Summary
Pest insects lead to excessive agricultural and therefore economical losses on crops worldwide. These insects have to withstand toxic molecules that are inherent to plant defences, as well as those that are produced and introduced by humans in the form of insecticides. In recent years, research on insect–microbe symbioses has recognized that microbial symbionts may play a role protecting against these toxins, leading to a form of defensive symbiosis between the pest insect and different types of microorganisms that we term detoxifying symbioses. In this minireview, we will highlight well‐characterized and emerging insect model systems of detoxifying symbioses and assess how the microorganisms influence the host's success.
Introduction
Insects are very successful eukaryotic life forms on earth and have evolved into a stunning diversity of lineages. In agriculture, many insects are beneficial because they pollinate crops, disperse seeds or prey on herbivores (Dillon and Dillon, 2004). On the other hand, there are also detrimental insects that feed on crops and challenge food security worldwide, and are therefore regarded as pest insects. Less than 0.5% of the known species of insects are considered pests, yet several estimates of worldwide losses caused by insects indicate a staggering 7.9–15.1% of the world's annual crop production (Oliveira et al., 2014). To summarize which insect species are the most harmful is a futile task as the pest status is highly variable, dependent on year, weather, geography and insect biology and ecology. Furthermore, the monitoring and evaluation of damage caused by pest insects is poorly documented on a worldwide scale, and estimates of losses often have to be made based on limited data. With this in mind, we exemplify economic losses reported in Brazil in 2014, where greatest crop losses due to insects per area were observed for apples (4281 $/ha), tomatoes (3806 $/ha), tobacco (2729 $/ha), garlic (2655 $/ha), peanut (1679 $/ha), rubber (1242 $/ha) and grapes (1004 $/ha) (Oliveira et al., 2014).
There are several mechanisms known for counteracting pest insects, e.g. sterile insect techniques (SIT), crop rotation, chemical insecticides or biological pest control exploiting predators and parasitoids (Douglas, 2007). The former employs the release of male insects that are not able to produce fertile offspring, yet mate with female wild‐type insects, yielding a reduced amount of pest insects in the next generation. The use of chemical insecticides has been under debate in the past decades as many insecticides have harmful effects on humans (Costa et al., 2007), ecosystems and non‐target insects (Pimentel, 2005). Release of parasitoids is now a commercially available biocontrol strategy with varying success (Smith, 1996; Bokonon‐Ganta et al., 2013). A largely untapped resource that may be used in pest management is the manipulation of microorganisms that live in symbiosis with insects. It has been recognized for a long time that microorganisms play key roles in biogeochemical element cycles (Rousk and Bengtson, 2014), ecosystem functioning (Graham et al., 2016), host nutrition (Hacquard et al., 2015) and human health (Sun and Chang, 2014). Microbial symbionts provide an especially diverse range of benefits in insect nutrition, e.g. by providing essential amino acids (Douglas, 1998), digestive enzymes (Brune, 2014) or vitamins (Salem et al., 2014). One field that has received less attention is the roles that microbes play in protecting insects from toxic plant compounds and insecticides. This is despite the fact that it is known that many microorganisms contain enzymatic degradation mechanisms for a variety of plant secondary metabolites such as terpenes (Marmulla and Harder, 2014), caffeine (Summers et al., 2012, 2015), nicotine (Brandsch, 2006; Li et al., 2010), cocaine (Narasimhan et al., 2012), isothiocyanates (Fan et al., 2011) and even phosphorus‐ or sulfur‐containing insecticides (Kertesz et al., 1994). Oftentimes the interactions between microbe and insect are difficult to disentangle, and the relative contribution of insect versus microbial defence mechanisms is not yet known. In this minireview, we will focus on hitherto described pest insects where symbiotic microorganisms may play a crucial role in detoxifying detrimental compounds for their respective hosts, and touch upon possible routes for pest insect management strategies.
Defining detoxifying symbiosis
The ecological definition of a symbiosis is broad, encompassing commensalism, parasitism and mutualism between any two dissimilar organisms. In insect–microbe interactions, the bacterial counterpart is generally referred to as ‘symbiont’ and the insect as ‘host’. As studies in microbe–insect interactions oftentimes also include the involved physiology of the plant that hosts an herbivorous insect, the plant may be referred to as ‘plant host’ or ‘host diet’. Symbionts can be distinguished as primary or secondary, which was particularly useful during the early days of research on the now well‐studied symbiosis between aphids and their primary symbionts Buchnera aphidicola. Primary symbionts that are important for proper functioning of the host are generally maternally transmitted and have remained unculturable even with modern culturing techniques. This fastidious nature is caused by drastic reduction in genome size (Moran and Bennett, 2014). Generally, this type of symbiosis, involving reduced symbiont genomes, is reserved for nutritional symbioses, but recent findings indicate that some primary symbionts may also have a defensive function in psyllids and stinkbugs (Hosokawa et al., 2006; Nakabachi et al., 2013). Primary symbionts are indispensable for both partners, resulting in perfect host infection rates. These symbioses, where essential nutrients are synthesized by an intracellular endosymbiont with a highly reduced genome, are particularly widespread in aphids, tsetse flies and psyllids (Douglas, 1998; Nikoh et al., 2011; Jing et al., 2014).
Secondary symbionts are generally ubiquitous in many types of insects. They show less fidelity towards one specific host, and not all individuals of a population necessarily carry the symbiont. Loss of a secondary symbiont by an insect is a common occurrence, especially in lab‐reared cultures where the natural driving force behind a sustained symbiosis may be inevitably or inadvertently removed (Kellner, 1999; Rani et al., 2009; Estes et al., 2012). The biological effects of most facultative symbionts are unknown, but findings regarding their defensive capabilities imply that their roles will be understandable only in the context of complex natural environments (Moran et al., 2008). Examples of defensive symbionts conferring beneficial traits include, but are not limited to; increased thermal tolerance, production of antipredator toxins and protection against pathogens (Flórez et al., 2015). Many insect–microbe symbioses were recently extensively reviewed by several authors (Douglas, 2013, 2015; Hansen and Moran, 2014), but one aspect in particular has not yet been well evaluated, namely symbiont‐mediated detoxification (Fig. 1). As the definition of a defensive symbiosis does not fully capture the concept of symbiont‐mediated detoxification, we suggest the term ‘detoxifying symbiosis’ for this type of mechanism, where insect‐associated microorganisms are the main factor responsible for the detoxification of plant toxins or insecticides. It should be kept in mind that categories like ‘nutritional’, ‘defensive’ or ‘detoxifying’ symbiosis are not mutually exclusive for any given symbiosis and refer to only one elucidated or hypothesized aspect of a complex partnership. Recent developments in the field are providing increasingly more evidence for the natural occurrence of gut‐associated microorganisms that aid or possibly are crucial to detoxification. Here, we compile recent studies that indicate microbial components involved in the detoxification of toxic plant metabolites and man‐made insecticides (Adams et al., 2013; Ben‐Yosef et al., 2014; Ceja‐Navarro et al., 2015; Berasategui et al., 2016; Welte et al., 2016a).
