Summary
Dopamine neurons in the ventral tegmental area (VTA) were previously found to express vesicular glutamate transporter 2 (VGLUT2) and to co-transmit glutamate in the ventral striatum (VStr). This capacity may play an important role in reinforcement learning. While it is known that activation of the VTA-VStr dopamine system readily reinforces behavior, little is known about the role of glutamate co-transmission in such reinforcement. By combining electrode recording and optogenetics, we found that stimulation of VTA dopamine neurons in vivo evoked fast excitatory responses in many VStr neurons of adult mice. Whereas conditional knockout of the gene encoding VGLUT2 in dopamine neurons largely eliminated fast excitatory responses, it had little effect on the acquisition of conditioned responses reinforced by dopamine neuron activation. Therefore, glutamate co-transmission appears dispensable for acquisition of conditioned responding reinforced by DA neuron activation.
Keywords: Intracranial self-stimulation, reward, reinforcement learning, ventral striatum, nucleus accumbens, mesolimbic dopamine system, glutamate co-transmission
eTOC
Wang et al find that targeted deletion of the gene encoding VGLUT2 in dopamine neurons largely eliminates fast excitatory responses caused by glutamate co-transmission in the ventral striatum in vivo. However, it has little effect on acquisition of conditioned responses reinforced by the activation of VTA dopamine neurons.
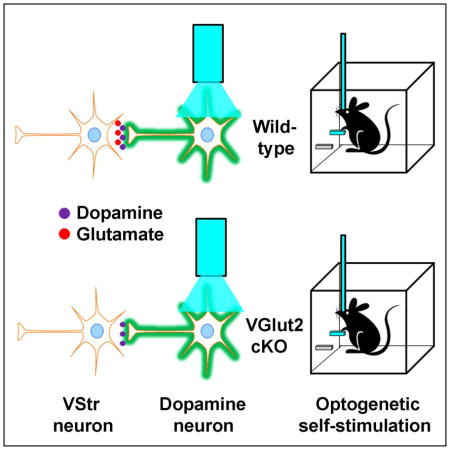
Introduction
Recent studies found that dopamine (DA) neurons in the ventral tegmental area (VTA) transmit both DA and glutamate (Chuhma et al., 2014; Chuhma et al., 2004; Dal Bo et al., 2004; Kawano et al., 2006; Koos et al., 2011; Morales and Root, 2014; Stuber et al., 2010; Sulzer et al., 1998; Tecuapetla et al., 2010; Trudeau et al., 2014; Zhang et al., 2015). The capacity of DA neurons to release glutamate and excite postsynaptic cells may play an important role in reinforcement learning, for which the activity of DA neurons is crucial. Phasic activity of DA neurons is said to indicate errors in predicting future rewards, and such signals provide vital information in acquiring conditioned responding (Montague et al., 1996; Schultz et al., 1997). Indeed, optogenetic activation of the VTA-VStr DA system readily reinforces behavior that is contingently paired with such activation, thereby supporting intracranial self-stimulation (ICSS) (Ilango et al., 2014a; 2014b; Steinberg et al., 2014; Witten et al., 2011). Similarly, rodents learn to self-administer drugs that potentiate DA transmission such as cocaine and amphetamines both intravenously and intracranially, directly into the VStr; such self-administration is abolished by the administration of intra-VStr DA receptor antagonists or by 6-hydroxydopamine lesions (Ikemoto and Wise, 2004; Koob, 1992; Pierce and Kumaresan, 2006; Wise and Bozarth, 1987). However, little is known about the role of glutamate release from DA neurons in such reinforcement learning.
It is well known that the sooner a reward follows a behavior, the quicker the behavior becomes conditioned (Keesey,1964). Given that glutamate release excites post-synaptic neurons on the order of milliseconds, whereas DA transmission takes at least several tens of milliseconds, it is possible that the rapid action of glutamate co-transmission of DA neurons is important in acquiring reinforced behavior. The present study aims to test this by utilizing two separate conditional knockout mouse lines in which Slc17a6, the gene encoding vesicular glutamate transporter type 2 (VGLUT2), was deleted in dopamine neurons (Birgner et al., 2010; Hnasko et al. 2010). Our results show that optogenetic stimulation of VTA DA neurons indeed triggered fast excitatory responses in VStr neurons in vivo, and the conditional deletion of VGLUT2 diminished such excitatory responses. However, the conditional deletion of VGLUT2 had little or no effect on acquisition of conditioned responding reinforced with optogenetic stimulation of VTA DA neurons.
