Abstract
In this study we investigate the relationship between maternal age at the time of birth and a variety of health behaviours and measures of health amongst young adults in contemporary Sweden. Previous research has shown that those born to younger and older mothers tend to have worse perinatal outcomes, and worse health in middle- and later adulthood. However, previous work has not examined health in early adulthood, and no studies have explored whether maternal age is related to health behaviours. Using survey data on 1236 19-year olds born in Sweden in 1990, we find that those born to older mothers have lower self-rated health, are more likely to smoke, more likely to drink alcohol regularly, and less likely to exercise regularly. We discuss potential explanations for these findings, such as older parents exerting lower social control due to greater levels of workplace responsibilities and time demands, long-term consequences of the poor peri-natal outcomes of those born to older mothers, as well as the potential role of parental health behaviours. Our findings suggest that health behaviours may play an important mediating role in explaining the worse long-term health of those born to younger and older mothers.
Keywords: Sweden, Maternal age, Adolescents, Health, Self-rated health, Smoking, Alcohol, Exercise
Highlights
-
•
Maternal age is related to self-rated health in early adulthood.
-
•
Maternal age is also related to health behaviours in early adulthood.
-
•
Children of older and younger mothers are more likely to smoke and drink.
-
•
Children of older and younger mother have worse self-rated health.
Introduction
Over the past decades there has been an increase in the mean age of childbearing across much of the developed world (OECD, 2014). In Sweden the mean age of first birth increased by approximately four years, to age 28, between 1970 and 2011, and in 2013 the majority of all births were to women aged 30 or older (Statistics Sweden). Research that has attempted to isolate the impact of maternal age at the time of birth on long-term offspring outcomes has generally shown a non-linear relationship where the children of younger and older mothers are shorter, have higher rates of obesity, lower self-rated health, higher mortality (Myrskylä & Fenelon, 2012), and higher rates of diabetes (Cardwell et al., 2010), and cancer (Hemminki & Kyyrönen, 1999). Both physiological and social explanations have been developed to account for this relationship, including a decline in oocyte quality with increasing maternal age (Navot et al., 1991), as well as how children of older mothers are at an increased risk of losing their mother to death at a young age, thereby potentially receiving less support and investment from their parents (Myrskylä, Elo, Kohler, & Martikainen, 2014).
While most previous research has examined either birth outcomes, or health outcomes in middle and older adulthood, this study examines the relationship between maternal age at the time of birth and both health and health behaviours of the offspring in the late teenage years, at age 19. While health measures such as height and obesity have been examined before, we are not aware of previous research that has examined the relationship between maternal age and offspring health behaviours. The measures we examine include height, being overweight, being obese, alcohol consumption, smoking behaviour, taking regular exercise, and self-rated health. In this study we show that health and health behaviours in late adolescence are associated with maternal age, with the offspring of both younger and older mothers typically exhibiting worse outcomes. This pattern of results indicates that shorter lifespan overlap is not the sole explanation for the relationship between advanced maternal age and offspring mortality in adulthood.
The physiological explanations for why advanced maternal age carries an increased risk are well documented. Female fecundity declines with age, and the difficulty of conceiving, rates of spontaneous abortion, and adverse perinatal outcomes such as stillbirth, pre-term birth, and low birth weight increase exponentially with maternal age (Schwartz and Mayaux, 1982, Andersen et al., 2000). Both pre-term birth and low birth weight are associated with lower cognitive ability in adulthood as well as other negative sequelae (Black et al., 2007, Saigal and Doyle, 2008). Childbearing at young ages is also associated with worse offspring outcomes. While this may be attributable to physiological underdevelopment (Fraser, Brockert, & Ward, 1995), it may also be due to the fact that teenage mothers tend to be drawn from low socioeconomic status backgrounds, and that early childbearing disrupts opportunities to increase socioeconomic attainment (Furstenberg, 2003).
The long-term socioeconomic consequences of being born to an older mother are not entirely clear. For one, older parents tend to have greater financial and social resources, which would benefit their offspring. On the other hand, older parents will typically have a shorter lifespan overlap with their children, as a mother who is 40 at the time of birth would ceteris paribus die twenty years before a woman who was age 20 at the time of birth. Recent research has indicated that a shorter lifespan overlap between the child and the mother may be the main explanation for the relationship between advanced maternal age and higher offspring mortality (Myrskylä and Fenelon, 2012, Myrskylä et al., 2014). A shorter lifespan overlap means that children may receive less parental investment, and some may be scarred by the trauma of losing a parent at a relatively young age (Rostila & Saarela, 2011). Alternatively, a short lifespan overlap may be evidence of shared frailty within the family, or a shared hazardous environmental exposure.
In a comparative perspective, life expectancy is high in Sweden, meaning that even a mother who gives birth at the very advanced age of 50 would be overwhelmingly likely to still be alive when her child is aged 19. Over 80% of women born in Sweden in the 1940 cohort were still alive by age 70 (Statistics Sweden, 2010). By examining self-reported health, biomarkers of health, and the health behaviours of the offspring in late adolescence we are able to test whether maternal age is associated with health before considerations regarding lifespan overlap are relevant for most respondents. Although the parents of most teenage respondents will not have died, it is likely that the oldest mothers and fathers would already have declining health. Seeing one’s parent with cancer or developing Alzheimer’s disease could clearly be a stressful and distressing experience that could in turn have a negative impact on the child. Even if there was not a direct impact upon the physical health of the child, such an experience might increase the likelihood of the offspring suffering from anxiety or depression (Compas et al., 1994, Armistead et al., 1995), which may increase the risk of engaging in negative health behaviours like excessive alcohol consumption (Dixit & Crum, 2000).
Parenting style is likely to vary by the age of the mother, even if this is largely explained by selection. Older parents are more likely to have elected to have a child, and may also be happier than younger parents (Myrskylä & Margolis, 2014). On the other hand, research indicates that older parents spend less time with their children (Sayer, Bianchi &, Robinson, 2004). Time use data from the United States suggests that mothers who are aged 45–54 spend 30 min less time per day with their children than parents who are aged 25–34, and parents who are aged 55–64 spend 40 min less time per day after adjusting for number of children and the presence of pre-school children (Sayer et al., 2004). These are quite substantial differences when considering that parental time and attention are critically important dimensions of investment in children (McLanahan & Sandefur, 1994), and will be related to the ability of the parents to encourage healthy behaviours, and discourage unhealthy ones (Barnes, Reifman, Farrell, & Dintcheff, 2000).
Smoking, excessive alcohol consumption, and poor cardiovascular fitness are all strongly associated with mortality (Blair et al., 1996, Erikson et al., 1979, Fuller, 2011). Furthermore, due to their addictive nature, patterns of cigarette and alcohol consumption are correlated over the life course, and cohort studies often show a rise in consumption with increasing age (Grant and Dawson, 1997, Faggiano et al., 2001). Patterns of exercise and sedentary activities are also correlated over the life course (Biddle et al., 2010, Midlöv et al., 2014). This previous research suggests that habits and behaviours can become ingrained over time. Although we do not have longitudinal data, this means that if maternal age is associated with health behaviours in late adolescence we can speculate that these behaviours may mediate the relationship between maternal age at the time of birth and health outcomes in later adulthood for the offspring.
