Abstract
Human health is dependent on the ability of the body to extract nutrients, fluids, and oxygen from the external environment while at the same time maintaining a state of internal sterility. Therefore, the cell layers that cover the surface areas of the body such as the lung, skin, and gastrointestinal mucosa provide vital semipermeable barriers that allow the transport of essential nutrients, fluid, and waste products, while at the same time keeping the internal compartments free of microbial organisms. These epithelial surfaces are highly specialized and differ in their anatomic structure depending on their location to provide appropriate and effective site-specific barrier function. Given this important role, it is not surprising that significant disease often is associated with alterations in epithelial barrier function. Examples of such diseases include inflammatory bowel disease, chronic obstructive pulmonary disease, and atopic dermatitis. These chronic inflammatory disorders often are characterized by diminished tissue oxygen levels (hypoxia). Hypoxia triggers an adaptive transcriptional response governed by hypoxia-inducible factors (HIFs), which are repressed by a family of oxygen-sensing HIF hydroxylases. Here, we review recent evidence suggesting that pharmacologic hydroxylase inhibition may be of therapeutic benefit in inflammatory bowel disease through the promotion of intestinal epithelial barrier function through both HIF-dependent and HIF-independent mechanisms.
Keywords: Epithelial Barrier, Inflammatory Bowel Disease, Hypoxia, Hypoxia-Inducible Factor (HIF) Hydroxylases
Abbreviations used in this paper: CD, Crohn’s disease; DMOG, dimethyloxalylglycine; DSS, dextran sodium sulfate; FIH, factor inhibiting hypoxia-inducible factor; HIF, hypoxia-inducible factor; IBD, inflammatory bowel disease; IL, interleukin; NF-κB, nuclear factor-κB; PHD, hypoxia-inducible factor–prolyl hydroxylases; TFF, trefoil factor; TJ, tight junction; TLR, Toll-like receptor; TNF-α, tumor necrosis factor α; UC, ulcerative colitis; ZO, zonula occludens
Summary.
An effective epithelial barrier is key to intestinal homeostasis and barrier dysfunction underpins inflammatory bowel disease. Here, we review the role of oxygen-sensing hydroxylases in the regulation of intestinal barriers and their potential as novel therapeutic targets.
The human body constantly is exposed to a range of environmental threats including primary and opportunistic microbial pathogens that are capable of causing infectious disease. Although components of the immune system determine the organism’s ability to respond to microbial invasion, the first level of defense is the ability to prevent microbes from entering the body in the first place. This role is performed primarily by epithelial barriers, which are an important component of the innate immune system. In addition to providing barrier function, epithelia also are responsible for the absorption of nutrients and other macromolecules, and therefore epithelial barriers in different parts of the body differ significantly in their degree of permeability. For example, an occlusive and stratified epithelium is found in the skin, where protection is more critical than absorption, whereas more permeable monolayers are found in the gastrointestinal tract or the lung, where nutrient and/or fluid transport is critical.1, 2, 3, 4 The intestinal epithelium is a selectively permeable monolayer that allows the transport of appropriate solutes and nutrients from the diet to facilitate nutrition.1, 5, 6 A further characteristic of the intestinal epithelial surface is its direct contact with large numbers of bacteria. Interaction between the intestinal epithelium and the gut microbiota now is appreciated to play an important role in the maintenance of intestinal homeostasis, although the mechanisms underpinning this are complex and remain poorly understood.7, 8 In this review, the composition and functions of the intestinal epithelial barrier and how its malfunction is related to the development of inflammatory bowel disease (IBD).
The Intestinal Epithelial Barrier
The intestinal epithelium permits the traffic of nutrients from the lumen to the blood, while at the same time restricting the passage of potentially harmful microorganisms and toxins.9, 10 Two main characteristics are key in establishing and maintaining the epithelial barrier. First, a capacity for controlled cell renewal, which is achieved through a tightly regulated balance between epithelial cell proliferation and apoptosis, and, second, the presence of effective intercellular junctions.
Cell renewal is key for the maintenance of the epithelial barrier. Intestinal epithelial cells are replaced every 4–5 days,11 an impressive feat considering that the adult intestinal epithelium has a surface area of approximately 300 m2.12 The control of epithelial cell renewal is based on a continuous balance between proliferation and apoptosis-dependent cell death.11, 13, 14 Epithelial cells are generated from stem cell progenitors in the intestinal crypts and migrate from the crypt to the lumen-facing mucosal surface. The process of intestinal cell proliferation and migration is regulated through complex signaling events in which the Wnt pathway is a key player.11, 15 An equally balanced rate of epithelial cell death also is essential to maintain controlled renewal of the epithelial barrier. Alterations in the balance between proliferation and apoptosis are known to be involved in barrier dysfunction, which leads to disease. For example, in the context of IBD, the primed inflammatory environment can lead to the generation of pro-apoptotic signals and, consequently, to the activation of apoptotic pathways in epithelial cells.16, 17, 18 Ultimately, this compromises the capacity of the epithelium to maintain barrier integrity. This is in contrast to intestinal epithelial cancers in which epithelial proliferation exceeds the rate of apoptosis and promotes the development of intestinal tumors.19
Effective sealing of the intercellular space is a second factor necessary to fulfill the provision of a continuous intestinal epithelial barrier. This sealing property is defined primarily by the tight junctions, adherens junctions, and desmosomes.20 Adherens junctions and desmosomes are responsible for the maintenance of the proximity between cells through intercellular molecular connections, whereas tight junctions are responsible for sealing the paracellular space. Tight junctions consist of complexes formed through the interaction of proteins including occludin, zonula occludens (ZO), and claudins (of which multiple isoforms exist).21, 22 Although occludin and claudins are transmembrane proteins, ZO acts as an anchoring component that binds transmembrane tight junction proteins.23, 24 In addition to these constituent proteins, regulatory enzymes can play an important role in controlling the permeability of the tight junction.25 An example of this is myosin light-chain kinase, which regulates tight junction permeability.26, 27, 28 Thus, in addition to transcellular transport, the paracellular space defines an alternative transport route.2 Therefore, the dynamic regulation of the tight junction is critical for the maintenance of effective barrier function while allowing the absorption of nutrients and other macromolecules. Importantly, inflammatory mediators such as tumor necrosis factor-α (TNF-α) or interleukin (IL)13 are capable of causing alterations in the structure of the tight junction (TJ) complexes and changes in permeability.29, 30 As discussed later, such alterations represent another mechanism of induction of barrier dysfunction-related intestinal disease.