Results
Optogenetic stimulation of VTA DA neurons evokes fast phasic activity in the VStr
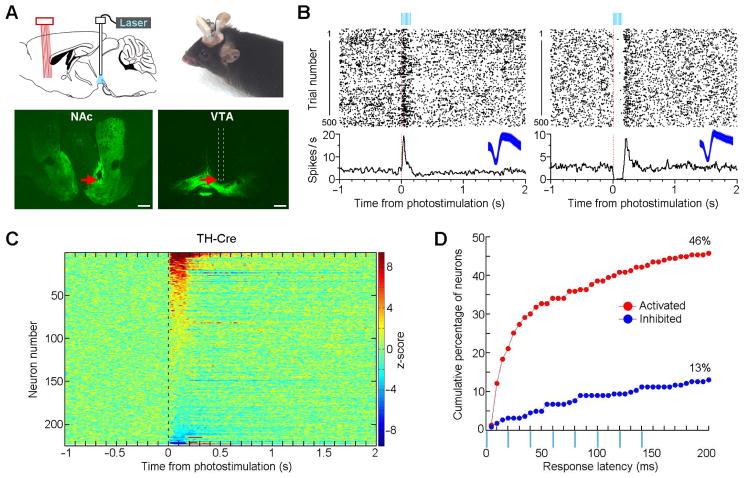
We used VTA DA-specific optogenetic stimulation combined with VStr tetrode recording to examine how activation of VTA DA neurons affects single neuron activity of VStr neurons in freely-behaving TH-Cre mice (Figures 1A and S1). We delivered VTA optogenetic stimulation, using a parameter (a 50-Hz 8-pulse train; 8-mW intensity and 1-ms pulse width) known to support ICSS (Ilango et al. 2014a), with variable intervals of 5–10 s (repeated 500 trials or more), while continuously recording VStr neural activity in freely-behaving mice in their home cages. Many VStr neurons displayed fast excitatory or inhibitory responses to VTA optogenetic stimulation (Figures 1B, 1C, and S2). In total, 46% of the recorded VStr neurons displayed excitation within 200 ms upon VTA optogenetic stimulation, while a smaller percentage of VStr neurons (13%) displayed inhibition (Figure 1D). Moreover, most of these fast VStr responses evoked by VTA optogenetic stimulation were insensitive to DA D1 or D2 receptor antagonist (Figure S3).
Figure 1. Optogenetic stimulation of VTA DA neurons evokes fast phasic responses in the Str of freely-behaving TH-Cre mice.
(A) Placements of an optic fiber above VTA DA neurons and an electrophysiological recording probe in the VStr. The red arrows in the bottom photomicrograms show a representative VTA stimulation site (right) and a VStr recording site (left). Scale bars, 0.5 mm. Green fluorescence indicates YFP expression.
(B) Perievent rasters and histograms showing examples of VStr neurons excited (left) and inhibited (right) upon VTA optogenetic stimulation. Insets, 1000 representative overlapping spike waveforms (blue traces; 1 ms) of the two neurons.
(C) Summary of the z-scored perievent histogram of all the recorded VStr neurons (n = 223) upon VTA optogenetic stimulation in TH-Cre mice (n = 8). Perievent histograms (bin size = 5 ms) were smoothed with a Gaussian filter (filter width = 3 bins).
(D) Cumulative percentage of the activated (red) and inhibited (blue) VStr neurons with response latencies of 200 ms or less.
Conditional knockout of VGLUT2 attenuates VStr fast phasic responses
We hypothesized that fast activations, particularly those occurring within 50 ms upon VTA stimulation, are mediated by glutamate co-transmission of DA neurons. To address this, we used a mouse line with conditional knockout (cKO) of VGLUT2 (Slc17a6flox/flox; Slc6a3+/IRESCre) in DA neurons (Birgner et al., 2010), which allowed selective activation of DA neurons without glutamate co-transmission. We injected a Cre-dependent AAV-ChR2 into the VTA of cKO and control littermates (Slc17a6+/flox; Slc6a3+/IRESCre), and implanted an optic fiber above the injection site. These mice were then tested behaviorally for acquisition of conditioned responding in an ICSS procedure, which we describe later, and thereafter a subset were further prepared to examine striatal neuron activity as a function of optogenetic stimulation of VTA DA neurons, which we describe immediately below.
