Abstract
In contrast to an obsolete notion that erythrocytes, or red blood cells (RBCs), play a passive and minor role in hemostasis and thrombosis, over the past decades there has been increasing evidence that RBCs have biologically and clinically important functions in blood clotting and its disorders. This review summarizes the main mechanisms that underlie the involvement of RBCs in hemostasis and thrombosis in vivo, such as rheological effects on blood viscosity and platelet margination, aggregation and deformability of RBCs; direct adhesion and indirect biochemical interactions with endothelial cells and platelets, etc. The ability of stored and pathologically altered RBCs to generate thrombin through exposure of phosphatidylserine has been emphasized. The procoagulant and prothrombotic potential of RBC-derived microparticles transfused with stored RBCs or formed in various pathological conditions associated with hemolysis has been described along with prothrombotic effects of free hemoglobin and heme. Binding of fibrinogen or fibrin to RBCs may influence their effects on fibrin network structure, clot mechanical properties, and fibrinolytic resistance. Recent data on platelet-driven clot contraction show that RBCs compressed by platelets pulling on fibrin form a tightly packed array of polyhedral erythrocytes, or polyhedrocytes, which comprises a nearly impermeable barrier important for hemostasis and wound healing. RBCs may perform dual roles, both helping to stem bleeding but at the same time contributing to thrombosis in a variety of ways.
Introduction
Until recently, little attention has been paid to the potential involvement of erythrocytes, or red blood cells (RBCs), in hemostasis and thrombosis. Moreover, most scientists and clinicians have assumed that they play a largely passive and relatively unimportant role. However, in the past few decades there has been increasing evidence that RBCs have a variety of active functions in hemostasis and thrombosis that are significant and need to be taken into account in assessing health and disease. This review will summarize the main mechanisms that underlie the involvement of RBCs in blood coagulation and its disorders, including the effects of stored and transfused RBCs.
Indirect evidence for the influence of RBCs on hemostasis and thrombosis
More than a hundred years ago it was reported that in anemia patients the bleeding time was prolonged irrespective of the platelet count [1]. Fifty years later a more general correlation between low hematocrit and prolonged bleeding times was found, including correction of the hemostatic parameters by blood transfusion [2]. It was also established that many bleeding disorders can be treated by an increase of RBC count despite low or normal platelet levels [3]. On the other hand, an abnormally high hematocrit is associated with thrombosis, and patients with polycythemia vera or taking erythropoietin are more susceptible to thrombosis and thromboembolism [4, 5]. These and other observations have provided indirect but strong evidence that RBCs are important players in hemostasis and thrombosis and can act as a procoagulant and prothrombotic blood component.
Rheological effect of RBCs
High hematocrit results in an increase in blood viscosity that impedes the blood flow [6, 7]. These hemorheological effects of RBCs can be a strong prothrombotic factor since the impaired blood flow is a component of Virchow’s triad that explains the pathophysiological mechanisms of thrombosis by a combination of hypercoagulability, disturbance of blood flow, and endothelial damage [8]. The hematocrit-related blood viscosity may have physical effects on the interaction between platelets and blood vessel surfaces. Under flow conditions, platelet adhesion increases greatly with hematocrit. Thus the volume fraction of red cells may have a significant impact on hemostasis and thrombosis, with the nature of the effect related to the flow conditions [9].
A remarkable rheological effect of RBCs that affects platelets in hemostasis and thrombosis is that RBCs preferentially move down the center of blood vessel, causing margination of platelets, so that they are poised to adhere preferentially to the site of vessel-wall injury [10]. In addition to platelets, the peripheral layer formed by the axial accumulation of RBCs contains plasma (with clotting factors) and neutrophils. Formation of the RBC-free layer next to the endothelial lining changes local viscosity, such that the viscosity gets smaller with decreasing vessel diameter (known as the Fahraeus–Lindqvist effect), down to 5–7 µm. In capillaries that are smaller than RBCs, the viscosity of the RBC-free layer increases due to the presence of platelets that have a greater viscosity than RBCs [11]. In the presence of RBCs, the distribution of platelets is changed by a few orders of magnitude compared to uniform Brownian diffusivity, resulting in a 3–8× platelet accumulation near the vessel wall [12, 13]. An elevated hematocrit predisposes one to platelet interactions with the activated endothelium, thus promoting hemostasis or thrombosis [14]. Another consequence of the axial RBC accumulation followed by reduction in local viscosity is a decrease of the wall shear stress causing a lesser local release of nitric oxide [15]. Because nitric oxide is known to prevent activation of endothelial cells and platelets, this nitric oxide deficiency promotes cellular activation.