Data
The data used for this study come from a panel survey collected by telephone interview in Sweden in 2009. The survey was collected as part of a project called LIFEINCON. The main focus of this project has been on contextual factors, such as social networks and neighbourhoods, explaining differences in young adults’ life chances in a longitudinal perspective. According to a consulting statement (2008/580-31), the Ethical Review Board (EPN) in Stockholm approved the application for ethical approval. The sample is based on three different groups of Swedes, differentiated by the parental country of birth, born in 1990: (a) all individuals with at least one parent born in Iran, (b) 50% of all individuals with at least one parent born in the former Yugoslavia, and (c) a random sample of 2500 individuals with two Swedish born parents. In Sweden immigrants of Iranian and Yugoslavian descent make up a substantial portion of the population of non-Nordic origin. The goal of this sampling approach was to be able to examine the life opportunities of these specific groups of immigrants, or descendants of immigrants, more carefully.
The survey sampled 5695 Swedish youths, who were aged 19 in 2009, and a total of 2942 interviews were successfully conducted by Statistics Sweden, giving a response rate of 51.7%. The most common reason for non-response, 77%, was that the interviewers could not get in contact with the individual, primarily due to the prevalence of pay-as-you-go phone users in this age group. In these cases, names are not registered to particular phone numbers. The effective sample was slightly biased. Information provided by Statistics Sweden showed that the rate of response was lower amongst those living in urban areas, with lower grades, no upper-secondary education, those who had not completed secondary education, and those whose parents’ had lower levels of educational attainment.
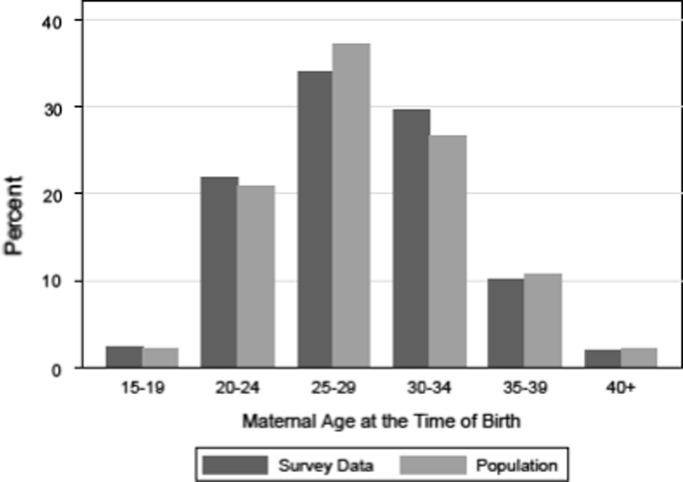
In this study we restrict our analyses to the random sample of 2500 individuals with two Swedish born parents. After considering non-response on some items, we have data on 1236 individuals for our analyses. We choose to focus upon respondents with two Swedish born parents so that we could comprehensively adjust for the socioeconomic status of the parents; that data was less available for respondents with an Iranian or Yugoslavian national origin. To this effect the survey data is bolstered by linkages to the Swedish administrative registers, including the occupational class of the father in 1990, the educational attainment of the mother in 2009 (the year of the survey), and the income of the father in 2009. Adjusting for parental SES is important as studies consistently demonstrate that early life investment is associated with long-term outcomes (Campbell et al., 2014). Our main explanatory variable is maternal age at the time of birth. Data on the year and month of birth of the mother and survey respondents were drawn from the population registers. Fig. 1 shows how the distribution of maternal age in our analytical sample compares to the distribution of maternal age in Sweden as a whole in 1990.
Fig. 1.
Distribution of maternal age at the time of birth in full population in 1990 and amongst analytical sample of survey respondents born in 1990 (N=1236).
Outcome variables
In this study we examine seven different outcome variables, which are height, being overweight, being obese, alcohol consumption, smoking behaviour, exercise behaviour, and self-rated health. All of these measures are self-reported. Height is measured in centimetres. Respondents also self-reported their weight, which made it possible to calculate BMI. Respondents with a BMI equal to or greater than 25 are classified as overweight, while those with a BMI equal to or greater than 30 are classified as obese. The variable for alcohol consumption is a six-point scale, based upon responses to the question “approximately how many times in the last 12 months have you been drinking enough alcohol to become drunk?”. Respondents could answer [1] 3 times/week or more, [2] 1–2 times/week, [3] 2–3 times/month, [4] once/month, [5] less often, or [6] never. The variable for smoking behaviour is based upon two binary questions. Respondents were asked both whether they smoke daily, as well as whether they smoke occasionally. If they responded that they smoked daily, they were classified as a daily smoker; if they responded no to the question of whether they smoked daily, but yes to the question about whether they smoked occasionally, they were classified as an occasional smoker; and, if they replied no to both questions, they were classified as a non-smoker. The variable for regular exercise is based upon a binary question concerning whether they train regularly at least once a week for at least 30 min, to which they could reply yes or no. The variable for self-rated health is based upon a likert scale. Respondents were asked: “how would you say your general health status is? Very good, good, average [“so-so”], bad, or very bad?”.
Covariates
In our analyses we adjust for a range of covariates, some of which are potential confounders for the relationship between maternal age at the time of birth, and others that are potentially mediating factors. Broadly we adjust for three groups of covariates. The first are demographic variables that include the gender and birth order of the respondent, and the size of the respondent’s sibling group. Gender is a critical component of health, and there is some evidence that the secondary sex ratio varies slightly by maternal age (James, 1987). Birth order is also related to maternal age, and has been shown to be related to both health behaviours and long-term health (Barclay and Myrskylä, 2014, Barclay and Kolk, 2015). The size of the sibling group will also be related to the mother’s age, and sibling group size has been shown to be related to both health and health behaviours (Hatton & Martin, 2010).
The second group of variables concern the socioeconomic status of the respondent’s parents, including the occupational class of the father in 1990, which is the birth year of the respondents, maternal educational attainment measured when the respondent was 19, and the logged income of the father, also measured in 2009. As described above, these measures are drawn from the administrative registers. Maternal educational attainment is coded into four categories, which are basic lower secondary education (grundskola), upper secondary education (gymnasium), less than two years of tertiary education, and 2 years or more tertiary education. The variable for paternal occupational class is taken from the 1990 census, and is a Swedish variant of the Erikson, Goldthorpe, Portacarero class measure (Erikson et al., 1979). Although there were originally 14 categories in the census, we converted this to an 8 category measure due to the small numbers in some categories by combining closely related categories. For example, we combined ‘unskilled in goods production’ and ‘unskilled in service production’ into one category, ‘unskilled’. Parental socioeconomic status and educational attainment are both related to maternal age at the time of birth (McLanahan, 2004), which is explained at least in part by the fact that individuals tend to delay childbearing until after completing their educational careers (Blossfeld & Huinink, 1991).
