.65
Epigenetic Mechanisms
Epigenetic mechanisms play important roles in the pathogenesis of GC, including DNA methylation (regional hypermethylation and global hypomethylation), histone modification, chromatin remodeling, and dysregulation of noncoding RNAs (eg, miRNAs or lncRNAs). Gene silencing mediated by regional hypermethylation in gene promoter regions, almost exclusively associated with CpG islands, has been widely studied in GC.66, 67, 68 Some silenced genes play key roles in malignant transformation, affecting expression of various proteins or noncoding RNAs. Tumor-suppressor genes including hMLH1, MGMT, p15, p16, CDH1, CDKN2A, APC, RUNX3, DAPK, and BNIP3, which are involved in DNA repair, cell-cycle control, cell adhesion/invasion, cell proliferation, and apoptosis, are inactivated by promoter methylation.66, 69 hMLH1 inactivation by promoter hypermethylation is a predominant cause of DNA mismatch repair deficiency and results in frequent MSI in sporadic cancers.9, 66 The majority of MSI-H GCs have hMLH1 hypermethylation, whereas this alteration is uncommon in MSS GCs.66 Methylation profiles also differ between the diffuse and intestinal types of GC; for example, CDH1 and p14 hypermethylation occurred more frequently in the diffuse type, whereas p16 hypermethylation occurred mostly in the intestinal type.66 Several tumor-suppressor miRNAs, including miR-124a, miR-199a, miR-34b, and miR-129, were silenced by DNA hypermethylation of their promoter CpG islands in GC.70, 71, 72 Hypermethylation may also determine GC prognosis. In one study, methylation of GFRA1, SRF, and ZNF382 was associated with a low risk of metastasis and longer overall survival in GC patients.73 Another study that used genome-wide methylation analysis in the gastric CIMP phenotype demonstrated that CIMP GCs were associated with widespread hypermethylation, young patient age, and worse survival outcomes, independent of tumor stage.68 CIMP GCs tended to harbor mutations in the oncogenes CTNNB1, ERBB2, KRAS, and PIK3CA.74 Similarly, reduced expression of hypermethylated BRINP1, SGCE, and MLH1 was significantly associated with favorable survival in GC.75 Moreover, infectious agents such as H pylori, EBV, and the infection-associated inflammatory response all induce altered DNA methylation.76, 77, 78 EBV-positive GCs exhibited extreme CIMP, confirmed by the TCGA study.8
Histone modifications including methylation, acetylation, phosphorylation, and ubiquitination can affect gene expression in several cancer types.79, 80 The tumor suppressor RUNX3 is hypoxically silenced by histone modification during GC progression.81 In one study comparing H3K27me3 variations at the genome-wide level by ChIP-chip, 128 genes displayed significant H3K27me3 differences between GC and adjacent normal tissue.82 Similarly, hundreds of altered promoters and predicted enhancers were identified by comparing multiple histone modifications in 5 GCs and matched normal tissues, on the basis of nanoscale chromatin immunoprecipitation sequencing.83 Finally, high levels of H3K9me3 trimethylation were associated with poor outcome in GC patients.84
There is accumulating evidence suggesting that epigenetic and genetic defects in noncoding RNAs play crucial roles in tumor initiation, progression, invasion, and metastasis. This finding is particularly evident for miRNAs and lncRNAs. Overexpressed oncogenic microRNAs that target tumor suppressor genes can promote progression, resist apoptotic signals, and promote cell invasion and tumor metastasis.11, 71 Upregulation of the miR-106b-25 cluster dysregulates E2F1 activity and impairs TGF-β–dependent cell-cycle arrest and apoptosis by suppressing p21 and Bim in GC.85 Similarly, by directly inhibiting expression of RECK and PTEN, overexpressed miR-21 leads to enhanced cell proliferation and invasion.86, 87 Overexpression of miR-27a is associated with metastasis of GC to lymph nodes.88 In contrast, tumor suppressor miRNAs such as miR-29c, miR-375, miR-148a, and miR-125b are downregulated in GC, targeting ITGB1, JAK2, ROCK1, and MCL1, respectively.89, 90, 91, 92, 93