Figure 1.
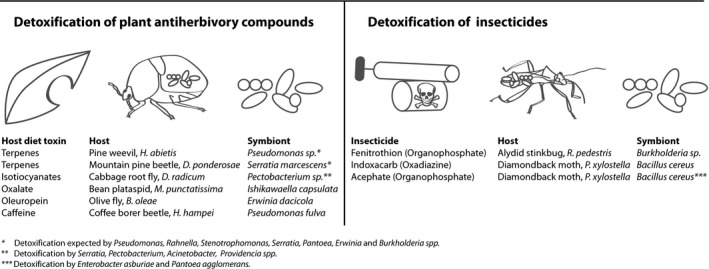
An overview of emerging models for symbiont‐mediated detoxification in pest insects.
Detoxifying symbionts confer resistance to plant allelochemicals
As a result of their sessile lifestyle, plants have developed an impressive arsenal of defensive mechanisms, even when disregarding physical defences such as trichomes, prickles or thorns. They have evolved to produce a multitude of antiherbivorous compounds to deter feeding insects that we will simply refer to as allelochemicals. Many herbivorous insects specialize on one or just a few closely related host plants and thus have to overcome the defensive compounds that these plants produce. In many cases, defence against allelochemicals is an intrinsic part of insect physiology, mediated by insect‐produced enzymes like cytochrome P450 enzymes, glutathione S‐transferases or esterases (Douglas, 2015). In other cases, the mechanism with which an insect copes with the toxicity of its diet has remained unclear. In this chapter, we discuss studies that suggest that microbial symbionts aid their hosts in the detoxification of antiherbivorous compounds and supply limited economic context for these pest insects.
Detoxifying symbionts of legume pests
Plataspids (or kudzu bugs, Megacopta spp.) are a notorious pest of peas, soya beans and other legumes, and received international notoriety after spreading from Asia to the United States in 2009 (Ruberson et al., 2012). Data on economic losses caused by the plataspid are scarce. However, occasional soya bean yield losses of up to 50% have been reported in China, and large infestations are now consistently found in the U.S.A., where smell and sheer number of plataspids can cause public annoyance (Ruberson et al., 2012). Plataspidae are known to vertically transmit their obligate Candidatus Ishikawaella capsulata symbiotic microbes by depositing brown symbiont‐containing capsules with their eggs that are ingested by newborn nymphs (Nikoh et al., 2011).
Sequencing of Ca. I. capsulata revealed an ode gene on one of its plasmids (Hosokawa et al., 2006; Nikoh et al., 2011). The ode gene codes for oxalate decarboxylase, which can break down oxalate; a plant secondary metabolite that provides defence against herbivory (Franceschi and Nakata, 2005). Soluble oxalic acid is a strong acid and is toxic to animals, while the crystallized calcium oxalate is reported to serve as a mechanical defence, as they have a striking abrasive effect on the mandibles of chewing insects (Korth et al., 2006). Feeding‐choice tests have shown that beet armyworms (Spodoptera exigua) avoid calcium oxalate containing wild‐type Medicago truncatula leaves in the presence of mutants that lack calcium oxalate. Breakdown of oxalate was already reported in gut bacteria found in humans, pigs and sheep (Allison et al., 1985). Future studies on the plataspid symbiont should consider the possibility of a similar detoxifying role for this insect. Nikoh and colleagues (Nikoh et al., 2011) thus suggested that Ca. I. capsulata may be giving not only nutritional but also protective benefits to its host.
Ca. I. capsulata shows remarkable resemblance to the Buchnera and Wigglesworthia genera that are found in obligate nutritional symbiosis with aphids and tsetse flies, respectively. A genetic analysis on the Ca. I. capsulata symbionts of pest plataspid Megacopta punctatissima revealed that the symbionts have a highly reduced genome (~746 kb) that contains genes involved in amino acid metabolism, indicative of obligate symbionts of sap‐feeding insects. Just like aphids, the stinkbug M. punctatissima feeds on phloem sap that contains little amino acids and its diet is supplemented by microbes that provide essential amino acids (Nikoh et al., 2011). It thus seems that Buchnera and Ishikawaella symbionts serve similar purposes for their respective hosts, but Ca. I. capsulata is not an endosymbiont. Instead, it can be found extracellularly in an enlarged portion of the posterior midgut of the adult stinkbug (Hosokawa et al., 2006). It is still considered an obligate symbiont because the experimental removal of Ca. I. capsulata from the insect caused retarded growth, a higher mortality rate and increased sterility, among other negative effects. Interestingly, when two species of plataspids with high and low pest status (M. punctatissima and Megacopta cribraria, respectively) had their obligate symbionts experimentally exchanged, their performances on a crop legume were also reversed (Hosokawa et al., 2007). This finding is particularly interesting with regard to possible insect control methods involving symbiotic microorganisms; if the pest status of an insect depends critically on symbiont genotype, this would provide a basis for identifying and/or selecting for genotypes geared towards specific pest management priorities. The next important (yet long outstanding) question is whether this type of selection is feasible with low‐tech management strategies like manipulation of the nutrition or abiotic factors experienced by insect pests (Douglas, 2007).
