Abstract
Canine and human lymphoma share similar characteristics in disease development and response to therapy. Translational research can be furthered using tools such as canine cell lines to model therapeutic compounds and strategies. We developed 5 B-cell lymphoma cell lines from dogs with confirmed large B-cell lymphoma. These cell lines were CD3, CD18, CD20, and CD90 positive with variable CD79a, CD1c and CD34 expression. All cell lines were tumorigenic in Nu/nu mice and were wild type for p53. Canine lymphoma cell lines serve as an important resource for translational lymphoma research.
Keywords: Canine, B-cell lymphoma, Cell line, Non-Hodgkin's
1. Introduction
Lymphoma is the most common hematological cancer in dogs with an estimated incidence of 1/100,000 dogs per year, with non-Hodgkin's diffuse large B-cell lymphoma representing the most common type [1]. Dogs are emerging as important models of human cancer because of the shared genome and polygenetic development of spontaneous cancers [2–4]. In fact, the canine genome, recently sequenced, is reported to be more homologous to humans than that of mice [5]. Dogs are affected by naturally occurring lymphoma that closely resembles the human disease with respect to the World Health Organization's (WHO) classification of lymphoma types, incidence rate, response to chemotherapy, and survival time [1,6,7]. Canine hematologic malignancies have also been shown to have evolutionarily conserved chromosome aberrations that are similar to equivalent diseases in humans, and also to have similar effects in both species [3]. Additionally, due to intense selection pressure, the genome of some pure bred dogs is more homogeneous than the outbred genome of most human populations. This homogeneity can facilitate the identification of key molecular events involved in lymphomagenesis, which can then be interrogated in humans. Canine lymphoma has been used to model the P53 pathway, and has been shown to have increased expression of RNPC1 with downregulation of P53, which may contribute to development of lymphoma [8]. Companion animals also have accelerated aging compared to humans, and are kept into old age where development of spontaneous cancers is more common [4]. The study of this naturally occurring tumor in dogs, is therefore likely to provide additional and useful information regarding lymphoma in people upon which to base human clinical trials.
While several canine cell lines have been generated and at least partially characterized, only one B-cell lymphoma line has been established and well characterized [9], likely due to the known difficulty in establishing lymphoid cell lines [10]. Because canine lymphoma appears to be a useful model of human non-Hodgkin's lymphoma, development of canine cell lines will provide greater insight into the molecular mechanisms of the disease and will allow in vitro testing of promising agents and therapies.
Therefore, the purpose of this study was to develop and characterize 5 distinct canine cell lines in order to provide more tools for research into both canine and human lymphoma.
2. Materials and methods
2.1. Canine lymphoma sample collection
Fresh tissue samples were obtained from a series of dogs presenting to the William R. Pritchard Veterinary Medical Teaching hospital for the diagnosis and/or treatment of lymphoma. Owner consent was obtained, and the procedure was approved by the institution's Animal Care and Use Committee.
Dogs were staged according to the WHO's classification of lymphoma in domestic animals. A 23-gauge needle was inserted into the lymph node and redirected through the node 2–3 times. The needle was attached to a 12mL syringe, and the cells of the needle were expelled into a tube containing 1 mL 1 × TBS buffer. A 2nd needle was introduced into the same lymph node, the sampling procedure was repeated, and the two samples were pooled.
2.2. Preparation of primary canine lymphoma cell cultures
The canine lymph node aspirated cells were suspended in TBS buffer and washed twice. 3 mL of red blood cell lysis buffer (155 mM ammonium chloride, 12mM potassium bicarbonate in purified water (Millipore Corporation, Billerica, MA), pH 7.2) were added, and the sample was incubated for 5min at room temperature until clear. The sample was then centrifuged for 5 min at 220 × g. The supernatant was removed, and the cells were washed in RPMI-1640 medium (ATCC, Cat. 30-2001).
