Abstract
Nivolumab is a novel chemotherapy currently approved for the treatment of multiple metastatic malignancies. This class of therapy, known as immune checkpoint inhibitors, is notable for an autoimmune-related adverse event profile. Diarrhea and colitis are common gastrointestinal adverse events, however, upper gastrointestinal events are rarely reported. We present a case of nivolumab-associated esophagitis and gastritis that has yet to be reported in the published literature.
Introduction
A new class of chemotherapy, known as checkpoint inhibitors, has been developed to enhance T-cell antitumor activity.1 Programmed death-1 (PD-1) is a receptor that is expressed on T-cells, and its activation serves as a physiological brake, or checkpoint, to autoimmunity. Fully humanized monoclonal antibodies targeting the PD-1 receptor allow for unregulated T-cell destruction of tumor cells and have had dramatic results in the treatment of a variety of metastatic malignancies.2-6 The adverse event profile of this class of therapies is related to the autoimmune nature of their mechanism of action, with rash and lower gastrointestinal (GI) side effects being the most common.7 To date, only two serious adverse events of upper GI involvement have been reported as part of ongoing clinical trials, however, no endoscopic or histopathological findings have been presented in the literature.8,9
Case Report
A 93-year-old woman presented to our institution with a 2-month history of progressive dysphagia and a 1.5-month history of diarrhea. Her medical history was notable for a low-grade B-cell lymphoma and classical Hodgkin’s lymphoma with known mediastinal, splenic, and epidural soft-tissue mass involvement. She had been treated with nivolumab for approximately 6 months prior to her symptom onset. Her other medical history was notable for gastroesophageal reflux disease managed with pantoprazole (40 mg twice daily), as well as lymphocytic colitis, diagnosed 5 years prior to nivolumab initiation and managed with longstanding oral budesonide (9 mg).
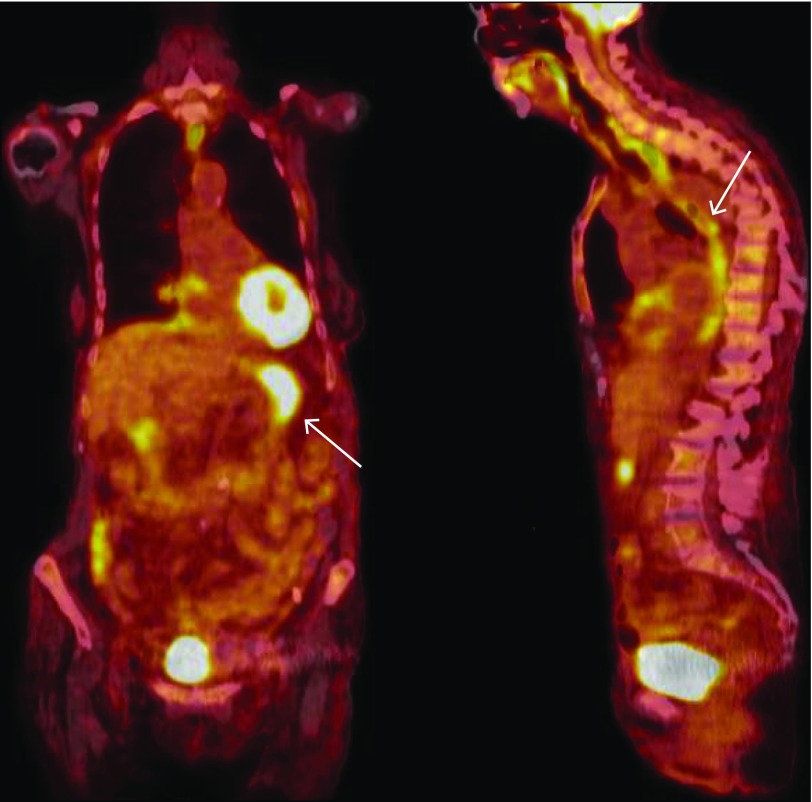
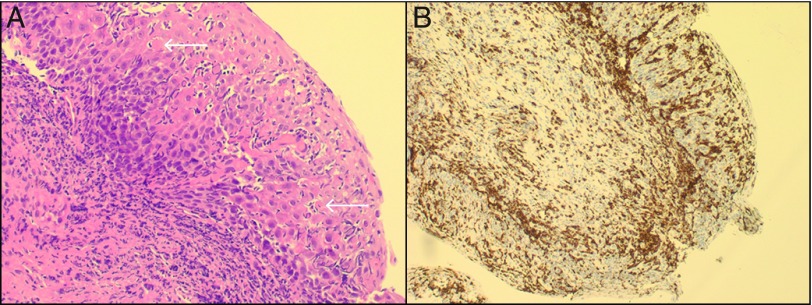
On the day of admission, a staging positron emission tomography with computed tomography (PET-CT) demonstrated limited metabolic activity at the previous sites of her known lymphoma. There was, however, significant abnormal fluorodeoxyglucose uptake in the esophagus and stomach, consistent with significantly increased metabolic activity (Figure 1). A subsequent esophagogastroduodenoscopy (EGD) revealed thick mucoid secretions, diffuse mucosal congestion with edema, erythema, and friability of the esophagus. Similarly, the stomach had thick mucosal exudates with underlying erythema (Figure 2). The duodenum was endoscopically normal. Biopsies of the squamous esophagus demonstrated marked intraepithelial lymphocytic infiltrate with scattered dyskeratotic keratinocytes (Figure 3). The gastric and duodenal biopsies also demonstrated infiltrating lymphocytes and plasma cells in the lamina propria and epithelial layers. There was no morphologic evidence of Hodgkin’s lymphoma in any of the submitted biopsies, and additional immunostains showed a predominance of CD3+ with only rare CD20+ lymphocytes, ruling out GI tract infiltration by the patient's Hodgkin’s lymphoma and low-grade B-cell lymphoma, respectively (Figure 3). There was no histological evidence of fungal elements, Helicobacter pylori infection, or cytomegalovirus (CMV) infection on biopsies. Further workup included a flexible sigmoidoscopy that was endoscopically normal with normal random biopsies and unremarkable infectious stool studies including stool culture, ova and parasite, Clostridium difficile toxin polymerase chain reaction (PCR), CMV stool PCR, and norovirus PCR.
