Abstract
Background: Postprandial hyperlipidemia is associated with impaired endothelial function. Peanut consumption favorably affects the lipid and lipoprotein profile; however, the effects on endothelial function remain unclear.
Objective: The purpose of the study was to evaluate the effects of acute peanut consumption as part of a high-fat meal on postprandial endothelial function.
Methods: We conducted a randomized, controlled, crossover postprandial study to evaluate the effect of acute peanut consumption on postprandial lipids and endothelial function as assessed by flow-mediated dilatation (FMD) of the brachial artery in 15 healthy overweight or obese men [mean age: 26.7 y; mean body mass index (in kg/m2): 31.4]. Participants consumed, in a randomized order, a peanut meal containing 3 ounces (85 g) ground peanuts (1198 kcal; 40.0% carbohydrate, 47.7% fat, 19.4% saturated fat, 13.2% protein) and a control meal matched for energy and macronutrient content. Meals were in the form of a shake, scheduled ≥1 wk apart. Lipids, lipoproteins, glucose, and insulin were measured at baseline (0 min) and at 30, 60, 120, and 240 min after shake consumption. FMD was measured at baseline and at 240 min.
Results: Acute peanut consumption blunted the serum triglyceride (TG) response 120 and 240 min after consumption compared with the control meal (means ± SEMs—120 min: 188.9 ± 19.4 compared with 197.5 ± 20.7 mg/dL; 240 min: 189.9 ± 24.3 compared with 197.3 ± 18.4 mg/dL; P < 0.05 for both). Total, LDL, and HDL cholesterol and glucose and insulin responses were similar between the test meals. Compared with baseline, only the control meal significantly decreased FMD at 240 min (control: −1.2% ± 0.5%; P = 0.029; peanut: −0.6% ± 0.5%; P = 0.3). Participants with higher baseline total (>150 mg/dL) and LDL (>100 mg/dL)-cholesterol concentrations showed a significant decrease in FMD after the control meal (−1.8%, P = 0.017; −2.0%, P = 0.038), whereas the peanut meal maintained endothelial function in all participants irrespective of total- and LDL-cholesterol concentrations.
Conclusion: The inclusion of 85 g peanuts (3 ounces) as part of a high-fat meal improved the postprandial TG response and preserved endothelial function in healthy overweight or obese men. This trial was registered at clinicaltrials.gov as NCT01405300.
Keywords: peanuts, nuts, postprandial responses, triglycerides, vascular function, overweight
Introduction
Cardiovascular disease (CVD)7 remains the leading cause of morbidity and mortality in the United States and globally. Several prospective studies have shown that the postprandial increase in nonfasting serum TGs increases the risk of atherosclerosis and ischemic stroke and is an independent risk factor for coronary artery disease (1–4). Evidence from the Women’s Health Study, a prospective study in 26,509 participants followed for a median of 11.4 y, reported that TGs measured 2–4 h after the last meal was a stronger predictor of events than were fasting TG concentrations (5). In addition, postprandial hypertriglyceridemia is involved in proinflammatory cytokine production and oxidative stress, thereby impairing vascular endothelial function, which also contributes to increased CVD risk (6–8).
Nuts contain many bioactive compounds with cardiometabolic benefits. Epidemiologic evidence from 3 large cohort studies (total n = 206,029) showed that nut and peanut consumption was inversely associated with total mortality (9). In the Nurses’ Health Study, the consumption of peanuts ≥2 times/wk led to a 34% reduction in relative risk of coronary artery disease (10). Peanuts are a popular snack in the United States and a source of unsaturated FAs, plant protein, and bioactive compounds (resveratrol, arginine, vitamin E, magnesium, fiber, and other antioxidants) (11, 12). Moderate [1–1.5 ounces/d (28–42 g/d)] consumption of peanuts has been shown to improve glucose metabolism (13, 14) and the blood lipid and lipoprotein profile (15), with no increase in body weight (16). A recent clinical study in obese men showed that acute consumption of conventional peanuts and high-oleic peanuts (56 g, providing 25% of total energy) attenuated postprandial inflammatory and insulin responses compared with a control meal with no peanuts (17).
The cardioprotective effects of nuts are also thought to be achieved by maintaining endothelial function. Endothelial dysfunction is a precursor for atherosclerosis and an independent predictor of cardiac events (18). Endothelial dysfunction can limit the production and/or availability of NO, leading to impaired vasodilation (18). A previous study reported that the consumption of an ad libitum diet that included 56 g (366 kcal) walnuts/d for 8 wk improved endothelial function measured by flow-mediated dilatation (FMD) compared with a control diet without walnuts in individuals with type 2 diabetes (change in FMD: 2.2% ± 1.7% compared with 1.2% ± 1.6%; P = 0.04) (19). In a randomized crossover trial, FMD improved in hypercholesterolemic subjects after 4 wk of consumption of walnuts (contributing 32% of total energy) compared with a Mediterranean-type control diet without walnuts (change in FMD: 5.9% ± 3.3% compared with 3.6% ± 3.3%; P = 0.04) (20).