Detoxifying symbionts of pine pests
Pine plantations are heavily impacted by mountain pine beetles (Dendroctonus ponderosae) and pine weevils (Hylobius abietis), two serious pests in the cultivation of pine seedlings and conifers causing losses of up to 80% (Petersson and Örlander, 2003; Boone et al., 2013). Beetles attempting to colonize living conifers will encounter a diverse array of toxic terpenes, produced by the tree for protection (Hamberger et al., 2011). Bacteria associated with D. ponderosae were first shown to be able to reduce concentrations of terpenes (Boone et al., 2013), and subsequent metagenomic sequencing of the microbiome of D. ponderosae revealed that its bacterial community is highly enriched in genes contributing to terpene metabolism (Adams et al., 2013). Recently, a study by Berasategui and co‐workers (Berasategui et al., 2016) found that the gut microbiome of pine weevils is also highly similar to closely related weevils and bark beetles feeding on similar food sources, raising the question whether those terpene‐degrading bacteria enable the pine weevil to overcome host plant defences (Berasategui et al., 2016). Similarly, a Rhodococcus species isolated from the generalist gypsy moth (Lymantria dispar) was closely related to the Rhodococcus erythropolis DCL14 that produces an enzyme responsible for monoterpene degradation (Broderick et al., 2004).
Detoxifying symbionts of olive pests
The olive fly Bactrocera oleae is a major pest insect found on olive trees worldwide. In the Mediterranean basin, where 98% of olive trees are found, the pest has occurred for over 2000 years. In heavily infested regions, the olive fruit fly can cause crop losses of up to 100% for table olives and up to 80% for olive oil variants due to lower quality requirements (Zalom, 2009; Hamdan, 2016). Yearly economic losses caused by the olive fruit fly are estimated at 800 million $, despite a yearly investment of 100 million $ combating olive tree pests (El‐Hadi, 1996). Recent efforts for finding effective biocontrol strategies included screening for wild parasitoids in Himalayan Asia (Bon et al., 2016), and the braconid wasp Psyttalia ponerophaga shows favourable results in experimental setups (Sime et al., 2007). Currently, the primary control method employed by olive growers in California entails intensive spraying of spinosad insecticides (Vossen et al., 2004). Unripe olives are rich in oleuropein, a bitter tasting phenolic glycoside that is the main secondary metabolite of the unripe fruit. When activated by glycosylation, it cross‐links foliar proteins into high molecular weight aggregates and binds lysine residues, effectively reducing the nutritional value of dietary protein in the affected plant (Konno et al., 1999). Olive fly larvae have to overcome this defence strategy, and one possible mechanism is the association with symbiotic microorganisms. Several recent studies (Estes et al., 2009; Ben‐Yosef et al., 2014, 2015; Andongma et al., 2015) highlighted the possible involvement of bacteria even though they do not provide definitive proof of bacterial detoxification of the oleuropein secondary metabolite. When olive flies with and without (aposymbiotic) the bacterium Ca. Erwinia dacicola were reared on unripe olives, aposymbiotic larvae did not develop beyond the second instar. Conversely, oleuropein concentration is drastically lower in ripe olives, and aposymbiotic larvae were able to complete their development to adulthood on these fruits, although it took significantly longer and the larvae were significantly lighter than their symbiotic counterparts (Ben‐Yosef et al., 2015). Flies commonly associate with free‐living, rot‐inducing bacteria like those of the genus Erwinia that can be inoculated into the fruit by ovipositing females, subsequently enhancing larval nutrition with proteins and essential nutrients. These bacteria can usually grow on fruits independently of the host insect (Ben‐Yosef et al., 2015). The olive fly symbiont Ca. E. dacicola, however, has so far not been obtained in pure culture but has been studied by a variety of culture‐independent methods. The association of the olive fly with Ca. E. dacicola combines characteristics that were once thought to be exclusive for either obligate or facultative symbionts (Moran et al., 2008). Its genome size and GC‐content are not representative of classical primary endosymbionts and are more indicative of free‐living or phytopathogenic bacteria. On the other hand, Ca. E. dacicola is vertically transmitted and shows a clear history of co‐evolution with its host like classical primary endosymbionts (Capuzzo et al., 2005). Ca. E. dacicola was found in all life stages and in all wild populations of B. oleae in both the New and Old World sampled over 2 years with a significant difference in relative abundance across life stages (Estes et al., 2012). Abundances were highest in ovipositing females and low in eggs and pupae (Estes et al., 2009). It was hypothesized that the high abundance of symbiotic Ca E. dacicola in ovipositing females causes a higher chance of vertical transmission to progeny and may possibly provide dietary supplementation during the nutritionally demanding period of oviposition. The association of Ca. E. dacicola with all olive fly life stages suggests that these bacteria can persist through metamorphosis and dietary changes. About 10% of the adult population appeared to lack the bacterium, so even though Ca. E. dacicola is tightly associated with the olive fly it may either not be obligate in terms of host survival or was present below the detection limit of the chosen method. Larvae void their intestine prior to pupation and might therefore contain a very low amount of symbiotic gut bacteria, leading to too low amounts of template DNA to detect the symbiont with 16S rRNA gene targeting methods (Estes et al., 2009). Ca. E. dadicola is maintained extracellularly in the adult host microbiome in the gut lumen, the gastric caeca (Ben‐Yosef et al., 2015) and intracellularly in the midgut epithelial cells of larvae (Estes et al., 2009). A significant nutritional role has been demonstrated for this bacterium, as it provides the capacity to utilize non‐essential amino acids and urea as a nitrogen source (Ben‐Yosef et al., 2014). The studies have indicated that it enables the olive fly to overcome plant defences, and it is implied that detoxification of plant oleuropein constitutes a major part in this defensive mechanism. Whether Ca. E. dacicola is the responsible element for the detoxification of oleuropein should be further investigated. The exact mechanism remains elusive and is difficult to validate without cultivation of Ca. E. dacicola, but hypotheses include symbiotic excretion of proteins that degrade, bind, or are resistant to polyphenols. The study further emphasizes the added value of being able to isolate a symbiotic bacterium for experimental purposes, which is still a major hurdle in many symbiotic models.