Figure 1.
PET-CT demonstrating hypermetabolic activity in the stomach (left) and esophagus (right).
Figure 2.
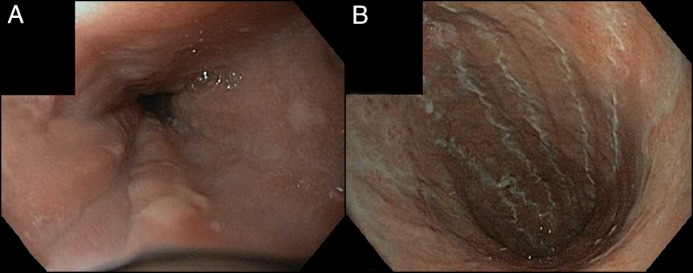
EGD showing (A) mucosal edema and diffuse erythema, and (B) exudates with diffuse underlying erythema and gastric atrophy mucosal edema.
Figure 3.
High-power view of the squamous esophagus showing (A) marked intraepithelial lymphocytic infiltrates with dyskeratotic keratinocytes (arrows), and (B) T lymphocyte infiltration confirmed with CD3+ immunohistochemical staining.
We suspected the endoscopic and biopsy findings were an adverse event related to the nivolumab therapy. Treatment was initiated with intravenous prednisone (1.0 mg/kg daily) along with continuing proton pump inhibitor therapy. Her dysphagia and diarrhea improved dramatically after 2 days of steroid therapy. Unfortunately, she developed an aspiration event 14 days into her hospitalization and ultimately expired from subsequent complications related to that event.
Discussion
Checkpoint inhibitors, including nivolumab and pembrolizumab, are novel therapies that have demonstrated significant efficacy in the management of metastatic malignancy.10 The immunological adverse event profile related to these therapies is broad, with rates of enterocolitis as high as 30%.7,11 The median time of onset of symptoms is 6–7 weeks from initiation of therapy but can vary, with some reports as late as 6 months, as in our case.11 The colitis is manifested by diarrhea, defined as greater than 4 stools per day, with blood or mucous, abdominal discomfort or cramping, and radiographic or endoscopic evidence of inflammation.12 The pathophysiology is characterized by lymphocytic infiltration in the lamina propria and epithelium of the involved mucosa. This inflammation can be transmural, and spontaneous bowel perforations have been reported.13 Interestingly, the remainder of the GI tract is rarely involved. Management includes exclusion of other infectious etiologies followed by initiation of corticosteroid therapy and cessation of chemotherapy in severe cases. For refractory cases of colitis, recommendations include infliximab dosed similarly for induction of moderate-severe colitis in inflammatory bowel disease.14 To date, only two reports of esophagitis related to these therapies has been described as adverse events during the pivotal phase 3 clinical trials, however, no endoscopic or histological findings have been presented in the published literature.
We were curious if the patient’s use of oral budesonide had afforded her with some protection against the more common adverse event of colitis. Interestingly, a randomized placebo-controlled trial investigating possible prophylaxis for colitis in those initiating immune checkpoint inhibitor therapy had already demonstrated no clinical reduction in the rates of diarrhea in those treated with oral budesonide (9 mg daily) for 16 weeks when compared to placebo.15
Gastroenterologists should be vigilant for drug-associated toxicities as a new class of checkpoint inhibitor chemotherapies will likely become more commonplace with the emergence of additional formulations and indications.
Disclosures
Author contributions: Both authors contributed equally to the manuscript. J. Boike is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained from the patient's next of kin for this case report.
References
- 1.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansell SM, Lesokhin AM, Borrello I, et al. . PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015; 372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris RL, Blumenschein GJ, Fayette J, et al. . Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016; 375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, et al. . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015; 373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Brady B, et al. . Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015; 372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 7.Eigentler TK, Hassel JC, Berking C, et al. . Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016; 45:7–18. [DOI] [PubMed] [Google Scholar]
- 8.ClinicalTrials.gov. Protocol CA209-153. https://clinicaltrials.gov/ct2/show/study/NCT02066636. Accessed December 20, 2016.
- 9.ClinicalTrials.gov. Protocol CA209-214. https://clinicaltrials.gov/ct2/show/NCT02231749. Accessed December 20, 2016.
- 10.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016; 16(5):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016; 44:51–60. [DOI] [PubMed] [Google Scholar]
- 12.Berman D, Parker SM, Siegel J, et al. . Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010; 11:10. [PMC free article] [PubMed] [Google Scholar]
- 13.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015; 4(5):560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014; 48(6):806–10. [DOI] [PubMed] [Google Scholar]
- 15.Weber J, Thompson JA, Hamid O, et al. . A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009; 15(17):5591–8. [DOI] [PubMed] [Google Scholar]