Postprandial hyperglycemia and hyperlipemia can initiate a cascade of biochemical events that lead to endothelial dysfunction (21). With the evidence we have to date that peanuts improve postprandial glucose (22, 23), lipid (17), and inflammatory (17) responses, it is possible that acute peanut consumption may improve vascular responses. However, there is limited evidence about whether peanut consumption affects postprandial vascular function. Therefore, in the present study we evaluated the effect of acute peanut consumption as part of a high-fat meal on postprandial vascular function, plasma glucose, insulin, and lipid and lipoprotein profiles. We hypothesized that acute peanut consumption would result in an improved lipid and lipoprotein profile and vascular function as assessed by FMD, a commonly used noninvasive assessment of vascular endothelial function.
Methods
Participants.
We recruited generally healthy, overweight or obese [BMI (in kg/m2) 28–39] men (to avoid hormone cycle effects on vascular function in women) between 20 and 65 y of age, with fasting TGs <350 mg/dL, LDL cholesterol <160 mg/dL, glucose <99 mg/dL, and blood pressure ≤140/90 mm Hg. Participants were free of diabetes, CVD, kidney disease, and other metabolic diseases. The study was approved by the Ethics Committee of The Pennsylvania State University and carried out in accordance with the Helsinki Declaration (clinicaltrials.gov, NCT01405300).
Study design.
A randomized crossover trial was conducted. Participants were evaluated on 2 occasions separated by a washout period of a minimum of 7 d between interventions. Participants were randomly assigned to consume the control or peanut shake during visit 1 and the alternate shake was consumed during visit 2. On each occasion, fasting (10–12 h) blood samples were collected at baseline (t = 0 min) and then at 30, 60, 120, and 240 min after the ingestion of either a control shake or a peanut shake (consumed within 15 min). Participants were asked not to consume alcohol 48 h before the blood draw and to avoid vigorous exercise 2 h before the blood draw. FMD and blood pressure were measured at baseline (t = 0 min) and at 240 min.
Nutrient profile and composition of the peanut and control shakes.
The nutrient profile of the test shakes is presented in Table 1. The control and peanut shakes were matched for macronutrient content. The control shake provided 24.8% carbohydrate, 64.7% fat (27.6% SFAs, 26.0% MUFAs, 11.1% PUFAs), 8.4% protein, 8.2 g fiber, and 1229 kcal. The peanut shake provided 25.9% carbohydrate, 66.2% fat (28.2% SFAs, 26.7 MUFAs, 11.3% PUFAs), 8.5% protein, 8.2 g fiber, and 1198 kcal. The ingredient composition of the test shakes is presented in Table 1. Sunflower oil, safflower oil, powdered egg white, and fiber were used in the control shake to match the nutrient profile of 3 ounces (85 g) of peanuts. The amount of whipping cream and glucose in the peanut and control shakes was adjusted to ensure that all shakes were matched for carbohydrate and SFA contents. The control shake provided 34.8 g glucose, 150 g heavy whipping cream, 39 g chocolate syrup, 20 g sunflower oil, 17 g safflower oil, 27 g powered egg white, and 9.6 g fiber supplement (Benefiber; Novartis Consumer Health, Inc.). The peanut shake provided 34.8 g glucose, 137 g heavy whipping cream, 39 g chocolate syrup, and 85 g (3.0 ounces) ground peanuts with skins. Ingredients were blended with 150 g water and 60 g crushed ice in a Vitamix blender (Vita-Mix Corporation).
TABLE 1.
Nutrient profile and composition of the test meals
| Control | Peanut1 | |
| Macronutrients | ||
| Carbohydrate, % | 24.8 | 25.9 |
| Protein, % | 8.4 | 8.5 |
| FAs, % | 64.7 | 66.2 |
| SFAs | 27.6 | 28.2 |
| MUFAs | 26.0 | 26.7 |
| PUFAs | 11.1 | 11.3 |
| Fiber, g | 8.2 | 8.2 |
| Total energy, kcal | 1229 | 1198 |
| Composition, g | ||
| Glucose | 34.8 | 34.8 |
| Heavy whipping cream | 150 | 137 |
| Chocolate syrup | 39 | 39 |
| Sunflower oil | 20 | |
| Safflower oil | 17 | |
| Powdered egg white | 27 | |
| Fiber supplement | 9.6 |
Provided 3.0 ounces of ground peanuts with skins.
Blood sampling.
A flexible catheter was inserted in the antecubital vein of the left arm, and blood samples were collected into evacuated tubes. Blood samples were collected in the fasted state (0 min) and at 30, 60,120, and 240 min after consumption of the test meals. Serum samples were sent to Quest Diagnostics (Chem 24 panel; Quest Diagnostics) for analysis. Lipids and lipoproteins, glucose, insulin, and C-reactive protein were assayed at all time points.