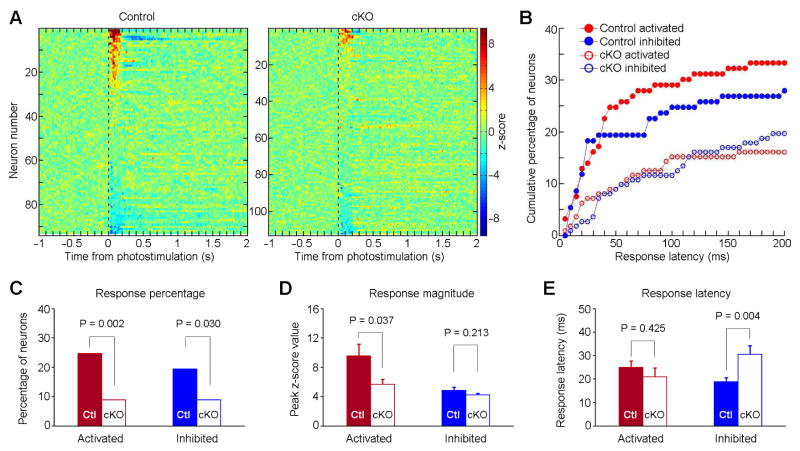
We recorded 112 neurons from cKO (n = 6) and 93 neurons from control mice (n = 6) as a function of VTA stimulation (a 50-Hz 8-pulse train; 8-mW intensity and 1-ms pulse width) (Figure 2A). Twenty-five percent (23/93) of VStr neurons displayed excitatory responses within 50 ms during which VStr neurons have likely not yet been affected by activities of G-protein coupled DAergic receptors (Marcott et al., 2014). Only 9% (10/112) of the VStr neurons displayed such responses in cKO mice (Figures 2B and 2C). Moreover, the magnitude of activation was greater in the control compared to cKO mice (Figure 2D, left) although response latency did not differ between the two groups (Figure 2E, left). VTA optogenetic stimulation inhibited some VStr neurons; the cKO mice had significantly less neurons inhibited and greater inhibition latency than the control mice, while the two groups did not differ in unit inhibition magnitude (Figure 2C and 2D, right).
Figure 2. Conditional knockout of VGLUT2 from DA neurons reduces VStr phasic response.
(A) Summary of the z-scored perievent histogram of all the recorded VStr neurons upon VTA optogenetic stimulation in Control (n = 93; left panel) and cKO mice (n = 112; right). Perievent histograms (bin size = 5 ms) were smoothed with a Gaussian filter (filter width = 3 bins). Color bars represent z-scored firing frequency.
(B) Cumulative percentages of the activated (red) and inhibited (blue) VStr neurons with response latencies of 200 ms or less, recorded from Control (filled circles) and cKO mice (open circles).
(C) Percentages of the same four groups of neurons (as shown in B) that responded with latencies of 50 ms or less. A chi-square test was used for statistics. Ctl, Control.
(D) and (E) Response amplitude (D; Mean ± SEM) and response latency (E; Mean ± SEM) of the same four groups of neurons that responded with latencies of 50 ms or less. An unpaired two-tailed t test was used for statistics.
Conditional knockout of VGLUT2 reduces glutamate release in vivo and eliminates glutamate co-transmission in vitro
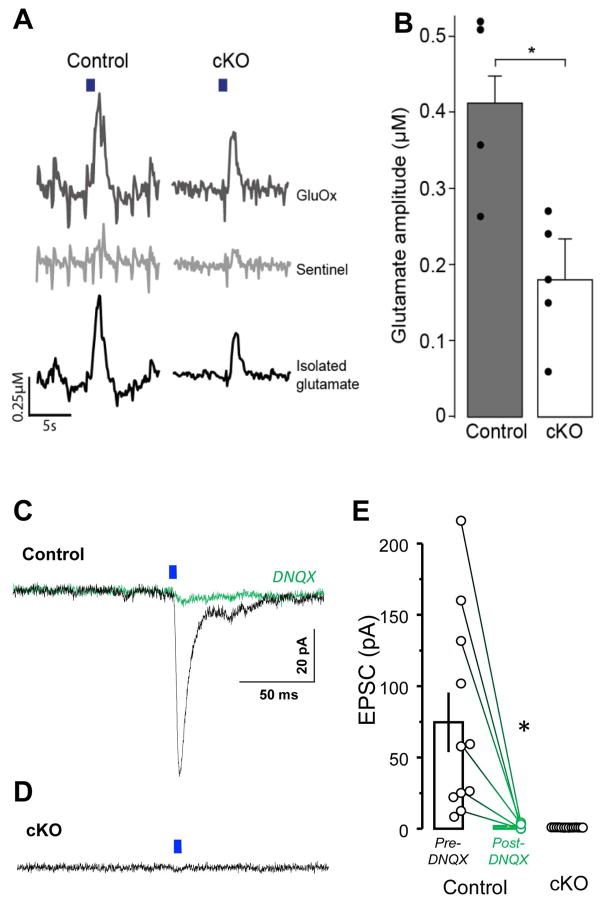
To verify attenuated glutamate release in cKO mice, we performed amperometric measurements of VStr glutamate evoked by terminal stimulation of VTA-VStr DA neurons. We observed reduced glutamate release in cKO mice (Figures 3A and 3B; Tables S1 and S2), although optogenetic stimulation still evoked some glutamate release in cKO mice.