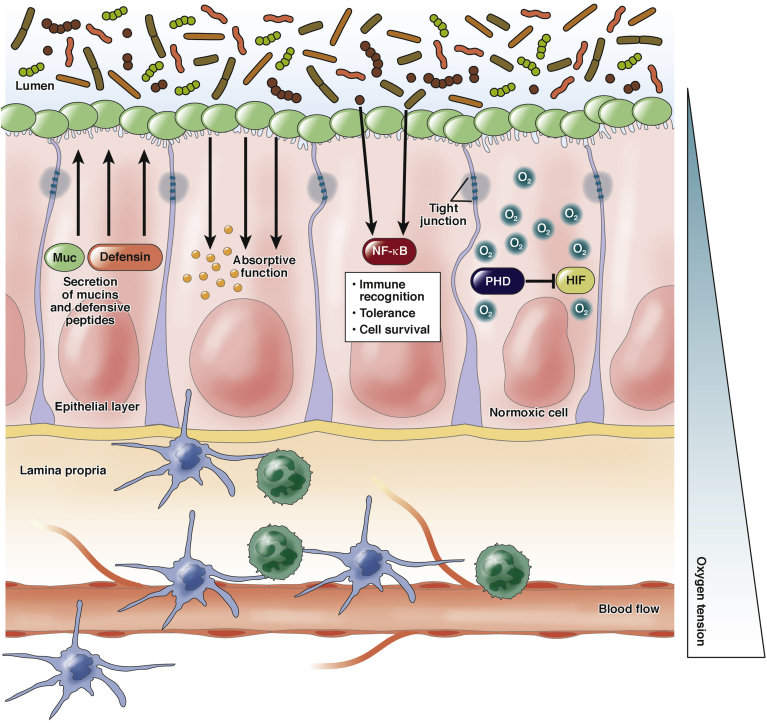
The intestinal epithelium is in direct contact with a large number of microorganisms that are part of the normal intestinal microbiota.31, 32 At the same time, the epithelium separates the microbiota from the intestinal immune system that is resident in the lamina propria (Figure 1). Therefore, the intestinal epithelium is a highly regulated and interactive surface that communicates signals between the mucosal immune system and the gut microbiota.31, 33, 34
Figure 1.
Representation of the intestinal epithelium in homeostatic conditions. In physiologic conditions, the intestinal epithelium forms a barrier that separates the anoxic and microorganism-rich lumen from the perfused and sterile lamina propria. Epithelial cells accomplish a variety of tasks: production of mucins, TFFs, and defensins; absorption of nutrients; sealing of the paracellular space; immune recognition; tolerance; and adaptation to oxygen tension changes. The external surface is covered by a layer of mucins that represent a nest for the intestinal microbiota. The inner surface is in contact with the resident immune system.
The number of bacterial cells that reside in the intestinal tract exceeds the number of human cells in the body.7, 35 This rich intestinal microenvironment has important interactions with the epithelial cells that form the intestinal barrier. Intestinal epithelial cells express pattern recognition receptors such as Toll-like receptors (TLRs).36 Furthermore, there is evidence that supports a role for the microbiome in both digestive and immune functions.37 For example, the gut microbiome is implicated in the absorption of fats.38, 39 Moreover, components of the microbiome also are required for the degradation of complex polysaccharides that cannot be absorbed into simpler carbohydrates that can be absorbed.40, 41 Furthermore, the gut microbiome is known to regulate mucins derived from intestinal goblet cells.42 Secreted mucins form a mucous layer that covers the epithelial surface (Figure 1). This mucous layer serves as a defense system against physical and chemical injury and aids the clearance of pathogenic microorganisms.43, 44 Components of the microbiome are involved in both mucus production as well as degradation.45, 46, 47, 48, 49 This implicates a mechanism of cross-talk between the intestinal epithelium and microbiota that contributes to both digestion and barrier function.
Beyond barrier formation, the intestinal epithelium actively regulates the immune response. In fact, as explained earlier, intestinal epithelial cells express multiple TLRs, including TLR-4 and TLR-3, and are responsive to microbial stimuli.50, 51, 52 These receptors are common immune receptors responsible for the recognition of bacterial components such as lipopolysaccharide. In response to such stimuli, human intestinal epithelial cells are capable of producing a variety of inflammatory cytokines including IL8,53 C-X-C motif chemokine ligand 2,54 or C-C motif chemokine ligand 20.52 These mediators actively recruit different components of the immune system and participate in the activation of inflammatory responses.
A final mechanism whereby the intestinal epithelium plays a role in the maintenance of homeostasis is through the production of mediators such as trefoil factors (TFFs) and defensins.55, 56, 57, 58 For example, in a study comparing the expression of TFF isoforms between healthy and intestinal biopsy specimens from patients with IBD, the messenger RNA levels of the TFFs analyzed were found to be correlated to the pathologic state.55 In specimens from patients with Crohn’s disease (CD) in remission, TFFs were up-regulated, showing an inverse correlation between TFF expression and inflammation.55 Moreover, local delivery of recombinant TFFs ameliorates dextran sodium sulfate (DSS)-induced colitis, providing further evidence for the beneficial role of these peptides in intestinal physiology.59 In line with these results, Podolsky et al60 showed that the deleterious effects of a mutation of TLR-2 were caused by altered production of TFF3. Thus, the role of TFFs in epithelial healing contribute to intestinal homeostasis.
Defensins are antimicrobial peptides, which in the gut are produced mainly by Paneth cells. These peptides participate in the response against infectious microorganisms and also are involved in the control of the intestinal microbiota.57, 58 Salzman et al58 showed that transgenic expression of human defensin-5 in mice makes them resistant to Salmonella typhimurium infection. In addition, the expression of these defensive peptides is altered in IBD.61, 62
In summary, the intestinal epithelium has critical interactions with both the luminal microenvironment and the immune system, as well as active roles in the immune response. These functions highlight the importance of an effective epithelial barrier in normal intestinal physiology and health.
Oxygen Tension and the Intestinal Epithelium
Atmospheric oxygen has contributed substantially to the evolution of metazoan life on earth.63 Aerobic organisms have developed systems for the absorption and utilization of molecular oxygen for metabolic purposes. The success of this metabolic approach has rendered most aerobic metazoans fully dependent on a constant supply of molecular oxygen for survival. The human body is working constantly to maintain an adequate oxygen supply from its absorption in the lungs, its distribution via blood vessels, and its transport and consumption at the cellular level. The intestinal epithelium is in a continuous state of “physiologic hypoxia” as a result of 2 main events.64, 65 First, it is juxtaposed between the largely anoxic intestinal lumen and the well-perfused lamina propria (Figure 1).66 Second, the oxygen pressure fluctuates in the lamina propria depending on blood volume in the gut. During fasting, blood flow to the intestine is relatively low and, as a result, so is the oxygen tension. However, when food is ingested, there is an increase in intestinal blood flow with the purpose of facilitating the absorption of nutrients.65 Thus, under physiologic conditions, the intestinal epithelium often is subjected to states of transient oxygen deprivation. This ability of the intestinal epithelial cells to tolerate transient periods of hypoxia in physiologic conditions has led to the concept of physiologic hypoxia.”67
Given the critical dependence of mammalian cells for oxygen, the development of adaptive mechanisms to hypoxia have been key to our survival. As discussed in the previous section, the intestinal epithelium is exposed constantly to low concentrations of oxygen, and thus represents a paradigm environment in which adaptation to hypoxia is key.66 At the cellular level, our ability to adapt to hypoxia depends on the activation of the hypoxia-inducible factor (HIF) signaling pathway.68, 69 HIF is a ubiquitously expressed family of heterodimeric transcription factors formed by the binding of HIF-α and HIF-β subunits. Although only 1 β subunit has been described, 3 different HIF-α isoforms exist. The mechanisms underpinning the regulation of HIF-1α and HIF-2α are well characterized and recently were reviewed extensively.69, 70, 71, 72, 73, 74 HIF-1β is expressed constitutively and is present in the nucleus, whereas HIF-α subunits are expressed constitutively in the cytoplasm. Under hypoxic conditions, the formation of functional HIF transcription factors in the nucleus triggers a reprogramming of gene expression that controls cell fate, activates alternative mechanisms of energy generation, or enhances oxygen absorption among many other functions.74, 75, 76, 77, 78 Thus, HIF responses are critical in the control of cell survival, metabolism, and other functions under low oxygenation.