The third group of variables are those that we consider to be potential mediators for the relationship between maternal age and the outcomes that we study. In the analyses of more objective health outcomes such as height, being overweight, and obesity, we include covariates for the health behaviours that we otherwise study as outcome variables, meaning alcohol consumption, smoking, and exercise behaviour, to see whether they mediate the relationship to any extent. This is less likely for height, but very possible for being overweight or obese. Finally, in the analyses of alcohol consumption, smoking, and taking regular exercise, we adjust for self-reported depression and anxiety. Although we have no indication of whether the depression or anxiety experienced by the respondents’ approaches a threshold that would obtain a clinical diagnosis, they are a useful indicator of the self-perceived mental health of the respondents. The two questions for depression and anxiety were asked as follows: “during the last 12 months, have you experienced (depression/anxiety)?” Respondents could answer that they were (a) much affected, (b) slightly affected, or (c) not affected. The motive for the inclusion of these variables is to attempt to address a hypothesised scenario where respondents who are born to older mothers begin to drink more heavily, for example, in response to depression or anxiety precipitated by the declining health of their older mother.
Statistical analyses
We estimate one model for each health outcome. For the physical markers of health, as well as self-rated health we estimate model 1, while for the health behaviour outcomes we estimate model 2:
| (1) |
| (2) |
where Y is the outcome variable; MAB is the maternal age at the time of birth for the respondent; MAB2 is a quadratic term for maternal age at the time of birth; BO is the variable for the birth order of the respondent; SEX is the variable for the gender of the respondent; DEM_SES is the vector of demographic and parental socioeconomic status characteristics (size of the sibling group of origin of the respondent, paternal occupational class at the time of birth of the respondent, maternal educational attainment when respondent was aged 19, paternal logged income when respondent was aged 19); HEALTH_BEHAV is the vector of health behaviour variables (alcohol consumption, smoking, exercise behaviour); and MENTALHEALTH is the vector of the respondent’s self-reported experience of depression and anxiety in the past 12 months. Although we also estimated models without a quadratic term for maternal age at the time of birth, previous research indicates that there is a U-shaped relationship between maternal age at the time of birth and health outcomes (Myrskylä & Fenelon, 2012), and these models were also of a better fit than the models that we estimated without the quadratic term.
In this study we use seven different outcome variables that have a range of different distributions. For examining height, alcohol consumption, and self-rated health we used ordinary linear regression. For examining smoking behaviour we used a multinomial logistic regression as the proportional odds assumption was violated when we estimated the models using an ordered logistic regression model. For examining the binary variables for being overweight, being obese, and exercising regularly, we used logistic regression. For each outcome variable we also estimated a semi-parametric regression model (Lokshin, 2006) so as to graphically illustrate the relationship between maternal age at the time of birth and the outcome measure for the various models that were described above. The non-parametric part of the regression is the association between maternal age and the outcome variable, and the parametric part involves the adjustment for the control variables included in the model. This semi-parametric regression model imposes no shape on the association between maternal age at the time of birth and the outcome variable, instead basing the shape of the function on locally weighted data to smooth the curve. In these analyses we therefore do not impose any assumption about the shape of the relationship between maternal age and the outcome variables, and the plotted line is completely data driven.
In our analyses using this semi-parametric regression model we standardise the outcome variables so that the results show how a change in maternal age is related to a standard deviation change in the outcome. For these analyses we reverse coded the variables for height, alcohol consumption, and regular exercise, so that a positive standard deviation change in all of the outcome variables represents a worse outcome for the index person.
Results
Summary statistics
Table 1 shows how the various outcome variables that we examine in this study are distributed according to maternal age at the time of birth. Amongst the survey respondents height does not vary much by maternal age, with the exception of those born to mothers aged 40 or older, who are on average 2 cm shorter. The proportion of overweight individuals is highest amongst those born to teenage mothers and mothers aged 40 or older. The proportion of obese individuals is also highest amongst those born to mothers aged 40 or older. The proportion of those who never drink is highest amongst those born to mothers aged 40 or older, while the proportion of those who report drinking 1–2 times per week or more is highest amongst those born to mothers aged 20–24. Regular exercise is least common amongst those born to mothers aged 40 or older, while the proportion who report ‘very good’ self-rated health is lowest amongst those born to teenage mothers, followed by those born to mothers aged 40 or older.
Table 1.
Summary statistics for outcome variables by maternal age at the time of birth.
|
Maternal age at birth |
||||||||
|---|---|---|---|---|---|---|---|---|
| Total | 15-19 | 20-24 | 25-29 | 30-34 | 35-39 | 40+ | ||
| Respondents | N | 1,236 | 30 | 270 | 420 | 366 | 125 | 25 |
| % | 100.0 | 2.4 | 21.8 | 34.0 | 29.6 | 10.1 | 2.0 | |
| Height | Mean | 174.6 | 175.5 | 174.7 | 174.6 | 174.5 | 174.6 | 172.5 |
| SD | 9.5 | 8.2 | 9.3 | 9.5 | 9.6 | 9.6 | 9.5 | |
| Overweight | Yes (%) | 19.3 | 26.7 | 21.1 | 19.1 | 18.6 | 16.0 | 24.0 |
| No (%) | 80.7 | 73.3 | 78.9 | 81.0 | 81.4 | 84.0 | 76.0 | |
| Obese | Yes (%) | 5.7 | 6.7 | 6.7 | 4.5 | 6.3 | 5.6 | 8.0 |
| No (%) | 94.3 | 93.3 | 93.3 | 95.5 | 93.7 | 94.4 | 92.0 | |
| Smoking | Daily (%) | 61.4 | 36.7 | 54.8 | 64.1 | 64.8 | 64.0 | 56.0 |
| Occasional (%) | 24.9 | 36.7 | 28.5 | 23.3 | 23.2 | 24.0 | 28.0 | |
| Never (%) | 13.7 | 26.7 | 16.7 | 12.6 | 12.0 | 12.0 | 16.0 | |
| Becoming drunk | 3 times per week or more (%) | 0.9 | 0.0 | 1.9 | 0.2 | 1.1 | 0.8 | 0.0 |
| 1–2 times per week (%) | 13.4 | 6.7 | 15.6 | 11.2 | 13.4 | 17.6 | 16.0 | |
| 2–3 times per month (%) | 30.3 | 40.0 | 28.5 | 31.2 | 28.7 | 32.0 | 36.0 | |
| Once per month (%) | 25.4 | 20.0 | 27.0 | 24.5 | 27.9 | 20.0 | 20.0 | |
| Less often (%) | 19.9 | 30.0 | 17.8 | 23.8 | 16.7 | 19.2 | 16.0 | |
| Never (%) | 10.1 | 3.3 | 9.3 | 9.1 | 12.3 | 10.4 | 12.0 | |
| Regular exercise | Yes (%) | 76.3 | 76.7 | 73.3 | 78.3 | 77.6 | 76.0 | 56.0 |
| No (%) | 23.7 | 23.3 | 26.7 | 21.7 | 22.4 | 24.0 | 44.0 | |
| Self-rated health | Very good (%) | 34.7 | 13.3 | 31.9 | 37.1 | 38.3 | 29.6 | 24.0 |
| Good (%) | 50.2 | 73.3 | 50.7 | 49.8 | 45.9 | 56.0 | 56.0 | |
| Average (%) | 13.2 | 10.0 | 14.8 | 10.7 | 15.3 | 12.0 | 16.0 | |
| Bad (%) | 1.7 | 3.3 | 2.2 | 2.1 | 0.3 | 2.4 | 4.0 | |
| Very bad (%) | 0.2 | 0.0 | 0.4 | 0.2 | 0.3 | 0.0 | 0.0 | |
Table 2 provides details on how the various covariates are distributed according to maternal age at the time of birth. The proportion of females is very low amongst those born to teenage mothers and very high amongst those born to mothers aged 40 or older, but this can be explained by random variation due to the relatively small numbers in those categories. Birth order increases with increasing maternal age, as does the size of the sibling group. The pattern of maternal education level by maternal age generally shows that older mothers have higher educational attainment, though this pattern is not completely consistent across all categories of educational attainment. Since all of the survey respondents were born in the same year, maternal age at the time of birth also necessarily reflects the birth cohort from which the mothers are drawn. Mothers aged 40 or older are the most likely to have a lower secondary education, but this is because the 20th century has seen rapid educational expansion in Sweden (Breen & Jonsson, 2007). Paternal occupational class is highest amongst those born to older mothers. The highest proportion of those who report being much affected by depression and anxiety are those born to teenage mothers and mothers aged 40 or older.
