ABSTRACT
Herpangina (HA) and hand, foot, and mouth disease (HFMD) are common infectious diseases caused by human enteroviruses and frequently occurr in young children. Previous published studies have mainly focused on HFMD, while the HA epidemiological and etiological characteristics in mainland China have not been described. From June, 2013 to March, 2014, HA and HFMD patients were monitored in participants from clinical trial of EV-A71 vaccine conducted during 2012–2013. A total of 95 HA patients and 161 HFMD patients were defined. Enteroviruses of HA samples were differentiated into 17 serotypes (EV-A71, CV-A16, CV-A24, E6, CV-B5, CV-A22, CV-A6, CV-A10, CV-B3, E9, CV-A9, CV-B4, CV-B2, E1, E7, E21 and CV-A20), the most common serotypes were EV-A71(10/95,10.5%), CV-A16(4/95,4.2%) and CV-A24(4/95,4.2%); while enteroviruses detected from HFMD samples were classfied into 21 serotypes ( EV-A71, CV-A16, CV-A10, CV-A6, E6, CV-B3, CV-B5, CV-A9, E9, CV-B2, CV-B4, E3, E11, E15, E16, CV-A1, EV-A69, E5, CA22, CA24 and EV99), the most common serotypes were EV-A71(28/161,17.4%), CV-A16(7/161,4.4%) and CV-A10(5/161,3.1%). The first HA epidemic peak occurred in summer and a second smaller peak occurred in January. In HA patients, the body temperature (P < 0.0001) and the incidence of fever (P < 0.05) were significant higher than those in HFMD patients. Between HA and HFMD patients infected with EV-A71, no significant differences were found in age, sex, circulating season, and the viral genome diversity. In summary, we firstly reported the epidemiological and etiological characteristics of HA in mainland China. Developing a multivalent vaccine will be helpful for the control of the HA/HFMD epidemic.
KEYWORDS: clinical manifestations, enterovirus spectrum, hand, foot, and mouth disease, herpangina, human enteroviruses
Introduction
Herpangina (HA) and hand foot and mouth disease (HFMD) are common infectious diseases caused by human enterovirus (HEVs) and frequently occurring in young children. HA are characterized with fever and oral ulcers without any progression of vesicular eruption on the skin. HFMD is a common childhood disorder that typically presents as a brief, febrile illness characterized by the association of oral enanthema and vesicular rash on palms, soles and/or buttocks.1,2
HEVs, which belong to the Picornaviridae family, contain a single positive-strand genomic RNA with a molecular weight of 7.4 kb. The genome of HEVs includes a single open reading frame that encodes 4 structural proteins (VP1, VP2, VP3, and VP4) and 7 non-structural proteins (2A, 2B, 2C, 3A, 3B, 3C, and 3D). The part encoding VP1 protein contains the major neutralization epitope which has been used for virus serotype identification and evolution studies.3,4 HEV types are currently classified into 4 groups, human enterovirus A-D (HEV-A-D) based on the genome and antigenic characterizations.5
HFMD cases were reported by the National Notifiable Diseases Surveillance System (NNDSS) in many countries. Many studies were carried out to investigate of HFMD and showed that EV-A71 and CV-A16 are the most frequent viral pathogens.6,7 In contrast to HFMD, there were a few studies on HA reported in Japan,8 Brazil9 and Taiwan,10 which indicated that HEV-A, were major serotypes associated with HA, including CV-A4, CV-A6, CV-A10 and EV-A71. In Japan,8 the CV-A4, CV-A6 and CV-A10 were the most common enterovirus caused HA during 2001–2007. In Korea, EV-A71 and CV-A16 were the third common enterovirus, followed by the CV-A5 and CV-A2 in 2009.28 Not like HFMD, HA is not included in the data reported to NNDSS in many countries. Therefore, the public health burden of HA diseases may be underestimated. As far as we know, there is no study on the etiological Characteristics of HA in mainland China has been reported.