RBC aggregation
At low shear rates or with stasis of blood, RBCs tend to form linear arrays of stacked cells (roleaux) or three-dimensional aggregates [16]. Such aggregates are difficult to disperse and they tend to increase the blood viscosity and hydrodynamic resistance in larger blood vessels with low shear, such as the veins in the lower limbs. RBC aggregation promotes thrombosis in veins, confirming the pathogenic importance of locally altered blood rheology in the development of venous thrombosis [17].
RBC deformability
RBCs are remarkably deformable, which is important to minimize their resistance to flow and to allow them to fit through blood vessels smaller than their size. The great deformability of RBCs is primarily a consequence of their biconcave shape, specifically the high surface area to volume ratio. More rigid RBCs may be less able to squeeze through the capillaries and they also increase platelet margination described above, both of which increase the susceptibility to thrombosis [18]. Increased rigidity can be caused by either a decrease in membrane deformability, determined primarily by the cytoskeleton and the cellular metabolic energy, or the cytoplasmic viscosity, determined mainly by the hemoglobin concentration [19].
A major, clinically significant feature of some inherited diseases is RBCs with reduced deformability. RBCs of patients with sickle cell disease have membranes that are stiffer than that of normal cells [20, 21]. In addition, the cells themselves become strikingly stiffer when the mutated hemoglobin polymerizes inside and sickles the cells [22]. Other diseases, including β-thalassemia, hemolytic anemias caused by RBC antibodies, and hereditary stomatocytosis, also commonly have RBCs with stiff membranes [23]. Some diseases, such as diabetes, hypertension, lower limb vein thrombosis, coronary heart disease, can secondarily alter the properties of RBCs, making them stiffer and prothrombotic [24]. Decreased RBC deformability reduces permeability of blood clots and thrombi, which may have implications for the penetration of fibrinolytic agents [25]. Stored RBCs exhibit altered biophysical characteristics, including higher cell rigidity that accounts in part for impaired blood flow hemodynamics and adverse effects of RBC transfusion [26].
Interaction of RBCs with platelets
RBCs can modulate platelet reactivity directly through either chemical signaling or adhesive RBC-platelet interactions. RBCs promote platelet aggregation and degranulation by releasing ATP and ADP under low pO2, low pH and in response to mechanical deformation [27, 28]. Another mechanism for platelet activation by RBC lysate is extracellular hemoglobin, which enhances platelet activation by lowering NO bioavailability [29]. Cell-free hemoglobin acts as a strong NO scavenger, preventing NO-mediated suppression of activated platelets [30]. Damaged RBCs also release arginase that cleaves L-arginine, a substrate for NO production [29]. In acute coronary syndrome, RBC transfusion increases platelet reactivity [31]. In the presence of RBCs, platelets are less responsive to aspirin, even when synthesis of thromboxane A2 is inhibited [32]. When RBCs are damaged by high shear in continuous flow ventricular assist devices, free hemoglobin induces platelet aggregation, contributing to high risk of thrombotic complications [33].
RBCs can play a role in thrombus formation under flowing conditions at venous shear rates by direct adhesive interactions with platelets [34, 35]. The RBC-platelet adhesive interaction may be important in pathological conditions associated with a high incidence of thrombosis, such as thalassemia [36] or sickle cell disease [37]. Interestingly, in the widely used ferric chloride in vivo model of thrombosis, platelets bind to the wall-associated RBC-derived material rather than the endothelium [38].
Interactions of RBCs with the endothelium
There is increasing evidence that RBCs can be incorporated into thrombi via specific interactions with activated endothelial cells and/or exposed sub-endothelial matrix. Normal mature RBCs do not interact with endothelium but they become highly sticky under certain pathological conditions, and this adhesion of abnormal and/or stimulated RBCs to vascular endothelium can contribute substantially to microvascular occlusions associated with thrombosis. The most common pathological states in which RBCs interact with the endothelium include sickle cell disease [39], malaria [40], and diabetes [41]. Structurally and metabolically altered RBCs, which are present in higher numbers in RBCs that have been stored longer, have greater strength of adhesion to the endothelium [26].
Phosphatidylserine exposure in RBC membrane
Efficient blood coagulation requires sufficient prothrombotic surfaces for the proper assembly of the prothrombinase complex and generation of thrombin to initiate clotting. These surfaces are provided by cells that expose phosphatidylserine, a negatively charged phospholipid, which is normally on the cytoplasmic side of the membrane to separate this procoagulant surface from plasma coagulation factors [42]. Much of the focus on exposure of phosphatidylserine in coagulation has been on activated platelets, but recently it has been shown that RBCs also are involved. Under conditions of apoptosis or RBC damage, such as high shear rates, inflammation, or oxidative stress, RBCs can lose membrane asymmetry and expose phosphatidylserine [43]. Phosphatidylserine externalization and shedding are mediated by increased cellular Ca-flux and play an important role in natural RBC senescence [44]. Because of the large numbers of RBCs present in the blood, even a small fraction of RBCs with phosphatidylserine exposure can result in prothrombotic conditions. Even in healthy individuals about 0.5–0.6% of the RBC population expresses phosphatidylserine and provides an active surface for prothrombin activation. This subpopulation of RBCs might account for up to 40% of the thrombin-generating potential of whole blood [45].