LncRNAs comprise another type of RNA that contributes to cancer, with oncogenic or tumor-suppressive functions. Knockdown of the oncogenic lncRNA HOTAIR reduces invasiveness and reverses epithelial-mesenchymal transition in GC cells, and high expression levels of HOTAIR are significantly associated with advanced tumor stage, lymph node metastasis, and poor survival in GC patients.94 Similarly, overexpression of the lncRNA H19 enhances carcinogenesis and metastasis mediated by direct upregulation of ISM1 and indirect suppression of CALN1 expression via miR-675 in GC.95 LncRNA H19, which induces cell proliferation, can also function via TP53 inactivation and apoptosis inhibition.96 Moreover, high ANRIL expression correlated significantly with advanced TNM stage and poor overall survival, promoting proliferation of GC cells by epigenetically silencing miR-99a/miR-449a.97 Finally, decreased expression of the tumor-suppressive lncRNAs GAS5 and MEG3 indicated a poor prognosis and promoted cell proliferation in GC,98, 99 whereas conversely, ectopic expression of MEG3 inhibited GC cell proliferation, promoted apoptosis, and modulated TP53 expression.99
More recently, circular RNAs, characterized by the formation of a covalently closed continuous RNA loop, are drawing renewed attention in cancer research.100, 101 Moreover, noncoding RNAs are stable in bodily fluids such as serum, plasma, gastric juice, and even in exosomes, making them promising GC biomarkers.102, 103, 104, 105
Preclinical Models of Gastric Cancer
The development of targeted anti-tumor drugs has been hampered by a paucity of effective preclinical models that can reliably reflect the complexity and heterogeneity of human tumors. In this context, PDX and organoids are attractive as offering effective tools for genetic evolution studies, biomarker identification, drug screening, and preclinical evaluation of personalized medicine strategies.16, 106
PDX models of human GC constructed by using subcutaneous or orthotopic implantation of surgical tissues or gastroscopic biopsies have the advantage of recapitulating most of the histology and somatic genetics of primary patient tumors.107, 108, 109, 110 Moreover, orthotopic implantation of intact GC tissue can lead to primary and metastatic tumor growth mimicking that seen in patients.107 In one study using PDX models generated by subcutaneous implantation, CD44v8-10 was verified as a GC stem cell marker.111 In another study, in vivo high-throughput screening using a 1 × 1 × 1 experimental design (a “one animal per model per treatment” approach) with PDX models assessed population responses to 62 treatments across 6 indications including GC112; these latter data demonstrated the reproducibility and clinical translatability of PDX clinical trials by identifying associations between a genotype and a drug response and established mechanisms of resistance.112 Similarly, on the basis of genomically defined GC PDX models, combination therapy of irinotecan with a BCL2L1-targeted drug was confirmed to effectively reduce tumor size.113 Moreover, inhibition of fibroblast growth factor receptor 2 signaling by AZD4547 induced dose-dependent GC regression in FGFR2-amplified PDX models (SGC083).114 PDX models can also be used to provide a surrogate assessment of efficacy and histologic investigation for tumor immunotherapy. In PDX models with transferred peripheral blood mononuclear cells from the same patient, combination therapy with anti-hCD137 and anti-hPD1 antibodies (urelumab and nivolumab, respectively) significantly slowed tumor growth.115 Although these data are promising, practical challenges remain to improving use of PDX models in cancer research, including delay between murine engraftment time and patient treatment schedule, lymphomagenesis of human tumors in mice, and cost.16, 116 In one large breast cancer PDX model study, even within the first murine passage, all models showed moderate drift to dramatic clonal selection during tumor growth.117
Organoids are miniature replicas of tissues cultured three-dimensionally in a semi-solid extracellular matrix and growth factor–enriched medium.118, 119 Organoids sustain high levels of architectural and physiological similarity to native organ systems, superior to traditional two-dimensional homogeneous cell lines.120 Additional advantages of organoids are that they are self-organizing, easy to handle, acceptable in cost, accessible to genetic engineering, and amenable to large-scale drug screening with shorter turnaround times.14, 121