FMD.
Participants were asked to lie down for 15 min before the FMD assessment. Endothelial function was assessed by FMD with high-frequency ultrasound in a quiet, temperature-controlled room (21–24°C). Longitudinal images of the brachial artery 5–10 cm above the elbow of the right arm were acquired with external B mode ultrasound imaging (Acuson Aspen 128XP equipped with a 10-mHz linear array transducer; Acuson) and recorded during the following procedure: baseline (1 min), arterial occlusion (5 min) via inflation of a cuff on the forearm (distal to the segment being imaged) set at 250 mm Hg (Hokanson), and reactive hyperemia (2 min). Images were graded by using R-wave detection so that scans were assessed at the end of diastole. Two independent, blinded technicians used automated edge detection software (Brachial Analyzer; MIA) to quantify artery diameter continuously throughout the test. Resting diameters were the average of all images collected over the 1-min baseline period. Peak artery diameter was determined as the largest diameter recorded in the 2-min reactive hyperemia segment. The reported FMD score was calculated as the percentage change from resting arterial diameter to peak reactive hyperemia diameter. Scores presented are the average of the 2 technicians’ scores.
Statistical analyses.
Statistical analysis was performed by using SAS (version 9.2; SAS Institute). The mixed-models procedure (PROC MIXED) was used to test the effects of treatment, time, and treatment by time interactions on each outcome. Subject was included as a random effect, and the remaining factors were fixed effects. Tukey-Kramer–adjusted P values were used for post hoc comparisons. The 4-h incremental AUC (iAUC) of postprandial concentrations of TGs, glucose, and insulin were analyzed by using a mixed model with treatment as a fixed effect. Changes were calculated by using values from the 240-min time point minus the baseline value (0 min).
As part of a secondary analysis, we categorized participants into 2 groups on the basis of their baseline total cholesterol (TC) concentration at 150 mg/dL (mean of baseline TC) and baseline LDL-cholesterol concentration at 100 mg/dL (optimal LDL-cholesterol concentration: ≤100 mg/dL). For this stratification, we determined baseline concentrations to be the value taken at 0 min on the first day of testing. Baseline lipid concentrations were added as a categorical variable to the mixed model as a fixed effect to determine whether participants initially presenting with elevated cholesterol responded differently to peanut and control meals than did participants with lower baseline cholesterol concentrations. Means are reported as least-squares means ± SEMs. A histogram and residual plots were used to verify normality of data. The study sample size was estimated with power set to 0.90 and α set to 0.05, which predicted a sample size of 15 participants to detect a 1% change in FMD (24).
Results
Participants and baseline characteristics.
Fifteen nonsmoking male participants met all of the inclusion criteria and completed the study. None of the enrolled participants withdrew from the study. Participant baseline characteristics are presented in Table 2. In the present study, 40% of participants were overweight and 60% were obese.
TABLE 2.
Anthropometric and metabolic characteristics of the men at screening1
| Clinical measurements | Value |
| Age, y | 26.7 ± 1.6 |
| Weight, kg | 100.0 ± 3.7 |
| BMI, kg/m2 | 31.4 ± 0.8 |
| SBP, mm Hg | 125 ± 2 |
| DBP, mm Hg | 83 ± 1 |
| Serum biochemistry | |
| Glucose, mg/dL | 92.5 ± 0.7 |
| Total cholesterol, mg/dL | 159.7 ± 7.1 |
| HDL cholesterol, mg/dL | 42.4 ± 1.9 |
| LDL cholesterol, mg/dL | 99.1 ± 6.4 |
| TGs, mg/dL | 90.6 ± 8.9 |
| TG-to-HDL-cholesterol ratio | 2.26 ± 1.15 |
Values are means ± SEMs. n = 15. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Postprandial insulin, glucose, and lipids.