A group of 3 prolyl hydroxylases (PHD), PHD-1, 2, and 3, and an asparaginyl hydroxylase known as factor inhibiting HIF (FIH), provide an efficient mechanism by which to control HIF-dependent responses.79, 80 HIF-α subunits are synthesized constitutively at high levels in all cells. Under normoxic conditions, HIF-α subunits are hydroxylated on 2 prolyl residues (pro402 and pro564 for HIF-1α and pro405 and pro531 for HIF-2α) within their oxygen-dependent degradation domain by PHD1-3.81, 82, 83, 84 Prolyl hydroxylation makes HIF-α a target for the E3 ubiquitin ligase von Hippel-Lindau protein that ubiquitinates HIF-α, marking it for proteosomal degradation. FIH hydroxylates HIF on an asparagine residue in its carboxy terminal–transactivation domain (Asn803 in HIF-1α), blocking its binding to the cofactor CREB-binding protein/p300 and preventing HIF-mediated transcriptional activation.79, 85, 86, 87 In hypoxia, hydroxylases no longer can hydroxylate HIF-α subunits (because of the lack of availability of molecular oxygen as a co-factor). In this situation, HIF-α is stabilized and translocates to the nucleus where it binds HIF-1β and forms transcriptionally active complexes.
Inflammatory Bowel Disease: The Involvement of Barrier Dysfunction
IBD is a chronic intestinal inflammatory disorder that comprises CD and ulcerative colitis (UC). Both pathologies are characterized by transition between periods of relapse and remission.88 IBD affects millions of people worldwide and is highly penetrant in developed countries.89 Furthermore, IBD progression is associated with the development of a number of common complications. For example, inflammation associated with CD often leads to fibrosis.90 Other complications such as anemia also are associated with IBD.
Current therapies for IBD are focused primarily on controlling inflammation using drugs such as mesalamine and/or corticosteroids.91, 92 However, these therapeutics often are insufficient to maintain patients in remission. In these cases, immunomodulator therapies such as azathioprine or methotrexate are used to prevent relapse.91 More recently developed approaches are focused on the use of antibodies targeting cytokines that are known to play critical roles in the development of the pathology. Among those, TNF-α is one of the most important drivers of inflammation in IBD, and thus therapies such as infliximab that specifically target TNF-α are common in maintenance therapy.91, 93, 94
Unwanted side effects of current IBD treatments are common. For example, the use of azathioprine is related to a higher risk of malignancy.92, 95 Anti-TNF therapy can lead to immune-compromised states and is related to a higher risk of infectious disease as well as a higher risk of malignancy.93 Moreover, complications such as fibrosis still can appear and represent major indication for surgical interventions owing to the lack of pharmacologic options.90 This emphasizes the need for new therapeutic approaches that can prolong remission and control the development of complications.
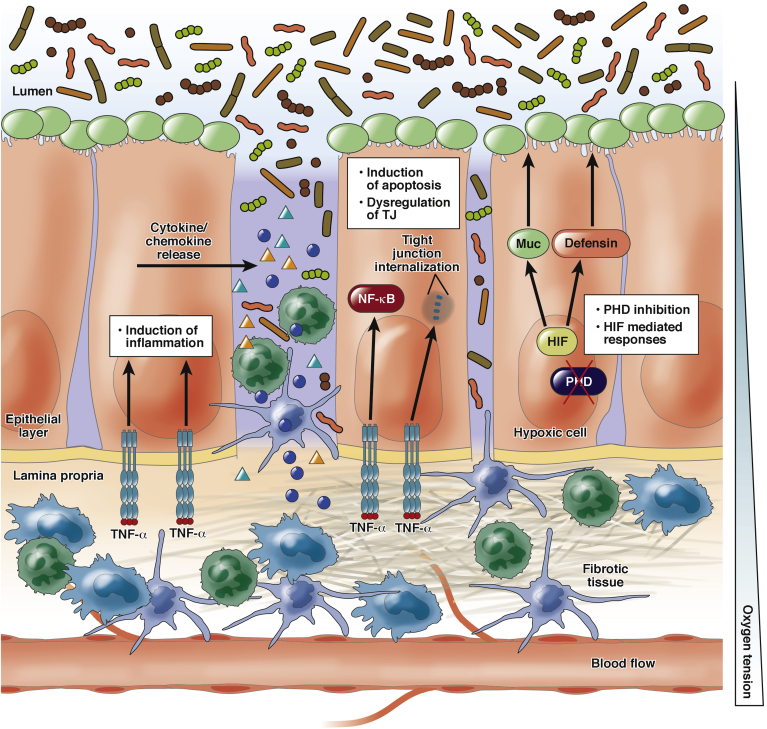
IBD is the result of a combination of environmental factors leading to recurrent inflammatory insults, which, in genetically predisposed patients, leads to the development of chronic inflammation.91, 92, 96 The development of chronic unresolved inflammation has been linked to intestinal epithelial barrier dysfunction including both disruption of the physical barrier as well as loss of tolerance against components of the microbiota.1, 97 First, the loss of the physical barrier function increases permeability, facilitating contact between luminal antigens and the mucosal immune cells (Figure 2). Second, the intestinal epithelial cells actively participate in the recognition of pathogens and recruitment of other immune cells and the initiation of inflammation (Figure 2).
Figure 2.
Alterations of barrier function are common in inflammatory bowel disease. The scheme represents the loss of barrier function mediated through increased apoptosis and induced TJ internalization. In pathologic situations, the intestinal epithelium becomes hypoxic as a result of the imbalance between oxygen supply and consumption. The intestinal epithelium also participates in the recruitment of immune cells through cytokine/chemokine release. Excessive activation of the immune system can in some cases lead to complications such as excessive extracellular matrix production, leading to tissue fibrosis.