Our previous study was carried out to investigate HFMD associated enterovirus spectrum in the Phase III clinical trial of inactivated EV-A71 vaccine in Jiangsu Province, in 2012–2013.11 The extended follow-up study was then carried on. HA and HFMD patients were monitored and in the partipants. Samples of throat and rectal swabs were collected during the course of disease. The clinical symptoms and enterovirus spectrum associated with HA and HFMD in Jiangsu Province, mainland China were further analyzed in this study.
Results
The characteristics of HA and HFMD patients
From June, 2013 to March, 2014, a total of 95 HA patients and 161 HFMD patients were defined. The median age of HA patients was 3.5 y old (95% CI: 2.2–4.9–y), while the median age of HFMD patients was 3.2 y (95% CI: 2.1–4.8 y). In the 2.0–3.0–y old group, HA cases was significantly less than HFMD cases (p < 0.05) (Fig. 1). The M/F ratio in HA and HFMD patients was 1.4 (56/41) and 1.7 (100/59), respectively.
Figure 1.
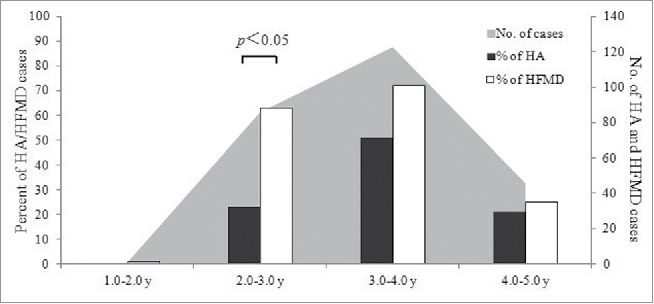
The age distribution of HA and HFMD in Jiangsu, China.
In HA patients, 10 EV-A71 infected cases were all in the placebo group. Four cases were infected with CV-A16 and 32 cases were infected with Non-EV-A71/CV-A16 HEVs. There is no significant difference between vaccine and placebo group in the patients infected with CV-A16 and Non-EV-A71/CV-A16 HEVs. In HFMD patients, all of the 28 cases infected with EV-A71 were also in the placebo group. Seven cases were infected with CV-A16 and 49 cases were infected with Non-EV-A71/CV-A16 HEVs. There is also no significant difference between vaccine and placebo group in the patients infected with CV-A16 and Non-EV-A71/CV-A16 HEVs (Table 1).
Table 1.
The HFMD/HA patients in vaccine group and placebo group.
| HA |
HFMD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine group | Placebo group | Chi-square | P valuec | Vaccine group | Placebo group | Chi-square | P valuec | |
| Non-HEVsa | 31 | 18 | 4.65 | P < 0.05 | 37 | 40 | 0.06 | P > 0.05 |
| CV-A16 | 2 | 2 | 0 | P > 0.05 | 4 | 3 | 0.15 | P > 0.05 |
| Non-EV-A71/CV-A16 HEVsb | 15 | 17 | 0.15 | P > 0.05 | 27 | 22 | 0 | P > 0.05 |
| EV-A71 | 0 | 10 | 10.56 | P < 0.01 | 0 | 28 | 30.67 | P < 0.01 |
| Total | 48 | 47 | 0.02 | P > 0.05 | 68 | 93 | 7.76 | P < 0.01 |
HFMD patients who were not associated with enterovirus.
HFMD patients who were associated with enterovirus but not EV-A71 or CV-A16.
Exact P value by Chi-square test for patients in placebo group compared with vaccine group.
The serotypes of HA/HFMD patients infected with HEVs
In 95 HA patients, 46 cases were infected with HEVs and 49 cases were infected with non-HEVs. Among the 46 cases infected with HEVs, 17 HEVs serotypes were detected. The most common HEVs serotypes were EV-A71, CV-A16, CV-A24, E6 and CV-B5, with the ratio of 10.5%(10/95), 4.2%(4/95), 4.2%(4/95), 3.2%(3/95), 3.2%(3/95), respectively.
In 161 HFMD patients, 84 cases were infected with HEVs and 77 cases were infected with non-HEVs. Among the 84 cases infected with HEVs, 21 HEVs serotypes were detected. The most common HEVs serotypes were EV-A71, CV-A16, CV-A10, CV-B5, CV-A6, with the ratio of 17.4%(28/161), 4.4%(7/161), 3.1%(5/161), 3.1%(5/161), 2.5%(4/161), respectively. (Table 2, Fig. 2).