Gastric organoids can be developed from pyloric glands and Lgr5+ve stem cells,122 from corpus glands and Troy+ chief cells,123 or from human pluripotent stem cells (hPSCs).13 Most recently, Wnt/β-catenin signaling was shown to promote gastric fundus specification in organoids developed from hPSCs.17 In one study using microinjection, gastric organoids mounted a nuclear factor kappa B–driven inflammatory response to H pylori infection, and the strength of this response depended on the differentiated cell types contained within the organoids.124 A quasi-immortal tissue culture model has also been developed in the form of gastric spheroids, which can serve as a suitable model of H pylori infection because of the formation of dense planar cultures of polarized epithelial cells after being transferred into two-dimensional culture.125 In a study using pluripotent stem cell–derived gastric organoids to model human disease pathogenesis, H pylori induced robust activation via tyrosine phosphorylation of c-Met and a 2-fold increase in epithelial cell proliferation. Cytotoxin-associated gene A played a pivotal role in this process, forming a complex with the c-Met receptor.13 In another related study, gastric organoids exhibited dysplasia and readily generated adenocarcinomas in mice characterized by activating mutations in KRAS or loss of TP53.126 The potential metastatic role of TGFBR2 loss-of-function mutations was shown in CDH1–/–;TP53–/– murine epithelial-mesenchymal organoids used to model hereditary GC, with short hairpin RNA knockdown of TGFBR2.127 A critical role of RHOA function in mediating anoikis in diffuse-type gastric carcinogenesis was confirmed in mouse intestinal organoids containing stably expressed RHOA mutations.44 Thus, organoids constitute a robust model system that may facilitate personalized therapy development by enabling high-throughput drug screening to identify gene-drug associations, as well as by testing specific individual responses to different therapeutic agents.128
Precision Medicine in Gastric Cancer
Although thus far only 2 targeted molecular therapeutic agents, trastuzumab and ramucirumab, have been approved by the Food and Drug Administration, a better and deeper understanding of genomic and epigenomic characteristics of GC, coupled with analyses of cancer phenotypes by using novel preclinical models, will hopefully lead to treatment optimization in the appropriate patient at the appropriate time (Figure 3). New molecular classification systems now provide a critical basis for the design of precision medicine clinical trials. Similarly, continuously updated molecular genetic profiling of GC has yielded promising new therapeutic targets such as RTKs or RAS and PI(3)-kinase signaling proteins.8, 9, 129 Although various new agents are still being investigated for targeted GC therapy, several ongoing clinical trials are already targeting STAT3, c-MET, mTOR, CLDN18.2, and PD-1/PD-L1.52, 130, 131 Some early results of these trials have been encouraging, particularly the multicenter phase 1b trial of pembrolizumab (KEYNOTE-012), which showed durable remissions in a subset of patients with PD-L1–positive advanced GC identified by using a prototype assay (sequencing was not performed in that study).52 Meanwhile, targeting epigenetic regulators such as methyltransferases, demethylases, and miRNAs for cancer therapy are also under investigation. One ongoing clinical trial will evaluate combination therapy with vorinostat (a histone deacetylase inhibitor) and radiotherapy in GC (ClinicalTrials.gov identifier: NCT01045538).132 Furthermore, integration of GC genotype and phenotype by using PDX and patient-derived organoid models promises to assist clinical trial design and personalized medicine strategies in the future.16, 106 However, much work remains to be done, considering that trastuzumab is thus far the only biomarker-driven therapy in clinical practice and especially in view of numerous negative clinical trials such as the LOGiC and TyTan trials of lapatinib (a tyrosine kinase inhibitor targeting HER2 and EGFR),133, 134 the RILOMET-1 trial of rilotumumab, and the METGastric trial of onartuzumab (targeting c-MET).135, 136 Notably, in this context, it should be emphasized that ramucirumab is not a biomarker-driven drug.137
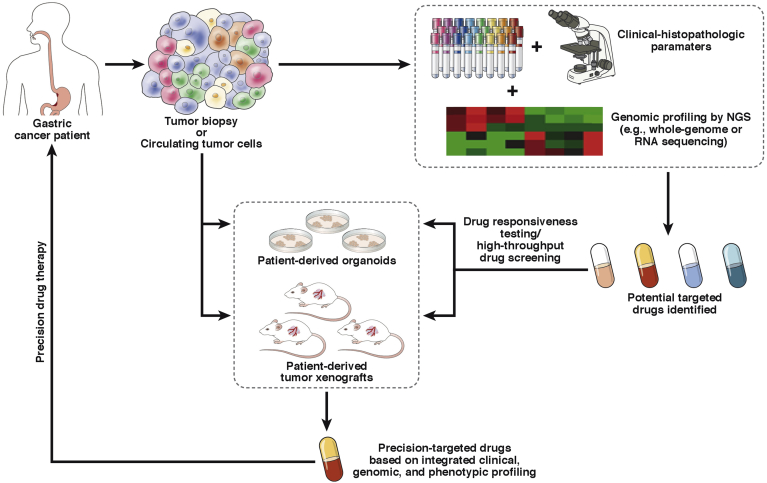
Figure 3.