Figure 3. Conditional knockout of VGLUT2 reduces extracellular glutamate release in vivo and eliminates glutamate co-transmission in vitro.
(A) Optogenetic stimulation (20 pulses at 20 Hz; blue bar) of VTA DA neuron terminals in the VStr evoked glutamate release in control (left) and cKO (right). Raw signals obtained from the GluOx-coated (top) and sentinel channel (middle) were subtracted from one another to obtain an isolated glutamate signal (bottom).
(B) The cKO mice (n = 5) had significantly lower glutamate amplitude than the control mice (n = 4) (t = 3.384, df = 7, P = 0.0117; unpaired two-tailed t-test).
(C and D) Optogenetic light-pulse (5 ms) delivered at DA neuron terminals evoked EPSCs from VStr neurons (Vh= −70 mV) of control (C), but not cKO (D), mice. The EPSCs present in controls were completely blocked by the AMPA receptor antagonist DNQX (C; green trace).
(E) Summary of EPSC peak amplitudes recorded from control (n = 11 neurons; n = 2 mice) and cKO (n = 14 neurons; n = 2 mice) mice.
To determine whether the residual responses in the VStr of the cKO mice (Figure 2) may be mediated by incomplete knockout of VGLUT2 in DA neurons, we performed whole cell recordings from VStr medium spiny neurons using acute brain slice preparations. Optogenetic stimulation of ChR2-expressing DA terminals evoked excitatory post-synaptic currents (EPSCs) in all recorded neurons of control mice, and the evoked EPSCs, measured at Vh −70 mV, were blocked by the AMPA receptor antagonist DNQX (Figures 3C and 3E). By contrast, we did not detect light-evoked EPSCs in any VStr recorded neurons of cKO mice (Figures 3D and 3E). These results suggest that, as shown for other cKO lines (Stuber 2010, Tritsch 2012), the co-transmission of glutamate is eliminated after the cKO of VGLUT2. Therefore, the fast-excitatory responses in striatal neurons of cKO mice in vivo are mediated by mechanisms distinct from glutamate co-release, and the evoked glutamate release detected over 1–2 seconds likely explained by network effects induced by release of DA and co-transmitters such as GABA and cholecystokinin (CCK) from DA neurons.
Glutamate co-transmission has little effect on the acquisition of conditioned behavior
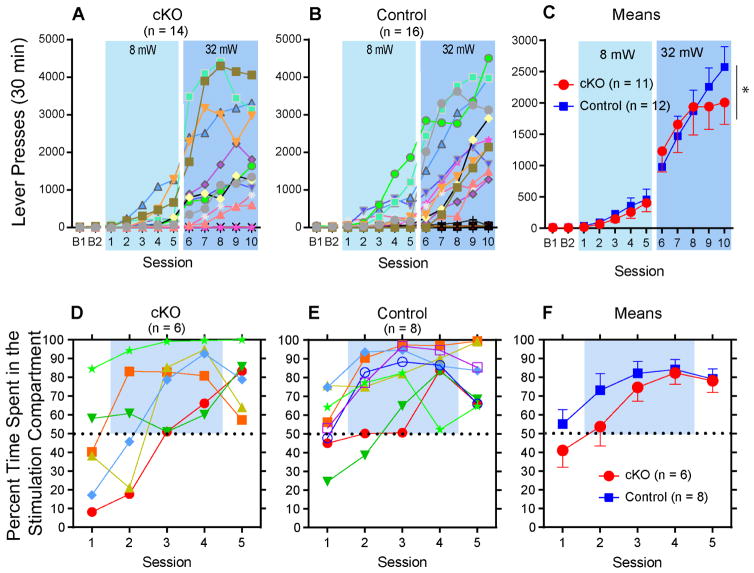
We used naïve mice to examine whether glutamate co-transmission is important in acquisition of behavior reinforced by phasic activation of VTA DA neurons in an ICSS procedure. Mice significantly increased lever presses reinforced by VTA optogenetic stimulation (a 50-Hz 8-pulse train; 8-mW intensity and 1-ms pulse width) over the first 5 sessions (session effect: F4,84 = 11.60, P < 0.00001 with a 2group x 5session mixed ANOVA), while cKO and control mice increased lever presses in a similar manner (group effect: F1,21 = 0.58, P = 0.58; group x session interaction: F4,84 = 0.096, P = 0.98) (Figures 4A–C). To recruit more neurons for greater response rates, we let mice lever-press for optogenetic stimulation of greater intensity (32 mW and 3-ms pulse width) over sessions 6–10. Although lever presses further increased (session effect: F4,84 = 35.65, p < 0.00001 with a 2group x 5session mixed ANOVA) without a main group effect (F1,21 = 0.025, P = 0.88), a significant group x session interaction was found (F4,84 = 5.03, P = 0.006). In addition, we examined how the mice change response rates as a function of optogenetic stimulation value. We used three different parameters of optogenetic stimulation (3, 5 and 8 pulses delivered at 14, 29 and 50 Hz, respectively). cKO and control mice displayed similar patterns of responding as a function of optogenetic stimulation value, with higher response rates for stimulations with greater pulse number-frequency (Figure S4). These results suggest that the loss of glutamate co-transmission from DA neurons does not affect sensitivity to reward value produced by DA neuron activation.