Table 2.
Summary statistics for control variables by maternal age at the time of birth.
|
Maternal age at birth |
||||||||
|---|---|---|---|---|---|---|---|---|
| Total | 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40+ | ||
| Respondents | N | 1236 | 30 | 270 | 420 | 366 | 125 | 25 |
| % | 100.0 | 2.4 | 21.8 | 34.0 | 29.6 | 10.1 | 2.0 | |
| Gender | Female (%) | 50.2 | 30.0 | 50.4 | 47.6 | 53.0 | 52.8 | 60.0 |
| Birth Order | Mean | 1.8 | 1.1 | 1.3 | 1.7 | 2.2 | 2.5 | 2.6 |
| Size of Sibling Group | Mean | 2.8 | 3.5 | 2.8 | 2.7 | 2.9 | 3.1 | 3.3 |
| Maternal | Lower secondary (%) | 7.8 | 23.3 | 7.0 | 6.9 | 5.7 | 10.4 | 28.0 |
| Education | Upper secondary (%) | 52.5 | 46.7 | 67.8 | 51.9 | 49.7 | 33.6 | 40.0 |
| <2 years tertiary (%) | 3.7 | 6.7 | 2.6 | 4.5 | 3.3 | 3.2 | 8.0 | |
| 2+ years tertiary (%) | 36.0 | 23.3 | 22.6 | 36.7 | 41.3 | 52.8 | 24.0 | |
| Paternal | Unskilled (%) | 21.6 | 33.3 | 33.3 | 24.1 | 13.4 | 12.8 | 4.0 |
| occupational class | Skilled (%) | 27.0 | 43.3 | 38.9 | 28.6 | 21.3 | 12.0 | 12.0 |
| Assistant non-manual (%) | 9.1 | 0.0 | 5.2 | 11.9 | 8.7 | 11.2 | 8.0 | |
| Intermediate non-manual (%) | 16.3 | 0.0 | 12.2 | 13.3 | 20.8 | 23.2 | 28.0 | |
| Professional/Managerial (%) | 15.5 | 0.0 | 3.0 | 13.1 | 24.0 | 28.0 | 20.0 | |
| Self-employed (%) | 4.1 | 3.3 | 3.0 | 3.3 | 4.1 | 8.0 | 12.0 | |
| Farmers (%) | 1.5 | 3.3 | 0.7 | 1.9 | 1.6 | 0.8 | 4.0 | |
| Unclassified/no record (%) | 4.9 | 16.7 | 3.7 | 3.8 | 6.0 | 4.0 | 12.0 | |
| Paternal log | Mean | 7.85 | 7.77 | 7.76 | 7.89 | 7.93 | 7.75 | 7.75 |
| income | S.D. | 0.60 | 0.23 | 0.65 | 0.48 | 0.63 | 0.78 | 0.65 |
| Depression | Much affected (%) | 6.3 | 10.0 | 7.8 | 6.4 | 4.9 | 4.8 | 12.0 |
| Slightly affected (%) | 10.1 | 6.7 | 13.0 | 9.5 | 8.7 | 11.2 | 8.0 | |
| Not affected (%) | 83.6 | 83.3 | 79.3 | 84.1 | 86.3 | 84.0 | 80.0 | |
| Anxiety | Much affected (%) | 10.4 | 10.0 | 12.6 | 9.8 | 9.6 | 10.4 | 12.0 |
| Slightly affected (%) | 28.5 | 36.7 | 26.7 | 26.0 | 29.8 | 34.4 | 32.0 | |
| Not affected (%) | 61.1 | 53.3 | 60.7 | 64.3 | 60.7 | 55.2 | 56.0 | |
Regression analyses
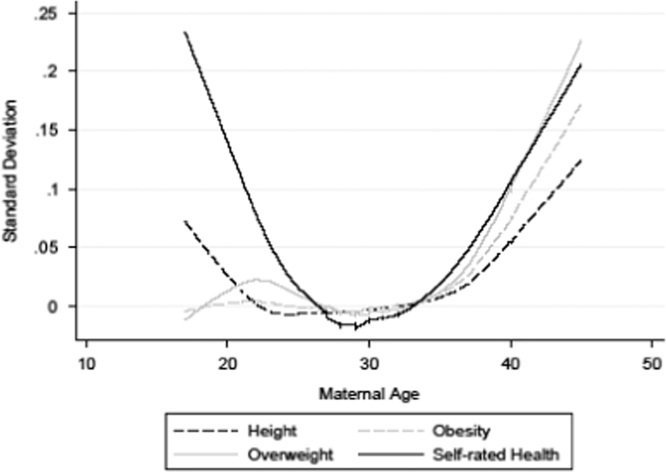
Fig. 2, Fig. 3, and Table 3, Table 4, show the results from our analyses examining the relationship between maternal age at the time of birth and the various outcomes that we study. The results for the relationship between maternal age and height, being obese, being overweight, and self-rated health can be seen in Table 3 and Fig. 2. Although the results in Table 3 do not show a statistically significant relationship, the results from the semi-parametric analysis, seen in Fig. 2, show that individuals born to teenage mothers are approximately 0.075 standard deviations shorter than those born to mothers aged 25–29, while individuals born to mothers aged 40 or older are approximately 0.1 standard deviations shorter, equivalent to approximately 1 cm.
