Abstract
Objective
To evaluate changes in characteristics of feline injection-site sarcomas (ISSs) from 1990 through 2006.
Design
Retrospective case series.
Animals
392 cats with a histologic diagnosis of soft tissue sarcoma, osteosarcoma, or chondrosarcoma at potential injection sites.
Procedures
Classification and anatomic location of tumors and signalment of affected cats were compared between ISSs diagnosed before and after publication of the Vaccine Associated Feline Sarcoma Task Force vaccination recommendations in 1996.
Results
From before to after publication of the vaccination recommendations, proportions of ISSs significantly decreased in the interscapular (53.4% to 39.5%) and right and left thoracic (10.2% to 3.6% and 9.1% to 1.3%, respectively) regions. On the other hand, proportions of ISSs significantly increased in the right thoracic limb (1.1% to 9.5%) and the combined regions of the right pelvic limb with right lateral aspect of the abdomen (12.5% to 25.0%) and the left pelvic limb with left lateral aspect of the abdomen (11.4% to 13.8%). Patterns of tumor classification and signalment did not change.
Conclusions and Clinical Relevance
Despite publication of the vaccination recommendations, a high proportion of tumors still developed in the interscapular region. There was also an increase in lateral abdominal ISSs, which are more difficult to treat and are likely attributable to aberrant placement of injections intended for the pelvic limbs. Veterinarians are complying with vaccination recommendations to some extent, but they need to focus on administering vaccines as distally as possible on a limb to allow for complete surgical margins if amputation of a limb is required.
An association between vaccine injections and the development of sarcomas in cats was first suggested in October 1991.1 Since that time, results of epidemiologic research and histologic evaluations have supported a causal relationship between injections and the development of sarcomas. Vaccines against the rabies virus and FeLV have been most commonly implicated.2–4 Other agents, such as FVRCP±C, long-acting penicillin, methyl prednisolone, and suture material, have also been implicated.5,6
Fibrosarcomas are the most common type of tumor to develop in an injection site. Other types of sarcomas, including osteosarcoma, chondrosarcoma, myxo-sarcoma, malignant fibrous histiocytoma, and rhabdomyosarcoma, have also been reported.7 These tumors are highly locally invasive, with up to 24% resulting in distant metastasis.8 Because of the locally aggressive nature of ISSs, wide or radical surgical excisions in combination with radiation therapy are required to obtain local control of these tumors.
In November 1996, the VAFSTF was formed; one of its goals was to identify methods of prevention of and treatment for these tumors.9 At their first meeting, the group formulated recommendations for the administration of vaccines to cats to standardize vaccination sites and to facilitate the treatment of the tumors that occur at those sites. Recommendations were to administer the rabies vaccine in the right rear leg as distally as possible, the FeLV vaccine in the left rear leg as distally as possible, and the FVRCP±C vaccine in the right shoulder.9
Prior to release of the recommendations, most SC injections, including vaccinations, were administered in the dorsal interscapular region. One study10 revealed that after excision of tumors at this location, local recurrence was detected at a median of 66 days when surgery was performed in a general practice or 274 days when a more aggressive surgery was performed at a referral institution. Aggressive surgical excision of ISSs in the interscapular region often requires removal of vertebral dorsal spinous processes and partial scapulectomies to provide a chance for an extended disease-free interval. However, such extensive procedures can result in morbidity associated with wound dehiscence.11 Even with adjuvant radiation therapy, there is a high rate of local recurrence of tumors.
At the Veterinary Medical Teaching Hospital of the University of California, ISSs that extend from the proximal pelvic limbs to the abdominal body wall or pelvises of cats are common. Unfortunately, curative-intent surgery for these tumors requires more than a routine amputation. The purpose of the study reported here was to evaluate changes in anatomic location and histologic classification of ISSs and signalment of affected cats from before to after publication of the VAFSTF recommendations. We hypothesized that since the vaccination recommendations were published, there would be a change in anatomic location of ISSs from the interscapular region to the pelvic limbs. A secondary hypothesis was that proportions of ISSs affecting the lateral aspect of the abdomen and pelvic regions would increase during the same period.