At the cellular level, the loss of epithelial barrier function seen in IBD can be caused by either enhanced apoptosis98, 99, 100 or alterations in the expression and assembly of the TJ proteins (or a combination of both).101, 102 This is supported, for example, by evidence linking TNF-α to epithelial cell apoptosis and TJ regulation.99, 101, 102, 103 Studies have shown that anti-TNF therapies reduce intestinal epithelial cell apoptosis in models of colitis and CD patients.98, 104, 105 TNF-α down-regulates expression and alters localization of ZO-1 and promotes occludin endocytosis, actively regulating the architecture of the TJ.101, 102 TNF-mediated effects on intestinal epithelial TJ occur via the activation of myosin light-chain kinase signaling.29, 106, 107 Thus, alterations in the regulation of epithelial barrier function appear to be determinants in the development of chronic inflammation in the intestinal tract.
Tissue oxygen levels are reduced in different pathologic foci including tumors and inflammatory environments such as IBD.64, 65, 66, 108, 109, 110 In the case of IBD, inflamed areas of the colon experience an increase in activity and subsequently oxygen consumption as a result of the increased presence of activated immune cells, including neutrophils, as well as the activation of reparative processes (Figure 2).65, 111, 112 Furthermore, the epithelial surface, which is in direct contact with the profoundly hypoxic intestinal lumen, is damaged in IBD and becomes leaky, allowing infiltration of luminal contents into the mucosa and lamina propria (Figure 2). The presence of profound hypoxia on the epithelial surface was shown by Karhausen et al112 using 2-(2-nitro-1H-imidazol-1yl)-N-(2,2,3,3,-pentafluoropropyl) acetamide-based measurement of tissue oxygenation in murine colitis. In this study, the presence of hypoxia was restricted to the epithelial surface in healthy colons but became penetrant in diseased colons.65, 112 Another factor that accounts for the poor tissue oxygenation is the vasculopathy that affects inflamed tissues and reduces blood perfusion and, as a result, compromises oxygen supply.66, 112 Therefore, hypoxia is a common feature of inflammatory bowel disease. In the following section, we discuss evidence showing the involvement of HIF and oxygen-sensing hydroxylases in epithelial barriers.
Hydroxylase Inhibitors: A New Therapeutic Approach in IBD
The knowledge that hypoxia is common in disease environments led to the interest in unravelling the consequences of low oxygenation in different pathologies. In the context of IBD, a key observation was the increased presence of HIF in the colon from both murine colitis specimens and IBD patients.112, 113 These observations led to the investigation of the effects of pharmacologic and genetic manipulation of the hypoxia-inducible factor pathway to analyze its involvement in disease.
Multiple studies now have shown that hydroxylase inhibition is protective in murine colitis.114 Initial studies have shown that 2 hydroxylase inhibitors, dimethyloxalylglycine (DMOG) and FG-4497, reduce inflammation in different models of murine IBD (DSS- and trinitrobenzene sulfonic acid–induced colitis, respectively).115, 116 Subsequently, multiple studies have confirmed the anti-inflammatory actions of these drugs.91 By using PHD-deficient mice for the 3 main PHD isoforms (PHD1–3), it was found that PHD1 deficiency was protective in DSS-induced colitis. Interestingly, this protective effect was mediated through reduced epithelial cell apoptosis.16 This provided evidence that the protective effects of hydroxylase inhibitors may be mediated via protection of the intestinal barrier. Hindryckx et al117 subsequently showed HIF-dependent reduction of intestinal epithelial cell apoptosis in DMOG-treated mice in a model of TNF-induced colitis. Moreover, a study investigating the expression of PHD1–3 in UC and CD biopsy specimens showed an up-regulation of PHD1 in IBD.118 Altogether these data suggest that the hydroxylase inhibition–mediated protection against intestinal inflammation relies at least in part on barrier-protective effects. In line with this hypothesis, other barrier-protective mechanisms have been found that implicate the participation of HIF. In this regard, HIF has been linked to the production of trefoil factors,119 mucins,120, 121 β-defensins,12 and the regulation of mucosal immune responses.121, 122 Through the regulation of these factors HIF provides a barrier-protective mechanism in intestinal disease. However, in some studies, indirect negative effects of HIF in colitis also have been reported. Knock-out of intestinal von Hippel-Lindau tumor suppressor protein, the ubiquitin ligase responsible for HIF degradation, led to increased severity of colitis indirectly correlating HIF to increased inflammation.123 However, the protective effects of pharmacologic hydroxylase inhibition now has been shown to be reproducible in multiple models of colitis.114
Another potential mechanism whereby hydroxylase inhibitors may be implicated in barrier protection is through the regulation of the tight junction. In 2 different reports, claudin-1 expression and occludin stabilization were found to be regulated by HIF and PHD3, respectively.124, 125 Interestingly, in 2 studies using an in vitro model of neural vasculature and an in vivo model of brain ischemic stroke, hypoxia and pharmacologic hydroxylase inhibition were found to modulate permeability via regulation of the tight junction.126, 127 In addition, a HIF-2α–mediated increase in caveolin 1 has been reported to increase intestinal permeability via down-regulation of occludin and disruption of tight junction.128 Further research is required to fully elucidate the role of hydroxylases in the regulation of the tight junction in the intestinal epithelium.
Beyond their fundamental role in the regulation of HIF, HIF hydroxylases have been shown to have important functions in the regulation of other processes. For example, there is a close relationship between HIF, HIF hydroxylases, and the nuclear factor-κB (NF-κB) pathway. Initial evidence for this comes from the observation that inflammatory cytokines such as IL1β, lipopolysaccharide, and TNF-α up-regulate HIF-1α at both RNA and protein levels.129, 130, 131 Indeed, these and other studies also have shown the involvement of different subunits of the NF-κB pathway in the regulation of HIF-1α expression and stabilization,131, 132 as well as in the expression of HIF-dependent genes.132, 133 Similar NF-κB–dependent regulatory mechanisms have been described for the expression of HIF-1β, giving further insights into the NF-κB–mediated HIF regulation.134 The interdependence between HIF and NF-κB also is regulated in the opposite direction.135 Cummins et al136 reported PHD1 silencing to up-regulate the NF-κB response, and Fitzpatrick et al137 showed a PHD1-dependent regulation of NF-κB–mediated apoptosis. Moreover, knock-down of PHD1 and FIH in combination regulates IL1β-mediated inflammatory gene expression via reduced NF-κB activity.138 In addition to this PHD1-dependent regulation, a number of studies have described an important role for PHD3 in the regulation of NF-κB signaling.139, 140, 141 Thus, there is now significant evidence supporting the involvement of PHDs in the regulation of NF-κB–dependent inflammatory pathways.
IBD often is associated with severe complications. One of these complications is a deficiency in iron, causing low levels of hemoglobin and leading to anemia.142 Anemia is common in IBD and is linked to poor prognosis.143 The causes of anemia in IBD could be linked to intestinal bleeding or malabsorption, which turns IBD into a debilitating disorder.142 As a regulator of hypoxic responses, HIF is a strong inducer of the expression of erythropoietin, which stimulates erythrocyte proliferation, contributing to increased oxygen transport in the blood.144, 145, 146, 147 This erythropoietin-inducing property now is being investigated in clinical trials for the treatment of anemia.146, 148 This could represent a further beneficial effect of hydroxylase inhibition in anemic IBD patients.