2.3. Establishment of canine lymphoma cell lines
Cells were cultured in T25 flasks in RPMI-1640 medium supplemented with 20% heat–inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin, and l-glutamine. The cultures were incubated at 37 °C in a humidified atmosphere of 5% CO2. For culture maintenance, a small volume of fresh growth medium was added once every 2–3 days, and the medium was thoroughly changed after centrifugation of the culture cells weekly. For the first 4 weeks, both adherent and non-adherent cells were passaged together weekly. After 4 weeks, the non-adherent cell population grew as a suspension culture consisting primarily of single cells and small aggregates of cells. After cell growth became apparent, the cells were passaged at 4 or 5 day intervals by adjusting the cell density to 2 × 105–1 × 106 cells/mL. For storage, aliquots of cells were kept at a concentration of 1–5 × 106 cells/mLin90% RPMI 1640 (supplemented with 10% FBS) and 10% DMSO and stored in liquid nitrogen. The cell lines were subsequently maintained in continuous cultures for over 1 year.
For generation of growth curves, cells were plated to achieve a plating density of 5–8 × 104 cells suspended in 1 mL of culture medium per well of a 48-well plate. Cells were incubated at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Cells in triplicate wells were counted every 24 h. The values obtained were plotted on a log-linear scale. The population-doubling time was determined from the exponential phase of the growth curve.
2.4. Flow cytometry
Lymph node aspirates were analyzed within 24 h of collection and were unfixed. For monoclonal antibody (MAb) binding to the indicated antibodies in Table 1, aliquots of approximately 1 × 106 cells were incubated with 25 μL of MAb tissue culture fluid supernatant for30minat room temperature. Cells were then spun and washed twice with flow buffer (1 mM Mg2+ in 1 × TBS, 1% horse serum). For indirect immunofluorescence, these cells were detected by incubation with 50 μl of a 1:100 dilution of FITC-conjugated horse anti-mouse IgG (Vector Laboratories, Carpenteria, CA) for 15 min. Cells were then spun, washed and resuspended in 500 μL of flow buffer. A negative control consisting of an isotype matched irrelevant MAb was included. Fluorescence was measured in 10,000 cells using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Data was analyzed with commercial software (Flowjo, Treestar Inc., Ashland, OR).
Table 1.
Antigen, clone number and targets of antibodies used to immunophenotype the canine lymphoma cell lines by flow cytometry and immunocytochemistry.
| Reagent | Clone # | Source |
|---|---|---|
| CD1ac allotype | Ca9.AG5 | P.F. Moore, UC Davis |
| CD1ca | Ca13.9H11 | P.F. Moore, UC Davis |
| CD3b | CD3-12 | Serotec, Oxford, UK |
| CD3c | Ca17.2A12 | P.F. Moore, UC Davis |
| CD4c | Ca13. 1E4 | P.F. Moore, UC Davis |
| CD5c | YKIX.322 | Serotec, Oxford, UK |
| CD11ac | Ca11.4D3 | P.F. Moore, UC Davis |
| CD11ac | Ca16.1B11 | P.F. Moore, UC Davis |
| CD11bc | Ca16.3E10 | P.F. Moore, UC Davis |
| CD11cc | Ca11.6A1 | P.F. Moore, UC Davis |
| CD11dc | Ca11.8H2 | P.F. Moore, UC Davis |
| CD14c | TÜK4 | CALTAG, Burlingame, CA |
| CD18c | Ca1.4E9 | P.F. Moore, UC Davis |
| CD20b | RB-9013 | Neomarkers, Fremont, CA |
| CD21a | Ca2.1D6 | P.F. Moore, UC Davis |
| CD34c | 1H6 | Peter McSweeney & Richard Nash, Fred Hutchinson Cancer Research Center, Seattle, WA |
| CD45a | Ca12.10C12 | P.F. Moore, UC Davis |
| CD45RAc | Ca4.1D3 | P.F. Moore, UC Davis |
| CD49Dc | Ca4.5B3 | P.F. Moore, UC Davis |
| CD54c | CL18.1D8 | P.F. Moore, UC Davis |
| CD79ab | HM57 | Dako, Carpenteria, CA |
| CD90c | Ca1.4G8 | P.F. Moore, UC Davis |
| Granulocytic (unclustered)c | DM5 | Brenda Sandmaier Fred Hutchinson Cancer Research Center, Seattle, WA |
| MHCIIa | Ca2.1C12 | P.F. Moore, UC Davis |
Antibody used for flow cytometry and immunocytochemistry.