Materials and Methods
Case selection
Medical records of cats evaluated at the Veterinary Medical Teaching Hospital of the University of California from 1990 through 2006 were retrospectively reviewed for a histologic diagnosis of sarcoma. Cats with a histologic diagnosis of heman-giosarcoma or round cell tumors with names that included sarcoma were excluded. Cats with an unspecified tumor location or with tumors that originated from sites such as from bone, within the abdominal or thoracic cavities, and on the head, paws, or ventrum were excluded from the study because these tumors were unlikely related to the administration of injections.
Medical records review
Information obtained from each medical record included signalment, body weight, biopsy date, and tumor histologic classification and anatomic location. Tumors were categorized on the basis of their site of origin. The categories included the left thoracic limb from the carpus to the scapula, right thoracic limb from the carpus to the scapula, left pelvic limb from the tarsus to the proximal femur, right pelvic limb from the tarsus to the proximal femur, dorsal inter-scapular and neck region, left lateral aspect of the thorax, right lateral aspect of the thorax, left lateral aspect of the abdomen, right lateral aspect of the abdomen, and tail. Tumor histologic classification and location as well as age, sex, breed, and body weight of affected cats were recorded. All biopsy reports were reviewed to confirm that the histologic diagnosis recorded was consistent with that recorded in the pathology reports. The histologic classification of other soft tissue sarcoma was used to represent unspecified sarcomas as well as types of soft tissue sarcomas diagnosed in < 4 cats. A date of December 31, 1996, was used to differentiate between tumors diagnosed before and after publication of the vaccination recommendations.
Statistical analysis
A Pearson χ2 test was used to evaluate differences between time points with respect to the distribution of categoric variables (ie, sex, breed, tumor location, and histologic diagnosis) unless values in the associated contingency table were < 5, in which situation a Fisher exact test was used. Continuous variables were assessed for normality. A Student t test was used to compare time points with respect to mean values of continuous variables (ie, age and body weight). A value of P < 0.05 was considered significant for all analyses. All analyses were performed by use of commercially available software.a,b
Results
Animals
Four hundred thirty cats were identified with soft tissue sarcomas at potential injection sites. Thirty-eight cats were excluded because biopsy reports were not available; therefore, 392 cats fulfilled the inclusion criteria for the study. The mean age of all cats was 9.6 years (range, 1.4 to 18.8 years) at the time of biopsy. There were 370 (94.4%) mixed-breed cats and 22 (5.6%) purebred cats. Of the purebred cats, 7 were Siamese, 4 were Persian, 3 were Manx, and there were < 3 each of the following breeds: Himalayan, Maine Coon, Balinese, Birman, Norwegian Forest Cat, and Scottish Fold. Most cats were neutered, and there was an equal distribution of males and females (Table 1). Mean body weight of the cats was 4.9 kg (10.8 lb; range, 2.0 to 9.0 kg [4.4 to 19.8 lb]). Types of tumors according to histologic diagnosis included 311 (79.3%) fibrosarcomas, 71 (18.1%) other soft tissue sarcomas, 5 (1.3%) liposarcomas, 3 (0.8%) osteosarcomas, and 2 (0.5%) chondrosarcomas. Most tumors were detected in the interscapular region (n = 167; 42.6%), followed by the right pelvic limb (61; 15.6%), right thoracic limb (30; 7.7%), left pelvic limb (30; 7.7%), right lateral aspect of the abdomen (26; 6.6%), left lateral aspect of the abdomen (22; 5.6%), left thoracic limb (21; 5.4%), right lateral aspect of the thorax (20; 5.1%), left lateral aspect of the abdomen (12; 3.1%), and the tail (3; 0.8%).
Table 1.