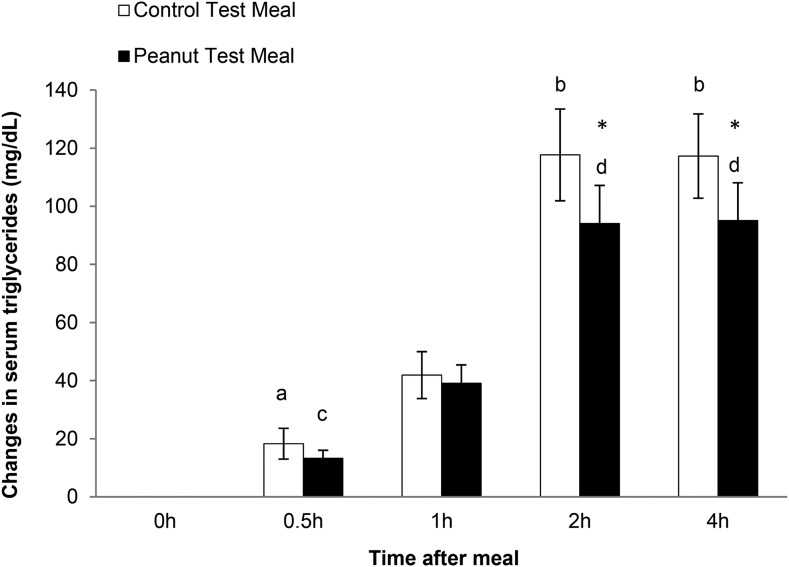
Postprandial glucose, insulin, lipid, and lipoprotein concentrations in response to the test meals are presented in Table 3. A mixed linear model showed a treatment by time interaction for TGs (P < 0.0001). Both control and peanut meals increased postprandial TGs after 60 min of ingestion compared with baseline (P < 0.05 for both treatments). Peak TG responses to the 2 treatments were observed at 120 and 240 min (P < 0.05 for both treatments compared with baseline). At these time points, postprandial changes in plasma TG concentrations were greater (P = 0.033) after the control meal than after the peanut meal (Figure 1). The postprandial changes in plasma TG concentrations were also calculated as iAUCs. There was a 31.9% reduction in the TG iAUC after consumption of the peanut meal compared with the control meal (P = 0.047).
TABLE 3.
Serum glucose, insulin, lipid, and lipoprotein responses to the control and peanut test meals in overweight or obese men1
| Control test meal |
Peanut test meal |
||||||||||
| 0 min | 30 min | 60 min | 120 min | 240 min | 0 min | 30 min | 60 min | 120 min | 240 min | P-meal | |
| Glucose, mg/dL | 88.7 ± 1.2a | 103.2 ± 2.3b | 99.8 ± 4.1b | 88.2 ± 3.9 | 93.4 ± 3.14 | 86.3 ± 1.73c | 103.2 ± 2.3d | 99.8 ± 4.1d | 99.9 ± 3.61d | 88.9 ± 1.9 | 0.38 |
| Insulin, μU/mL | 6.3 ± 0.82a | 30.78 ± 6.24b | 36.7 ± 6.9b | 24.8 ± 4.2b | 13.2 ± 1.1b | 5.2 ± 0.82c | 46.3 ± 8.7d | 35.2 ± 5.9d | 29.4 ± 4.5d | 12.9 ± 1.7 | 0.11 |
| TGs, mmol/L | 82.8 ± 7.4a | 100.5 ± 9.5 | 124.1 ± 12.1 | 197.5 ± 20.7b | 197.3 ± 18.4b | 93.0 ± 11.4c | 106.3 ± 10.5 | 132.1 ± 10.8 | 188.9 ± 19.4d* | 189.9 ± 24.3d* | 0.04 |
| HDL-C, mmol/L | 41.7 ± 2.1 | 42.9 ± 2.1 | 42.7 ± 2.1 | 40.4 ± 2.1 | 38.9 ± 2.1 | 41.1 ± 1.8 | 43.1 ± 2.1 | 41.7 ± 2.1 | 40.1 ± 2.1 | 39.1 ± 2.1 | 0.32 |
| LDL-C, mmol/L | 95.1 ± 6.6 | 98.2 ± 6.7 | 92.3 ± 6.7 | 80.6 ± 6.3 | 82.8 ± 6.9 | 95.1 ± 6.8 | 96.5 ± 7.8 | 92.7 ± 6.8 | 80.9 ± 6.6 | 86.4 ± 8.2 | 0.36 |
| CRP, mg/dL | 1.4 ± 0.4 | 1.4 ± 0.5 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.5 ± 0.4 | 1.7 ± 0.7 | 1.7 ± 0.7 | 1.8 ± 0.7 | 1.7 ± 0.7 | 1.6 ± 0.6 | 0.34 |
| Non-HDL-C, mmol/L | 111.5 ± 7.1 | 118.5 ± 7.2 | 117.1 ± 7.1 | 120.3 ± 6.9 | 122.5 ± 7.2 | 113.8 ± 7.8 | 117.8 ± 8.5 | 119.4 ± 7.6 | 118.9 ± 8.1 | 124.4 ± 8.2 | 0.37 |
| Total cholesterol, mmol/L | 153.1 ± 7.1 | 161.2 ± 7.3 | 159.8 ± 7.1 | 160.5 ± 6.9 | 161.2 ± 7.2 | 154.8 ± 7.7 | 160.8 ± 8.4 | 160.9 ± 7.6 | 158.9 ± 8.1 | 163.6 ± 8.2 | 0.28 |
| TG:HDL-C | 2.09 ± 0.23 | 2.48 ± 0.29 | 3.12 ± 0.42 | 5.25 ± 0.69 | 5.37 ± 0.57 | 2.42 ± 0.35 | 2.66 ± 0.34 | 3.37 ± 0.35 | 5.06 ± 0.58 | 5.30 ± 0.8 | 0.37 |
Values are means ± SEMs. n = 15. *Different from the control meal at that time, P < 0.05. Within-meal: means in a row with different superscript letters differ, P < 0.05. Unlabeled means are intermediate and not significantly different. CRP, C-reactive protein; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
FIGURE 1.