Another common complication in IBD is intestinal fibrosis, which is more common in CD patients although still is significant in UC.149, 150 Fibrosis is the consequence of the overactivation of wound healing responses leading to the deposition of excessive extracellular matrix and the formation of scar tissue (Figure 2).111, 150 Intestinal fibrosis is the main cause of surgical intervention in CD patients owing to the lack of pharmacologic tools to prevent it.151 The role of hypoxia and hydroxylases in wound healing and fibrosis has been investigated in many fibrotic pathologies and has generated variable results.152, 153, 154, 155, 156 However, little is known about the effects of hydroxylase inhibitors in intestinal fibrosis. In recently published work, we described a role of hydroxylase inhibition as a negative regulator of transforming growth factor-β1–induced intestinal fibrosis. These effects were HIF independent and appear to be caused by hydroxylase-dependent modulation of the noncanonical transforming growth factor–extracellular regulated kinase signaling pathway.157 Given the relevance of the extracellular regulated kinase pathway in fibrotic pathology, these observations may represent a first step toward investigating hydroxylase inhibition as antifibrotic therapy in IBD.158, 159, 160
Some side effects of hydroxylase inhibitors can be expected and are related mainly to the ability of these drugs to activate the HIF pathway. As discussed in the previous section, HIF activation can mediate erythropoietin production and lead to increased erythropoiesis. Although this effect can be exploited in anemic patients, the up-regulation of erythropoietin generally would be undesired. A potential solution to this side effect would be the formulation of hydroxylase inhibitors in targeted release forms. We recently described that the use of a colonic release form can diminish the systemic exposure of DMOG and allows dose reduction without affecting its intestinal protective effects.161
Other potential source of negative effects come from the observation that HIF is activated in tumors and may contribute to tumor survival and growth.162, 163, 164, 165 HIF induces vascular endothelial growth factor in different cancer models.166, 167, 168, 169 This effect is related to the HIF-driven response that enhances oxygen supply, in this case via promotion of angiogenesis. Vascular endothelial growth factor angiogenic properties are a contributing factor to tumor growth and could represent a negative side effect in hydroxylase inhibition therapy.170 Furthermore, other cancer-related processes such as metastasis or cancer stem cell differentiation also have been related to hypoxia and HIF, representing other potential side effects.171, 172
Conclusions
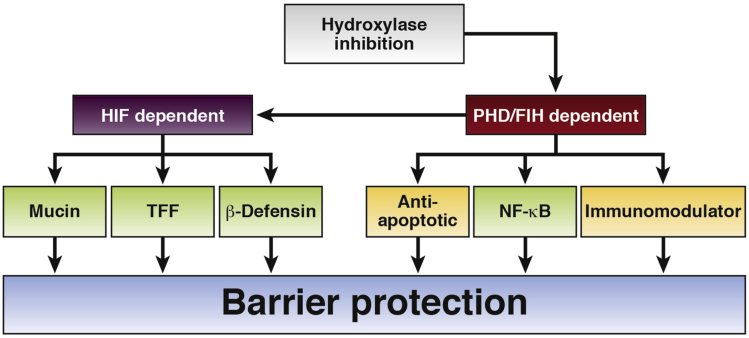
The intestinal epithelial barrier is crucial for the maintenance of homeostatic conditions. This single layer of epithelial cells is involved in both nutrition and defense, playing fundamental roles in the transport of nutrients, the formation of the external mucosal environment, the tolerance toward microbiota components, and the control of inflammatory responses. Epithelial disruption is one of the key drivers of inflammatory bowel diseases and restoring the epithelial permeability is seen as one of the main challenges in the development of novel therapies. Hypoxia is another central feature of IBD as a result of the excessive oxygen consumption combined with the reduced supply that is typical of chronic inflamed environments. Pharmacologic targeting of oxygen-sensing hydroxylases represents a promising therapeutic approach in multiple models of IBD. This protective effect likely is related to both HIF-dependent and HIF-independent mechanisms that contribute to barrier function (Figure 3). Given the reported beneficial effects of the inhibition of single subunits of the oxygen-sensing hydroxylase family, the possibility of developing specific drugs represents a promise toward the improvement of this potential new therapeutic approach. Therefore, future research should focus on a more detailed characterization of the actions of single oxygen-sensing hydroxylases in epithelial function and on the development and test of specific inhibitors.
Figure 3.
Mechanisms of hydroxylase inhibition mediated intestinal anti-inflammatory effects. Described mechanisms include both HIF-dependent and -independent functions. HIF-dependent mechanisms include regulation of TFFs, defensins, and mucins. PHD-mediated actions include immune modulation and apoptosis regulation, implicating the NF-κB pathway.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the Science Foundation Ireland (SFI PI award 11/PI/1005) and the ERACoSYSMed first translational call.
References
- 1.Colgan S.P., Taylor C.T. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham K.E., Turner J.R. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34–42. doi: 10.1111/j.1749-6632.2012.06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svoboda M., Bilkova Z., Muthny T. Could tight junctions regulate the barrier function of the aged skin? J Dermatol Sci. 2016;81:147–152. doi: 10.1016/j.jdermsci.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Iwai I., Han H.M., den Hollander L. The human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety. J Invest Dermatol. 2012;132:2215–2225. doi: 10.1038/jid.2012.43. [DOI] [PubMed] [Google Scholar]
- 5.Nalle S.C., Turner J.R. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 2015;8:720–730. doi: 10.1038/mi.2015.40. [DOI] [PubMed] [Google Scholar]
- 6.Taylor C.T. Regulation of intestinal epithelial gene expression in hypoxia. Kidney Int. 2004;66:528–531. doi: 10.1111/j.1523-1755.2004.761_12.x. [DOI] [PubMed] [Google Scholar]
- 7.Gareau M.G., Barrett K.E. Fluid and electrolyte secretion in the inflamed gut: novel targets for treatment of inflammation-induced diarrhea. Curr Opin Pharmacol. 2013;13:895–899. doi: 10.1016/j.coph.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Davies J.M., Abreu M.T. Host-microbe interactions in the small bowel. Curr Opin Gastroenterol. 2015;31:118–123. doi: 10.1097/MOG.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett K.E. Epithelial biology in the gastrointestinal system: insights into normal physiology and disease pathogenesis. J Physiol Lond. 2012;590:419–420. doi: 10.1113/jphysiol.2011.227058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch S., Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60. doi: 10.1146/annurev-pathol-011811-120905. [DOI] [PubMed] [Google Scholar]
- 11.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 12.Kelly C.J., Glover L.E., Campbell E.L. Fundamental role for HIF-1 alpha in constitutive expression of human beta defensin-1. Mucosal Immunol. 2013;6:1110–1118. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran A., Madesh M., Balasubramanian K.A. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol. 2000;15:109–120. doi: 10.1046/j.1440-1746.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 14.Gregorieff A., Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 15.Sansom O.J., Reed K.R., Hayes A.J. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tambuwala M.M., Cummins E.P., Lenihan C.R. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 17.Hall P.A., Coates P.J., Ansari B. Regulation of cell number in the mammalian gastrointestinal-tract - the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 18.Wen Y.A., Li X., Goretsky T. Loss of PHLPP protects against colitis by inhibiting intestinal epithelial cell apoptosis. Biochim Biophys Acta. 2015;1852:2013–2023. doi: 10.1016/j.bbadis.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemieux E., Cagnol S., Beaudry K. Oncogenic KRAS signalling promotes the Wnt/beta-catenin pathway through LRP6 in colorectal cancer. Oncogene. 2015;34:4914–4927. doi: 10.1038/onc.2014.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giepmans B.N.G., van Ijzendoorn S.C.D. Epithelial cell-cell junctions and plasma membrane domains. Biochim Biophys Acta. 2009;1788:820–831. doi: 10.1016/j.bbamem.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Shen L., Weber C.R., Raleigh D.R. Tight, junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunzel D., Yu A.S.L. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanning A.S., Jameson B.J., Jesaitis L.A. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 24.Muller S.L., Portwich M., Schmidt A. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- 25.Yu D., Marchiando A.M., Weber C.R. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci U S A. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berglund J.J., Riegler M., Zolotarevsky Y. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1487–G1493. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- 27.Turner J.R., Rill B.K., Carlson S.L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol Cell Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 28.Clayburgh D.R., Dirisina R., Barrett T.A. In vivo redistribution of tight junction proteins in mouse intestine following T cell-dependent TNF release. Gastroenterology. 2004;126:A109. [Google Scholar]