Characteristics of cats with ISSs diagnosed before (1990 to 1996; n = 88) and after (1997 to 2006; 304) publication of VAFSTF recommendations.
| Variable | 1990–1996 | 1997–2006 | P value* |
|---|---|---|---|
| Age (y) | 9.0 ± 3.1 | 9.8 ± 3.3 | 0.06 |
| Body weight (kg)† | 4.7 ± 1.1 | 4.9 ± 1.4 | 0.30 |
| Breed | 0.58 | ||
| Mixed | 82 (93.2) | 288 (94.7) | |
| Pure | 6 (6.8) | 16 (5.3) | |
| Sex | 0.68 | ||
| Neutered male | 39 (44.3) | 141 (46.4) | |
| Spayed female | 46 (52.3) | 158 (52.0) | |
| Sexually intact male | 1 (1.1) | 2 (0.7) | |
| Sexually intact female | 2 (2.3) | 2 (0.7) | |
| Unknown | 0 (0) | 1 (0.3) | |
| Histologic diagnosis | 0.88 | ||
| Fibrosarcoma | 68 (77.3) | 243 (79.9) | |
| Other soft tissue sarcoma‡ | 18 (20.5) | 53 (17.4) | |
| Osteosarcoma | 1 (1.1) | 2 (0.7) | |
| Chondrosarcoma | 0 (0) | 2 (0.7) | |
| Liposarcoma | 1 (1.1) | 4 (1.3) |
Values for age and body weight are reported as mean ± SD. Values for breed, sex, and histologic diagnosis are reported as number (%).
Values of P < 0.05 were considered significant.
To convert kilograms to pounds, multiply value by 2.2.
Other soft tissue sarcomas include nonspecific sarcomas and those represented by < 4 each of giant cell sarcoma, leiomyosarcoma, lymphangiosarcoma, malignant fibrous histiocytoma, myofibroblastic sarcoma, myxofibrosarcoma, myxosarcoma, nerve sheath tumor, and synovial cell sarcoma.
Changes in tumor locations
Prior to December 31, 1996, most ISSs were detected in the interscapular region (53.4%), followed by the right pelvic limb (10.2%), right lateral aspect of the thorax (10.2%), left lateral aspect of the thorax (9.1%), and left pelvic limb (8.0%; Table 2). After December 31, 1996, the proportion of tumors in the interscapular region, right lateral aspect of the thorax, and left lateral aspect of the thorax significantly decreased to 39.5% (P = 0.02), 3.6% (P = 0.01), and 1.3% (P < 0.001), respectively. The percentages of tumors that were detected at the right thoracic limb and right lateral aspect of the abdomen were originally 1.1% and 2.2%, respectively, then significantly increased to 9.5% (P = 0.009) and 7.9% (P = 0.04), respectively. There were also apparent, albeit insignificant, increases in proportions of tumors located at the left thoracic limb (from 2.2% to 6.3%; P = 0.15), the right pelvic limb (from 10.2% to 17.1%; P = 0.12), the left lateral aspect of the abdomen (from 3.4% to 6.3%; P = 0.31), and the tail (0% to 0.9%; P = 0.35).
Table 2.
Number (%) of ISSs at various anatomic locations in cats before (1990 to 1996; n = 88) and after (1997 to 2006; 304) publication of VAFSTF recommendations.
| Anatomic location | 1990–1996 | 1997–2006 | P value* |
|---|---|---|---|
| Left thoracic limb | 2 (2.2) | 19 (6.3) | 0.15 |
| Right thoracic limb | 1 (1.1) | 29 (9.5) | 0.009 |
| Left pelvic limb | 7 (8.0) | 23 (7.5) | 0.90 |
| Right pelvic limb | 9 (10.2) | 52 (17.1) | 0.12 |
| Left lateral aspect of the thorax | 8 (9.1) | 4 (1.3) | < 0.001 |
| Right lateral aspect of the thorax | 9 (10.2) | 11 (3.6) | 0.01 |
| Left lateral aspect of the abdomen | 3 (3.4) | 19 (6.3) | 0.31 |
| Right lateral aspect of the abdomen | 2 (2.2) | 24 (7.9) | 0.04 |
| Interscapular | 47 (53.4) | 120 (39.5) | 0.02 |
| Tail | 0 (0) | 3 (0.9) | 0.35 |
| Left pelvic limb and left lateral aspect of the abdomen | 10 (11.4) | 42 (13.8) | < 0.001 |
| Right pelvic limb and right lateral aspect of the abdomen | 11 (12.5) | 76 (25.0) | < 0.001 |
Values of P < 0.05 were considered significant.