Changes in serum TGs in overweight or obese men in response to control and peanut test meals. Values are means ± SEMs. n = 15. *Different from the control meal at that time, P < 0.05. Within-meal: means with different letters differ, P < 0.05. Unlabeled means are intermediate and not significantly different.
The TG-to-HDL-cholesterol ratio was used as a surrogate marker for insulin resistance (25). At 120 and 240 min, there was a trend for an increased TG-to-HDL-cholesterol ratio in response to the control meal compared with the peanut meal (P = 0.09). There were no treatment, time, or treatment-by-time interactions for TC, LDL cholesterol, or HDL cholesterol. As expected, glucose and insulin increased immediately after shake consumption (effect of time, P < 0.001), but no difference between treatments was observed.
Endothelial function.
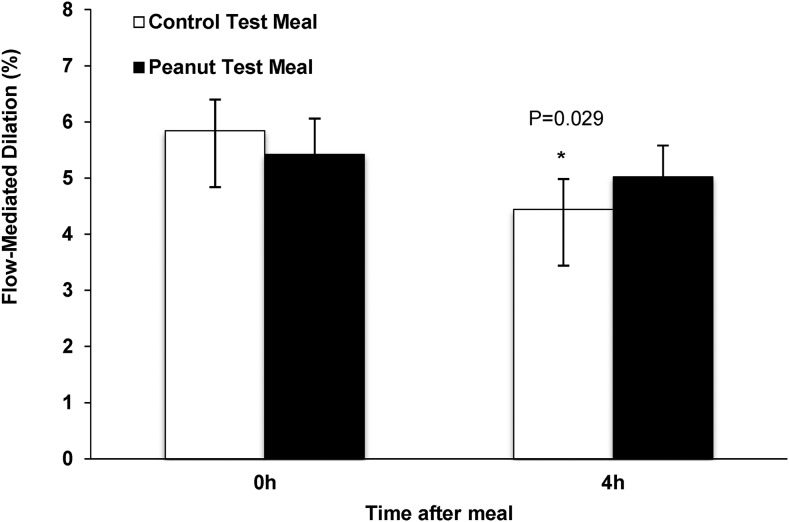
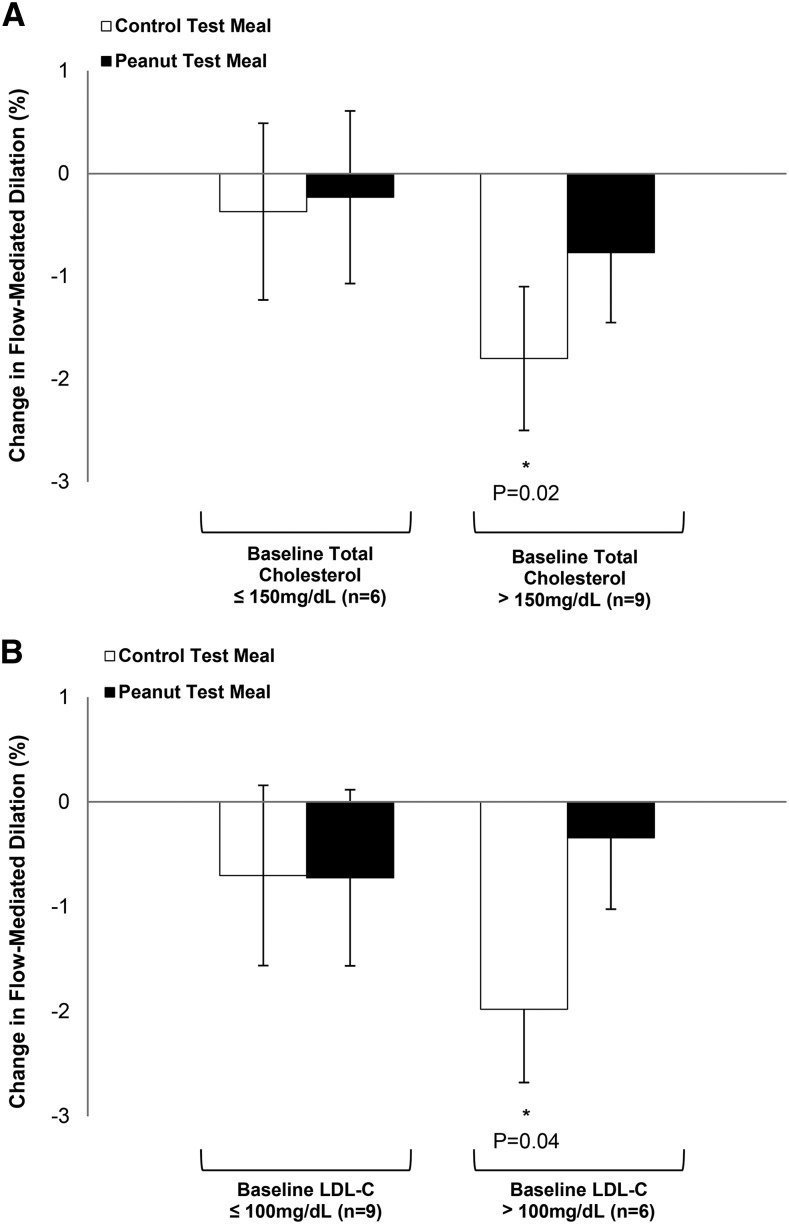
The control shake significantly reduced FMD by 1.2% at 240 min postconsumption compared with baseline (P = 0.029). There were no significant differences in FMD between baseline and 240 min postconsumption of the peanut shake (Figure 2). Baseline (0 min) cholesterol concentration predicted change in FMD. Participants with higher baseline TC concentrations (>150.0 mg/dL; n = 9) showed a significant decrease in FMD (−1.8%; P = 0.017) 240 min after consumption of the control meal compared with baseline (Figure 3A). In contrast, FMD in response to the peanut meal remained unchanged in participants with both lower and higher baseline TC concentrations. Similar results were observed in participants with a higher baseline LDL-cholesterol concentration. Participants (n = 6) with a higher baseline LDL-cholesterol concentration (>100 mg/dL) showed a significant decrease in FMD (−2.0%; P = 0.038) 240 min after the control meal (Figure 3B). Furthermore, the peanut meal did not decrease FMD compared with baseline in participants, irrespective of baseline LDL cholesterol concentration.
FIGURE 2.
FMD responses of overweight or obese men 4 h after the control and peanut test meals. Values are means ± SEMs. n = 15. *Different from 0 h, P < 0.05. FMD, flow-mediated dilatation.
FIGURE 3.
FMD responses of overweight or obese men by baseline TC (A) or LDL cholesterol (B) concentration 4 h after the control and peanut test meals. Values are least-squares means ± SEMs. *Different from 0 min, P < 0.05. FMD, flow-mediated dilatation; LDL-C, LDL cholesterol; TC, total cholesterol.
Discussion