Some remarkable examples of phosphatidylserine exposure in RBC membranes are sickle cell disease and thalassemia [46, 47]. The abnormal phosphatidylserine exposure in sickle cell disease is thought to result from the repeated cell sickling and unsickling that are linked to polymerization and depolymerization of mutated hemoglobin [48]. An increase in RBC phosphatidylserine exposure in β-thalassemia patients has been shown to be connected with eryptosis, the suicidal death of RBCs [49].
RBC-derived microparticles
Activation, aging, and apoptosis of various cells, including RBCs, are accompanied by formation of microscopic extracellular membranous structures named microvesicles or microparticles (MPs). The ability of cells to generate MPs in vivo is an important regulatory mechanism of physiologic reactions, a means for intercellular communications and a pathogenic component in many diseases that impact hemostasis and thrombosis [50, 51]. Formation of RBC-derived MPs is typical during the ex vivo storage of whole blood [52] and accumulation of MPs is thought to be responsible for an increased incidence of deep vein thrombosis after transfusion of “old” red cells [53]. An increase in the number of circulating RBC-derived MPs has been found in RBC-related prothrombotic states, such as sickle cell disease and hemolytic anemia [54]. Irrespective of their source, elevated plasma levels of MPs are associated with a reduced clotting time and a dose- and time-dependent increase in thrombin generation, suggesting that the MPs enhance hypercoagulability.
The ability of RBC-derived MPs to enhance thrombin generation has been associated with expression of phosphatidylserine [55] and possibly also tissue factor [56]. RBC-derived MPs are capable of activating coagulation by other clotting factors or supporting anticoagulant reactions. The circulating MPs can internalize free heme and transfer it to vascular endothelium, promoting vaso-occlusion, or amplify systemic inflammation via thrombin-mediated activation of the complement system [57]. Given the broad procoagulant activity of RBC-derived MPs, they are considered a potential agent for treatment of hemostatic disorders [58].
RBC storage
Stored RBCs undergo a complex structural and metabolic impairment that includes leakage of hemoglobin from the cells and hemolysis, reduced energy and NO production, formation of toxic products, such as lysophospholipids and free iron, phosphatidylserine exposure and shedding MPs [59]. All these and other changes that occur to RBCs during storage make infusions of RBCs a procedure with frequent side effects and complications, including an increased incidence of deep vein thrombosis [53]. During the storage of RBCs, the MP concentration increases and the number of those that express phosphatidylserine also increases [60], which represents a mechanism by which stored RBCs could promote thrombotic complications after infusion. Another potential mechanism that underlies deleterious effects upon RBC infusion is NO scavenging by hemoglobin released from RBCs during storage [61].
RBC destruction (hemolysis)
More or less massive in vivo hemolysis with release of free, extracellular hemoglobin into the blood is the pathogenic basis of hereditary and acquired hemolytic anemias of various etiologies, of which immune hemolysis is the most common. These conditions are accompanied by (pro)thrombotic disorders that vary from laboratory signs of hypercoagulability to life-threatening complications, such as disseminated intravascular coagulation [62] and venous thromboembolism [63]. There are several pathogenic mechanisms by which hemolysis may lead to intravascular coagulation. First, damaged RBCs release free hemoglobin and heme that are toxic to many cells and tissues. Extracellular hemoglobin sequesters NO and thus promotes activation of endothelial cells and adhesion/aggregation of platelets [64]. Free heme upregulates heme oxygenase activity, generates reactive oxygen species, and activates endothelial cells and macrophages directly [65]. Second, immune hemolysis is accompanied by production of TNF-α which induces tissue factor expression in endothelial cells and also decreases the endothelial expression of thrombomodulin, a potent modulator of thrombin activity [62]. Third, hemolysis results in a massive release of procoagulant RBC-derived MPs [66].
Interactions of RBCs and fibrinogen
The tendency of RBCs to form roleaux under low shear conditions requires fibrinogen [67]. An increase of fibrinogen concentration can result in greater RBC aggregation, which is associated with a higher incidence of thrombosis. Such RBC aggregation has generally been considered to be caused by the nonspecific binding of fibrinogen to RBC membrane. However, there is now some evidence for the existence of specific interactions between fibrinogen and an integrin receptor on the RBC membrane [68], either a β3 integrin [69] or CD47 (integrin-associated protein) [70] or both. Interestingly, RBCs from a Glanzmann thrombastenia patient (a rare hereditary bleeding disease caused by αIIbβ3 mutation) show impaired fibrinogen binding [69]. The probability of binding interactions of RBC and fibrinogen progressively decrease with in vivo cell aging, likely associated with the loss of sialic acid on older RBCs [71]. The administration of fibrinogen concentrate, which is critical for the formation of a fibrin clot in the perioperative setting and in massive hemorrhage, may include interaction with RBCs followed by hemostatic effects of RBC aggregates.