- 29.Wang F.J., Schwarz B.T., Graham W.V. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller F., Florian P., Bojarski C. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L., Kelly C., Campbell E. Microbe-host crosstalk between short-chain fatty acids and intestinal epithelial HIF provides a new mechanism to augment tissue barrier function. FASEB J. 2015:29. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Round J.L., Masmanian S.K. The gut microbiome shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oppong G.O., Rapsinski G.J., Newman T.N. Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica Serovar typhimurium curli fibrils in the gut. Infect Immun. 2013;81:478–486. doi: 10.1128/IAI.00453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 35.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 37.Rios-Covian D., Ruas-Madiedo P., Margolles A. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semova I., Carten J.D., Stombaugh J. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12:277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato H., Zhang L.S., Martinez K. Antibiotics suppress activation of intestinal mucosal mast cells and reduce dietary lipid absorption in Sprague-Dawley rats. Gastroenterology. 2016;151:923–932. doi: 10.1053/j.gastro.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ze X.L., Duncan S.H., Louis P. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsbrink J., Rogers T.E., Hemsworth G.R. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature. 2014;506:498–502. doi: 10.1038/nature12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Vliet M.J., Harmsen H.J.M., de Bont E.S.J.M. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. Plos Pathog. 2010;6:e1000879. doi: 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Sluis M., De Koning B.A.E., De Bruijn A.C.J.M. Muc2-deficient mice spontaneously develop colitis, indicating that Muc2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Johansson M.E.V., Phillipson M., Petersson J. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruas-Madiedo P., Gueimonde M., Fernandez-Garcia M. Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Appl Environ Microbiol. 2008;74:1936–1940. doi: 10.1128/AEM.02509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derrien M., Collado M.C., Ben-Amor K. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersson J., Schreiber O., Hansson G.C. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sommer F., Backhed F. The gut microbiota - masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 49.Barcelo A., Claustre J., Moro F. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dheer R., Santaolalla R., Davies J.M. Intestinal epithelial Toll-like receptor 4 signaling affects epithelial function and colonic microbiota and promotes a risk for transmissible colitis. Infect Immun. 2016;84:798–810. doi: 10.1128/IAI.01374-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukata M., Michelsen K.S., Eri R. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 52.Skovdahl H.K., Granlund A.V.B., Ostvik A.E. Expression of CCL20 and its corresponding receptor CCR6 is enhanced in active inflammatory bowel disease, and TLR3 mediates CCL20 expression in colonic epithelial cells. Plos One. 2015;10:e0141710. doi: 10.1371/journal.pone.0141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steiner T.S., Lima A.A.M., Nataro J.P. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 54.Ohtsuka Y., Lee J., Stamm D.S. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hensel K.O., Boland V., Postberg J. Differential expression of mucosal trefoil factors and mucins in pediatric inflammatory bowel diseases. Sci Rep. 2014;4:7343. doi: 10.1038/srep07343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taupin D., Podolsky D.K. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4 doi: 10.1038/nrm1203. 721-U3. [DOI] [PubMed] [Google Scholar]
- 57.Wehkamp J., Koslowski M., Wang G. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol. 2008;1:S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 58.Salzman N.H., Ghosh D., Huttner K.M. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 59.Vandenbroucke K., Hans W., Van Huysse J. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502–513. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 60.Podolsky D.K., Gerken G., Eyking A. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009;137:209–220. doi: 10.1053/j.gastro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuding S., Fellermann K., Wehkamp J. Reduced mucosal antimicrobial activity in Crohn's disease of the colon. Gut. 2007;56:1240–1247. doi: 10.1136/gut.2006.118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aldhous M.C., Noble C.L., Satsangi J. Dysregulation of human beta-defensin-2 protein in inflammatory bowel disease. PLoS One. 2009;4:e6285. doi: 10.1371/journal.pone.0006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor C.T., McElwain J.C. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology. 2010;25:272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 64.Vaupel P., Schlenger K., Knoop C. Oxygenation of human tumors - evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 65.Taylor C.T., Colgan S.P. Hypoxia and gastrointestinal disease. J Mol Med (Berl) 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 66.Kruschewski M., Foitzik T., Perez-Canto A. Changes of colonic mucosal microcirculation and histology in two colitis models - an experimental study using intravital microscopy and a new histological scoring system. Dig Dis Sci. 2001;46:2336–2343. doi: 10.1023/a:1012334727509. [DOI] [PubMed] [Google Scholar]
- 67.Zheng L., Kelly C.J., Colgan S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C350–C360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via denovo protein-synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G.L., Jiang B.H., Rue E.A. Hypoxia-inducible factor-1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular O-2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pugh C.W., ORourke J.F., Nagao M. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- 71.Blancher C., Moore J.W., Talks K.L. Relationship of hypoxia-inducible factor (HIF)-1 alpha and HIF-2 alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2000;60:7106–7113. [PubMed] [Google Scholar]