Fig. 2.
Maternal age and height, obesity, overweight, and self-rated health. Estimates are based upon semi-parametric lowess regression.
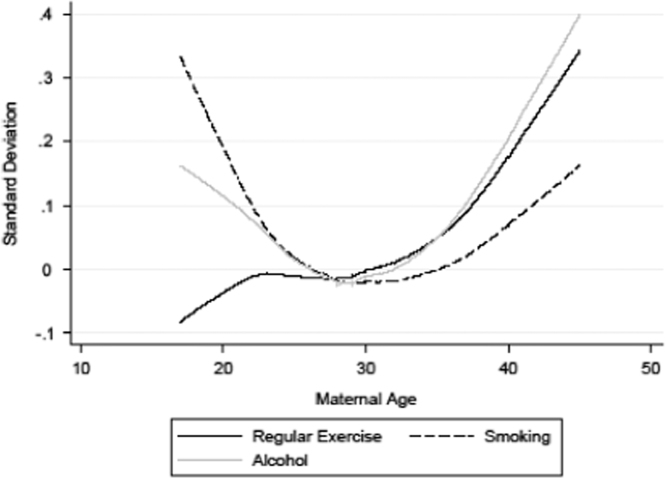
Fig. 3.
Maternal age and regular exercise, smoking, and alcohol consumption behaviours. Estimates are based upon semi-parametric lowess regression.
Table 3.
Results: maternal age at the time of birth (MAB) and height, self-rated health, overweight, and obesity.
|
Heighta |
SRHa |
Overweightb |
Obeseb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| b | se | b | se | b | se | b | se | ||
| Maternal age | 0.299 | 0.297 | −0.059+ | 0.035 | −0.173 | 0.123 | −0.107 | 0.210 | |
| Maternal age Sq | −0.005 | 0.005 | 0.001+ | 0.001 | 0.003 | 0.002 | 0.002 | 0.004 | |
| Gender | Male | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Female | −14.305⁎ | 0.363 | 0.199 | 0.041 | −0.232 | 0.147 | 0.796⁎ | 0.273 | |
| Size of sibling group | 1 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| 2 | −0.781 | 0.941 | −0.054 | 0.117 | 0.533 | 0.495 | 0.239 | 0.734 | |
| 3 | −1.447 | 0.958 | 0.001 | 0.117 | 0.739 | 0.494 | 0.849 | 0.715 | |
| 4 | −0.863 | 0.996 | −0.052 | 0.125 | 0.674 | 0.514 | 0.483 | 0.787 | |
| 5+ | −1.561 | 1.044 | 0.091 | 0.130 | 0.885+ | 0.524 | 1.068 | 0.771 | |
| Maternal education | Basic Education | −1.162 | 1.047 | −0.016 | 0.143 | −0.196 | 0.448 | −0.635 | 0.913 |
| Gymnasium | −0.233 | 0.899 | −0.023 | 0.129 | −0.125 | 0.381 | 0.494 | 0.720 | |
| <2 Years Tertiary | 0.000 | 0.000 | 0.000 | . | 0.000 | ||||
| 2+ Years Tertiary | 0.052 | 0.911 | −0.076 | 0.133 | −0.383 | 0.393 | 0.009 | 0.756 | |
| Paternal occupational class | Unskilled | −1.443⁎ | 0.629 | −0.016 | 0.071 | 0.217 | 0.258 | −0.149 | 0.423 |
| Skilled | −1.041+ | 0.580 | 0.087 | 0.068 | 0.409+ | 0.249 | −0.018 | 0.401 | |
| Assistant non-manual | −1.400+ | 0.730 | 0.049 | 0.085 | 0.501 | 0.305 | 0.127 | 0.492 | |
| Intermediate non-manual | 0.000 | 0.000 | 0.000 | . | 0.000 | ||||
| Professional/Managerial | −0.165 | 0.638 | 0.033 | 0.071 | −0.102 | 0.299 | −0.905 | 0.596 | |
| Self-employed | 0.365 | 1.032 | −0.124 | 0.125 | 0.129 | 0.438 | 0.077 | 0.699 | |
| Farmers | 1.490 | 1.751 | 0.110 | 0.140 | 0.670 | 0.567 | 0.000 | ||
| Unclassified/no record | −2.504⁎ | 1.044 | −0.125 | 0.107 | 0.847⁎ | 0.351 | 0.540 | 0.536 | |
| Paternal log income | Log Income of Father | 0.267 | 0.322 | −0.019 | 0.039 | −0.078 | 0.109 | −0.117 | 0.161 |
| Regular exercise | No | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Yes | 0.290 | 0.434 | −0.257⁎ | 0.051 | 0.098 | 0.181 | −0.259 | 0.291 | |
| Smoking | Daily | −0.078 | 0.447 | −0.076 | 0.051 | −0.107 | 0.185 | −0.013 | 0.357 |
| Occasional | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Never | 0.328 | 0.581 | 0.217⁎ | 0.075 | −0.045 | 0.255 | 0.533 | 0.425 | |
| Becoming drunk | 3 times per week or more | −1.986 | 2.057 | 0.360 | 0.258 | 0.066 | 0.843 | 1.567+ | 0.941 |
| 1-2 times per week | −0.630 | 0.565 | 0.076 | 0.069 | −0.359 | 0.266 | −0.340 | 0.482 | |
| 2-3 times per month | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Once per month | −0.492 | 0.468 | −0.037 | 0.054 | 0.025 | 0.200 | 0.221 | 0.380 | |
| Less often | −0.918+ | 0.535 | 0.045 | 0.060 | 0.080 | 0.213 | 0.221 | 0.388 | |
| Never | −1.291+ | 0.728 | 0.112 | 0.076 | 0.333 | 0.260 | 1.139 | 0.409 | |
| Constant | 177.850 | 5.039 | 2.924 | 0.595 | 1.021 | 2.098 | −1.887 | 3.544 | |
| N | 1236 | 1236 | 1236 | 1236 | |||||
Note:
OLS.
Logistic regression model. Statistical significance indicators.
p<0.1,
p<0.05.
Table 4.