Antibody used for immunocytochemistry only.
Antibody used for flow cytometry only.
For the original patient samples, single- and double-labeled tubes of MAb to CD3, CD21 and CD49d were used followed by the FITC-conjugated secondary of horse anti-mouse IgG (Vector Laboratories, Carpenteria, CA) were prepared and a maximum of 20,000 cells were analyzed with the flow cytometer. Cell line preparations were prepared in single-labeled tubes. Monoclonal antibodies used in immunophenotyping of the cell lines are shown in Table 1.
2.5. Immunocytochemistry and immunohistochemistry
The expression of antigens was assessed on one original patient sample, cytospin smears of lymphoma cell lines, and xenograft tissue, as previously described [11]. To address the complication of using murine monoclonal antibodies in mouse tissue, the Dako ARK method was used for assessing the xenograft tissue in mice (Dako, Carpenteria, USA).
2.6. PCR test for clonality
PCR assays for clonality assessment were performed on DNA extracted from original patient samples (aspirate smears or blood from leukemia patients), cytospin smears of lymphoma cell lines, and xenograft tissue as described previously [12]. Two additional primer sets, directed against 2 additional canine Ig loci, FR2 and Kde, were also run [13]. All assays included a negative control (10 μL water instead of gDNA template), polyclonal control, and appropriate clonal controls. DNA samples were prepared using the QIAamp DNA mini kit (Qiagen Inc., Valencia, CA). For amplification (in triplicate), approximately 100 ng of DNA was amplified with 200–400 nM of each primer in a 50 μL reaction volume, including 1 × reaction buffer, 0.5 mM MgCl2, 50 mM each dNTP and 0.25 μL Qiagen HotStart Taq Polymerase. PCR products were analyzed on an eGene capillary electrophoresis machine (HDA-GT12 system, eGene, Inc., Irvine, CA). A reaction was considered clonal if one or more reproducible, dominant and discrete bands were present after electrophoresis. A reaction was considered negative if no bands, a diffuse smear or a ladder of faint bands was observed.
2.7. Tumor cell growth in mice
All mouse studies were conducted in accordance with the principles and procedures outlined in the NIH Guide forthe Care and Use of Animals and were approved by the Animal Care and Use Committee of the National Cancer Institute. 8- to 10-week old immunodeficient athymic NCr-nu/nu female mice (NCI Animal Production Program, Frederick, MD) were used for all in vivo studies. Mice were housed in the animal facility at the National Institutes of Health in cages of 5 or fewer mice and fed animal chow and water ad libitum.
Each cell line was tested for viral contaminants prior to injection into mice. When cells of each cell line reached near confluence in vitro, a single cell suspension of 10 × 106 cells was implanted on the lateral aspect of the rear leg of 10–14 week old mice. Mice were injected with a tumor cell suspension, and subsequent tumors were passaged into new mice 2–4 times as cell suspensions. When tumors reached 1000 mm3 or if mice showed signs of systemic illness, mice were euthanized and a full necropsy was performed by a veterinary pathologist. Masses from the inoculation site were excised and divided with 1/3 fixed in 10% neutral buffered formalin for histopathology, 1/3 embedded and frozen in optimal cutting temperature compound for immunohistochemistry, and 1/3 snap frozen for future studies.
2.8. P53 status
mRNA was purified from each cell line using TRIzol Reagent (Invitrogen), and then transcribed using oligo(dT)18 primer (Fermentas) and M-MLV reverse transcriptase (Promega). The upper p53 fragment (nt 18–932) was amplified from cDNA with primers 5′AAGTCCAGAGCCACCATCC and 5′TCAAAGCTGTTGCGTCCC, and the lower fragment (nt 727–1362) was amplified with primers 5′-GCCAAGTACCTGGACGACA and 5′-CAGGGAAGGAGGACGAGA. Both fragments were sequenced.