Table 2.
Comparison between frequency of enterovirus serotypes in HA/HFMD patients.
| HA |
HFMD |
||||
|---|---|---|---|---|---|
| Virus | No. of case | % | No. of case | % | |
| EV-A71 | 10 | 10.5 | 28 | 17.4 | |
| CV-A16 | 4 | 4.2 | 7 | 4.4 | |
| CV-A10 | 1 | 1.1 | 5 | 3.1 | |
| CV-A6 | 1 | 1.1 | 4 | 2.5 | |
| E6 | 3 | 3.2 | 3 | 1.9 | |
| CV-B3 | 1 | 1.1 | 2 | 1.2 | |
| CV-B5 | 3 | 3.2 | 2 | 1.2 | |
| CV-A9 | 1 | 1.1 | 2 | 1.2 | |
| E9 | 1 | 1.1 | 2 | 1.2 | |
| CV-B2 | 1 | 1.1 | 1 | 0.6 | |
| CV-B4 | 1 | 1.1 | 1 | 0.6 | |
| E1 | 1 | 1.1 | 0 | 0 | |
| E3 | 0 | 0 | 1 | 0.6 | |
| E7 | 1 | 1.1 | 0 | 0 | |
| E11 | 0 | 0 | 1 | 0.6 | |
| E15 | 0 | 0 | 1 | 0.6 | |
| E21 | 1 | 1.1 | 0 | 0 | |
| CV-A22 | 2 | 2.1 | 5 | 3.1 | |
| CV-A24 | 4 | 4.2 | 2 | 1.2 | |
| CV-A20 | 1 | 1.1 | 0 | 0 | |
| EV-C99 | 0 | 0 | 1 | 0.6 | |
| Non-HEVs | 49 | 51.6 | 77 | 47.8 | |
| Untypable | 9 | 9.5 | 8 | 5.0 | |
| Co-infection | 0 | 0 | 8 | 5.0 | |
| Total | 95 | 100 | 161 | 100 | |
Figure 2.
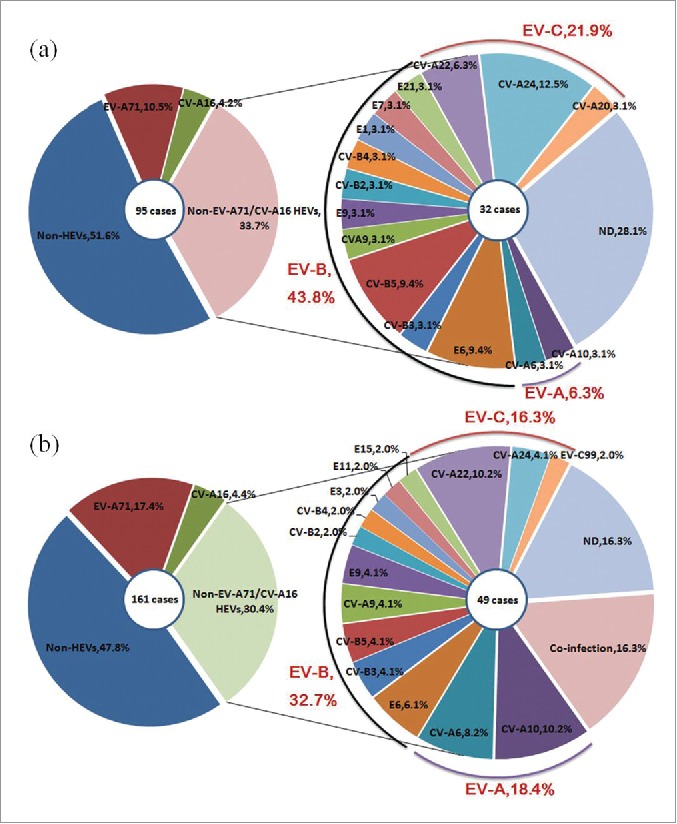
Distribution of human enterovirus serotypes in Jiangsu, China, 2012–2013.(A) Percentage of 4 different groups of HA patients (left). Percentage of each serotype in HA patients with infected the Non-EV-A71/CV-A16, including co-infection and ND ones (right).(B) Percentage of 4 different groups of HFMD patients (left). Percentage of each serotype in HFMD patients with infected the Non-EV-A71/CV-A16, including co-infection and ND ones (right).