- 72.Wykoff C.C., Beasley N.J.P., Watson P.H. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 73.Hu C.J., Wang L.Y., Chodosh L.A. Differential roles of hypoxia-inducible factor 1 alpha (HIF-1 alpha) and HIF-2 alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rankin E.B., Biju M.P., Liu Q.D. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maxwell P.H., Wiesener M.S., Chang G.W. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 76.Rey S., Semenza G.L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochiai D., Goda N., Hishiki T. Disruption of HIF-1 alpha in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem Biophys Res Commun. 2011;415:445–449. doi: 10.1016/j.bbrc.2011.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halligan D.N., Murphy S.J., Taylor C.T. The hypoxia-inducible factor (HIF) couples immunity with metabolism. Semin Immunol. 2016;28:469–477. doi: 10.1016/j.smim.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Mahon P.C., Hirota K., Semenza G.L. FIH-1: a novel protein that interacts with HIF-1 alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meneses A.M., Wielockx B. PHD2: from hypoxia regulation to disease progression. Hypoxia (Auckl) 2016;4:53–67. doi: 10.2147/HP.S53576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hewitson K.S., Schofield C.J., Ratcliffe P.J. Hypoxia-inducible factor prolyl-hydroxylase: purification and assays of PHD2. Methods Enzymol. 2007;435:25–42. doi: 10.1016/S0076-6879(07)35002-7. [DOI] [PubMed] [Google Scholar]
- 82.Ohh M., Park C.W., Ivan N. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 83.Huang L.E., Gu J., Schau M. Regulation of hypoxia-inducible factor 1 alpha is mediated by an O-2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirsila M., Koivunen P., Gunzler V. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 85.Koivunen P., Hirsila M., Gunzler V. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279:9899–9904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- 86.Lando D., Peet D.J., Whelan D.A. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 87.McNeill L.A., Hewitson K.S., Claridge T.D. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the beta-carbon of asparagine-803. Biochem J. 2002;367:571–575. doi: 10.1042/BJ20021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liverani E., Scaioli E., Digby R.J. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016;22:1017–1033. doi: 10.3748/wjg.v22.i3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loftus E.V. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 90.Rieder F., Fiocchi C., Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2016;152:340–350.e6. doi: 10.1053/j.gastro.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cummins E.P., Doherty G.A., Taylor C.T. Hydroxylases as therapeutic targets in inflammatory bowel disease. Lab Invest. 2013;93:378–383. doi: 10.1038/labinvest.2013.9. [DOI] [PubMed] [Google Scholar]
- 92.Podolsky D.K. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 93.Billiet T., Rutgeerts P., Ferrante M. Targeting TNF-alpha for the treatment of inflammatory bowel disease. Expert Opin Biol Ther. 2014;14:75–101. doi: 10.1517/14712598.2014.858695. [DOI] [PubMed] [Google Scholar]
- 94.Stack W.A., Mann S.D., Roy A.J. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-alpha in Crohn's disease. Lancet. 1997;349:521–524. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]
- 95.Kandiel A., Fraser A.G., Korelitz B.I. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abraham C., Cho J.H. Mechanisms of disease: inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glover L.E., Colgan S.P. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–1755. doi: 10.1053/j.gastro.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiu W., Wu B., Wang X.W. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722–1732. doi: 10.1172/JCI42917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nenci A., Becker C., Wullaert A. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y.H., Srinivasan K., Siddiqui M.R. A novel role for villin in intestinal epithelial cell survival and homeostasis. J Biol Chem. 2008;283:9454–9464. doi: 10.1074/jbc.M707962200. [DOI] [PubMed] [Google Scholar]
- 101.Marchiando A.M., Shen L., Graham W.V. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–U160. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma T.Y., Iwamoto G.K., Hoa N.T. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 103.Frey M.R., Edelblum K.L., Mullane M.T. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology. 2009;136:217–226. doi: 10.1053/j.gastro.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marini M., Bamias G., Rivera-Nieves J. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A. 2003;100:8366–8371. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeissig S., Bojarski C., Buergel N. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F.J., Graham W.V., Wang Y.M. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma T.Y., Boivin M.A., Ye D.M. Mechanism of TNF-alpha modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 108.Evans S.M., Hahn S., Pook D.R. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–2024. [PubMed] [Google Scholar]
- 109.Etherington P.J., Winlove P., Taylor P. VEGF release is associated with reduced oxygen tensions in experimental inflammatory arthritis. Clin Exp Rheumatol. 2002;20:799–805. [PubMed] [Google Scholar]
- 110.Cummins E.P., Crean D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017;19:210–221. doi: 10.1016/j.micinf.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 111.Manresa M.C., Godson C., Taylor C.T. Hypoxia-sensitive pathways in inflammation-driven fibrosis. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1369–R1380. doi: 10.1152/ajpregu.00349.2014. [DOI] [PubMed] [Google Scholar]
- 112.Karhausen J., Furuta G.T., Tomaszewski J.E. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giatromanolaki A., Sivridis E., Maltezos E. Hypoxia inducible factor 1 alpha and 2 alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor C.T., Doherty G., Fallon P.G. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J Clin Invest. 2016;126:3716–3724. doi: 10.1172/JCI84433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cummins E.P., Seeballuck F., Keely S.J. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 116.Robinson A., Keely S., Karhausen J. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hindryckx P., De Vos M., Jacques P. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J Immunol. 2010;185:6306–6316. doi: 10.4049/jimmunol.1002541. [DOI] [PubMed] [Google Scholar]
- 118.Van Welden S., Laukens D., Ferdinande L. Differential expression of prolyl hydroxylase 1 in patients with ulcerative colitis versus patients with Crohn's disease/infectious colitis and healthy controls. J Inflamm (Lond) 2013;10:36. doi: 10.1186/1476-9255-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Furuta G.T., Turner J.R., Taylor C.T. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Louis N.A., Hamilton K.E., Canny G. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–1627. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 121.Fluck K., Breves G., Fandrey J. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 2016;9:379–390. doi: 10.1038/mi.2015.67. [DOI] [PubMed] [Google Scholar]
- 122.Clambey E.T., McNamee E.N., Westrich J.A. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shah Y.M., Ito S., Morimura K. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–2048. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saeedi B.J., Kao D.J., Kitzenberg D.A. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell. 2015;26:2252–2262. doi: 10.1091/mbc.E14-07-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Y., Zhang H.S., Fong G.H. PHD3 stabilizes the tight junction protein occludin and protects intestinal epithelial barrier function. J Biol Chem. 2015;290:20580–20589. doi: 10.1074/jbc.M115.653584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamagata K., Tagami M., Takenaga F. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol Dis. 2004;17:491–499. doi: 10.1016/j.nbd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Reischl S., Li L.X., Walkinshaw G. Inhibition of HIF prolyl-4-hydroxylases by FG-4497 reduces brain tissue injury and edema formation during ischemic stroke. PLoS One. 2014;9:e84767. doi: 10.1371/journal.pone.0084767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xie L.W., Xue X., Taylor M. Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol Cell Biol. 2014;34:3013–3023. doi: 10.1128/MCB.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sharma V., Dixit D., Koul N. Ras regulates interleukin-1 beta-induced HIF-1 alpha transcriptional activity in glioblastoma. J Mol Med (Berl) 2011;89:123–136. doi: 10.1007/s00109-010-0683-5. [DOI] [PubMed] [Google Scholar]