Effects of RBCs on clot structure
The presence of RBCs affects the structure of fibrin clots. Intermediate RBC concentrations cause heterogeneity in the fiber network with pockets of densely packed fibers alongside regions with few fibers [72]. With high levels of RBCs, fibers are arranged more uniformly but loosely around the cells. There is also a significant increase in fiber diameter upon RBC incorporation and the viscoelastic properties of the clot are influenced. Besides the effects of intact RBCs, free extracellular hemoglobin prolongs clotting time of fibrinogen due to impaired polymerization [73]. Therefore, intact or damaged RBCs trigger variability in fibrin network structure, individual fiber characteristics, and overall clot viscoelasticity, which has important implications for in vivo clot formation, maturation, stability, embolization, and the efficacy of prophylactic anticoagulation and therapeutic fibrinolysis [72, 74]. It has been shown that RBC retention within clots determines thrombus size dependent on factor XIIIa activity [75], a plasma transglutaminase that crosslinks fibrin polymer covalently increasing its mechanical stability, via crosslinking of the fibrin α chains [76]. RBCs are incorporated into all types of clots and thrombi formed in whole blood, both in vitro and in vivo, either venous [77] or arterial [31].
RBCs and fibrinolysis
RBCs are an important component of the complex reactions in clot formation and thus determine the ultimate physical and biological properties of fibrin, which affect profoundly the course of its dissolution [78]. As a generality, incorporation of RBCs increases the lytic resistance and decreases the permeability of fibrin in a dose-dependent manner [79, 80]. In addition, the RBC-induced retardation of fibrinolysis correlates with mechanical stabilization and strengthening of fibrin clots, which was shown for thrombi in experimental cerebral ischemia [81].
RBCs in clot contraction
Clot contraction, or retraction, has been proposed to be involved in hemostasis to form a tighter seal to stem bleeding, to pull clots or thrombi closer to the vessel wall so that they are less obstructive, and in wound healing. Clot contraction requires platelets and fibrin or fibrinogen. Non-muscle myosin IIa inside the platelet interacts with actin filaments attached to the cell membrane integrin αIIbβ3 via talin and kindlin. Fibrin or fibrinogen binds to αIIbβ3 outside the platelet to link other platelets [82, 83].
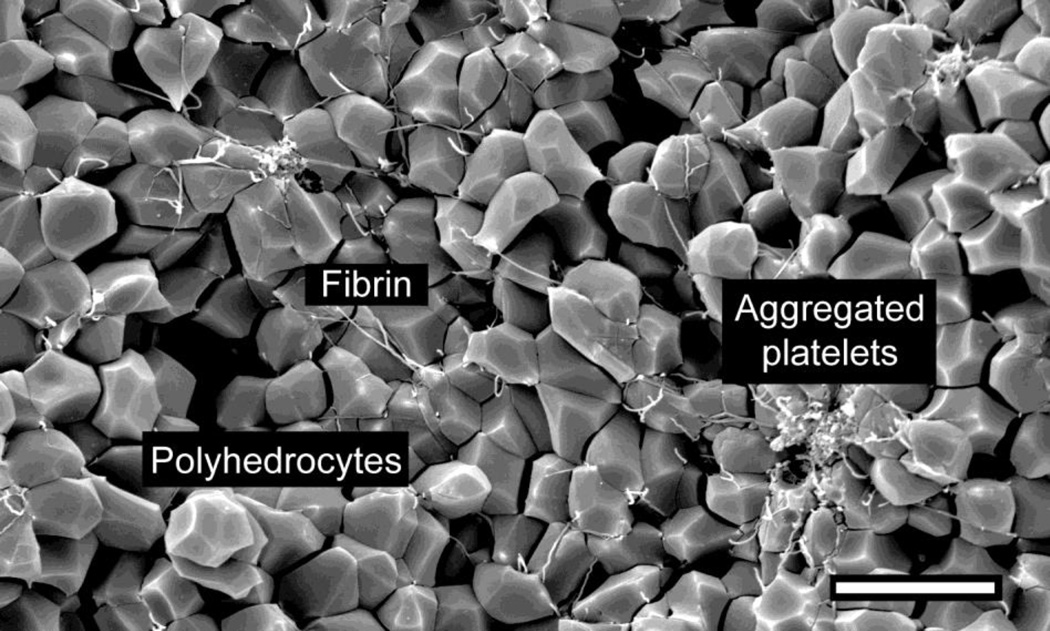
Contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes, named polyhedrocytes, within (Fig. 1). Platelets (with their cytoskeletal motility proteins) and fibrin(ogen) (as the substrate bridging platelets for contraction) are required to generate the forces necessary to segregate platelets/fibrin from RBCs and to compress these cells into a tightly packed array [84].