Figure 4. Conditional knockout of VGLUT2 from DA neurons does not affect the acquisition of ICSS or place preference.
(A) and (B) Active lever pressing was reinforced with VTA optogenetic stimulation (50 Hz, 8 pulses with 1 ms pulse width; 8 mW) in sessions 1–5 and then with optogenetic stimulation of increased intensity (3 ms pulse width; 32 mW) in sessions 6–10. In session 10, 11 out of 14 cKO and 12 out of 16 control mice responded 500 times or more.
(C) Mean responses with SEM are shown with the data of responders with 500 responses or more. The cutoff of 500 responses is to focus on differences in responders of the two groups and is justified by the fact that similar ratios of mice were excluded from the analyses between the two groups. * P < 0.05, a significant group x session interaction.
(D) and (E) While no optogenetic stimulation was delivered in sessions 1 or 5, mice received VStr optogenetic stimulation in one of two compartments during sessions 2–4. Depicted are percent time spent in the stimulation compartment of each mouse.
(F) Percentage of time (Mean ± SEM) spent in the stimulation compartment.
We also examined whether cKO mice acquire real-time place preference induced by optogenetic stimulation of VStr terminals of VTA DA neurons. Such stimulation (continuous 20 Hz) did not produce a significant difference in real-time place preference between the cKO and littermate control mice (Figures 4D–F) (session effect: F1,12 = 333.41, P < 0.00001, with a 2group x 5session mixed ANOVA; group effect: F1,12 = 1.27, P = 0.28; group x session interaction: F1,12 = 0.99, P = 0.42). These results suggest that the loss of glutamate co-transmission from DA neurons does not affect the acquisition of place preference reinforced by the stimulation of DA terminals in the VStr.
Discussion
We discuss three notable methodological issues here. First, the use of DAT-Cre mice, especially the controls (Slc17a6+/flox; Slc6a3+/IRESCre), served as a complement for the experiment where TH-Cre mice were used, and vice versa. This is because the TH-Cre line expresses Cre in some non-DA neurons (Lammel et al., 2015; Lindenberg, 2004; Nordenankar et al., 2015; Pupe and Wallen-Mackenzie, 2015), and some TH-expressing VTA neurons, particularly those located in the medial VTA, do not express DAT (Lammel et al., 2015; Viereckel et al., 2016). Second, a 50-Hz train employed in the present study was adequate for examining fast activation of striatal neurons despite the reports that 50-Hz optogenetic stimulation does not trigger reliable depolarization at each pulse in vitro (Tsai et al., 2009; Witten et al., 2011). This is because (a) many DA neurons can spontaneously fire 50 Hz or more (Wang and Tsien, 2011); (b) 50-Hz optogenetic stimulation can trigger a spike for each pulse in vivo and supports vigorous ICSS (Ilango et al. 2014a); (c) 40-Hz optogenetic stimulation triggers greater DA release than 20- or 30-Hz stimulation in vivo (Bass et al., 2010). Finally, although the VTA AAV injections resulted in the expression of ChR2 in some medial SNc DA neurons, optogenetic stimulation most likely activated selectively VTA DA neurons. This is because optogenetic stimulation only excites neurons just below the tip. Even if it recruited nigral DA neurons, fast excitatory activation must have been mediated by VTA DA neurons because nigral DA cells do not express detectable levels of VGLUT2 (Hnasko et al., 2010) or project densely to the VStr (Ikemoto, 2007).