Of the 84 HFMD patients infected with HEVs, 68 patients (81.0%) were infected with single serotype, 8 patients (9.5%) were co-infected with multiple serotypes, while the rest 8 patients (9.5%) were infected with serotypes that could not be determined. It is worthwhile to note that no co-infection cases were detected in HA patients.
The temporal distribution of HA and HFMD
The predominant epidemic peak of HA and HFMD seemed to occur during the summer season (Fig. 3). As for HA, an increase in infection incidence was found in the summer season, 29.7% in Jul, 21.6% in Aug, and 10.8% in Sep, while a smaller peak occurred in Jan. As for HFMD, the epidemic season is similar to that of HA and the epidemic peak is also in summer season, 22.4% in Jul, 26.3% in Aug, and 15.8% in Sep, however the second peak occurred in Nov. The results showed that EV-A71 circulated in Jul-Sep and Nov-Jan. CV-A16 mainly circulated in Jun-Aug.
Figure 3.
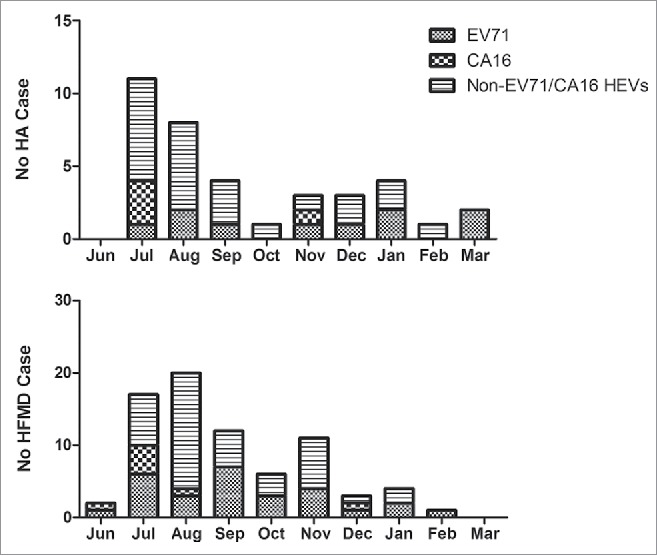
The temporal distribution of HA and HFMD in Jiangsu, China, from June, 2013 to March, 2014.
The clinical symptoms of HA and HFMD
Fever developed in 77.9% (74/95) of HA patients, and in 47.8% (77/161) of HFMD patients. The incidence of fever in HA patients was significant higher than that of HFMD patients (P < 0.05). The highest temperature in HA patient was 40.2°C, the average temperature was 38.6°C (95% CI: 37.5°C-39.6°C), while the highest temperature in HFMD patient was 40.0°C, the average temperature was 38.2°C (95% CI: 37.0°C-39.4°C). The temperature of HA patients was found significant higher than that of HFMD patients (P < 0.0001).
In the HA case, 74.7% patients (71/95) developed symptoms of respiratory system; 5.3% patients (5/95) developed symptoms of digestion; one patient presented symptoms of nervous and one patient presented symptoms of circulatory. In the HFMD case, 39.8% patients (64/161) developed symptoms of respiratory system; 4.3% patients (7/161) developed symptoms of digestion; 2.5% patients (4/161) presented symptoms of nervous, such as headache and dysphoria; 0.6% patients (1/161) presented cardio acceleration.
The incidence of symptoms of respiratory system in HA patients were significant higher than that of HFMD patients (P < 0.01). The incidence of sore throat in HA patients were significant higher than that of HFMD patients (P < 0.05). (Table 3, Table S1)
Table 3.