Figure 1. The structure of contracted whole blood clot.
Scanning electron micrograph of the interior of a contracted whole blood clot activated by thrombin following recalcification. The red blood cells are compressed by platelets pulling on fibrin to change shape from biconcave to polyhedral, and are tightly packed; hence they are named polyhedrocytes. A few fibrin strands and platelet aggregates are visible, but most of the platelets and fibrin are on the exterior of the contracted clot. Magnification bar = 10 µm.
The structure and properties of contracted clots and the kinetics of contraction vary depending on the relative amounts of platelets, fibrinogen and RBCs and the conditions of clotting. Clot contraction occurs in three sequential phases, each characterized by a distinct rate constant. Thrombin, calcium ions, the integrin αIIbβ3, non-muscle myosin IIa, factor XIIIa crosslinking, and platelet count all promote one or more phases of the clot contraction process. In contrast, RBCs impair contraction and reduce stiffness, while increasing the overall contractile stress generated by the platelet-fibrin meshwork [75, 76, 85].
Polyhedrocytes are the major component of venous clots, demonstrating that clot contraction occurs in vivo and suggesting that polyhedrocytes may play a role in hemostasis, at least on the venous side. Polyhedrocytes have also been observed in human arterial and especially venous thrombi, and pulmonary emboli, taken from patients [84, 86]. Moreover, the kinetics of contraction and extent of contraction can be different in patients with sickle cell disease, ischemic stroke, and deep vein thrombosis [87]. Such experiments suggest that the extent of clot contraction and the prevalence of polyhedrocytes may be associated with thrombosis and could be a marker of prothrombotic conditions.
Conclusions
The best-known effects of RBCs in clotting in vivo are rheological, involving laminar shearing with platelet margination plus aggregation and deformability of RBCs. In addition, RBCs interact directly and indirectly with endothelial cells and platelets, which may be involved in thrombosis. Both the stiffness of RBCs and the extent to which they form a procoagulant surface to generate thrombin through exposure of phosphatidylserine appear to play an important role. RBC-derived MPs transfused with stored RBCs or formed in various pathological conditions associated with hemolysis have strong procoagulant potential along with prothrombotic effects of the extracellular hemoglobin and heme. RBCs directly interact with fibrin(ogen) and affect the structure, mechanical properties, and lytic resistance of clots and thrombi. Finally, the results on clot contraction demonstrate how contracted clots form an impermeable barrier made of tessellated polyhedral RBCs (polyhedrocytes) important for hemostasis and wound healing and to restore flow past obstructive thrombi. In summary, RBCs may perform a dual role, both helping to stem bleeding but at the same time contributing to thrombosis in several ways.
Acknowledgments
Funding:
Some of the authors’ work mentioned here was supported by NIH grants NHLBI HL090774 and UO1HL116330, and NSF grant DMR1505662.
References
- 1.Duke WW. The relation of blood platelets to hemorrhagic disease. JAMA. 1910;60:1185–1192. [PubMed] [Google Scholar]
- 2.Hellem AJ, Borchgrevink CF, Ames SB. The role of red cells in haemostasis: the relation between haematocrit, bleeding time and platelet adhesiveness. Br J Haematol. 1961;7:42–50. doi: 10.1111/j.1365-2141.1961.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 3.Weiss HJ, Lages B, Hoffmann T, et al. Correction of the platelet adhesion defect in delta-storage pool deficiency at elevated hematocrit--possible role of adenosine diphosphate. Blood. 1996;87:4214–4222. [PubMed] [Google Scholar]
- 4.Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29:215–221. doi: 10.1016/j.blre.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 6.Andrews DA, Low PS. Role of red blood cells in thrombosis. Curr Opin Hematol. 1999;6:76–82. doi: 10.1097/00062752-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol. 1992;263:H1770–H1778. doi: 10.1152/ajpheart.1992.263.6.H1770. [DOI] [PubMed] [Google Scholar]
- 8.Wolberg AS, Aleman MM, Leiderman K, et al. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth Analg. 2012;114:275–285. doi: 10.1213/ANE.0b013e31823a088c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207:541–543. doi: 10.1126/science.7352265. [DOI] [PubMed] [Google Scholar]
- 10.Flamm MH, Diamond SL. Multiscale systems biology and physics of thrombosis under flow. Ann Biomed Eng. 2012;40:2355–2364. doi: 10.1007/s10439-012-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dintenfass L. Inversion of the Fahraeus-Lindqvist phenomenon in blood flow through capillaries of diminishing radius. Nature. 1967;215:1099–1100. doi: 10.1038/2151099a0. [DOI] [PubMed] [Google Scholar]