For detection of p53 protein, 3 cell lines were treated with or without 0.1 and 0.15 μg/mL doxorubicin, or 5 or 10 μM nutlin-3 for 18h. Protein was extracted, and Western blot analysis was done, as previously described [14]. Briefly, cell lysates were made with 2× SDS sample buffer and boiled for 5 min. Proteins were then resolved in SDS-PAGE gels and transferred onto nitrocellulose membranes. Membranes were then subjected to blocking, washing, antibody incubation, and detection by enhanced chemoluminescence. Antibodies against p53 (FL-393), MDM2(SMP14 and 2A10), p21 (C–19) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (FL-335), the loading control, were purchased from Santa Cruz Biotechnology.
3. Results
3.1. Original tissue specimens
The dogs whose tissue samples produced stable cell lines were large breed, middle aged to older, with a predominance of male neutered animals (Table 2). Prior to tissue culture, fine needle aspirates of affected lymph nodes in each dog were cytologically diagnosed as high grade, large cell lymphoma. When assessed with flow cytometry, neoplastic lymphocytes from the original specimens were all CD21 positive and CD3 negative (Dog 2) or CD21 positive and dim CD3 positive (Dogs 1, 3, 4, and 5), indicating a mature B cell lineage. Four of the 5 samples were assayed for CD49d, the alpha4 integrin, expression, and all samples weakly expressed CD49d. Immunohistochemistry was performed on formalin fixed, paraffin-embedded tissue of Dog 2 postmortem, and was CD79a, CD20, CD45, and CD45r positive, and CD3 negative, consistent with B cell lymphoma.
Table 2.
Patient and initial tumor characteristics of the samples that led to the generation of the five canine B-cell lymphoma cell lines.
| Cell line | Dog | Type | Grade | Cell | Stage | Breed | Sex | Age (years) |
|---|---|---|---|---|---|---|---|---|
| UCDK9B1 | 1 | B | High | Large | Vb | Bull Mastiff | Female spayed | 5 |
| UCDK9B2 | 2 | B | High | Large | IVb | German Short haired Pointer | Male castrated | 11 |
| UCDK9B3 | 3 | B | High | Large | IIIa | Labrador | Male castrated | 11 |
| UCDK9B4 | 4 | B | High | Large | IVb | Bassett | Male castrated | 12 |
| UCDK9B5 | 5 | B | High | Large | IV | Greyhound | Male castrated | 7 |
Clonality assessment was done on DNA extracted from lymph node aspirate smears or leukemic blood from the affected dogs. The lymphoma samples from all 5 dogs had clonal Ig gene rearrangements, confirming B-cell lymphoma. Two of the lymphoma samples (dogs 2 and 3) also had concurrent clonal TCRG gene rearrangements.
3.2. Cell line analysis
The tissue sample from dogs 1 to 5 produced stable cell lines. Tumor cells grew even without the addition of growth factors. The canine cell line has a doubling time of approximately 24 h during exponential growth under standard culture conditions. The cell line shows a growth pattern of single large, round suspension cells that sometimes grow in small clusters.
The morphology of the cell lines was homogeneous, and comprised of large, immature, round cells consistent with high grade lymphoma of large cell type. The cells had high N/C ratios with marked anisokaryosis. The nuclei were round to indented or lobed with occasional multilobated and multinucleate forms. Rare, large bi-nucleate cells were also present. Chromatin was fine and stippled, and nuclei had multiple large, prominent nucleoli. Numerous mitotic figures were present. Cells had a moderate volume of deep blue cytoplasm with prominent perinuclear clearing. Many cells had low numbers of large, clear cytoplasmic vacuoles (Fig. 2A).
Fig. 2.
