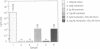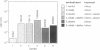Abstract
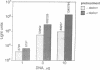
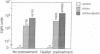
We have subverted a receptor-mediated endocytosis event to transport genes into human leukemic cells. By coupling the natural iron-delivery protein transferrin to the DNA-binding polycations polylysine or protamine, we have created protein conjugates that bind nucleic acids and carry them into the cell during the normal transferrin cycle [Wagner, E., Zenke, M., Cotten, M., Beug, H. & Birnstiel, M. L. (1990) Proc. Natl. Acad. Sci. USA 87, 3410-3414]. We demonstrate here that this procedure is useful for a human leukemic cell line. We enhanced the rate of gene delivery by (i) increasing the transferrin receptor density through treatment of the cells with the cell-permeable iron chelator desferrioxamine, (ii) interfering with the synthesis of heme with succinyl acetone treatment, or (iii) stimulating the degradation of heme with cobalt chloride treatment. Consistent with gene delivery as an endocytosis event, we show that the subsequent expression in K-562 cells of a gene included in the transported DNA depends upon the cellular presence of the lysosomotropic agent chloroquine. By contrast, monensin blocks "transferrinfection," as does incubation of the cells at 18 degrees C.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Hsi B. L., Stevens P. J. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980 Aug 23;2(8191):390–392. doi: 10.1016/s0140-6736(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebers H. A., Finch C. A. The physiology of transferrin and transferrin receptors. Physiol Rev. 1987 Apr;67(2):520–582. doi: 10.1152/physrev.1987.67.2.520. [DOI] [PubMed] [Google Scholar]
- Khazaie K., Dull T. J., Graf T., Schlessinger J., Ullrich A., Beug H., Vennström B. Truncation of the human EGF receptor leads to differential transforming potentials in primary avian fibroblasts and erythroblasts. EMBO J. 1988 Oct;7(10):3061–3071. doi: 10.1002/j.1460-2075.1988.tb03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. M., O'Dowd T., Driver M., Tee D. E. Demonstration of an epitope of the transferrin receptor in human cervical epithelium--a potentially useful cell marker. J Clin Pathol. 1984 Feb;37(2):131–135. doi: 10.1136/jcp.37.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- Muller-Eberhard U., Liem H. H., Grasso J. A., Giffhorn-Katz S., DeFalco M. G., Katz N. R. Increase in surface expression of transferrin receptors on cultured hepatocytes of adult rats in response to iron deficiency. J Biol Chem. 1988 Oct 15;263(29):14753–14756. [PubMed] [Google Scholar]
- Petrini M., Pelosi-Testa E., Sposi N. M., Mastroberardino G., Camagna A., Bottero L., Mavilio F., Testa U., Peschle C. Constitutive expression and abnormal glycosylation of transferrin receptor in acute T-cell leukemia. Cancer Res. 1989 Dec 15;49(24 Pt 1):6989–6996. [PubMed] [Google Scholar]
- Rossiello R., Carriero M. V., Giordano G. G. Distribution of ferritin, transferrin and lactoferrin in breast carcinoma tissue. J Clin Pathol. 1984 Jan;37(1):51–55. doi: 10.1136/jcp.37.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N. H., Maxfield F. R. Fusion accessibility of endocytic compartments along the recycling and lysosomal endocytic pathways in intact cells. J Cell Biol. 1989 Nov;109(5):2097–2104. doi: 10.1083/jcb.109.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Marshall J., Hayman M. J., Doderlein G., Beug H. Monoclonal antibodies to novel erythroid differentiation antigens reveal specific effects of oncogenes on the leukaemic cell phenotype. Leuk Res. 1986;10(3):257–272. doi: 10.1016/0145-2126(86)90023-8. [DOI] [PubMed] [Google Scholar]
- Shindelman J. E., Ortmeyer A. E., Sussman H. H. Demonstration of the transferrin receptor in human breast cancer tissue. Potential marker for identifying dividing cells. Int J Cancer. 1981 Mar 15;27(3):329–334. doi: 10.1002/ijc.2910270311. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Bensch K. G., Sussman H. H. Complete inhibition of transferrin recycling by monensin in K562 cells. J Biol Chem. 1984 Dec 10;259(23):14762–14772. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Testa U. Transferrin receptors: structure and function. Curr Top Hematol. 1985;5:127–161. [PubMed] [Google Scholar]
- Trowbridge I. S., Shackelford D. A. Structure and function of transferrin receptors and their relationship to cell growth. Biochem Soc Symp. 1986;51:117–129. [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. H., Jordan I., Kushner J. P., Kaplan J. Heme regulation of HeLa cell transferrin receptor number. J Biol Chem. 1984 Nov 10;259(21):13235–13240. [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988 Oct 15;263(29):14621–14624. [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987 Apr 5;262(10):4429–4432. [PubMed] [Google Scholar]
- Zenke M., Steinlein P., Wagner E., Cotten M., Beug H., Birnstiel M. L. Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3655–3659. doi: 10.1073/pnas.87.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]