The present study observed that stimulation of VTA DA neurons activates many VStr neurons within 50 ms in freely-moving adult mice (Figures 1D and 2B), and this observation is most likely explained by glutamate co-transmission of DA neurons. First, these excitatory responses were not mediated by DA, because DA receptors are G-protein coupled, with relatively slow activation kinetics (> 50 ms) (Dong and White, 2003; Marcott et al., 2014). Consistently, these evoked fast responses were still present after the administration of DA D1 or D2 receptor antagonist (Figure S3). Second, optogenetic stimulation of VTA DA neurons excited fewer VStr neurons, and resulted in less glutamate release (with VTA-VStr terminal stimulation) in cKO mice than control mice.
While the residual fast-excitatory responses and glutamate release in response to light-evoked DA neuron stimulation raised a concern as to whether the cKO of VGLUT2 was complete, whole cell recordings from VStr medium-sized spiny neurons show clearly the complete removal of glutamate co-transmission in cKO mice. Therefore, any remaining fast excitation must be explained by other factors that may include a) background/signal noise, b) alternate excitatory signal/transmitter, and c) polysynaptic mechanism. Similarly, the evoked glutamate release, which took place on the scale of seconds must be due to secondary, tertiary, and quaternary consequences mediated by DA and other co-transmitters such as GABA or neuropeptides. This is consistent with our amperometic measurements did not detect synaptic glutamate release, but extra-synaptic levels of glutamate over 1–2 s.
We unexpectedly observed that optogenetic stimulation of DA neurons inhibited a smaller percentage of VStr neurons in the cKO mice than the control mice, and the latency of inhibitory responses was greater in the cKO mice than the control mice. These observations can be understood with consideration of previous findings. First, DA neurons have been shown to co-transmit GABA (Tritsch et al., 2012; Tritsch et al., 2014). In addition, 99% of striatal neurons are GABAergic, and they receive inhibitory inputs from not only interneurons, but also medium-sized spiny neurons, which send extensive collaterals to each other and engage in mutual inhibitory interactions (Bishop et al., 1982; Taverna et al., 2004). Accordingly, the elimination of fast excitation in GABAergic neurons in cKO mice could result in the elimination of secondary inhibitory responses among striatal neurons.
The present study showed that the cKO mice acquired conditioned responding reinforced by VTA DA neuron activation at similar rates as control mice. Similarly, previous studies found no clear difference between cKO and control mice in acquiring motor performance with rotarod or operant responding reinforced by sucrose pellets, or performing a radial maze baited with food (Birgner et al., 2010; Hnasko et al., 2010). While these tasks arguably depend, at least in part, on DA neuron activity (Alsio et al., 2011; Birgner et al., 2010; Hnasko et al., 2010), the present study demonstrated dispensability of glutamate co-transmission in trial-and-error learning with responding reinforced by DA neuron activation.
A subtle difference between cKO and control mice emerged after many sessions of ICSS. While both groups increased ICSS rates for each subsequent session, the rate of increase was lessened for cKO groups during the last several sessions when compared to controls. The difference may be explained by the role of glutamate in vesicular loading of DA (Hnasko et al., 2010; Hnasko and Edwards, 2012). Indeed, VGLUT2 cKO from DA neurons not only eliminates glutamate release, but also reduces DA release (Alsio et al., 2011; Fortin et al., 2012; Hnasko et al., 2010). Because DA transmission is important in energizing behavior (Hamid et al., 2016; Ilango et al., 2014a), the deficit in DA vesicular loading may have prevented the cKO mice from performing at high response rates. Consistently, previous studies found that cKO mice did not increase locomotor activity as much as control mice after the administration of a low dose of amphetamine or cocaine (Birgner et al., 2010; Hnasko et al., 2010). Moreover, cKO mice self-administer more cocaine than control mice (Alsio et al., 2011). The latter effect is explained by the fact that cocaine increases extracellular DA concentration by blocking DA uptake, and DA concentration regulates cocaine self-administration (Norman and Tsibulsky, 2006; Wise et al., 1995). Therefore, reduced dopamine concentrations in the cKO may cause mice to administer cocaine with shorter intervals (Ikemoto et al., 2015). Interestingly, cocaine is found to blunt glutamate co-transmission from DA neurons via enhanced activation of D2 autoreceptors (Adrover et al., 2014), corroborating the notion that glutamate co-transmission plays little role in vigor of behavior.
In sum, we provide direct evidence that glutamate co-release from dopamine terminals can trigger fast excitatory responses of VStr neurons in vivo. However, despite the asymptotic response difference between cKO and control mice in ICSS, acquisition rates of ICSS and real-time place-preference did not differ between the two groups. Therefore, glutamate co-transmission appears dispensable for the acquisition of conditioned responding reinforced by DA neuron activation.