The symptoms of patients HA and HFMD.
| HA |
HFMD |
||||
|---|---|---|---|---|---|
| Symptoms | No | % | No | % | |
| Male | 55 | 57.9 | 102 | 63.4 | |
| Female | 40 | 42.1 | 59 | 36.6 | |
| Fever | 74 | 77.9 | 77 | 47.8 | |
| Temperature(°C) | 38.55(95%CI: 37.52–39.57) | / | 38.19(95%CI: 37.03–39.35) | / | |
| Herpes, Rash | 0 | / | / | ||
| Hand | 0 | / | 94 | 58.4 | |
| Foot | 0 | / | 48 | 29.8 | |
| Mouth | 0 | / | 44 | 27.3 | |
| Buttock | 0 | / | 33 | 20.5 | |
| Limbs | 0 | / | 21 | 13.0 | |
| Trunk | 0 | / | 12 | 7.5 | |
| Neck and face | 0 | / | 4 | 2.5 | |
| Oral cavity | 76 | 80.0 | 57 | 35.4 | |
| Buccalis | 17 | 17.9 | 11 | 6.8 | |
| Isthmus fauces | 59 | 62.1 | 46 | 28.6 | |
| Tongue | 2 | 2.1 | 4 | 2.5 | |
| Lesion | / | / | |||
| Oral cavity | 23 | 24.2 | 16 | 9.9 | |
| Buccalis | 11 | 11.6 | 3 | 1.9 | |
| Isthmus fauces | 17 | 17.9 | 10 | 6.2 | |
| Tongue | 1 | 1.1 | 4 | 2.5 | |
| Respiratory system | 71 | 74.7 | 64 | 39.8 | |
| Running nose | 56 | 58.9 | 48 | 29.8 | |
| Cough | 59 | 62.1 | 44 | 27.3 | |
| Sore throat | 11 | 11.6 | 8 | 5.0 | |
| Digestive system | 5 | 5.3 | 7 | 4.3 | |
| Nausea | 2 | 2.1 | 5 | 3.1 | |
| Vomit | 2 | 2.1 | 3 | 1.9 | |
| Bellyache | 2 | 2.1 | 2 | 1.2 | |
| Diarrhe | 2 | 2.1 | 1 | 0.6 | |
| Nervous system | 1 | 1.1 | 4 | 2.5 | |
| Headache | 0 | / | 4 | 2.5 | |
| Dysphoria | 0 | / | 3 | 1.9 | |
| Hand and foot trembling | 1 | 1.1 | 0 | / | |
| Circulatory system | 1 | 1.1 | 1 | 0.6 | |
| Coolness of extremities | 1 | 1.1 | 0 | / | |
| Cardioacceleration | 0 | / | 1 | 0.6 | |
The HA and HFMD caused by EV-A71
All HA and HFMD patients caused by EV-A71 were in placebo group. Among 10 HA patients and 28 HFMD patients infected with EV-A71, the ratio of male and female was 1:1. The average age of HA patients was 43 months old (95%CI: 28 m–58 m), and the average age of HFMD patients was 39 months old (95%CI: 24 m–55 m). There is no significant difference between HA and HFMD patients in age and sex. (Table 4, Table S2)
Table 4.
The symptoms of HA patients infected with EV-A71.
| Patient No | Sex | Age(month) | Date onset | Symptoms |
|---|---|---|---|---|
| ZJW | M | 42 | 05-07-2013 | 38.5°C, herpes on isthmus fauces, symptom of the respiratory system, running nose |
| WQX | F | 41 | 27-08-2013 | 38.4°C, herpes on isthmus fauces, symptom of the respiratory system,running nose |
| LGG | F | 30 | 28-08-2013 | 38.5°C, herpes on isthmus fauces |
| LQ | M | 46 | 02-09-2013 | 38.7°C,herpes on isthmus |
| DX | F | 43 | 09-11-2013 | Herpes on isthmus fauces |
| XZX | F | 33 | 29-12-2013 | 38.7°C, herpes on isthmus fauces, symptom of the respiratory system, running nose, cough |
| WXL | M | 53 | 10-01-2014 | 38.9°C, herpes on isthmus, symptom of the respiratory system, running nose, cough |
| CW | M | 46 | 16-01-2014 | 38.8°C, herpes on isthmus, symptom of the respiratory system, running nose, cough |
| ZX | F | 50 | 01-03-2014 | 38.6°C, herpes on isthmus fauces, symptom of the respiratory system, running nose, cough |
| ZZ | M | 48 | 03-03-2014 | 38.1°C,herpes on isthmus fauces, symptom of the respiratory system, running nose, cough |
The clinical symptoms of enterovirus co-infection
In HFMD patients, 8 cases were co-infected with 2–3 HEV serotypes. CV-A6 and CV-A22 were the most common enteroviurs in co-infected HFMD patients. Three patients were co-infected with 3 enterovirus serotypes. The symptoms of 8 co-infected HFMD patients were vesicular rashes on the extensor surfaces of the hands, feet and mouth. Five patients were accompanied with symptom related with the respiratory system, such as running nose and cough. No severe clinical symptoms were found in these co-infected HFMD patients. (Table 5).