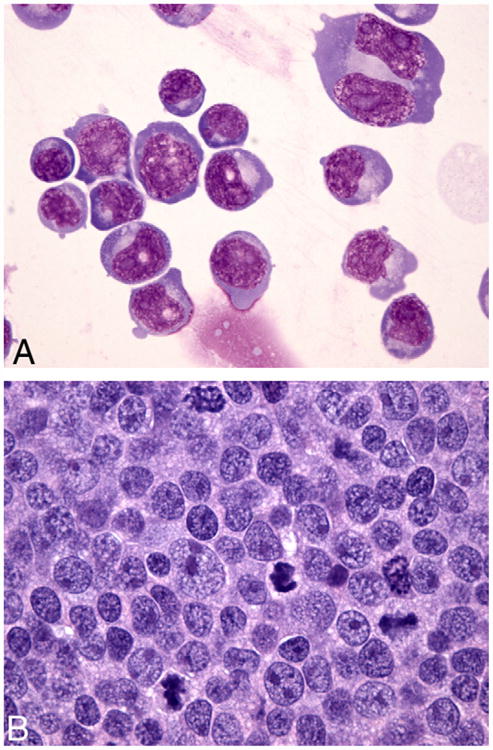
(A) Cytospin slide of cell line UCDK9B5. The lymphocytes have high N/C ratios with marked anisokaryosis and multiple, large prominent nucleoli. Rare large bi-nucleate cells with mirror image nuclei and large prominent nucleoli are also present. Cells have a variable volume of deep blue cytoplasm with prominent perinuclear clearing and occasional large, clear vacuoles. Wright–Giemsa stain, 60× objective. (B) Section of implanted tumor. The lymphocytes are large with multiple, prominent peripheralized nucleoli and a variable volume of amphophilic cytoplasm. Mitotic figures are frequent. This morphology, along with the immunophenotype and diffuse growth pattern is indicative of centroblastic diffuse large B cell lymphoma. HE stain, 60× objective.
Samples of the cultured cells were analyzed using an immunocytochemical panel on cytospin cell preparations. All cell lines expressed cytoplasmic CD20 and CD3. CD79a was variably expressed, from no staining to 45% staining of tumor cells. The remainder of the antigens assessed were negative (Table 3).
Table 3.
Immunophenotype of 5 canine lymphoma cell lines from a cell preparation as assayed by immunocytochemistry and flow cytometry. Only positive markers from the panel in Table 1 are shown.
| Immunocytochemistry | Flow Cytometry | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| CD1c | CD3 | CD20 | CD21 | CD45 | CD79a | MHCII | CD45r | CD1c | CD18 | CD90 | CD34 | |
| UCDK9B1 | – | + | + | – | – | 11% | – | – | (+) | + | + | – |
| UCDK9B2 | – | + | + | – | – | Rare | – | – | – | + | + | – |
| UCDK9B3 | – | + | + | – | – | – | – | – | – | + | + | – |
| UCDK9B4 | – | + | + | – | – | – | – | – | (+) | + | + | – |
| UCDK9B5 | – | + | + | – | – | 45% | – | – | + | + | (+) | |
The cell lines were analyzed with a standard flow cytometry panel (Table 1) with the exception of CD34 in UCDK9B2 and UCDK9B3. All cell lines were CD18 and CD90 positive (Fig. 1). UCDK9B1, UCDK9B3, and UCDK9B4 had weak CD1c expression, and UCDK9B4 had weak CD34 expression (Table 3). The cell lines did not express any other antigens assessed.
Fig. 1.
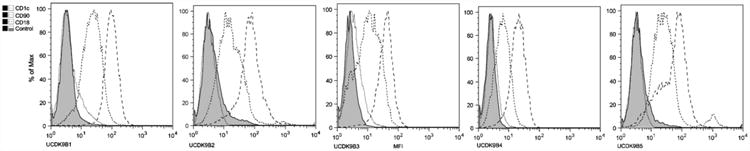
Overlaid flow cytometry histograms of cell lines. All cell lines expressed CD90 and CD18. UCDK9B1, UCDK9B3, and UCDK9B4 had weak CD1c expression.
Clonality of the cell lines was also evaluated. UCDK9B1 had a clonal TCRG gene rearrangement, and UCDK9B5 maintained a clonal Ig gene rearrangement (Fig. 3). Clonal gene rearrangements were no longer detected in the remaining cell lines.
Fig. 3.