In the present study, we assessed the effects of peanut consumption on metabolic responses and endothelial function in generally healthy overweight or obese men. Acute peanut consumption attenuated postprandial TG responses (31.9% reduction in TG iAUC) compared with a calorie and macronutrient-matched control meal. We showed that acute consumption of 3.0 ounces of peanuts as part of a high-fat meal preserved postprandial endothelial function, particularly in participants with unfavorable baseline lipid profiles. In addition, these findings suggest that the inclusion of peanuts in the context of a high-fat meal may have favorable postprandial cardiometabolic effects.
Most individuals spend the majority of their nonsleeping hours in a postprandial state. Postprandial lipemia, a well-recognized risk factor for atherosclerosis, is associated with impaired endothelial function. During the postprandial period, an increase in TG-rich lipoproteins after a high-fat load increases oxidative stress. This resulting reduced NO bioavailability leads to endothelial dysfunction, which is indicated by a suppressed FMD response (26). Previous studies have shown that increases in postprandial TG-rich lipoproteins after a high-fat meal temporarily impair endothelial function in young (23 ± 2 y) participants (27, 28). In the present study, the consumption of a high-fat control meal significantly decreased endothelial function, whereas it was maintained after the consumption of a macronutrient-matched meal with peanuts, and was accompanied by a blunted postprandial TG response. These findings suggest that peanut consumption preserves endothelial function in the presence of postprandial TG-rich lipoprotein increases, which may be related to a reduction in oxidative stress. We hasten to add that other mechanisms may be involved in mediating this effect, and additional research is needed to clarify this.
This cardioprotective effect may be due to the bioactive compounds in peanuts and peanut skins. For example, NO is an important vasodilator that is synthesized from l-arginine in endothelial cells (29). Peanuts are a rich source of l-arginine, which reflects their high protein content (25% of total energy) (30). Dietary l-arginine could enhance NO synthesis and protect the endothelium against postprandial oxidative damage. Marchesi et al. (24) reported that 10-d administration of a low-dose of l-arginine (6 g/d) attenuated postprandial endothelial dysfunction in young healthy men. Oral l-arginine supplementation (7 g, 3 times/d for 4 wk) improved endothelial dysfunction in hypercholesterolemic young adults (31). In our study, 85 g peanuts were provided to the participants, which provided 2.74 g l-arginine.