When results for the pelvic limbs were combined with those for adjacent lateral abdominal regions, the percentage of tumors on the caudal left side of the body significantly (P < 0.001) increased between time points from 11.4% to 13.8% and that on the caudal right side of the body significantly (P < 0.001) increased from 12.5% to 25.0%. Before December 31, 1996, 76.1% of ISSs were detected in locations cranial to the diaphragm, and 23.9% of ISSs were caudal to the diaphragm. After December 31, 1996, the proportion of tumors cranial to the diaphragm decreased significantly (P = 0.006) to 60.2% and caudal tumors increased to 39.8%. There was no significant difference in mean body weight, signalment, or tumor histologic classification for cats evaluated before and after December 31, 1996 (Table 1).
From 2003 to 2005, the total number of tumors caudal to the diaphragm surpassed the number of tumors cranial to the diaphragm. In 2006, there was an approximately equal number of cranial and caudal tumors (Figure 1).
Figure 1.
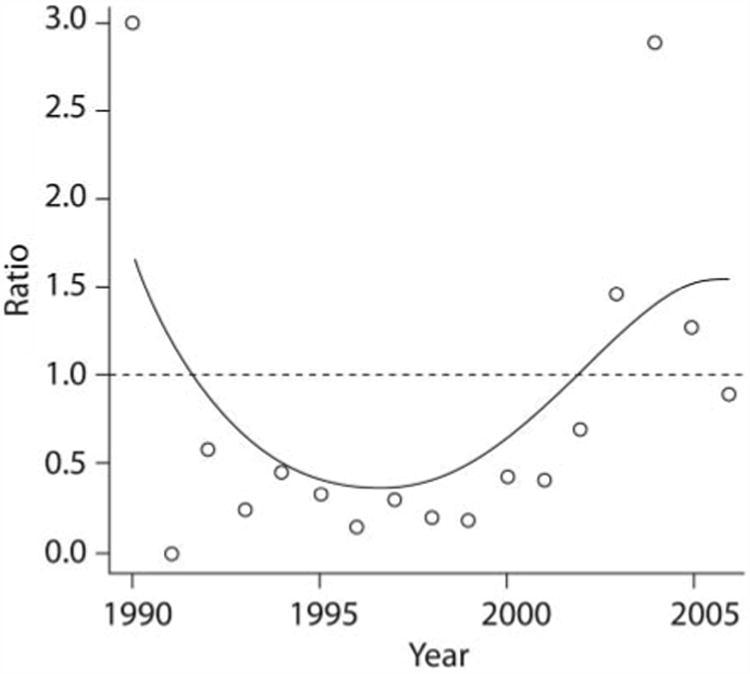
Graph of the ratio of the number of ISSs that were detected caudal versus cranial to the diaphragm in 392 cats evaluated for ISSs at various time points.
Discussion
Effective prevention of and treatment for ISS in cats is challenging. These tumors are characterized by cellular pleomorphism, high mitotic activity, inflammatory cell infiltrates, rapid growth, and local invasiveness into surrounding tissues.12,13 Local recurrence develops sooner after conservative versus wide or radical excision.10,14 Radiation therapy also has a role in the management of these tumors, but even with radiation therapy, local recurrence reportedly occurs with 28% to 45% of tumors.8,15–17 The VAFSTF recommendations were published with the aim of facilitating identification of the vaccinations that result in tumor development and facilitating treatment of cats with ISS by shifting the location of vaccine injections to a site at which radical surgical excision via amputation would be locally curative if a tumor developed.