Integrated precision medicine strategy for GC. Tumor specimens obtained by biopsy or circulating tumor cells are subjected to clinical-histopathologic examination and genomic analysis by NGS. Potential targetable genomic alterations are identified. Simultaneously, patient-derived tumor models (eg, xenografts or organoids) are established from the same specimens. Effective, safe therapies are then chosen by using high-throughput screening in these model systems. Finally, the patient receives optimized therapy based on this integrated strategy.
Conclusions
Because of the pronounced interpatient and intratumor heterogeneity of GC, the current uniform treatment strategy used in virtually all patients seems suboptimal. With the advent of NGS and novel preclinical model strategies, the study and treatment of GC are undergoing a radical shift toward precision medicine. Recent genomic and epigenomic profiling studies are now beginning to form a crucial foundation on which to build both improved molecular understanding of and better targeted therapies for GC. Combined with new PDX and organoid models, integrated traditional, genome-based and phenotype-based strategies promise to open new vistas for precision medicine applications in GC.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Dr Meltzer is an American Cancer Society Clinical Research Professor, the Myerberg/Hendrix Professor of Gastroenterology at Johns Hopkins, and is supported by USPHS grants CA190040 and CA211457. Xi Liu is supported by the Key Science and Technology Program of Shaanxi Province, China, 2015SF128.
References
- 1.Fitzmaurice C., Dicker D., Pain A. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre L.A., Bray F., Siegel R.L. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Anderson W.F., Camargo M.C., Fraumeni J.F. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauren P. The two histological main types of gastric carcinoma: diffuse and so called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Bang Y.J., Van Cutsem E., Feyereislova A. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs C.S., Tomasek J., Yong C.J. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 8.The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristescu R., Lee J., Nebozhyn M. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 10.Li X., Wu W.K., Xing R. Distinct subtypes of gastric cancer defined by molecular characterization include novel mutational signatures with prognostic capability. Cancer Res. 2016;76:1724–1732. doi: 10.1158/0008-5472.CAN-15-2443. [DOI] [PubMed] [Google Scholar]
- 11.Song J.H., Meltzer S.J. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143:35–47. doi: 10.1053/j.gastro.2012.05.003. e2. [DOI] [PubMed] [Google Scholar]
- 12.Fang X.Y., Pan H.F., Leng R.X. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett. 2015;356:357–366. doi: 10.1016/j.canlet.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 13.McCracken K.W., Catá E.M., Crawford C.M. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachs N., Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73. doi: 10.1016/j.gde.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Merker S.R., Weitz J., Stange D.E. Gastrointestinal organoids: how they gut it out. Dev Biol. 2016;420:239–250. doi: 10.1016/j.ydbio.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo M., Amant F., Biankin A.V. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCracken K.W., Aihara E., Martin B. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa P., Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19:S37–S43. [PubMed] [Google Scholar]
- 20.Bouvard V., Baan R., Straif K. A review of human carcinogens: part B—biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 21.Moss S.F. The clinical evidence linking Helicobacter pylori to gastric cancer. Cell Mol Gastroenterol Hepatol. 2017;3:183–191. doi: 10.1016/j.jcmgh.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plummer M., Franceschi S., Vignat J. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Wadhwa R., Song S., Lee J.S. Gastric cancer: molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham D.Y. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–731.e3. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton J.P., Meltzer S.J. A review of the genomics of gastric cancer. Clin Gastroenterol Hepatol. 2006;4:416–425. doi: 10.1016/j.cgh.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Chow W.H., Blaser M.J., Blot W.J. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–590. [PubMed] [Google Scholar]
- 28.Chen J.N., He D., Tang F. Epstein-Barr virus-associated gastric carcinoma: a newly defined entity. J Clin Gastroenterol. 2012;46:262–271. doi: 10.1097/MCG.0b013e318249c4b8. [DOI] [PubMed] [Google Scholar]