In addition to l-arginine, other bioactive compounds in peanuts may offer other cardioprotective effects. Wien et al. (32) reported that the daily consumption of peanuts (46 g/d) over 24 wk improved the nutrient profile of the diet (increased unsaturated fat, α-tocopherol, niacin, and magnesium) and reduced body weight in participants with type 2 diabetes. Each kilogram of weight loss was associated with a 0.11-mmol/L reduction in fasting blood glucose and a 0.07% reduction in glycated hemoglobin (32). Moreover, peanuts (including peanut skins) are a source of phenolic compounds that have high antioxidant capacity, which may prevent endothelial damage by blunting postprandial oxidative reactions associated with a high-fat meal. The phenolic compounds in peanuts and nuts may also contribute to an anti-inflammatory postprandial response (33). There is evidence that postprandial inflammation and oxidative stress can exaggerate postprandial lipemia, which leads to vascular dysfunction and damage (34). Moreira Alves et al. (17) reported that acute consumption of 56 g peanuts (with skins) significantly reduced the postprandial inflammatory biomarkers TNF-α and IL-10 3 h after consumption in overweight men. Thus, the bioactive compounds in peanuts may attenuate inflammation and the resulting oxidative stress (caused by meals high in SFAs), thereby benefiting endothelial health, especially in the postprandial state.
The role of antioxidants and l-arginine in improving FMD and the lipid and lipoprotein profile has been investigated in other nuts. Ros et al. (20) reported that the consumption of a walnut diet (18% of total energy) for 4 wk attenuated endothelial dysfunction in individuals with hypercholesterolemia. Acute consumption of a walnut meal (90 g walnuts) increased postprandial γ-tocopherol and resulted in a lower concentration of malondialdehyde postprandially, suggesting less lipid peroxidation (35). Hudthagosol et al. (36) reported that, after consumption of a pecan test meal (90 g pecans), concentrations of γ-tocopherol doubled and oxidized LDL decreased by 26% at 8 h. Collectively, the findings of these studies indicate that bioactive constituents in nuts contribute to postprandial antioxidant defenses. Although walnuts are the most-studied nuts relative to the evaluation of vascular reactivity, other nuts and peanuts would be expected to have similar benefits.
In the present study, we performed a stratification analysis on the basis of participants’ baseline lipid and lipoprotein profile. Participants with higher baseline cholesterol and LDL-cholesterol concentrations are more prone to endothelial dysfunction after a high-fat meal. We have shown that peanut consumption maintained vascular function in participants who were at greater cardiometabolic risk on the basis of their lipid and lipoprotein profile.
This robust study design directly compared peanut consumption with the consumption of a macronutrient-matched control meal without peanuts. Thus, we were able to evaluate the contribution of the bioactive components in peanuts. In addition, the postprandial design allowed us to characterize the metabolic time course of the endpoints we assessed after peanut consumption. A limitation of this study is the small sample size (n = 15). The results from the present study need to be verified in a larger sample that includes both women and men. In addition, the fat content of the test meal may have been greater than the typical fat content of a Western diet, which may limit the generalizability of the study results. However, it is not uncommon that milkshakes that are high in calories are consumed in the context of a high-fat meal. The use of shakes as a delivery vehicle may somewhat represent the unhealthy diet pattern that may contribute to the postprandial hyperlipidemia responses that have deleterious effects on endothelial function. Postprandial effects also may be more apparent in individuals with diabetes who have impaired glycemic control.
In conclusion, the acute consumption of 3.0 ounces (85 g) of peanuts as a part of a high-fat meal reduced the postprandial TG response and maintained endothelial function in overweight and obese men, particularly in those who were at greater cardiometabolic risk due to elevated baseline TC and LDL-cholesterol concentrations. Therefore, frequent consumption of peanuts may improve CVD risk via mechanisms that involve postprandial cardiometabolic responses, including those that affect vascular function.
Acknowledgments
We thank Ann Skulas-Ray for her contributions to the study design. AMH, SGW, and PMK-E designed the research; XL, RMG, and JAF conducted the research; XL and CEM performed the statistical analysis; and XL and PMK-E wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; FMD, flow-mediated dilatation; iAUC, incremental AUC; TC, total cholesterol.