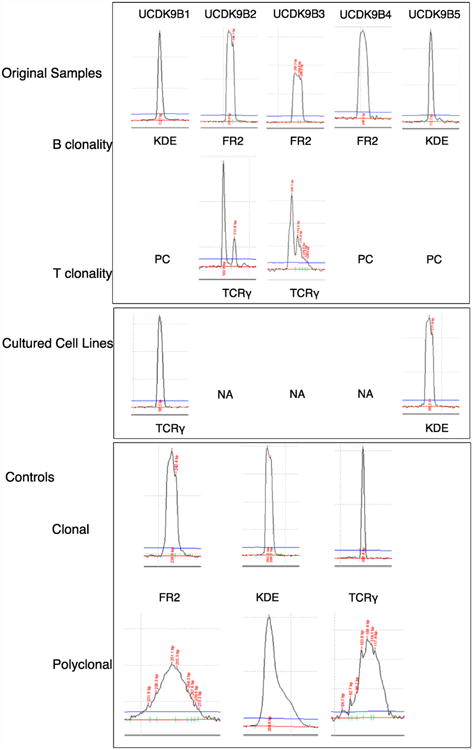
Capillary electrophoresis and BioCalculator software analysis plots of clonality PCR done on DNA extracted from original lymphomas and cultured cell lines. All results were reproducible in duplicate or triplicate but only single examples are shown. FR2, Framework 2 (immunoglobulin heavy chain locus); KDE, kappa deleting element (immunoglobulin light chain locus); TCRγ, T cell receptor gamma (T cell receptor gamma chain locus); PC, polyclonal; NA, no amplification.
3.3. Xenograft analysis
Each cell line had negative test results for viral contamination. After 3–4 passages of the cells through mice, UCDK9B1 and UCDK9B5 had growth times of approximately 4 weeks, and the remaining cell lines had growth times of 6–8 weeks. While all of the cell lines produced large, local masses, none of them was metastatic at the time of euthanasia. Tumors consisted of large, immature round cells that formed dense sheets and a diffuse growth pattern. These cells frequently invaded into surrounding skeletal muscle. Neoplastic lymphocytes had multiple, prominent peripheralized nucleoli and a variable volume of amphophilic cytoplasm. Mitotic figures were frequent (Fig. 2B). The morphology, along with the initial immunophenotype and diffuse growth pattern was indicative of centroblastic diffuse large B cell lymphoma in all instances. Immunohistochemical immunophenotyping was done using an extensive panel of antibodies (CD1, CD3, CD4, CD8a, CD8b, CD18, CD20, CD21, CD45, CD79a, MHCII), and all implanted tumors failed to express any of the antigens assessed.
3.4. P53 status
Sequencing of p53 mRNA revealed no mutations in the coding region of the dog p53 gene in any of the cell lines. To further investigate the p53 status of these lymphoma cell lines, they were either mock-treated or exposed to doxorubicin or nutlin-3 for 18h. Doxorubicin is a DNA damaging agent which is known to induce double-stranded breaks and result in p53 stabilization in human cells [15]. Nutlin-3 on the other hand is an MDM2 inhibitor which blocks MDM2-mediated p53 degradation [16]. All cell lines expressed p53 protein (Fig. 4). To test if this accumulated p53 was transcriptionally active, Western blotting for p21 and MDM2 was done on these same treated cell lines. p21 is induced by p53 and is required for p53-induced G1 arrest [17]. MDM2 is a E3 ubiquitin ligase which targets p53 for proteosomal degradation [17]. Protein levels for p53 and p21 were moderately increased after exposure to either doxorubicin or nutlin-3, while MDM2 expression was moderately inhibited by exposure to doxorubicin but was not affected by exposure to nutlin-3 (Fig. 4). Together, these results indicate that all of the cell lines express wild type, functional p53.
Fig. 4.
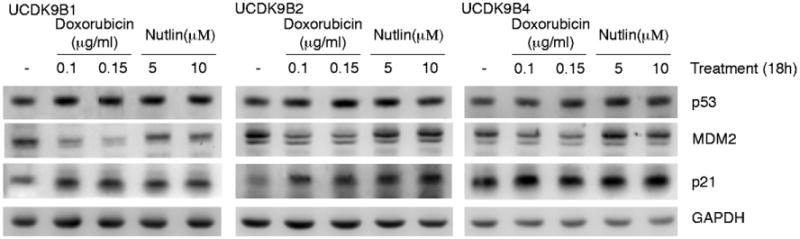
Western blot of protein extracted from 3 of the canine lymphoma cell lines either untreated (–) or treated with doxorubicin or nutlin-3 for 18h. GAPDH was used as a loading control.