The results of the present study indicated a high proportion of ISSs in the interscapular region of cats prior to publication of the VAFSTF recommendations, and this proportion significantly decreased after publication of the recommendations. A similar decrease in proportion of lateral thoracic ISSs suggest that the tumors in these locations were caused by interscapular injections that were aberrantly administered or injections that were purposely administered in the locations as an alternative to the interscapular region. The increase in the proportion of ISSs on the limbs of cats after December 31, 1996, also suggested that many veterinarians were complying with the VAFSTF recommendations. However, there was an increase in the proportion of ISSs that were detected on the lateral aspects of abdomens of cats. It is likely that this increase was caused by the aberrant placement of vaccine injections intended for the pelvic limbs. In our experience, cats that are anxious and fearful when handled for an injection are likely to crouch down with their pelvic limbs tucked up against their abdomen. Because the skin of cats is loose and elastic, an injection intended for a pelvic limb can easily be misdirected into the subcutaneous tissue that shifts over the lateral aspect of the abdomen when a cat stands from its crouching position.
Although there was a steady increase in the proportion of ISSs detected caudal to the diaphragm since publication of the VAFSTF recommendations, the absolute number of caudal tumors did not surpass the number of cranial tumors until 2003 (Figure 1). The delay in shift of location of these tumors may be explained by the time it took for the vaccination recommendations to be disseminated and widely practiced. Additionally, the time it takes for ISS to develop after vaccination ranges widely, from as short as 4 weeks to as long as 10 years.18,19 The higher caudal-to-cranial tumor ratio in 1990 and an approximately equal ratio in 2006 were likely attributable to the few data points available for those years.
The increase in the proportion of lateral abdominal ISSs over the period of the study is cause for concern. Cats with lateral abdominal tumors can be as challenging, if not more difficult, to treat than those with ISS in the interscapular region. Complete excision of lateral abdominal ISSs may require abdominal wall resection, often with abdominal wall reconstruction and extirpation of affected internal organs because of tumor invasion. Additionally, adjuvant radiation treatment applied to the lateral aspect of the abdomen for incompletely excised tumors may be challenging because of the vital underlying organs within the abdomen. In our experience, tumors that originate from the pelvic limbs often develop from the proximal aspect of the thigh, with the tumor extending onto the abdominal body wall or pelvis. In these situations, amputation of the affected limb alone is inadequate in controlling the local disease and abdominal wall resection or hemipelvectomy may be required to obtain complete excisional margins.
Despite the decrease in proportion of interscapular ISSs detected after December 31, 1996, there were still a large percentage of tumors that developed in that location. This finding may be explained by the results of a 2002 study,20 which revealed that ≤ 41% of FVRCP±C vaccines, some of which are administered in combination with rabies or FeLV vaccine, are still being administered in the interscapular region. The delay in tumor formation after vaccination or the injection of substances other than vaccines at that site may also contribute to continued tumor development in the interscapular region. If we attribute ISSs that arise from the limbs and lateral aspect of the abdomen to the corresponding vaccines recommended for those regions and we assume that the percentage of tumors caused by injections of substances other than rabies, FeLV, and FVRCP±C vaccines are negligible, then rabies vaccine is responsible for 51.7%, FeLV vaccine is responsible for 28.6%, and FVRCP±C is responsible for 19.7% of all ISSs detected after December 31, 1996.
Limitations of the present study include those inherent to retrospective studies. Proof of vaccination at the tumor location could not be attained because of the large number of referral records that were unavailable (most historical medical records were destroyed after a limited number of years). Microscopic descriptions of most biopsy specimens processed by external laboratories were not requested, so the presence of characteristic features of ISS could not be assessed, making it difficult to ascertain that each tumor was truly induced by an injection. However, because the goal of our study was to characterize the change in distribution of ISSs that coincided with publication of the VAFSTF recommendations, the proportion of naturally developing sarcomas theoretically should not be affected by the VAF-STF recommendations and should remain constant in anatomic distribution before and after publication. In 1999 and 2000, recruitment of cats with ISS for a computed tomography study performed at our institution resulted in an increase in the number of cats with ISS that were evaluated during those years. This artificial increase precluded analysis of data for prevalence of the disease. Lastly, because all records came from a teaching hospital, the population of cats in the study may not have been representative of the general population of cats with ISS. Tumors may have been excised via amputation in general practice without need for referral, which would likely decrease the magnitude of the differences detected in our study.