- 12.Aarts PA, van den Broek SA, Prins GW, et al. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis. 1988;8:819–824. doi: 10.1161/01.atv.8.6.819. [DOI] [PubMed] [Google Scholar]
- 13.Yeh C, Eckstein EC. Transient lateral transport of platelet-sized particles in flowing blood suspensions. Biophys J. 1994;66:1706–1716. doi: 10.1016/S0006-3495(94)80962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsmith HL, Bell DN, Braovac S, et al. Physical and chemical effects of red cells in the shear-induced aggregation of human platelets. Biophys J. 1995;69:1584–1595. doi: 10.1016/S0006-3495(95)80031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baskurt OK, Yalcin O, Ozdem S, et al. Modulation of endothelial nitric oxide synthase expression by red blood cell aggregation. Am J Physiol Heart Circ Physiol. 2004;286:H222–H229. doi: 10.1152/ajpheart.00532.2003. [DOI] [PubMed] [Google Scholar]
- 16.Baumler H, Neu B, Donath E, et al. Basic phenomena of red blood cell rouleaux formation. Biorheology. 1999;36:439–442. [PubMed] [Google Scholar]
- 17.Yu FT, Armstrong JK, Tripette J, et al. A local increase in red blood cell aggregation can trigger deep vein thrombosis: evidence based on quantitative cellular ultrasound imaging. J Thromb Haemost. 2011;9:481–488. doi: 10.1111/j.1538-7836.2010.04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aarts PA, Banga JD, van Houwelingen HC, et al. Increased red blood cell deformability due to isoxsuprine administration decreases platelet adherence in a perfusion chamber: a double-blind cross-over study in patients with intermittent claudication. Blood. 1986;67:1474–1481. [PubMed] [Google Scholar]
- 19.Du VX, Huskens D, Maas C, et al. New insights into the role of erythrocytes in thrombus formation. Semin Thromb Hemost. 2014;40:72–80. doi: 10.1055/s-0033-1363470. [DOI] [PubMed] [Google Scholar]
- 20.Alapan Y, Little JA, Gurkan UA. Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Sci Rep. 2014;4:7173. doi: 10.1038/srep07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barabino GA, Platt MO, Kaul DK. Sickle cell biomechanics. Annu Rev Biomed Eng. 2010;12:345–367. doi: 10.1146/annurev-bioeng-070909-105339. [DOI] [PubMed] [Google Scholar]
- 22.Friederichs E, Meiselman HJ. Effects of calcium permeabilization on RBC rheologic behavior. Biorheology. 1994;31:207–215. doi: 10.3233/bir-1994-31208. [DOI] [PubMed] [Google Scholar]
- 23.Vaya A, Suescun M, Hernandez JL, et al. Rheological red blood cell behaviour in minor alpha-thalassaemia carriers. Clin Hemorheol Microcirc. 2011;48:241–246. doi: 10.3233/CH-2011-1416. [DOI] [PubMed] [Google Scholar]
- 24.Symeonidis A, Athanassiou G, Psiroyannis A, et al. Impairment of erythrocyte viscoelasticity is correlated with levels of glycosylated haemoglobin in diabetic patients. Clin Lab Haematol. 2001;23:103–109. doi: 10.1046/j.1365-2257.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 25.van Gelder JM, Nair CH, Dhall DP. Erythrocyte aggregation and erythrocyte deformability modify the permeability of erythrocyte enriched fibrin network. Thromb Res. 1996;82:33–42. doi: 10.1016/0049-3848(96)00048-5. [DOI] [PubMed] [Google Scholar]
- 26.Sparrow RL. Red blood cell storage duration and trauma. Transfus Med Rev. 2015;29:120–126. doi: 10.1016/j.tmrv.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Reimers RC, Sutera SP, Joist JH. Potentiation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood. 1984;64:1200–1206. [PubMed] [Google Scholar]
- 28.Sprague RS, Ellsworth ML, Stephenson AH, et al. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 29.Helms CC, Marvel M, Zhao W, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013;11:2148–2154. doi: 10.1111/jth.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gambaryan S, Subramanian H, Kehrer L, et al. Erythrocytes do not activate purified and platelet soluble guanylate cyclases even in conditions favourable for NO synthesis. Cell Commun Signal. 2016;14:16. doi: 10.1186/s12964-016-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvain J, Abtan J, Kerneis M, et al. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study (impact of transfusion of red blood cell on platelet activation and aggregation studied with flow cytometry use and light transmission aggregometry) J Am Coll Cardiol. 2014;63:1289–1296. doi: 10.1016/j.jacc.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Santos MT, Valles J, Aznar J, et al. Prothrombotic effects of erythrocytes on platelet reactivity. Reduction by aspirin. Circulation. 1997;95:63–68. doi: 10.1161/01.cir.95.1.63. [DOI] [PubMed] [Google Scholar]