- 130.Peyssonnaux C., Cejudo-Martin P., Doedens A. Cutting edge: essential role of hypoxia inducible factor-1 alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 131.van Uden P., Kenneth N.S., Rocha S. Regulation of hypoxia-inducible factor-1 alpha by NF-kappa B. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rius J., Guma M., Schachtrup C. NF-kappa B links innate immunity to the hypoxic response through transcriptional regulation of HIF-1 alpha. Nature. 2008;453 doi: 10.1038/nature06905. 807-U9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nam S.Y., Ko Y.S., Jung J. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-kappa B promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104:166–174. doi: 10.1038/sj.bjc.6606020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Uden P., Kenneth N.S., Webster R. Evolutionary conserved regulation of HIF-1 beta by NF-kappa B. PLoS Genet. 2011;7:e1001285. doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taylor C.T., Cummins E.P. The role of NF-kappa B in hypoxia-induced gene expression. Ann N Y Acad Sci. 2009;1177:178–184. doi: 10.1111/j.1749-6632.2009.05024.x. [DOI] [PubMed] [Google Scholar]
- 136.Cummins E.P., Berra E., Comerford K.M. Prolyl hydroxylase-1 negatively regulates I kappa B kinase-beta, giving insight into hypoxia-induced NF kappa B activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fitzpatrick S.F., Fabian Z., Schaible B. Prolyl hydroxylase-1 regulates hepatocyte apoptosis in an NF-kappa B-dependent manner. Biochem Biophys Res Commun. 2016;474:579–586. doi: 10.1016/j.bbrc.2016.04.085. [DOI] [PubMed] [Google Scholar]
- 138.Scholz C.C., Cavadas M.A.S., Tambuwala M.M. Regulation of IL-1 beta-induced NF-kappa B by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci U S A. 2013;110:18490–18495. doi: 10.1073/pnas.1309718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xue J., Li X.B., Jiao S. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKK beta independent of hydroxylase activity. Gastroenterology. 2010;138:606–615. doi: 10.1053/j.gastro.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 140.Fu J., Taubman M.B. Prolyl hydroxylase EGLN3 regulates skeletal myoblast differentiation through an NF-kappa B-dependent pathway. J Biol Chem. 2010;285:8927–8935. doi: 10.1074/jbc.M109.078600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fu J., Taubman M.B. EGLN3 Inhibition of NF-kappa B is mediated by prolyl hydroxylase-independent inhibition of I kappa B kinase gamma ubiquitination. Mol Cell Biol. 2013;33:3050–3061. doi: 10.1128/MCB.00273-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gasche C., Waldhoer T., Feichtenschlager T. Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am J Gastroenterol. 2001;96:2382–2387. doi: 10.1111/j.1572-0241.2001.04094.x. [DOI] [PubMed] [Google Scholar]
- 143.Koutroubakis I.E., Ramos-Rivers C., Regueiro M. Persistent or recurrent anemia is associated with severe and disabling inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:1760–1766. doi: 10.1016/j.cgh.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kapitsinou P.P., Liu Q.D., Unger T.L. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bernhardt W.M., Wiesener M.S., Scigalla P. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Besarab A., Chernyayskaya E., Motylev I. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hsieh M.M., Linde N.S., Wynter A. HIF-prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holdstock L., Meadowcroft A.M., Maier R. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27:1234–1244. doi: 10.1681/ASN.2014111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fiocchi C., Lund P.K. Themes in fibrosis and gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2011;300:G677–G683. doi: 10.1152/ajpgi.00104.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gordon I.O., Agrawal N., Goldblum J.R. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20:2198–2206. doi: 10.1097/MIB.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 151.Rieder F., Fiocchi C. Intestinal fibrosis in inflammatory bowel disease - current knowledge and future perspectives. J Crohns Colitis. 2008;2:279–290. doi: 10.1016/j.crohns.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 152.Higgins D.F., Kimura K., Bernhardt W.M. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fang Y., Yu X.F., Liu Y. miR-29c is downregulated in renal interstitial fibrosis in humans and rats and restored by HIF-alpha activation. Am J Physiol Renal Physiol. 2013;304:F1274–F1282. doi: 10.1152/ajprenal.00287.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kapitsinou P.P., Sano H., Michael M. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–2409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kalucka J., Ettinger A., Franke K. Loss of epithelial hypoxia-inducible factor prolyl hydroxylase 2 accelerates skin wound healing in mice. Mol Cell Biol. 2013;33:3426–3438. doi: 10.1128/MCB.00609-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zimmermann A.S., Morrison S.D., Hu M.S. Epidermal or dermal specific knockout of PHD-2 enhances wound healing and minimizes ischemic injury. PLoS One. 2014;9:e93373. doi: 10.1371/journal.pone.0093373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manresa M.C., Tambuwala M., Radhakrishnan P. Hydroxylases regulate intestinal fibrosis through the suppression of ERK mediated TGF-β1 signaling. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1076–G1090. doi: 10.1152/ajpgi.00229.2016. [DOI] [PubMed] [Google Scholar]
- 158.Lawrenz M., Visekruna A., Kuhl A. Genetic and pharmacological targeting of TPL-2 kinase ameliorates experimental colitis: a potential target for the treatment of Crohn's disease. Mucosal Immunol. 2012;5:129–139. doi: 10.1038/mi.2011.57. [DOI] [PubMed] [Google Scholar]
- 159.Pat B., Yang T., Kong C.Z. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Kidney Int. 2005;67:931–943. doi: 10.1111/j.1523-1755.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 160.Hu Y.B., Peng J.W., Feng D.Y. Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-beta 1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung. 2006;184:33–42. doi: 10.1007/s00408-005-2560-5. [DOI] [PubMed] [Google Scholar]
- 161.Tambuwala M.M., Manresa M.C., Cummins E.P. Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J Control Release. 2015;217:221–227. doi: 10.1016/j.jconrel.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 162.Chaturvedi P., Gilkes D.M., Chak C. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wong C.C.L., Gilkes D.M., Zhang H.F. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 165.Theodoropoulos V.E., Lazaris A.C., Sofras F. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004;46:200–208. doi: 10.1016/j.eururo.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 166.Jensen R.L., Ragel B.T., Whang K. Inhibition of hypoxia inducible factor-1 alpha (HIF-1 alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006;78:233–247. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- 167.Lin C.Z., McGough R., Aswad B. Hypoxia induces HIF-1 alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J Ortho Res. 2004;22:1175–1181. doi: 10.1016/j.orthres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 168.Cianfrocca R., Tocci P., Rosano L. Nuclear beta-arrestin1 is a critical cofactor of hypoxia-inducible factor-1 alpha signaling in endothelin-1-induced ovarian tumor progression. Oncotarget. 2016;7:17790–17804. doi: 10.18632/oncotarget.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carroll V.A., Ashcroft M. Role of hypoxia-inducible factor (HIF)-1 alpha-versus HIF-2 alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-1, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 170.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 171.Wang T., Gilkes D.M., Takano N. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111:E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang C.Z., Samanta D., Lu H.Q. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]