Results of the study reported here indicated that although there was a shift in the proportion of ISSs that were detected from cranial to the diaphragm to caudal to the diaphragm since the VAFSTF recommendations were published, ISS continued to be detected, most importantly at locations that remain a challenge to treat. There has been a decrease in interscapular and lateral thoracic tumors and an increase in tumors of the limbs since publication of the recommendations; however, there has also been an increase in the lateral abdominal tumors, which is most likely attributable to the aberrant placement of injections intended for the pelvic limbs. The VAFSTF recommendations should be adhered to more strictly, with emphasis on placement of injections in limbs as distally as possible to prevent misadministration of injections with subsequent tumor formation at the lateral aspect of the abdomen and proximal thigh, where complete surgical excision is more invasive than amputation alone and may result in a higher morbidity rate.
Acknowledgments
Supported by grant No. UL1 RR024146 from the National Center for Research Resources of the National Institutes of Health and the NIH Roadmap for Medical Research.
Abbreviations
- FVRCP±C
Multivalent vaccine against feline herpes virus, feline calicivirus, and feline pan-leukopenia virus, including or not including Chlamydophila felis
- ISS
Injection-site sarcoma
- VAFSTF
Vaccine Associated Feline Sarcoma Task Force
Footnotes
Stata 9.0, StataCorp, L P, College Station, Tex.
R software, version 2.4.1, R Foundation for Statistical Computing, Vienna, Austria.
Presented in part at the 27th Annual Veterinary Cancer Society Annual Meeting, Fort Lauderdale, Fla, November 2007.
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Contributor Information
Stephen C. Shaw, William R. Prichard Veterinary Medical Teaching Hospital, University of California, Davis, CA 95616.
Michael S. Kent, Department of Surgical and Radiological Sciences, University of California, Davis, CA 95616.
Ira K. Gordon, Department of Surgical and Radiological Sciences, University of California, Davis, CA 95616.
Cameron J. Collins, William R. Prichard Veterinary Medical Teaching Hospital, University of California, Davis, CA 95616.
Tamara A. Greasby, School of Veterinary Medicine, and the Department of Health Sciences, School of Medicine, University of California, Davis, CA 95616.
Laurel A. Beckett, School of Veterinary Medicine, and the Department of Health Sciences, School of Medicine, University of California, Davis, CA 95616.
Genevieve M. Hammond, William R. Prichard Veterinary Medical Teaching Hospital, University of California, Davis, CA 95616.
Katherine A. Skorupski, Department of Surgical and Radiological Sciences, University of California, Davis, CA 95616.
References
- 1.Hendrick MJ, Goldschmidt MH. Do injection site reactions induce fibrosarcomas in cats? (lett) J Am Vet Med Assoc. 1991;199:968. [PubMed] [Google Scholar]
- 2.Hendrick MJ, Shofer FS, Goldschmidt MH, et al. Comparison of fibrosarcomas that developed at vaccination sites and at non-vaccination sites in cats: 239 cases (1991–1992) J Am Vet Med Assoc. 1994;205:1425–1429. [PubMed] [Google Scholar]
- 3.Kass PH, Barnes WG, Jr, Spangler WL, et al. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats (Erratum published in J Am Vet Med Assoc 1993;203:1046) J Am Vet Med Assoc. 1993;203:396–405. [PubMed] [Google Scholar]