Experimental Procedures
Detailed protocols are described in the Supplemental Experimental Procedures.
Animals
TH-IRES-Cre mice, cKO mice (Slc17a6flox/flox; Slc6a3+/IRESCre) and control mice (Slc17a6+/flox; Slc6a3+/IRESCre) were used.
Intracranial self-stimulation (NIDA)
Two levers, an active and an inactive, were available in the self-stimulation chamber. During the acquisition-phase, press of the active lever triggered 8 pulses of 50-Hz optogenetic stimulation in the VTA (no timeout except for the duration of optogenetic stimulation), while press of the inactive lever had no consequence. A lower laser intensity (8 mW and 1-ms pulse width) was used between acquisition-sessions 1–5 (all three mouse lines), and a higher laser intensity (32 mW and 3-ms pulse width) was used between acquisition-sessions 6–10 (only DAT and cKO mice).
In vivo electrophysiology (NIDA)
We used a bundle of 8 tetrodes (32 channels) coupled to a moveable (screw-driven) microdrive assembly for in vivo VStr recording in freely-behaving mice. Neural signals were recorded using a Neuralynx Digital Lynx acquisition system. Spikes were digitized at 32 kHz and filtered at 600–6,000 Hz, using one recording electrode that lacked obvious spike signals as the reference.
In vitro electrophysiology (UCSD)
Acute brain slices were prepared and whole cell recordings made from visually identified VStr neurons held in voltage-clamp at −70 mV to record AMPAR EPSCs as previously described (Yoo et al., 2016). For whole-cell voltage-clamp recordings, single-pulse (5-ms) optogenetic stimuli were applied every 55 sec and 10 photo-evoked currents were averaged per neuron per condition.
Place preference (UCSD)
We used home built two-chamber acrylic apparatuses. Time spent in each chamber was assessed and mice were assigned active (i.e. optogenetic stimulation-paired) sides in an unbiased manner. Upon head entry to the pre-defined active side, software triggered TTL-pulses to activate the laser (20 Hz, 10-ms pulse width, ~10 mW at fiber tip), which discontinued upon head exit.
Data analysis
Tetrode recording
We used the Plexon OfflineSorter for separation of recorded spike waveforms. Sorted spikes were processed and analyzed in NeuroExplorer (Nex Technologies) and Matlab (Mathworks, Inc.). For z-score analysis, neural activity one sec before the optogenetic stimulation was used as the baseline. Response latency was defined as the latency of the first bin from at least five consecutive bins that exceeded the z-score of 2 (or −2 for inhibited neurons).
Histology
Mice were deeply anesthetized and intracardially perfused with PBS followed by 4% paraformaldehyde (PFA). Brains were sliced on a cryostat (50-μm coronal sections) or a vibratome (60-μm coronal sections). Sections were mounted with the Mowiol mounting medium mixed with DAPI.
Supplementary Material
Highlights.
Glutamate co-release from DA neurons drives fast excitatory responses in VStr in vivo.
Loss of VGLUT2 in DA neurons abolishes glutamate co-transmission.
Such loss does not disrupt DA neurons from reinforcing behavior.
Acknowledgments
We thank Marisela Morales, Roy A. Wise and Carlos Mejias-Aponte for their helpful discussion on the data and Hanna Pettersson for technical assistance. The work was supported by the Intramural Research Program of NIDA, NIH (S.I.), R01DA036612 (T.S.H.), Tobacco-Related Disease Research Program (J.H.Y.), Swedish Research Council (2013-4657, 2014-3804) (Å.M.), Swedish Brain Foundation (Å.M.), and Research Foundations of Bertil Hållsten, Åhlén and Parkinsonfonden (Å.M.).
Footnotes
AUTHOR CONTRIBUTIONS
D.V.W. and S.I. designed self-stimulation and electrophysiological experiments, analyzed the data, and drafted the manuscript; D.V.W., C.J.B., A.T. and A.J.K. performed the experiments. T.S.H., J.H.Y. and M.G. designed and performed the place preference experiment. T.S.H and V.Z. designed and performed the slice electrophysiology experiment. T.V., Å.K.G. and Å.M. designed amperometry experiments and analyzed data; T.V., E.A. and Å.K.G. performed the experiments.