Detoxifying symbionts of cabbage pests
Another fly species that may make use of detoxifying bacterial symbionts is the cabbage root fly, Delia radicum. It feeds on plants of the Brassicaceae family to which many well‐known vegetable crops belong like cabbages, cauliflower or brussels sprouts, as well as oilseed rape used for the production of vegetable oil and biodiesel. Although exact numbers are scarce, economical losses inflicted by D. radicum include crop losses of up to 80% but are highly dependent on the host plant and crop rotation. Yield reduction in three consecutive years of canola production, for example, amounted to economic losses of approximately 292–377 Can$/ha/year, mainly due to D. radicum infestation (Dosdall et al., 2012). Insecticide treatments using the organophosphate insecticide chlorpyrifos can be effective, but are banned in several countries. Cruciferous plants employ a common defence mechanism where toxic isothiocyanates (ITC) are produced by the breakdown of glucosinolates as catalysed by the plant enzyme myrosinase, as a response to insect damage. Most insect herbivores that specialize on glucosinolate‐containing plants avoid the production of ITC altogether (Winde and Wittstock, 2011). Infestation of the cabbage root fly, however, does lead to the emittance of ITC by plant roots (Crespo et al., 2012). Cabbage root fly larvae appear to lack any of the known avoidance mechanisms observed in other cabbage pests, such as phloem sucking to avoid glucosinolate disruption or enzymatically guided glucosinolate breakdown towards less toxic epithionitriles as an end product instead of ITC (Wittstock et al., 2003). A recent study shows that cabbage root fly larvae harbour gut bacteria capable of detoxifying the ITC themselves (Welte et al., 2016a). Four Gammaproteobacterial isolates (Serratia, Providencia, Pectobacterium and Acinetobacter) from the gut were able to degrade the ITC to less harmful compounds. Genome sequencing of these four isolates results in the assembly of several near‐identical plasmids recurring in the different genera, suggesting horizontal gene transfer. More specifically, the presence of tra genes indicates the occurrence of conjugation events. One plasmid, designated as Drgb3, contains the gene saxA (Fan et al., 2011) that encodes a novel ITC hydrolase (Welte et al., 2016b) which hydrolyses several aromatic ITC. The respective bacterial species were dominant players in the gut microbial community as judged by a metagenome analysis, and may therefore reduce the levels of toxic isothiocyanate in the host gut, benefiting host fitness.
Detoxifying symbionts in coffee pests
The coffee borer beetle or coffee berry borer (Hypothenemus hampei) is the primary pest for coffee bean farmers across the world. Over 20 million coffee‐growing families are economically affected by this pest, and infestation levels are particularly high in plantations in Tanzania (90%), Malaysia (50–90%), Uganda (80%), Colombia (60 %), Mexico (60%) and Jamaica (58–85%; Jaramillo et al., 2006). Yearly losses have been estimated at 500 million $ worldwide, but accumulative approximations of economic loss per country indicate that this is a very conservative estimate (Vega et al., 2015). Caffeine is an important substance that gives coffee its stimulating effects, but in an ecological context coffee beans use caffeine as a protective alkaloid allelochemical against herbivory. The coffee borer beetle overcomes the toxicity of caffeine using its gut microbes for caffeine degradation as demonstrated by Ceja‐Navarro et al. (2015). Beetles with an intact gut flora were able to fully deplete the caffeine in their diet, whereas beetles that had their gut flora disrupted with an antibiotic treatment lost their ability to degrade caffeine. Interestingly, the researchers were able to reinstate the caffeine degradation in H. hampei by transferring a pure culture of Pseudomonas fulva into the gut via inoculum of the beetles’ artificial diet. This P. fulva had beforehand been isolated from the digestive tract of H. hampei and showed strong caffeine degrading capabilities of its own. Although many of the core microorganisms from H. hampei were able to subsist on caffeine, only P. fulva isolates yielded positive amplification of the ndmA gene which codes for an enzyme that catalyses the first step in caffeine degradation (Summers et al., 2012). This gene was also expressed in situ, further implying the importance of the role of P. fulva in detoxifying caffeine for its host.
The study of Ceja‐Navarro et al. provides excellent insight into the importance of bacteria in the detoxification of plant secondary metabolites. However, like many studies in this field, it also raises several new questions. For instance, out of over 100 isolates over 12 different bacterial species that were able to subsist on caffeine as their sole carbon and nitrogen source, only P. fulva contained the ndmA gene that is associated with caffeine breakdown. What are the metabolic pathways of the others? And even though infecting H. hampei with pure P. fulva was enough to reinstate caffeine degradation, the abundance of other species in natural core microbiomes suggests additional unknown functions for these bacteria.
Symbiont‐mediated insecticide resistance
Plant‐produced toxins are not the only dangerous chemicals that insects encounter. Insecticides are employed to increase crop yields and control hygienic pests, contributing to worldwide agriculture, economy and health (Kikuchi et al., 2012). The rapid development of insecticide resistance by diverse organisms has raised concern and should be further investigated. Multiple mechanisms for insecticide resistance have been attributed to host‐level physiology (Kasai et al., 2000; Temeyer et al., 2008; Tabashnik and Carriere, 2010), but in recent years, some scientists argue that certain insecticide resistances may be attributable to detoxifying symbionts. Isolates of insect fungal symbionts have long been shown to be promising sources of detoxifying enzymes that target toxic allelochemicals as well as insecticides (Shen and Dowd, 1991; Dowd, 1992; Shen and Dowd, 1992). Evidence of insecticide resistance conferred by symbiont‐level physiology is still scarce, but particularly convincing results regarding a notorious Asian legume pest were recently put forward and will be discussed in the next paragraph.