4. Discussion
5 canine B-cell lymphoma cell lines were successfully developed and characterized. These cell lines were CD3, CD18, CD20, CD49d (alpha4-beta1 integrin) and CD90 positive with variable CD79a, CD1c and CD34 expression. All cell lines were tumorigenic in Nu/nu mice.
Clonal B-cell heavy chain gene rearrangements were evident in the original tissue samples, confirming that these were B-cell lymphomas. Two of the original samples had concurrent clonal TCRG rearrangements. Cross lineage gene rearrangements are not uncommon in B-cell lymphomas [12]. The cell lines also expressed CD3, a T-cell marker, intracytoplasmically, along with CD20, a B-cell marker. T-cell markers have been shown to be expressed in the canine B-cell line previously described, and may represent selection of a clone with an aberrant phenotype or clonal evolution [9].
Several markers were lost in the transition from fresh tissue to a cell line. Only UCDK9B1 retained a clonal TCRG gene rearrangement, and UCDK9B5 retained a clonal IgH gene rearrangement. It is likely that additional mutations occurred in culture resulting in subsequent lack of clonality.
The cell lines were tumorigenic in Nu/nu mice with typical morphologic characteristics of high grade, large cell lymphoma. We were unable to detect expression of CD markers after the cells were passaged through mice. The additional transformations that occurred likely resulted in altered expression or loss of expression of these CD antigens.
There were small discrepancies in results obtained for cell line immunophenotyping using antibodies tested with immunocytochemistry and flow cytometry. Some cell lines were weakly CD79a positive and strongly CD3 positive on immunocytochemistry, but negative on flow cytometry. Conversely, cell lines were weakly CD1c positive on flow cytometry and negative on immunocytochemistry. Some of the differences may lie in where the antigen is expressed. For example, the CD3 and CD20 antibodies used in immunocytochemistry target an intracellular antigen, while the antibodies used for flow cytometry target cell surface antigens. Differential method sensitivities, with flow cytometric detection being more sensitive than light microscopic immunocytochemical detection, might also explain some of these discrepancies.
We also found that these cell lines were p53 wild type by sequencing of mRNA and by functional analysis. Exposure to a DNA damaging agent or an MDM2 inhibitor resulted in accumulation of p53 protein and increased expression of the p53 effector gene p21. These finding are consistent with human lymphoma where the majority of human lymphoma, and diffuse B-cell lymphomas, in particular, express wild-type p53 [18].
MDM2 protein expression was decreased in the cell lines after exposure to doxorubicin, but not to nutlin-3. The reason for this remains unclear as most cell lines show increased MDM2 expression after exposure to DNA damaging agents. There are previous reports of decreased MDM2 protein expression in cell lines with wild type p53 in response to a DNA damaging agent [19]. As suggested in a recent study, it is possible that the MDM2 antibody fails to recognize the phosphorylated form of MDM2 due to epitope masking [19,20]. Other possibilities include either caspase or other proteolytic-induced cleavage of MDM2 in response to DNA damage orapoptosis [21,22].
This is the first report of canine lymphoma cell lines expressing the alpha4-beta1 integrin (CD49d) on the cell surface. This integrin has been shown to be present in an active form on human leukemia and lymphoma cell lines [23]. The similarity in expression of this integrin in human and canine lymphomas and its affinity to a peptide, in development, that may be used cross-species may be an important future tool in targeting neoplastic lymphocytes.
In conclusion, 5 new canine B-cell lymphoma cell lines were developed and characterized by morphologic assessment, immunophenotyping and clonality assessment. In addition, all 5 cell lines were tumorigenic in mice and were found to express wild-type p53. These new resources should prove valuable in the development of new therapeutic strategies to treat lymphoma in both dogs and people.
Acknowledgments
None.