Results: maternal age at the time of birth (MAB) and smoking behaviour, alcohol consumption, and regular exercise.
|
Smokinga |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Daily |
Occasionally |
Alcoholb |
Regular Exercisec |
||||||
| b | se | b | se | b | se | b | se | ||
| Maternal age | 0.313+ | 0.160 | 0.026 | 0.177 | 0.129⁎ | 0.060 | 0.142 | 0.118 | |
| Maternal age Sq | −0.005+ | 0.003 | 0.000 | 0.003 | −0.002⁎ | 0.001 | −0.003 | 0.002 | |
| Gender | Male | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Female | −0.386⁎ | 0.190 | −0.660⁎ | 0.212 | 0.240⁎ | 0.071 | 0.336⁎ | 0.146 | |
| Size of sibling group | 1 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| 2 | −0.007 | 0.493 | 0.046 | 0.566 | −0.059 | 0.206 | 0.665⁎ | 0.339 | |
| 3 | −0.184 | 0.498 | −0.185 | 0.571 | −0.026 | 0.208 | 0.863⁎ | 0.344 | |
| 4 | 0.095 | 0.533 | 0.354 | 0.605 | −0.053 | 0.223 | 0.552 | 0.364 | |
| 5+ | −0.448 | 0.538 | −0.215 | 0.611 | 0.047 | 0.228 | 0.472 | 0.381 | |
| Maternal education | Basic Education | −0.877 | 0.627 | −1.266+ | 0.666 | 0.321 | 0.220 | 0.132 | 0.431 |
| Gymnasium | −0.501 | 0.555 | −1.130+ | 0.587 | 0.209 | 0.176 | 0.168 | 0.361 | |
| <2 Years Tertiary | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| 2+ Years Tertiary | −0.340 | 0.562 | −0.890 | 0.594 | 0.089 | 0.178 | 0.271 | 0.366 | |
| Paternal occupational class | Unskilled | −0.593+ | 0.343 | −0.436 | 0.385 | −0.110 | 0.123 | −0.137 | 0.242 |
| Skilled | −0.845⁎ | 0.321 | −0.449 | 0.356 | −0.111 | 0.117 | 0.030 | 0.234 | |
| Assistant non-manual | −0.424 | 0.420 | −0.186 | 0.461 | −0.074 | 0.150 | 0.065 | 0.287 | |
| Intermediate non-manual | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Professional/Managerial | −0.420 | 0.374 | −0.231 | 0.420 | −0.168 | 0.130 | 0.170 | 0.259 | |
| Self-employed | −1.189 | 0.474 | −0.224 | 0.512 | −0.604⁎ | 0.192 | −0.939⁎ | 0.338 | |
| Farmers | 13.957⁎ | 0.408 | 14.250⁎ | 0.547 | −0.415+ | 0.229 | −0.537 | 0.504 | |
| Unclassified/no record | −1.489⁎ | 0.408 | −1.511⁎ | 0.492 | −0.288 | 0.199 | −0.129 | 0.344 | |
| Paternal log income | Log Income of Father | 0.218 | 0.149 | 0.387⁎ | 0.172 | −0.076 | 0.068 | 0.111 | 0.118 |
| Depression | Much affected | 0.087 | 0.397 | −0.251 | 0.433 | −0.084 | 0.192 | −0.315 | 0.327 |
| Slightly affected | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Not affected | 0.690⁎ | 0.291 | −0.015 | 0.306 | 0.108 | 0.128 | 0.475⁎ | 0.222 | |
| Anxiety | Much affected | −0.386 | 0.320 | −0.178 | 0.345 | −0.046 | 0.149 | −0.258 | 0.256 |
| Slightly affected | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Not affected | 0.300 | 0.217 | 0.163 | 0.240 | 0.103 | 0.082 | 0.155 | 0.169 | |
| Constant | −4.352 | 2.731 | −1.104 | 3.037 | 2.293 | 1.082 | −2.975 | 2.001 | |
| N | 1236 | 1236 | 1236 | 1236 | |||||
Note:
Multinomial logistic regression;
OLS;
Logistic regression. Statistical significance indicators:
p<0.1,
p<0.05.
The results for the relationship between maternal age and both being obese and overweight can also be seen in Fig. 2 and Table 3. Fig. 2 shows that individuals born to women 30 or younger are not at an increased risk of being obese, but that there is a between a 0.10 and 0.15 standard deviation increase in the probability of being obese amongst those who are born to mothers aged 40 or older. The pattern is similar for the probability of being overweight, though now those born to older mothers have between a 0.10 to 0.20 standard deviation increase in the probability of being overweight. As for self-rated health, Fig. 2 shows that there is a clear disadvantage for those born to younger or older mothers. Relative to those born to mothers aged 28–30, those born to the very youngest and the very oldest mothers have self-reported health that is 0.20 standard deviations lower.
The results from the analyses examining the relationship between maternal and health behaviours can be seen in Fig. 3 and Table 4. The results from the semi-parametric regression for the relationship between maternal age and taking regular exercise show that those born to a teenaged mother, or a mother in her early 20s, have a higher probability of exercising regularly. However, compared to those born to women in their late twenties, the probability of exercising regularly amongst those born to the oldest mothers is 0.30 standard deviations lower. The results for smoking behaviour, also shown in Fig. 3, indicate that the frequency of smoking is 0.30 standard deviations higher than the mean amongst those born to the youngest mothers, and over 0.10 standard deviations higher than the mean amongst those born to the very oldest mothers. Finally, the results for the relationship between maternal age and alcohol consumption can also be seen in Fig. 3 and Table 4. The results shown in Fig. 3 indicate that the self-reported alcohol consumption of those born to the youngest mothers is over 0.10 standard deviations higher than amongst those born to mothers aged 28–30, while self-reported alcohol consumption is over 0.20 standard deviations higher amongst those who are born to mothers in their late thirties and early forties.
Discussion
The results from this study show for the first time that maternal age at the time of birth is associated with the health behaviours of young adults in Sweden, and consistent with previous research, finds that those who are born to younger and older mothers have lower self-rated health. Furthermore, those born to the youngest and oldest mothers are shorter, and those born to older mothers are more likely to be obese or overweight. The results from the semi-parametric regressions indicate that this is true even after controlling for parental SES, various socio-demographic characteristics, as well as exercise behaviour and alcohol and smoking patterns. One explanation for these patterns of results could be a lasting disadvantage from poor peri-natal outcomes, as it is known that those born to younger and older mothers are at greater risk of low birth weight, pre-term delivery, and other pregnancy complications, which can have long-term negative consequences (Black et al., 2007). While most of the results from our various OLS, logistic regression, and multinomial regression analyses did not show a statistically significant relationship between maternal age and the various outcomes that we study, that is likely to be due to the relatively low statistical power of this study. This low statistical power makes it more difficult to pick up statistically significant differences where they are likely to be small, such as with height, and for outcomes that are relatively rare amongst young adults in Sweden, such as being obese and overweight. Although rates of being overweight and obese are increasing in Sweden, they still lag far behind the rates found in the trend leader, the United States. It may well be that as weight increases with age, those weight gains may be particularly concentrated amongst those born to younger and older parents.