- 29.Hino R., Uozaki H., Murakami N. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69:2766–2774. doi: 10.1158/0008-5472.CAN-08-3070. [DOI] [PubMed] [Google Scholar]
- 30.Shinozaki-Ushiku A., Kunita A., Fukayama M. Update on Epstein-Barr virus and gastric cancer (review) Int J Oncol. 2015;46:1421–1434. doi: 10.3892/ijo.2015.2856. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira C., Pinheiro H., Figueiredo J. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60–e70. doi: 10.1016/S1470-2045(14)71016-2. [DOI] [PubMed] [Google Scholar]
- 32.Hansford S., Kaurah P., Li-Chang H. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1:23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 33.Worthley D., Phillips K., Wayte N. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut. 2012;61:774–779. doi: 10.1136/gutjnl-2011-300348. [DOI] [PubMed] [Google Scholar]
- 34.McLean M.H., El-Omar E.M. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 35.Kakiuchi M., Nishizawa T., Ueda H. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–587. doi: 10.1038/ng.2984. [DOI] [PubMed] [Google Scholar]
- 36.Wong S.S., Kim K.M., Ting J.C. Genomic landscape and genetic heterogeneity in gastric adenocarcinoma revealed by whole-genome sequencing. Nat Commun. 2014;5:5477. doi: 10.1038/ncomms6477. [DOI] [PubMed] [Google Scholar]
- 37.Chen K., Yang D., Li X. Mutational landscape of gastric adenocarcinoma in Chinese: implications for prognosis and therapy. Proc Natl Acad Sci U S A. 2015;112:1107–1112. doi: 10.1073/pnas.1422640112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geddert H., Zur Hausen A., Gabbert H.E. EBV-infection in cardiac and non-cardiac gastric adenocarcinomas is associated with promoter methylation of p16, p14 and APC, but not hMLH1. Anal Cell Pathol (Amst) 2010;33:143–149. doi: 10.3233/ACP-CLO-2010-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H., Jove R. The STATs of cancer: new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Lane D.P. Cancer: p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 42.Zang Z.J., Cutcutache I., Poon S.L. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 43.Wang K., Kan J., Yuen S.T. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–1223. doi: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 44.Wang K., Yuen S.T., Xu J. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y., Wu Z., Guo W. Gene mutations in gastric cancer: a review of recent next-generation sequencing studies. Tumour Biol. 2015;36:7385–7394. doi: 10.1007/s13277-015-4002-1. [DOI] [PubMed] [Google Scholar]
- 46.Ott K., Vogelsang H., Mueller J. Chromosomal instability rather than p53 mutation is associated with response to neoadjuvant cisplatin-based chemotherapy in gastric carcinoma. Clin Cancer Res. 2003;9:2307–2315. [PubMed] [Google Scholar]
- 47.Kim K.M., Kwon M.S., Hong S.J. Genetic classification of intestinal-type and diffuse-type gastric cancers based on chromosomal loss and microsatellite instability. Virchows Arch. 2003;443:491–500. doi: 10.1007/s00428-003-0840-0. [DOI] [PubMed] [Google Scholar]
- 48.Wu M.S., Chang M.C., Huang S.P. Correlation of histologic subtypes and replication error phenotype with comparative genomic hybridization in gastric cancer. Genes Chromosomes Cancer. 2001;30:80–86. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1062>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Arakawa N., Sugai T., Habano W. Genome-wide analysis of DNA copy number alterations in early and advanced gastric cancers. Mol Carcinog. 2017;56:527–537. doi: 10.1002/mc.22514. [DOI] [PubMed] [Google Scholar]
- 50.Weiss M.M., Kuipers E.J., Postma C. Genomic alterations in primary gastric adenocarcinomas correlate with clinicopathological characteristics and survival. Cell Oncol. 2004;26:307–317. doi: 10.1155/2004/454238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng N., Goh L.K., Wang H. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muro K., Chung H.C., Shankaran V. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 53.Chia N.-Y., Deng N., Das K. Regulatory crosstalk between lineage-survival oncogenes KLF5, GATA4 and GATA6 cooperatively promotes gastric cancer development. Gut. 2015;64:707–719. doi: 10.1136/gutjnl-2013-306596. [DOI] [PubMed] [Google Scholar]
- 54.Palanisamy N., Ateeq B., Kalyana-Sundaram S. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nature Med. 2010;16:793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao J., Deng N.T., Ramnarayanan K. CD44-SLC1A2 gene fusions in gastric cancer. Sci Transl Med. 2011;3:77ra30. doi: 10.1126/scitranslmed.3001423. [DOI] [PubMed] [Google Scholar]