References
- 1.Ansar S, Koska J, Reaven PD. Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: focus on incretins. Cardiovasc Diabetol 2011;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 2008;300:2142–52. [DOI] [PubMed] [Google Scholar]
- 3.Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 4.Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med 2011;270:65–75. [DOI] [PubMed] [Google Scholar]
- 5.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker P. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309–16. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura K, Miyoshi T, Yunoki K, Ito H. Postprandial hyperlipidemia as a potential residual risk factor. J Cardiol 2016;67:335–9. [DOI] [PubMed] [Google Scholar]
- 7.van Oostrom AJ, Sijmonsma TP, Verseyden C, Jansen EH, de Koning EJ, Rabelink TJ, Castro Cabezas M. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J Lipid Res 2003;44:576–83. [DOI] [PubMed] [Google Scholar]
- 8.Norata GD, Grigore L, Raselli S, Redaelli L, Hamsten A, Maggi F, Eriksson P, Catapano AL. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis 2007;193:321–7. [DOI] [PubMed] [Google Scholar]
- 9.Luu HN, Blot WJ, Xiang YB, Cai H, Hargreaves MK, Li H, Yang G, Signorello L, Gao YT, Zheng W, et al. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern Med 2015;175:755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ 1998;317:1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alper CM, Mattes RD. Effects of chronic peanut consumption on energy balance and hedonics. Int J Obes Relat Metab Disord 2002;26:1129–37. [DOI] [PubMed] [Google Scholar]
- 12.Devitt AA, Kuevi A, Coelho SB, Lartey A, Lokko P, Costa N, Bressan J, Mattes RD. Appetitive and dietary effects of consuming an energy-dense food (peanuts) with or between meals by snackers and nonsnackers. J Nutr Metab 2011;2011:928352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovejoy JC, Most MM, Lefevre M, Greenway FL, Rood JC. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr 2002;76:1000–6. [DOI] [PubMed] [Google Scholar]
- 14.Josse AR, Kendall CW, Augustin LS, Ellis PR, Jenkins DJ. Almonds and postprandial glycemia—a dose-response study. Metabolism 2007;56(3):400–4. [DOI] [PubMed] [Google Scholar]
- 15.Lokko P, Lartey A, Armar-Klemesu M, Mattes RD. Regular peanut consumption improves plasma lipid levels in healthy Ghanaians. Int J Food Sci Nutr 2007;58(3):190–200. [DOI] [PubMed] [Google Scholar]
- 16.Bes-Rastrollo M, Wedick NM, Martinez-Gonzalez MA, Li TY, Sampson L, Hu FB. Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am J Clin Nutr 2009;89:1913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreira Alves RD, Boroni Moreira AP, Macedo VS, Bressan J, de Cassia Goncalves Alfenas R, Mattes R, Brunoro Costa NM. High-oleic peanuts: new perspective to attenuate glucose homeostasis disruption and inflammation related obesity. Obesity (Silver Spring) 2014;22:1981–8. [DOI] [PubMed] [Google Scholar]
- 18.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005;1:183–98. [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care 2010;33(2):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004;109:1609–14. [DOI] [PubMed] [Google Scholar]
- 21.O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol 2008;51:249–55. [DOI] [PubMed] [Google Scholar]
- 22.Johnston CS, Buller AJ. Vinegar and peanut products as complementary foods to reduce postprandial glycemia. J Am Diet Assoc 2005;105(12):1939–42. [DOI] [PubMed] [Google Scholar]
- 23.Reis CE, Ribeiro DN, Costa NM, Bressan J, Alfenas RC, Mattes RD. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: a randomised cross-over clinical trial. Br J Nutr 2013;109:2015–23. [DOI] [PubMed] [Google Scholar]
- 24.Marchesi S, Lupattelli G, Siepi D, Roscini AR, Vaudo G, Sinzinger H, Mannarino E. Oral L-arginine administration attenuates postprandial endothelial dysfunction in young healthy males. J Clin Pharm Ther 2001;26(5):343–9. [DOI] [PubMed] [Google Scholar]
- 25.Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, Pierpont B, Weiss R. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 2011;34:1869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002–12. [DOI] [PubMed] [Google Scholar]
- 27.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 1997;79(3):350–4. [DOI] [PubMed] [Google Scholar]
- 28.Marchesi S, Lupattelli G, Schillaci G, Pirro M, Siepi D, Roscini AR, Pasqualini L, Mannarino E. Impaired flow-mediated vasoactivity during post-prandial phase in young healthy men. Atherosclerosis 2000;153(2):397–402. [DOI] [PubMed] [Google Scholar]
- 29.Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun 1988;153(3):1251–6. [DOI] [PubMed] [Google Scholar]
- 30.Brufau G, Boatella J, Rafecas M.. Nuts: source of energy and macronutrients. Br J Nutr 2006;96(Suppl 2):S24–8. [DOI] [PubMed] [Google Scholar]
- 31.Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest 1996;97:1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wien M, Oda K, Sabate J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr J 2014;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang R, Jacobs DR Jr, Mayer-Davis E, Szklo M, Herrington D, Jenny NS, Kronmal R, Barr RG. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2006;163(3):222–31. [DOI] [PubMed] [Google Scholar]
- 34.Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012;220:22–33. [DOI] [PubMed] [Google Scholar]
- 35.Haddad EH, Gaban-Chong N, Oda K, Sabate J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr J 2014;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudthagosol C, Haddad EH, McCarthy K, Wang P, Oda K, Sabate J. Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. J Nutr 2011;141:56–62. [DOI] [PubMed] [Google Scholar]