Our analyses also show that those born to young and older mothers are more likely to smoke, and to consume alcohol regularly, while those born to older mothers are less likely to exercise regularly, even after adjusting for parental SES, socio-demographic characteristics, and self-reported depression and anxiety. This suggests that our hypothesis that alcohol consumption and smoking could be influenced by depression or anxiety related to the declining health of the parent does not explain the observed relationship. Although we can only speculate about the explanation for the remaining association, it could be related to the fact that previous studies have shown that older parents spend less time per day with their children (Sayer et al., 2004). Older parents may well be more invested in their careers, or simply have more workplace responsibilities since they will on average have more senior positions within the workplace. Less time for parent-child interaction decreases the opportunities for parents to exercise social control. In turn, less constrained adolescents will have more opportunities to experiment, and may be more likely to be influenced by peer behaviours.
Another potentially important explanation is the health behaviours of the parents themselves. As mentioned earlier, the fact that we focus on a particular birth cohort, individuals born in Sweden in 1990, means that maternal age is also mechanically related to maternal birth cohort. Patterns of smoking have varied by cohort, and smoking rates are higher in earlier born cohorts because public health campaigns and changes to taxation rates have progressively introduced stronger disincentives for smoking over time (Jha & Peto, 2014). Those who have been smoking for longer are less able to quit (Chen & Millar, 1998). Given that parental smoking is strongly correlated with child smoking uptake (Chassin, Presson, Todd, Rose, & Sherman, 1998), we can surmise that those born to older mothers are more likely to have parents who smoke. Unfortunately we do not have information on parental health behaviours in our data that would allow us to test that hypothesis. Although this may explain part of the relationship between maternal age and smoking, there are good reasons why it should not. Our analyses also control for parental socioeconomic status, educational level, and income, all of which are strongly correlated with smoking behaviours (Huisman, Kunst, & Mackenbach, 2005). Furthermore, educational levels explain a much greater proportion of the variance in smoking than does maternal age. In 1990 there was a 4 percentage point difference in regular smoking between mothers aged 25–29 and mothers aged 35–39 (Forey, Hamling, Hamling, Thornton, & Lee, 2011). However, the difference in daily smoking between women with a high or a low education was more than twice as large as that (Cavelaars et al., 2000, Giskes et al., 2005). Furthermore, rates of regular smoking are much lower amongst mothers than they are amongst women in the population as a whole, though young and lower educated mothers have the lowest rates of quitting (Dejin-Karlsson et al., 1996). Given these various factors, we consider the risk that our results are an artefact of differences in maternal smoking by maternal age to be small.
Although we cannot test whether our hypotheses are true or not, it is worthwhile speculating what the long-term consequences of higher rates of alcohol consumption and smoking for these adolescents would lead to in the future. Since nicotine and alcohol are addictive and habit-forming, consumption patterns tend to be maintained over time, or even to increase with age (Grant and Dawson, 1997, Faggiano et al., 2001). This gives some reason to believe that part of the relationship between being born to an older mother and worse long-term health that has been shown in other studies could be explained by these health behaviours. Higher levels of alcohol consumption and smoking would also be consistent with the specific negative long-term health outcomes that have been found to be associated with advanced maternal age, such as higher rates of obesity, lower self-rated health, higher mortality (Myrskylä & Fenelon, 2012), diabetes (Cardwell et al., 2010), and cancer (Hemminki & Kyyrönen, 1999). Having said that, we should be somewhat cautious with these speculations. Although alcohol consumption is correlated over time, the pattern is less stable for projections based upon samples of younger adults, and heavy drinkers (Greenfield & Kerr, 2003). Explanations for changes in consumption include a maturation effect related to age, a period effect, and there may also be cohort effects. A general limitation of our data is that it is impossible for us to begin to distinguish between age, period, and cohort influences since all of our respondents are drawn from the same birth year. Nevertheless, based on our findings we believe that future work on the influence of maternal age would benefit from research investigating how adolescents and young adults are affected rather than primarily focusing on health differences in late adulthood.
Funding Source
European Research Council Starting Grant to Mikko Myrskylä (COSTPOST): 336475.
Acknowledgements
Data collection was supported by the Swedish Council for Working Life and Social Research, the Stockholm University Linnaeus Center for Integration Studies, and by a European Research Council Starting Grant [grant no. 263422] awarded to Jens Rydgren.
Contributor Information
Kieron Barclay, Email: k.j.barclay@lse.ac.uk, kieron.barclay@sociology.su.se, barclay@demogr.mpg.de.
Mikko Myrskylä, Email: m.myrskyla@lse.ac.uk, myrskyla@demogr.mpg.de.
References
- Andersen A.-M.N., Wohlfahrt J., Christens P., Olsen J., Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320(7251):1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead L., Klein K., Forehand R. Parental physical illness and child functioning. Clinical Psychology Review. 1995;15(5):409–422. [Google Scholar]
- Barclay K., Myrskylä M. Birth order and physical fitness in early adulthood: evidence from Swedish military conscription data. Social Science Medicine. 2014;123:141–148. doi: 10.1016/j.socscimed.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Barclay K., Kolk M. Birth order and mortality: a population-based cohort study. Demography. 2015;52(2):613–639. doi: 10.1007/s13524-015-0377-2. [DOI] [PubMed] [Google Scholar]
- Barnes G.M., Reifman A.S., Farrell M.P., Dintcheff B.A. The effects of parenting on the development of adolescent alcohol misuse: a six-wave latent growth model. Journal of Marriage and Family. 2000;62(1):175–186. [Google Scholar]
- Biddle S.J., Pearson N., Ross G.M., Braithwaite R. Tracking of sedentary behaviours of young people: a systematic review. Preventive Medicine. 2010;51(5):345–351. doi: 10.1016/j.ypmed.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Black S.E., Devereux P.J., Salvanes K.G. From the cradle to the labor market? The effect of birth weight on adult outcomes. Quarterly Journal of Economics. 2007;122(1):409–439. [Google Scholar]
- Blair S.N., Kampert J.B., Kohl H.W., Barlow C.E., Macera C.A., Paffenbarger R.S. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. Journal of American Medical Association. 1996;276(3):205–210. [PubMed] [Google Scholar]
- Blossfeld H.-P., Huinink J. Human capital investments or norms of role transition? How women’s schooling and career affect the process of family formation. American Journal of Sociology. 1991;97(1):143–168. [Google Scholar]