- 56.Lee J., Lee S.E., Kang S.Y. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer. 2013;119:1627–1635. doi: 10.1002/cncr.27967. [DOI] [PubMed] [Google Scholar]
- 57.Yao F., Kausalya J.P., Sia Y.Y. Recurrent fusion genes in gastric cancer: CLDN18-ARHGAP26 induces loss of epithelial integrity. Cell Rep. 2015;12:272–285. doi: 10.1016/j.celrep.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 58.Hause R.J., Pritchard C.C., Shendure J. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342–1350. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 59.Kim T.M., Park P.J. A genome-wide view of microsatellite instability: old stories of cancer mutations revisited with new sequencing technologies. Cancer Res. 2014;74:6377–6382. doi: 10.1158/0008-5472.CAN-14-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mori Y., Yin J., Rashid A. Instabilotyping comprehensive identification of frameshift mutations caused by coding region microsatellite instability. Cancer Res. 2001;61:6046–6049. [PubMed] [Google Scholar]
- 61.Rhyu M.G., Park W.S., Meltzer S.J. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994;9:29–32. [PubMed] [Google Scholar]
- 62.Yoon K., Lee S., Han T.S. Comprehensive genome- and transcriptome-wide analyses of mutations associated with microsatellite instability in Korean gastric cancers. Genome Res. 2013;23:1109–1117. doi: 10.1101/gr.145706.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.David S., Meltzer S.J. Stomach-genetic and epigenetic alterations of preneoplastic and neoplastic lesions. Cancer Biomark. 2011;9:493–507. doi: 10.3233/CBM-2011-0169. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto H., Perez-Piteira J., Yoshida T. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology. 1999;116:1348–1357. doi: 10.1016/s0016-5085(99)70499-3. [DOI] [PubMed] [Google Scholar]
- 65.Le D.T., Uram J.N., Wang H. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato F., Meltzer S.J. CpG island hypermethylation in progression of esophageal and gastric cancer. Cancer. 2006;106:483–493. doi: 10.1002/cncr.21657. [DOI] [PubMed] [Google Scholar]
- 67.Toyota M., Ahuja N., Suzuki H. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 1999;59:5438–5442. [PubMed] [Google Scholar]
- 68.Zouridis H., Deng N., Ivanova T. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004504. 156ra140. [DOI] [PubMed] [Google Scholar]
- 69.Qu Y., Dang S., Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53–65. doi: 10.1016/j.cca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Ando T., Yoshida T., Enomoto S. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect. Int J Cancer. 2009;124:2367–2374. doi: 10.1002/ijc.24219. [DOI] [PubMed] [Google Scholar]
- 71.Ueda T., Volinia S., Okumura H. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai K.W., Wu C.W., Hu L.Y. Epigenetic regulation of miR-34b and miR-129 expression in gastric cancer. Int J Cancer. 2011;129:2600–2610. doi: 10.1002/ijc.25919. [DOI] [PubMed] [Google Scholar]
- 73.Liu Z., Zhang J., Gao Y. Large-scale characterization of DNA methylation changes in human gastric carcinomas with and without metastasis. Clin Cancer Res. 2014;20:4598–4612. doi: 10.1158/1078-0432.CCR-13-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim J.G., Takeshima H., Niwa T. Comprehensive DNA methylation and extensive mutation analyses reveal an association between the CpG island methylator phenotype and oncogenic mutations in gastric cancers. Cancer Lett. 2013;330:33–40. doi: 10.1016/j.canlet.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 75.Sepulveda J.L., Gutierrez-Pajares J.L., Luna A. High-definition CpG methylation of novel genes in gastric carcinogenesis identified by next-generation sequencing. Mod Pathol. 2016;29:182–193. doi: 10.1038/modpathol.2015.144. [DOI] [PubMed] [Google Scholar]
- 76.Niwa T., Tsukamoto T., Toyoda T. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–1440. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- 77.Matsusaka K., Kaneda A., Nagae G. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 2011;71:7187–7197. doi: 10.1158/0008-5472.CAN-11-1349. [DOI] [PubMed] [Google Scholar]
- 78.Liang Q., Yao X., Tang S. Integrative identification of Epstein-Barr virus-associated mutations and epigenetic alterations in gastric cancer. Gastroenterology. 2014;147:1350–1362. e4. doi: 10.1053/j.gastro.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 79.Gigek C.O., Chen E.S., Calcagno D.Q. Epigenetic mechanisms in gastric cancer. Epigenomics. 2012;4:279–294. doi: 10.2217/epi.12.22. [DOI] [PubMed] [Google Scholar]