Insecticide resistance in the Japanese legume pest Riptortus pedestris
The broad‐headed bugs of the Alydidae family, particularly Riptortus spp. and Leptocorisa spp., are major pests on soya beans and other legumes in parts of eastern Asia, including Japan. Riptortus clavatus houses a dense population of Burkholderia symbionts in their midgut crypts, which are acquired from the environment in every generation, rather than the ‘conventional’ method of vertical maternal transmission (Kikuchi et al., 2007). The symbiotic Burkholderia are able to degrade the insecticide fenitrothion, a prevalent organophosphorus agent in agriculture (Kikuchi et al., 2012). Moreover, individual bugs of Riptortus pedestris readily establish a symbiosis with fenitrothion‐degrading Burkholderia symbionts and subsequently show much higher survival rates on plants dipped in fenitrothion than bugs with non‐degrading Burkholderia symbionts (Kikuchi et al., 2012). Application of fenitrothion to a field results in a relative increase of fenitrothion‐degrading bacteria in the soil, which is hypothesized to affect the dynamics of transmission of symbiotic degrading Burkholderia from the soil to stinkbugs (Tago et al., 2015). These findings suggest that insecticide resistance might develop in a field even in the absence of pest insects, which could then quickly establish in a single insect generation (Kikuchi et al., 2012). The established Riptortus pedestris model provides a good opportunity for studying bacterial symbiotic factors at a molecular level, as the Burkholderia symbionts are cultivable and genetically manipulable (Kim et al., 2015). Development of an ecological insecticide by utilizing gut symbionts is one of the long‐term research goals in the field. The studies regarding fenitrothion may only represent the proverbial tip of the iceberg and could thus be applicable to many as of yet undiscovered mechanisms of insecticide resistance.
Insecticide breakdown by diamondback moth‐associated symbionts
The diamondback moth Plutella xylostella (sometimes also called the cabbage moth, not to be confused with Mamestra brassicae) is a major global pest of cruciferous crops, estimated to cost approximately 4 billion euros per year in lost production and management (Zalucki et al., 2012). Unlike the aforementioned D. radicum, P. xyolostella produces an enzyme that prevents formation of dietary isothiocyanates (Ratzka et al., 2002). P. xylostella is not only able to overcome host defences, but it has even been shown to be highly resistant to a large variety of chemical insecticides and it is one of the only three insect species to have developed resistance to Bacillus thuringiensis‐based insect control methods (Furlong et al., 2013). The rapid development of highly resistant phenotypes of P. xylostella is at least in part attributed to the insect's own physiology, and includes altered target sites for carbamates and organophosphates, metabolism of parathion via glutathione S‐transferases and detoxification of pyrethroids via microsomal P‐450 monooxygenases (Ramya et al., 2016). Isolated Bacillus cereus colonizing the moth's gut were able to break down the insecticide indoxacarb for use in metabolism and growth (Janmaat and Myers, 2003; Ramya et al., 2015). Another insecticide, acephate, was also readily broken down by bacteria isolated from the gut of the diamondback moth. These findings, combined with findings on symbiont‐mediated insecticide resistance in stinkbugs (Kikuchi et al., 2012), indicate that bacteria may play a larger role in insect resistance to insecticides than previously thought. However, in vivo breakdown of either insecticide by P. xylostella gut bacteria has not yet been demonstrated, so cause and effect remain unclear. Even if detoxifying symbiosis does not play a role in this association, further study can still be fruitful because organophosphorus‐degrading bacteria by themselves may prove of value in ecological and industrial applications (for an overview we refer to the review of Singh, 2009).
Conclusions and outlook
In agriculturally important pest insects, the microbiome offers potential for improvement of current methods in pest management. Primary opportunities include the prediction of host traits and thereby the efficacy of control strategies, targeting of the microbiome for direct pest control or targeting the microbiome to reduce the vector competence of the pest (Douglas, 2015). Even though many studies speculate on the contribution of microorganisms in detoxifying symbiosis it is – even with the current advances in culture‐independent microbiological techniques – still challenging to unravel specific contributions of microbial players in the complete metabolism. Many detoxifying symbioses are specific to a certain type of insect lifestyle, including the specialization to a host plant producing toxic allelochemicals. Microbes are known to break down a large array of allelochemicals and insecticides which offers many opportunities for insects to establish detoxifying symbioses. Evolution in microorganisms proceeds at a faster pace than in insects, which might lead to quick adaptation of pest insects to insecticides by the use of symbiotic microorganisms (Kikuchi et al., 2012). Insects are furthermore able to rapidly acquire novel metabolic functions and to invade new ecological niches by engaging in symbiotic relationships with microbes that already possess complete, well‐attuned metabolic pathways (Hosokawa et al., 2016).
An increased demand for novel insect pest management created by growing human populations and global climate change is anticipated, and symbiotic microorganisms offer one potential route to meet this demand (Douglas, 2007). Of the symbiont‐based pest control strategies, only sterile insect technique is currently routinely used, and developments in paratransgenesis are ongoing but require genetic modification, making it difficult to apply in agricultural systems. Further research efforts into (detoxifying) symbiosis may lead to environmentally friendly and sustainable methods to control major pest insect populations. For example, if insect pest status depends critically on symbiont genotype, it would provide a basis for identifying and/or selecting for genotypes geared towards specific pest management priorities, ideally using low‐tech management strategies. Isolation of detoxifying symbionts could lead to applications in bioremediation or treatments for insecticide poisonings. In this minireview, we have assembled relevant studies regarding detoxifying symbioses in agriculturally important pest insects and how they were investigated, thereby providing an array of experimental strategies to learn more about microbes and their role in detoxifying symbiosis.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors are thankful for financial support by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek through the SIAM Gravitation Grant 024.002.002 as well as for general support by Prof. Mike Jetten. Dr. Tobias Engl (University of Mainz) is kindly acknowledged for his input on the manuscript. We thank two anonymous reviewers, whose input helped strengthen this work.