- 33.Tran PL, Pietropaolo MG, Valerio L, et al. Hemolysate-mediated platelet aggregation: an additional risk mechanism contributing to thrombosis of continuous flow ventricular assist devices. Perfusion. 2016;31:401–408. doi: 10.1177/0267659115615206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermand P, Gane P, Huet M, et al. Red cell ICAM-4 is a novel ligand for platelet-activated alpha IIbbeta 3 integrin. J Biol Chem. 2003;278:4892–4898. doi: 10.1074/jbc.M211282200. [DOI] [PubMed] [Google Scholar]
- 35.Goel MS, Diamond SL. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood. 2002;100:3797–3803. doi: 10.1182/blood-2002-03-0712. [DOI] [PubMed] [Google Scholar]
- 36.Sirachainan N. Thalassemia and the hypercoagulable state. Thromb Res. 2013;132:637–641. doi: 10.1016/j.thromres.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 37.White J, Lancelot M, Sarnaik S, et al. Increased erythrocyte adhesion to VCAM-1 during pulsatile flow: Application of a microfluidic flow adhesion bioassay. Clin Hemorheol Microcirc. 2015;60:201–213. doi: 10.3233/CH-141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr JD, Chauhan AK, Schaeffer GV, et al. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121:3733–3741. doi: 10.1182/blood-2012-11-468983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaul DK, Finnegan E, Barabino GA. Sickle red cell-endothelium interactions. Microcirculation. 2009;16:97–111. doi: 10.1080/10739680802279394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JD, Rowe JA, Higgins MK, et al. Malaria's deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013;15:1976–1983. doi: 10.1111/cmi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossin N, Wautier MP, Wautier JL. Red blood cell adhesion in diabetes mellitus is mediated by advanced glycation end product receptor and is modulated by nitric oxide. Biorheology. 2009;46:63–72. doi: 10.3233/BIR-2009-0519. [DOI] [PubMed] [Google Scholar]
- 42.Schroit AJ, Zwaal RF. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim Biophys Acta. 1991;1071:313–329. doi: 10.1016/0304-4157(91)90019-s. [DOI] [PubMed] [Google Scholar]
- 43.Shi J, Shi Y, Waehrens LN, et al. Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death. Cytometry A. 2006;69:1193–1201. doi: 10.1002/cyto.a.20345. [DOI] [PubMed] [Google Scholar]
- 44.Freikman I, Fibach E. Distribution and shedding of the membrane phosphatidylserine during maturation and aging of erythroid cells. Biochim Biophys Acta. 2011;1808:2773–2780. doi: 10.1016/j.bbamem.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Whelihan MF, Zachary V, Orfeo T, et al. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–3845. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ataga KI, Cappellini MD, Rachmilewitz EA. Beta-thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. Br J Haematol. 2007;139:3–13. doi: 10.1111/j.1365-2141.2007.06740.x. [DOI] [PubMed] [Google Scholar]
- 47.Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: Relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 2016;30:245–256. doi: 10.1016/j.blre.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franck PF, Bevers EM, Lubin BH, et al. Uncoupling of the membrane skeleton from the lipid bilayer. The cause of accelerated phospholipid flip-flop leading to an enhanced procoagulant activity of sickled cells. J Clin Invest. 1985;75:183–190. doi: 10.1172/JCI111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ibrahim HA, Fouda MI, Yahya RS, et al. Erythrocyte phosphatidylserine exposure in beta-thalassemia. Lab Hematol. 2014;20:9–14. doi: 10.1532/LH96.12016. [DOI] [PubMed] [Google Scholar]
- 50.Brill A, Dashevsky O, Rivo J, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 52.Rubin O, Crettaz D, Canellini G, et al. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang. 2008;95:288–297. doi: 10.1111/j.1423-0410.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 53.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 54.van Beers EJ, Schaap MC, Berckmans RJ, et al. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–1519. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 56.Rubin O, Crettaz D, Tissot JD, et al. Microparticles in stored red blood cells: submicron clotting bombs? Blood Transfus. 2010;8(Suppl 3):s31–s38. doi: 10.2450/2010.006S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol. 2014;34:313–320. doi: 10.1161/ATVBAHA.113.302378. [DOI] [PubMed] [Google Scholar]
- 58.Jy W, Johansen ME, Bidot C, Jr, et al. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb Haemost. 2013;110:751–760. doi: 10.1160/TH12-12-0941. [DOI] [PubMed] [Google Scholar]
- 59.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 60.Gao Y, Lv L, Liu S, et al. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang. 2013;105:11–17. doi: 10.1111/vox.12014. [DOI] [PubMed] [Google Scholar]