Role of the funding source: Toni Wiebe Memorial Research Fund and the UCD Cancer Center Core Support Grant NIH CA093373. The above sponsors had no involvement in study design, data collection, analysis, interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
Contributions: All authors were involved in the conception and design of the study, data acquisition, interpretation of data, drafting and revision of the manuscript, and final approval of the version to be submitted (ALZ, WV, CS, WY, XC, IKG, MSK).
References
- 1.Valli V. Veterinary pathologists achieve 80% agreement in application of WHO diagnoses to canine lymphoma. Cancer Ther. 2008;6:221–6. [Google Scholar]
- 2.Hansen K, Khanna C. Spontaneous and genetically engineered animal models: use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–80. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Breen M, Modiano JF. Evolutionary conserved cytogenetic changes in hematological malignancies of dogs and humans—man and his best friend share more than companionship. Chromosome Res. 2008;16:145–54. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 4.Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–8. doi: 10.1016/j.molmed.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkness EF, Bafna V, Halpern AL, Levy S, Remington K, Rusch DB, et al. The dog genome: survey sequencing and comparative analysis. Science (New York, NY) 2003;301:1898–903. doi: 10.1126/science.1086432. [DOI] [PubMed] [Google Scholar]
- 6.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–92. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 7.Hahn KA, Bravo L, Adams WH, Frazier DL. Naturally occurring tumors in dogs as comparative models for cancer therapy research. In Vivo. 1994;8:133–43. [PubMed] [Google Scholar]
- 8.Zhang J, Cho SJ, Shu L, Yan W, Guerrero T, Kent M, et al. Translational repression of p53 by RNPC1, a p53 target overexpressed in lymphomas. Genes Dev. 2011;25:1528–43. doi: 10.1101/gad.2069311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutgen BC, Hammer SE, Gerner W, Christian M, de Arespacochaga AG, Willmann M, et al. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk Res. 2010;34:932–8. doi: 10.1016/j.leukres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Drexler HG. The leukemia-lymphoma cell line facts book. San Diego: Academic Press; 2001. [Google Scholar]
- 11.Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69:145–64. doi: 10.1016/s0165-2427(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 12.Valli VE, Vernau W, de Lorimier LP, Graham PS, Moore PF. Canine indolent nodular lymphoma. Vet Pathol. 2006;43:241–56. doi: 10.1354/vp.43-3-241. [DOI] [PubMed] [Google Scholar]
- 13.Bienzle D, Vernau W. The diagnostic assessment of canine lymphoma: implications for treatment. Clin Lab Med. 2011;31:21–39. doi: 10.1016/j.cll.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Bargonetti J, Prives C. p53, through p21 (WAF1/CIP1), induces cyclin D1 synthesis. Cancer Res. 1995;55:4257–63. [PubMed] [Google Scholar]
- 15.Nelson WG, Kastan MB. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994;14:1815–23. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science (New York, NY) 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 17.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Newcomb EW. P53 gene mutations in lymphoid diseases and their possible relevance to drug resistance. Leuk Lymphoma. 1995;17:211–21. doi: 10.3109/10428199509056825. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Q, Chen J. The phenotype of MDM2 auto-degradation after DNA damage is due to epitope masking by phosphorylation. Cell Cycle. 2011;10:1162–6. doi: 10.4161/cc.10.7.15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eischen CM. Decreased Mdm2 levels after DNA damage: antibody masking or protein degradation? Cell Cycle. 2011;10:1347. doi: 10.4161/cc.10.9.15437. [DOI] [PubMed] [Google Scholar]
- 21.Oliver TG, Meylan E, Chang GP, Xue W, Burke JR, Humpton TJ, et al. Caspase-2-mediated cleavage of Mdm2 creates a p53-induced positive feedback loop. Mol Cell. 2011;43:57–71. doi: 10.1016/j.molcel.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Marechal V, Moreau J, Levine AJ, Chen J. Proteolytic cleavage of the mdm2 oncoprotein during apoptosis. J Biol Chem. 1997;272:22966–73. doi: 10.1074/jbc.272.36.22966. [DOI] [PubMed] [Google Scholar]
- 23.Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha(4)beta(1) integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–9. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]