Microbial Biotechnology (2016) 10(3, 531–540
References
- Adams, A.S. , Aylward, F.O. , Adams, S.M. , Erbilgin, N. , Aukema, B.H. , Currie, C.R. , et al (2013) Mountain pine beetles colonizing historical and naïve host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl Environ Microbiol 79: 3468–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison, M.J. , Dawson, K.A. , Mayberry, W.R. , and Foss, J.G. (1985) Oxalobacter formigenes gen. nov., sp. nov.: oxalate‐degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol 141: 1–7. [DOI] [PubMed] [Google Scholar]
- Andongma, A.A. , Wan, L. , Dong, Y.‐C. , Li, P. , Desneux, N. , White, J.A. , and Niu, C.‐Y. (2015) Pyrosequencing reveals a shift in symbiotic bacteria populations across life stages of Bactrocera dorsalis . Sci Rep 5: 9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Yosef, M. , Pasternak, Z. , Jurkevitch, E. , and Yuval, B. (2014) Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J Evol Biol 27: 2695–2705. [DOI] [PubMed] [Google Scholar]
- Ben‐Yosef, M. , Pasternak, Z. , Jurkevitch, E. , and Yuval, B. (2015) Symbiotic bacteria enable olive fly larvae to overcome host defences. R Soc Open Sci 2: 150170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasategui, A. , Axelsson, K. , Schmidt, A. , Borg‐Karlson, A.‐K. , Gershenzon, J. , Terenius, O. , and Kaltenpoth, M. (2016) The gut microbiota of the pine weevil (Hylobius abietis, Coleoptera: Curculionidae) is similar across Europe and resembles that of other confer‐feeding beetles. Mol Ecol 24: 4014–4031. [DOI] [PubMed] [Google Scholar]
- Bokonon‐Ganta, A.H. , McQuate, G.T. , Messing, R.H. , and Jang, E.B. (2013) Release and establishment of the parasitoid Diachasmimorpha kraussii against the tephritid fruit fly Bactrocera latifrons in Hawaii. J Insect Sci 13: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon, M.C. , Hoelmer, K.A. , Pickett, C.H. , Kirk, A.A. , He, Y. , Mahmood, R. , and Daane, K.M. (2016) Populations of Bactrocera oleae (Diptera: Tephritidae) and its parasitoids in Himalayan Asia. Ann Entomol Soc Am 109: 81–91. [Google Scholar]
- Boone, C.K. , Keefover‐Ring, K. , Mapes, A.C. , Adams, A.S. , Bohlmann, J. , and Raffa, K.F. (2013) Bacteria associated with a tree‐killing insect reduce concentrations of plant defense compounds. J Chem Ecol 39: 1003–1006. [DOI] [PubMed] [Google Scholar]
- Brandsch, R. (2006) Microbiology and biochemistry of nicotine degradation. Appl Microbiol Biotechnol 69: 493–498. [DOI] [PubMed] [Google Scholar]
- Broderick, N.A. , Raffa, K.F. , Goodman, R.M. , and Handelsman, J. (2004) Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture‐independent methods. Appl Environ Microbiol 70: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune, A. (2014) Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol 12: 168–180. [DOI] [PubMed] [Google Scholar]
- Capuzzo, C. , Firrao, G. , Mazzon, L. , Squartini, A. , and Girolami, V. (2005) “Candidatus Erwinia dacicola”, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). Int J Syst Evol Microbiol 55: 1641–1647. [DOI] [PubMed] [Google Scholar]
- Ceja‐Navarro, J.A. , Vega, F.E. , Karaoz, U. , Hao, Z. , Jenkins, S. , Lim, H.C. , et al (2015) Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat Commun 6: 7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, C. , Silva, S. , Coelho, P. , Roma‐Torres, J. , Teixeira, J.P. , and Mayan, O. (2007) Micronucleus analysis in a Portuguese population exposed to pesticides: preliminary survey. Int J Hyg Environ Health 210: 415–418. [DOI] [PubMed] [Google Scholar]
- Crespo, E. , Hordijk, C.A. , de Graaf, R.M. , Samudrala, D. , Cristescu, S.M. , Harren, F.J.M. , and van Dam, N.M. (2012) On‐line detection of root‐induced volatiles in Brassica nigra plants infested with Delia radicum L. root fly larvae. Phytochemistry 84: 68–77. [DOI] [PubMed] [Google Scholar]
- Dillon, R.J. , and Dillon, V.M. (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49: 71–92. [DOI] [PubMed] [Google Scholar]
- Dosdall, L.M. , Harker, K.N. , O'Donovan, J.T. , Blackshaw, R.E. , Kutcher, H.R. , Gan, Y. , et al (2012) Crop sequence effects on root maggot (Diptera: Anthomyiidae: Delia spp.) infestations in canola. J Econ Entomol 105: 1261–1267. [DOI] [PubMed] [Google Scholar]
- Douglas, A.E. (1998) Nutritional interactions in insect‐microbial symbioses: aphids and their symbiotic bacteria Buchnera . Annu Rev Entomol 43: 17–37. [DOI] [PubMed] [Google Scholar]
- Douglas, A.E. (2007) Symbiotic microorganisms: untapped resources for insect pest control. Trends Biotechnol 25: 338–342. [DOI] [PubMed] [Google Scholar]
- Douglas, A.E. (2013) Microbial brokers of insect‐plant interactions revisited. J Chem Ecol 39: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, A.E. (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60: 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, P.F. (1992) Insect fungal symbionts: a promising source of detoxifying enzymes. J Ind Microbiol 9: 149–161. [Google Scholar]
- El‐Hadi, F.A. (1996) The economic damage caused by the olive fruit fly, Bactrocera oleae In Phytoparasitica 24 pp. 132–133. [Google Scholar]
- Estes, A.M. , Hearn, D.J. , Bronstein, J.L. , and Pierson, E.A. (2009) The olive fly endosymbiont, “Candidatus Erwinia dacicola”, switches from an intracellular existence to an extracellular existence during host insect development. Appl Environ Microbiol 75: 7097–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes, A.M. , Hearn, D.J. , Burrack, H.J. , Rempoulakis, P. , and Pierson, E.A. (2012) Erwinia dacicola in wild and laboratory olive fruit fly populations and across developmental stages. Environ Entomol 41: 265–274. [DOI] [PubMed] [Google Scholar]
- Fan, J. , Crooks, C. , Creissen, G. , Hill, L. , Fairhurst, S. , Doerner, P. , and Lamb, C. (2011) Pseudomonas sax genes overcome aliphatic isothiocyanate‐mediated non‐host resistance in Arabidopsis . Science 331: 1185–1188. [DOI] [PubMed] [Google Scholar]