Supplemental Information includes Supplemental Experimental Procedures, 4 figures, and 2 tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrover MF, Shin JH, Alvarez VA. Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:3183–3192. doi: 10.1523/JNEUROSCI.4958-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsio J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, et al. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci. 2011;31:12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Vance ZB, Sullivan RP, Bonin KD, Budygin EA. Optogenetic control of striatal dopamine release in rats. Journal of neurochemistry. 2010;114:1344–1352. doi: 10.1111/j.1471-4159.2010.06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, et al. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Chang HT, Kitai ST. Morphological and physiological properties of neostriatal neurons: an intracellular horseradish peroxidase study in the rat. Neuroscience. 1982;7:179–191. doi: 10.1016/0306-4522(82)90159-2. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Zhang H, Masson J, Zhuang XX, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. Journal of Neuroscience. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. Journal of neurochemistry. 2004;88:1398–1405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- Dong Y, White FJ. Dopamine D1-class receptors selectively modulate a slowly inactivating potassium current in rat medial prefrontal cortex pyramidal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:2686–2695. doi: 10.1523/JNEUROSCI.23-07-02686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, Arvidsson E, Rymar VV, Berube-Carriere N, Claveau AM, et al. Glutamate corelease promotes growth and survival of midbrain dopamine neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:17477–17491. doi: 10.1523/JNEUROSCI.1939-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, Tan A. Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behavioural brain research. 2015;290:17–31. doi: 10.1016/j.bbr.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Broker CJ, Wang DV, Ikemoto S. Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: parametric and reinforcement-schedule analyses. Front Behav Neurosci. 2014a;8:155. doi: 10.3389/fnbeh.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar Roles of Substantia Nigra and Ventral Tegmental Dopamine Neurons in Reward and Aversion. Journal of Neuroscience. 2014b;34:817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. The Journal of comparative neurology. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Keesey RE. Intracranial Reward Delay and the Acquisition Rate of a Brightness Discrimination. Science. 1964;143:702–703. doi: 10.1126/science.143.3607.702. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koos T, Tecuapetla F, Tepper JM. Glutamatergic signaling by midbrain dopaminergic neurons: recent insights from optogenetic, molecular and behavioral studies. Current opinion in neurobiology. 2011;21:393–401. doi: 10.1016/j.conb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Fӧldy C, Wall NR, Beier K, Luo L, Malenka RC. Diversity of transgenic mouse models for selective targeting of midbraine dopamine neurons. Neuron. 2015;85:429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Marcott PF, Mamaligas AA, Ford CP. Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron. 2014;84:164–176. doi: 10.1016/j.neuron.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D, Harrison NR, Gonzales CB, Schilstrom B, Konradsson-Geuken A. Effects of age and acute ethanol on glutamatergic neurotransmission in the medial prefrontal cortex of freely moving rats using enzyme-based microelectrode amperometry. PloS one. 2015;10:e0125567. doi: 10.1371/journal.pone.0125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282C:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenankar K, Smith-Anttila CJ, Schweizer N, Viereckel T, Birgner C, Mejia-Toiber J, Morales M, Leao RN, Wallen-Mackenzie A. Increased hippocampal excitability and impaired spatial memory function in mice lacking VGLUT2 selectively in neurons defined by tyrosine hydroxylase promoter activity. Brain Struct Funct. 2015;220:2171–2190. doi: 10.1007/s00429-014-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Tsibulsky VL. The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain research. 2006;1116:143–152. doi: 10.1016/j.brainres.2006.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and biobehavioral reviews. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pupe S, Wallen-Mackenzie A. Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends Neurosci. 2015;38:375–386. doi: 10.1016/j.tins.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PloS one. 2014;9:e94771. doi: 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. Journal of Neuroscience. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, van Dongen YC, Groenewegen HJ, Pennartz CMA. Direct physiological evidence for synaptic connectivity between medium-sized spiny neurons in rat nucleus accumbens in situ. Journal of neurophysiology. 2004;91:1111–1121. doi: 10.1152/jn.00892.2003. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Oh WJ, Gu CH, Sabatini BL. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. Elife. 2014:3. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S, Sulzer D. The multilingual nature of dopamine neurons. Progress in brain research. 2014;211:141–164. doi: 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viereckel T, Dumas S, Smith-Anttila CJ, Vlcek B, Bimpisidis Z, Lagerstrom MC, Konradsson-Geuken A, Wallen-Mackenzie A. Midbrain Gene Screening Identifies a New Mesoaccumbal Glutamatergic Pathway and a Marker for Dopamine Cells Neuroprotected in Parkinson’s Disease. Sci Rep. 2016;6:35203. doi: 10.1038/srep35203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PloS one. 2011;6:e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annual review of neuroscience. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong SC, Ramakrishnan C, et al. Recombinase-Driver Rat Lines: Tools, Techniques, and Optogenetic Application to Dopamine-Mediated Reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, Johnson AB, Fife KH, Faget L, Hnasko TS. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 2016;7:13697. doi: 10.1038/ncomms13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nature neuroscience. 2015;18:386–392. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.