- 80.Chi P., Allis C.D., Wang G.G. Covalent histone modifications: miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10:457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S., Kim J., Kim W. Hypoxic silencing of tumor suppressor RUNX3 by histone modification in gastric cancer cells. Oncogene. 2009;28:184–194. doi: 10.1038/onc.2008.377. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L., Zhong K., Dai Y. Genome-wide analysis of histone H3 lysine 27 trimethylation by ChIP-chip in gastric cancer patients. J Gastroenterol. 2009;44:305–312. doi: 10.1007/s00535-009-0027-9. [DOI] [PubMed] [Google Scholar]
- 83.Muratani M., Deng N., Ooi W.F. Nanoscale chromatin profiling of gastric adenocarcinoma reveals cancer-associated cryptic promoters and somatically acquired regulatory elements. Nat Commun. 2014;5:4361. doi: 10.1038/ncomms5361. [DOI] [PubMed] [Google Scholar]
- 84.Park Y.S., Jin M.Y., Kim Y.J. The global histone modification pattern correlates with cancer recurrence and overall survival in gastric adenocarcinoma. Ann Surg Oncol. 2008;15:1968–1976. doi: 10.1245/s10434-008-9927-9. [DOI] [PubMed] [Google Scholar]
- 85.Petrocca F., Visone R., Onelli M.R. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Z., Li Z., Gao C. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88:1358–1366. doi: 10.1038/labinvest.2008.94. [DOI] [PubMed] [Google Scholar]
- 87.Zhang B.G., Li J.F., Yu B.Q. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019–1026. doi: 10.3892/or.2012.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katada T., Ishiguro H., Kuwabara Y. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–542. [PubMed] [Google Scholar]
- 89.Han T.S., Hur K., Xu G. MicroRNA-29c mediates initiation of gastric carcinogenesis by directly targeting ITGB1. Gut. 2015;64:203–214. doi: 10.1136/gutjnl-2013-306640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding L., Xu Y., Zhang W. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 91.Zheng B., Liang L., Wang C. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 92.Wu S., Liu F., Xie L. miR-125b suppresses proliferation and invasion by targeting MCL1 in gastric cancer. Biomed Res Int. 2015;2015:365273. doi: 10.1155/2015/365273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X., Peng Y., Jin Z. Integrated miRNA profiling and bioinformatics analyses reveal potential causative miRNAs in gastric adenocarcinoma. Oncotarget. 2015;6:32878–32889. doi: 10.18632/oncotarget.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Z.Y., Yu Q.M., Du Y.A. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H., Yu B., Li J. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang F., Bi J., Xue X. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhang E.B., Kong R., Yin D.D. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5:2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun M., Jin F.Y., Xia R. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun M., Xia R., Jin F. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35:1065–1073. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 100.Guarnerio J., Bezzi M., Jeong J.C. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 101.Chen L.L. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17:205–211. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 102.Liu H., Zhu L., Liu B. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196–203. doi: 10.1016/j.canlet.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 103.Shao Y., Ye M., Jiang X. Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer. 2014;120:3320–3328. doi: 10.1002/cncr.28882. [DOI] [PubMed] [Google Scholar]
- 104.Zhou X., Yin C., Dang Y. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci Rep. 2015;5:11516. doi: 10.1038/srep11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y., Zheng Q., Bao C. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 107.Furukawa T., Kubota T., Watanabe M. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 1993;53:608–612. doi: 10.1002/ijc.2910530414. [DOI] [PubMed] [Google Scholar]
- 108.Furukawa T., Fu X., Kubota T. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204–1208. [PubMed] [Google Scholar]
- 109.Zhang L., Yang J., Cai J. A subset of gastric cancers with EGFR amplification and overexpression respond to cetuximab therapy. Sci Rep. 2013;3:2992. doi: 10.1038/srep02992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu Y., Tian T., Li Z. Establishment and characterization of patient-derived tumor xenograft using gastroscopic biopsies in gastric cancer. Sci Rep. 2015;5:8542. doi: 10.1038/srep08542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lau W.M., Teng E., Chong H.S. CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 2014;74:2630–2641. doi: 10.1158/0008-5472.CAN-13-2309. [DOI] [PubMed] [Google Scholar]