- Flórez, L.V. , Biedermann, P.H.W. , Engl, T. , and Kaltenpoth, M. (2015) Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat Prod Rep 32: 904–936. [DOI] [PubMed] [Google Scholar]
- Franceschi, V.R. , and Nakata, P.A. (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56: 41–71. [DOI] [PubMed] [Google Scholar]
- Furlong, M.J. , Wright, D.J. , and Dosdall, L.M. (2013) Diamondback moth ecology and management: problems, progress, and prospects. Annu Rev Entomol 58: 517–541. [DOI] [PubMed] [Google Scholar]
- Graham, E.B. , Knelman, J.E. , Schindlbacher, A. , Siciliano, S. , Breulmann, M. , Yannarell, A. , et al (2016) Microbes as engines of ecosystem function: when does community structure enhance predictions of ecosystem processes? Front Microbiol 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacquard, S. , Garrido‐Oter, R. , González, A. , Spaepen, S. , Ackermann, G. , Lebeis, S. , et al (2015) Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe 17: 603–616. [DOI] [PubMed] [Google Scholar]
- Hamberger, B.B. , Ohnishi, T. , Hamberger, B.B. , Séguin, A. , and Bohlmann, J. (2011) Evolution of diterpene metabolism: sitka spruce CYP720B4 catalyzes multiple oxidations in resin acid biosynthesis of conifer defense against insects. Plant Physiol 157: 1677–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan, A. (2016) Bionomics of olive fruit fly, Bactrocera oleae (Rossi) [Diptera: Tephritidae] infesting ten olive cultivars in the southern highlands of West‐Bank, Palestine. Int J Sci Basic Appl Res 27: 194–203. [Google Scholar]
- Hansen, A.K. , and Moran, N.A. (2014) The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol Ecol 23: 1473–1496. [DOI] [PubMed] [Google Scholar]
- Hosokawa, T. , Kikuchi, Y. , Nikoh, N. , Shimada, M. , and Fukatsu, T. (2006) Strict host‐symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol 4: 1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, T. , Kikuchi, Y. , Shimada, M. , and Fukatsu, T. (2007) Obligate symbiont involved in pest status of host insect. Proc Biol Sci 274: 1979–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, T. , Ishii, Y. , Nikoh, N. , Fujie, M. , Satoh, N. , and Fukatsu, T. (2016) Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat Microbiol 1: 15011. [DOI] [PubMed] [Google Scholar]
- Janmaat, A.F. , and Myers, J. (2003) Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni . Proc R Soc B Biol Sci 270: 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo, J. , Borgemeister, C. , and Baker, P. (2006) Coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): searching for sustainable control strategies. Bull Entomol Res 96: 223–233. [DOI] [PubMed] [Google Scholar]
- Jing, X. , Wong, A.C.N. , Chaston, J.M. , Colvin, J. , McKenzie, C.L. , and Douglas, A.E. (2014) The bacterial communities in plant phloem‐sap‐feeding insects. Mol Ecol 23: 1433–1444. [DOI] [PubMed] [Google Scholar]
- Kasai, S. , Weerashinghe, I.S. , Shono, T. , and Yamakawa, M. (2000) Molecular cloning, nucleotide sequence and gene expression of a cytochrome P450 (CYP6F1) from the pyrethroid‐resistant mosquito, Culex quinquefasciatus Say. Insect Biochem Mol Biol 30: 163–171. [DOI] [PubMed] [Google Scholar]
- Kellner, R.L.L. (1999) What is the basis of pederin polymorphism in Paederus riparius rove beetles? The endosymbiotic hypothesis. Entomol Exp Appl 93: 41–49. [Google Scholar]
- Kertesz, M.A. , Cook, A.M. , and Leisinger, T. (1994) Microbial metabolism of sulfur‐ and phosphorus‐containing xenobiotics. FEMS Microbiol Rev 15: 195–215. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y. , Hosokawa, T. , and Fukatsu, T. (2007) Insect‐microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73: 4308–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, Y. , Hayatsu, M. , Hosokawa, T. , Nagayama, A. , Tago, K. , and Fukatsu, T. (2012) Symbiont‐mediated insecticide resistance. Proc Natl Acad Sci USA 109: 8618–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.K. , Lee, J.B. , Huh, Y.R. , Jang, H.A. , Kim, C.H. , Yoo, J.W. , and Lee, B.L. (2015) Burkholderia gut symbionts enhance the innate immunity of host Riptortus pedestris . Dev Comp Immunol 53: 265–269. [DOI] [PubMed] [Google Scholar]
- Konno, K. , Hirayama, C. , Yasui, H. , and Nakamura, M. (1999) Enzymatic activation of oleuropein: a protein crosslinker used as a chemical defense in the privet tree. Proc Natl Acad Sci USA 96: 9159–9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth, K.L. , Doege, S.J. , Park, S.‐H. , Goggin, F.L. , Wang, Q. , Gomez, S.K. , et al (2006) Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol 141: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmulla, R. , and Harder, J. (2014) Microbial monoterpene transformations‐a review. Front Microbiol 5: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, N.A. , and Bennett, G.M. (2014) The tiniest tiny genomes. Annu Rev Microbiol 68: 195–215. [DOI] [PubMed] [Google Scholar]
- Moran, N.A. , McCutcheon, J.P. , and Nakabachi, A. (2008) Genomics and evolution of heritable bacterial symbionts. Ann Rev Genet 42: 165–190. [DOI] [PubMed] [Google Scholar]
- Nakabachi, A. , Ueoka, R. , Oshima, K. , Teta, R. , Mangoni, A. , Gurgui, M. , et al (2013) Defensive bacteriome symbiont with a drastically reduced genome. Curr Biol 23: 1478–1484. [DOI] [PubMed] [Google Scholar]
- Narasimhan, D. , Woods, J.H. , and Sunahara, R.K. (2012) Bacterial cocaine esterase: a protein‐based therapy for cocaine overdose and addiction. Future Med Chem 4: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh, N. , Hosokawa, T. , Oshima, K. , Hattori, M. , and Fukatsu, T. (2011) Reductive evolution of bacterial genome in insect gut environment. Genome Biol Evol 3: 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, C.M. , Auad, A.M. , Mendes, S.M. , and Frizzas, M.R. (2014) Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Prot 56: 50–54. [Google Scholar]