- 61.Liu C, Liu X, Janes J, et al. Mechanism of faster NO scavenging by older stored red blood cells. Redox Biol. 2014;2:211–219. doi: 10.1016/j.redox.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davenport RD. Pathophysiology of hemolytic transfusion reactions. Semin Hematol. 2005;42:165–168. doi: 10.1053/j.seminhematol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Thachil J. Autoimmune haemolytic anaemia--an under-recognized risk factor for venous thromboembolism. Transfus Med. 2008;18:377–378. doi: 10.1111/j.1365-3148.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 64.Rother RP, Bell L, Hillmen P, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 65.Camus SM, De Moraes JA, Bonnin P, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125:3805–3814. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, et al. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10:1355–1362. doi: 10.1111/j.1538-7836.2012.04758.x. [DOI] [PubMed] [Google Scholar]
- 67.Fahraeus R. The suspension stability of the blood. Physiol. Rev. 1929;9:241–274. [Google Scholar]
- 68.Lominadze D, Dean WL. Involvement of fibrinogen specific binding in erythrocyte aggregation. FEBS Lett. 2002;517:41–44. doi: 10.1016/s0014-5793(02)02575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carvalho FA, Connell S, Miltenberger-Miltenyi G, et al. Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano. 2010;4:4609–4620. doi: 10.1021/nn1009648. [DOI] [PubMed] [Google Scholar]
- 70.De Oliveira S, Vitorino de Almeida V, Calado A, et al. Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim Biophys Acta. 2012;1818:481–490. doi: 10.1016/j.bbamem.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 71.Carvalho FA, de Oliveira S, Freitas T, et al. Variations on fibrinogen-erythrocyte interactions during cell aging. PLoS One. 2011;6:e18167. doi: 10.1371/journal.pone.0018167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–1175. doi: 10.1160/TH09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brezniak DV, Moon DG, Beaver JA, et al. Haemoglobin inhibition of fibrin polymerization and clotting. Blood Coagul Fibrinolysis. 1994;5:139–143. doi: 10.1097/00001721-199402000-00016. [DOI] [PubMed] [Google Scholar]
- 74.Carr ME, Jr, Hardin CL. Fibrin has larger pores when formed in the presence of erythrocytes. Am J Physiol. 1987;253:H1069–H1073. doi: 10.1152/ajpheart.1987.253.5.H1069. [DOI] [PubMed] [Google Scholar]
- 75.Aleman MM, Byrnes JR, Wang JG, et al. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124:3590–3600. doi: 10.1172/JCI75386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Byrnes JR, Duval C, Wang Y, et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin alpha-chain crosslinking. Blood. 2015;126:1940–1948. doi: 10.1182/blood-2015-06-652263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tratar G, Blinc A, Podbregar M, et al. Characterization of pulmonary emboli ex vivo by magnetic resonance imaging and ultrasound. Thromb Res. 2007;120:763–771. doi: 10.1016/j.thromres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 78.Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–180. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- 79.Wohner N, Sotonyi P, Machovich R, et al. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol. 2011;31:2306–2313. doi: 10.1161/ATVBAHA.111.229088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varin R, Mirshahi S, Mirshahi P, et al. Whole blood clots are more resistant to lysis than plasma clots--greater efficacy of rivaroxaban. Thromb Res. 2013;131:e100–e109. doi: 10.1016/j.thromres.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 81.van der Spuy WJ, Pretorius E. Interaction of red blood cells adjacent to and within a thrombus in experimental cerebral ischaemia. Thromb Res. 2013;132:718–723. doi: 10.1016/j.thromres.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 82.Lam WA, Chaudhuri O, Crow A, et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10:61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Litvinov RI, Weisel JW. What Is the Biological and Clinical Relevance of Fibrin? Semin Thromb Hemost. 2016;42:333–343. doi: 10.1055/s-0036-1571342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cines DB, Lebedeva T, Nagaswami C, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123:1596–1603. doi: 10.1182/blood-2013-08-523860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tutwiler V, Litvinov RI, Lozhkin AP, et al. Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood. 2016;127:149–159. doi: 10.1182/blood-2015-05-647560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zabczyk M, Sadowski M, Zalewski J, et al. Polyhedrocytes in intracoronary thrombi from patients with ST-elevation myocardial infarction. Int J Cardiol. 2015;179:186–187. doi: 10.1016/j.ijcard.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Peshkova AD, Malyasev DV, Bredikhin RA, et al. Contraction of blood clots is impaired in deep vein thrombosis. BioNanoScience. 2016 [Google Scholar]