- 112.Gao H., Korn J.M., Ferretti S. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21:1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 113.Park H., Cho S.-Y., Kim H. Genomic alterations in BCL2L1 and DLC1 contribute to drug sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2015;112:12492–12497. doi: 10.1073/pnas.1507491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie L., Su X., Zhang L. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572–2583. doi: 10.1158/1078-0432.CCR-12-3898. [DOI] [PubMed] [Google Scholar]
- 115.Sanmamed M.F., Rodriguez I., Schalper K.A. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rγnull immunodeficient mice. Cancer Res. 2015;75:3466–3478. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 116.Zhang L., Liu Y., Wang X. The extent of inflammatory infiltration in primary cancer tissues is associated with lymphomagenesis in immunodeficient mice. Sci Rep. 2015;5:9447. doi: 10.1038/srep09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eirew P., Steif A., Khattra J. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518:422–426. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sato T., Stange D.E., Ferrante M. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 119.Engevik A.C., Feng R., Choi E. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol Gastroenterol Hepatol. 2016;2:605–624. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dedhia P.H., Bertaux-Skeirik N., Zavros Y. Organoid models of human gastrointestinal development and disease. Gastroenterology. 2016;150:1098–1112. doi: 10.1053/j.gastro.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hill D.R., Spence J.R. Gastrointestinal organoids: understanding the molecular basis of the host-microbe interface. Cell Mol Gastroenterol Hepatol. 2017;3:138–149. doi: 10.1016/j.jcmgh.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barker N., Huch M., Kujala P. Lgr5+ ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 123.Stange D.E., Koo B.-K., Huch M. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bartfeld S., Bayram T., van de Wetering M. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126–136. doi: 10.1053/j.gastro.2014.09.042. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schlaermann P., Toelle B., Berger H. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65:202–213. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li X., Nadauld L., Ootani A. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. 2014;20:769–777. doi: 10.1038/nm.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nadauld L.D., Garcia S., Natsoulis G. Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer. Genome Biol. 2014;15:428. doi: 10.1186/s13059-014-0428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van de Wetering M., Francies H.E., Francis J.M. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuboki Y., Yamashita S., Niwa T. Comprehensive analyses using next-generation sequencing and immunohistochemistry enable precise treatment in advanced gastric cancer. Ann Oncol. 2016;27:127–133. doi: 10.1093/annonc/mdv508. [DOI] [PubMed] [Google Scholar]
- 130.Shitara K., Ohtsu A. Advances in systemic therapy for metastatic or advanced gastric cancer. J Natl Compr Canc Netw. 2016;14:1313–1320. doi: 10.6004/jnccn.2016.0138. [DOI] [PubMed] [Google Scholar]
- 131.Becerra C., Stephenson J., Jonker D.J. Phase Ib/II study of cancer stem cell (CSC) inhibitor BBI608 combined with paclitaxel in advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2015;33(suppl; abstr 4069) [Google Scholar]
- 132.Abdelfatah E., Kerner Z., Nanda N. Epigenetic therapy in gastrointestinal cancer: the right combination. Therap Adv Gastroenterol. 2016;9:560–579. doi: 10.1177/1756283X16644247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hecht J.R., Bang Y.J., Qin S.K. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. J Clin Oncol. 2015;34:443–451. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 134.Satoh T., Xu R.H., Chung H.C. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32:2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 135.Cunningham D., Tebbutt N.C., Davidenko I. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol. 2015;33:4000. [Google Scholar]
- 136.Shah M.A., Bang Y.-J., Lordick F. METGastric: a phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC) J Clin Oncol. 2015;33:4012. [Google Scholar]
- 137.Pectasides E. Genomic alterations and targeted therapy in gastric and esophageal adenocarcinoma. Clin Ther. 2016;38:1589–1599. doi: 10.1016/j.clinthera.2016.03.016. [DOI] [PubMed] [Google Scholar]