- Petersson, M. , and Örlander, G. (2003) Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Can J For Res 33: 64–73. [Google Scholar]
- Pimentel, D. (2005) Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. Environ. Dev. Sustain. 7: 229–252. [Google Scholar]
- Ramya, S.L. , Venkatesan, T. , Srinivasa Murthy, K. , Jalali, S.K. , and Verghese, A. (2015) Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Brazilian J Microbiol 47: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya, S.L. , Venkatesan, T. , Murthy, K.S. , Jalali, S.K. , and Varghese, A. (2016) Degradation of acephate by Enterobacter asburiae, Bacillus cereus and Pantoea agglomerans isolated from diamondback moth Plutella xylostella (L), a pest of cruciferous crops. J Environ Biol 37: 611–618. [PubMed] [Google Scholar]
- Rani, A. , Sharma, A. , Rajagopal, R. , Adak, T. , and Bhatnagar, R.K. (2009) Bacterial diversity analysis of larvae and adult midgut microflora using culture‐dependent and culture‐independent methods in lab‐reared and field‐collected Anopheles stephensi‐an Asian malarial vector. BMC Microbiol 9: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzka, A. , Vogel, H. , Kliebenstein, D.J. , Mitchell‐Olds, T. , and Kroymann, J. (2002) Disarming the mustard oil bomb. Proc Natl Acad Sci USA 99: 11223–11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousk, J. , and Bengtson, P. (2014) Microbial regulation of global biogeochemical cycles. Front Microbiol 5: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberson, J.R. , Takasu, K. , Buntin, G.D. , Eger, J.E. , Gardner, W.A. , Greene, J.K. , et al (2012) From Asian curiosity to eruptive American pest: Megacopta cribraria (Hemiptera: Plataspidae) and prospects for its biological control. Appl Entomol Zool 48: 3–13. [Google Scholar]
- Salem, H. , Bauer, E. , Strauss, A.S. , Vogel, H. , Marz, M. , and Kaltenpoth, M. (2014) Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc R Soc B 281: 20141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, S.K. , and Dowd, P.F. (1991) Detoxification spectrum of the cigarette beetle symbiont Symbiotaphrina kochii in culture. Entomol Exp Appl 60: 51–59. [Google Scholar]
- Shen, S.K. , and Dowd, P.F. (1992) Detoxifying enzymes and insect symbionts. J Chem Educ 69: 796. [Google Scholar]
- Sime, K.R. , Daane, K.M. , Kirk, A. , Andrews, J.W. , Johnson, M.W. , and Messing, R.H. (2007) Psyttalia ponerophaga (Hymenoptera: Braconidae) as a potential biological control agent of olive fruit fly Bactrocera oleae (Diptera: Tephritidae) in California. Bull Entomol Res 97: 233–242. [DOI] [PubMed] [Google Scholar]
- Singh, B.K. (2009) Organophosphorus‐degrading bacteria : ecology and industrial applications. Nat Rev Microbiol 7: 156–164. [DOI] [PubMed] [Google Scholar]
- Smith, S.M. (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Annu Rev Entomol 41: 375–406. [DOI] [PubMed] [Google Scholar]
- Summers, R.M. , Louie, T.M. , Yu, C.L. , Gakhar, L. , Louie, K.C. , and Subramanian, M. (2012) Novel, highly specific N‐demethylases enable bacteria to live on caffeine and related purine alkaloids. J Bacteriol 194: 2041–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers, R.M. , Mohanty, S.K. , Gopishetty, S. , and Subramanian, M. (2015) Genetic characterization of caffeine degradation by bacteria and its potential applications. Microb Biotechnol 8: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , and Chang, E.B. (2014) Exploring gut microbes in human health and disease: pushing the envelope. Genes Dis 1: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik, B.E. , and Carriere, Y. (2010) Field‐evolved resistance to Bt Cotton: bollworm in the U.S and Pink Bollworm in India. Southwest Entomol 35: 417–424. [Google Scholar]
- Vega, F.E. and Dowd, P.F. (2005) The Role of Yeasts as Insect Endosymbionts In Insect‐Fungal Association: Ecology and Evolution. Vega F.E. and Blackwell M. (Eds.). Oxford University Press, UK. [Google Scholar]
- Vega, F.E. , Infante, F. and Johnson, A.J. (2015) The genus Hypothenemus, with emphasis on H. hampei, the coffee berry borer In Bark Beetles: Biology and Ecology of Native and Invasive Species. Vega F.E. and Hofstetter R. (eds.). Elsevier, pp. 427–494. [Google Scholar]
- Vossen, P. , Varela, L.G. and Devarenne, A. (2004) Olive fruit fly. UCCE Sonoma County, University of California URL http://fruitsandnuts.ucdavis.edu/files/74133.pdf
- Welte, C.U. , de Graaf, R.M. , van den Bosch, T.J.M. , Op den Camp, H.J.M. , van Dam, N.M. , and Jetten, M.S.M. (2016a) Plasmids from the gut microbiome of cabbage root fly larvae encode SaxA that catalyzes the conversion of the plant toxin 2‐phenylethyl isothiocyanate. Environ Microbiol 18: 1379–1390. [DOI] [PubMed] [Google Scholar]
- Welte, C.U. , Rosengarten, J.F. , de Graaf, R.M. , and Jetten, M.S.M. (2016b) SaxA‐mediated isothiocyanate metabolism in phytopathogenic pectobacteria. Appl Environ Microbiol 82: 2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winde, I. , and Wittstock, U. (2011) Insect herbivore counteradaptations to the plant glucosinolate‐myrosinase system. Phytochemistry 72: 1566–1575. [DOI] [PubMed] [Google Scholar]
- Wittstock, U. , Kliebenstein, D.J. , Lambrix, V. , Reichelt, M. , and Gershenzon, J. (2003) Glucosinolate hydrolysis and its impact on generalist and specialst insect herbivores. Recent Advances in Phytochemistry 37: 101–125. [Google Scholar]
- Zalom, F.G. (2009) Olive fruit fly. Univ. Calif. Agric. Nat. Resour. URL http://ipm.ucanr.edu/PMG/PESTNOTES/pn74112.htm.
- Zalucki, M.P. , Shabbir, A. , Silva, R. , Adamson, D. , Shu‐Sheng, L. , and Furlong, M.J. (2012) Estimating the economic cost of one of the world's major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J Econ Entomol 105: 1115–1129. [DOI] [PubMed] [Google Scholar]