- Breen R., Jonsson J.O. Explaining Change in Social Fluidity: Educational Equalization and Educational Expansion in Twentieth‐Century Sweden. American Journal of Sociology. 2007;112(6):1775–1810. [Google Scholar]
- Campbell F., Conti G., Heckman J.J., Moon S.H., Pinto R., Pungello E. Early childhood investments substantially boost adult health’. Science. 2014;343(6178):1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell C.R., Stene L.C., Joner G., Bulsara M.K., Cinek O., Rosenbauer J. Maternal age at birth and childhood type 1 diabetes: a pooled analysis of 30 observational studies. Diabetes. 2010;59(2):486–494. doi: 10.2337/db09-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelaars A.E., Kunst A.E., Geurts J.J., Crialesi R., Grötvedt L., Helmert U. Educational differences in smoking: international comparison. British Medical Journal. 2000;320(7242):1102–1107. doi: 10.1136/bmj.320.7242.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L., Presson C.C., Todd M., Rose J.S., Sherman S.J. Maternal socialization of adolescent smoking: the intergenerational transmission of parenting and smoking. Developmental Psychology. 1998;34(6):1189–1201. doi: 10.1037//0012-1649.34.6.1189. [DOI] [PubMed] [Google Scholar]
- Chen J., Millar W.J. Age of smoking initiation: implications for quitting. Health Reports Statistics Canada. 1998;9:39–48. [PubMed] [Google Scholar]
- Compas B.E., Worsham N.L., Epping-Jordan J.E., Grant K.E., Mireault G., Howell D.C. When mom or dad has cancer: markers of psychological distress in cancer patients, spouses, and children. Health Psychology. 1994;13(6):507. [PubMed] [Google Scholar]
- Dejin-Karlsson E., Hanson B.S., Ostergren P.O., Ranstam J., Isacsson S.O., Sjöberg N.O. Psychosocial resources and persistent smoking in early pregnancy-a population study of women in their first pregnancy in Sweden. Journal of Epidemiology and Community Health. 1996;50(1):33–39. doi: 10.1136/jech.50.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit A.R., Crum R.M. Prospective study of depression and the risk of heavy alcohol use in women. American Journal of Psychiatry. 2000;157(5):751–758. doi: 10.1176/appi.ajp.157.5.751. [DOI] [PubMed] [Google Scholar]
- Erikson R., Goldthorpe J.H., Portocarero L. Intergenerational class mobility in three Western European societies: England, France and Sweden. British Journal of Sociology. 1979;34(3):415–441. doi: 10.1111/j.1468-4446.2009.01246.x. [DOI] [PubMed] [Google Scholar]
- Ezzati M., Lopez A.D. Estimates of global mortality attributable to smoking in 2000. The Lancet. 2003;362(9387):847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- Faggiano F., Versino E., Lemma P. Decennial trends of social differentials in smoking habits in Italy. Cancer Causes Control. 2001;12(7):665–671. doi: 10.1023/a:1011247024979. [DOI] [PubMed] [Google Scholar]
- Forey, B., Hamling, J., Hamling, J., Thornton, A. & Lee, P. (2011). International smoking statistics, a collection of worldwide historical data: Sweden (accessed 23.10.15) 〈http://www.pnlee.co.uk/Downloads/ISS/ISS-Sweden_111024.pdf〉.
- Fraser A.M., Brockert J.E., Ward R.H. Association of young maternal age with adverse reproductive outcomes. New England Journal of Medicine. 1995;332(17):1113–1118. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- Fuller T.D. Moderate alcohol consumption and the risk of mortality. Demography. 2011;48(3):1105–1125. doi: 10.1007/s13524-011-0035-2. [DOI] [PubMed] [Google Scholar]
- Furstenberg F.E., Jr Teenage childbearing as a public issue and private concern. Annual Review of Sociology. 2003;29:23–39. [Google Scholar]
- Giskes K., Kunst A.E., Benach J., Borrell C., Costa G., Dahl E. Trends in smoking behaviour between 1985 and 2000 in nine European countries by education. Journal of Epidemiology and Community Health. 2005;59(5):395–401. doi: 10.1136/jech.2004.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B.F., Dawson D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Greenfield T.K., Kerr W.C. Tracking alcohol consumption over time. Alcohol Research and Health. 2003;27(1):30–38. [PMC free article] [PubMed] [Google Scholar]
- Hatton T.J., Martin R.M. The effects on stature of poverty, family size, and birth order: British children in the 1930s. Oxford Economic Papers. 2010;62(1):157–184. [Google Scholar]
- Hemminki K., Kyyrönen P. Parental age and risk of sporadic and familial cancer in offspring: implications for germ cell mutagenesis. Epidemiology. 1999;10(6):747–751. [PubMed] [Google Scholar]
- Huisman M., Kunst A.E., Mackenbach J.P. Educational inequalities in smoking among men and women aged 16 years and older in 11 European countries. Tobacco Control. 2005;14(2):106–113. doi: 10.1136/tc.2004.008573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W.H. The human sex ratio. Part 1: a review of the literature. Human Biology. 1987;59(5):721–752. [PubMed] [Google Scholar]
- Jha P., Peto R. Global effects of smoking, of quitting, and of taxing tobacco. New England Journal of Medicine. 2014;370(1):60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- Lokshin M. Difference-based semiparametric estimation of partial linear regression models. The Stata Journal. 2006;6:377–383. [Google Scholar]
- McLanahan S. Diverging destinies: how children are faring under the second demographic transition. Demography. 2004;41(4):607–627. doi: 10.1353/dem.2004.0033. [DOI] [PubMed] [Google Scholar]
- McLanahan S., Sandefur G. Growing up with a single parent: what hurts, what helps. Harvard University Press; Cambridge, MA: 1994. [Google Scholar]
- Midlöv P., Leijon M., Sundquist J., Sundquist K., Johansson S.-E. The longitudinal exercise trend among older Swedes aged 53-84 years-a 16-year follow-up study. BMC Public Health. 2014;14(1):1327. doi: 10.1186/1471-2458-14-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrskylä M., Fenelon A. Maternal age and offspring adult health: evidence from the health and retirement study. Demography. 2012;49(4):1231–1257. doi: 10.1007/s13524-012-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrskylä M., Margolis R. Happiness: before and after the kids. Demography. 2014;51(5):1843–1866. doi: 10.1007/s13524-014-0321-x. [DOI] [PubMed] [Google Scholar]
- Myrskylä M., Elo I.T., Kohler I.V., Martikainen P. The association between advanced maternal and paternal ages and increased adult mortality is explained by early parental loss. Social Science Medicine. 2014;119:215–223. doi: 10.1016/j.socscimed.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navot D., Bergh R., Williams M.A., Garrisi G.J., Guzman I., Sandler B. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. The Lancet. 1991;337(8754):1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- OECD . OECD; Paris: 2014. OECD family database. [Google Scholar]
- Rostila M., Saarela J.M. Time does not heal all wounds: mortality following the death of a parent. Journal of Marriage and Family. 2011;73(1):236–249. [Google Scholar]
- Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Sayer L.C., Bianchi S.M., Robinson J.P. Are parents investing less in children? Trends in mothers and fathers time with children. American Journal of Sociology. 2004;110(1):1–43. [Google Scholar]
- Schwartz D., Mayaux M.J. Female fecundity as a function of age results of artificial insemination in 2193 nulliparous women with azoospermic husbands. New England Journal of Medicine. 1982;306:404–406. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- Statistics Sweden . Vol. 1861. Statistics Sweden; Stockholm: 2010. Cohort mortality in Sweden. (Mortality statistics since). [Google Scholar]