- 4.Lester S, Clemett T, Burt A. Vaccine site-associated sarcomas in cats: clinical experience and a laboratory review (1982–1993) J Am Anim Hosp Assoc. 1996;32:91–95. doi: 10.5326/15473317-32-2-91. [DOI] [PubMed] [Google Scholar]
- 5.Buracco P, Martano M, Morello E, et al. Vaccine-associated-like fibrosarcoma at the site of a deep nonabsorbable suture in a cat. Vet J. 2002;163:105–107. doi: 10.1053/tvjl.2001.0617. [DOI] [PubMed] [Google Scholar]
- 6.Kass PH, Spangler WL, Hendrick MJ, et al. Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. J Am Vet Med Assoc. 2003;223:1283–1292. doi: 10.2460/javma.2003.223.1283. [DOI] [PubMed] [Google Scholar]
- 7.Hendrick MJ, Brooks JJ. Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Vet Pathol. 1994;31:126–129. doi: 10.1177/030098589403100121. [DOI] [PubMed] [Google Scholar]
- 8.Cronin K, Page RL, Spodnick G, et al. Radiation therapy and surgery for fibrosarcoma in 33 cats. Vet Radiol Ultrasound. 1998;39:51–56. doi: 10.1111/j.1740-8261.1998.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Morrison WB, Starr RM. Vaccine-associated feline sarcomas. J Am Vet Med Assoc. 2001;218:697–702. doi: 10.2460/javma.2001.218.697. [DOI] [PubMed] [Google Scholar]
- 10.Hershey AE, Sorenmo KU, Hendrick MJ, et al. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996) J Am Vet Med Assoc. 2000;216:58–61. doi: 10.2460/javma.2000.216.58. [DOI] [PubMed] [Google Scholar]
- 11.Withrow SJ, Vail DM. Withrow & MacEwen's small animal clinical oncology. 4th. St Louis: Saunders; 2007. p. 445. [Google Scholar]
- 12.Doddy FD, Glickman LT, Glickman NW, et al. Feline fibrosarcomas at vaccination sites and non-vaccination sites. J Comp Pathol. 1996;114:165–174. doi: 10.1016/s0021-9975(96)80005-3. [DOI] [PubMed] [Google Scholar]
- 13.Seguin B. Injection site sarcomas in cats. Clin Tech Small Anim Pract. 2002;17:168–173. doi: 10.1053/svms.2002.36605. [DOI] [PubMed] [Google Scholar]
- 14.Davidson EB, Gregory CR, Kass PH. Surgical excision of soft tissue fibrosarcomas in cats. Vet Surg. 1997;26:265–269. doi: 10.1111/j.1532-950x.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Hauck ML, Dodge R, et al. Preoperative radiotherapy for vaccine associated sarcoma in 92 cats. Vet Radiol Ultrasound. 2002;43:473–479. doi: 10.1111/j.1740-8261.2002.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen M, Wright JC, Brawner WR, et al. Use of surgery and electron beam irradiation, with or without chemotherapy, for treatment of vaccine-associated sarcomas in cats: 78 cases (1996–2000) J Am Vet Med Assoc. 2001;219:1582–1589. doi: 10.2460/javma.2001.219.1582. [DOI] [PubMed] [Google Scholar]
- 17.Bregazzi VS, LaRue SM, McNiel E, et al. Treatment with a combination of doxorubicin, surgery, and radiation versus surgery and radiation alone for cats with vaccine-associated sarcomas: 25 cases (1995–2000) J Am Vet Med Assoc. 2001;218:547–550. doi: 10.2460/javma.2001.218.547. [DOI] [PubMed] [Google Scholar]
- 18.Hendrick MJ, Goldschmidt MH, Shofer FS, et al. Postvaccinal sarcomas in the cat: epidemiology and electron probe microanalytical identification of aluminum. Cancer Res. 1992;52:5391–5394. [PubMed] [Google Scholar]
- 19.Burton G, Mason KV. Do postvaccinal sarcomas occur in Australian cats? Aust Vet J. 1997;75:102–106. doi: 10.1111/j.1751-0813.1997.tb14167.x. [DOI] [PubMed] [Google Scholar]
- 20.Gobar GM, Kass PH. World Wide Web-based survey of vaccination practices, postvaccinal reactions, and vaccine site-associated sarcomas in cats. J Am Vet Med Assoc. 2002;220:1477–1482. doi: 10.2460/javma.2002.220.1477. [DOI] [PubMed] [Google Scholar]