Table 5.
The symptoms of the HFMD patients with enterovirus co-infection.
| Patient No | Sex | Age(month) | Vaccine/Placebo Group | Serotype | Date onset | Symptoms |
|---|---|---|---|---|---|---|
| 3195 | M | 35 | Vaccine | E11/E9 | 24-11-2013 | 37.2°C, herpes, papula on buttock, symptom of the respiratory system, running nose, cough |
| 3336 | F | 45 | Vaccine | E16/CV-A1/CV-A22 | 19-08-2013 | 37.7°C,herpes, papula on buttock, symptom of the respiratory system, running nose, cough |
| 3450 | M | 46 | Control | E1/CV-A6 | 09-09-2013 | 38.7°C,herpes, papula on buttock, symptom of the respiratory system, running nose, cough |
| 3526 | M | 48 | Control | CV-B4/CV-A22 | 01-08-2013 | 39.0°C,herpes, papula on mouth, isthmus fauces |
| 3609 | M | 36 | Vaccine | E16/CV-A1/CV-A22 | 18-08-2013 | 37.8°C, herpes, papula on hand symptom of the respiratory system, running nose, cough |
| 4363 | M | 42 | Control | EV-B69/E25/CV-A24 | 08-01-2014 | 37.6°C, herpes, papula on buttock |
| 5935 | M | 33 | Vaccine | CV-A6/CV-A22 | 07-09-2013 | Herpes, papula on mouth, oral cavity |
| 9306 | M | 37 | Vaccine | CV-A6/CV-B3 | 31-10-2013 | Herpes, papula on hand, foot, mouth, buttock, isthmus fauces, symptom of the respiratory system, running nose |
The molecular epidemiology of enterovirus from 2012 to 2014
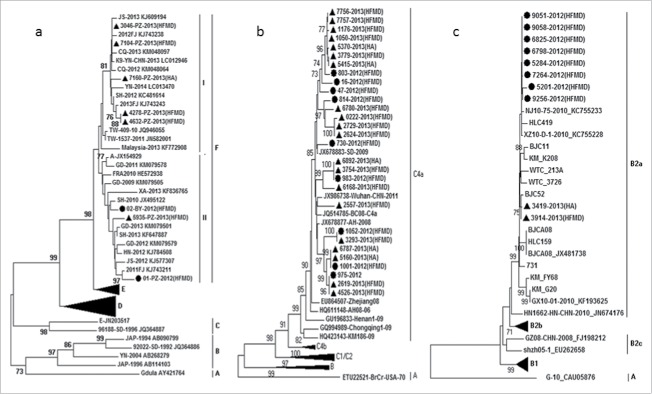
Based on the VP1 gene sequence, all of the EV-A71 strains, including 5 strains discovered in 2012–2013 and 6 strains in 2013–2014, were clustered into C4a sub-genotype. The EV-A71 strains isolated from Jiangsu shared 80.9–82.6% nucleotide identity with the prototype strain BrCr (Genebank no: ETU22521). While, these strains isolated from Jiangsu displayed a close genetic relationship with other strains from Anhui in 2008 (Genebank no: HQ611148), Shandong in 2009 (Genebank no: JX678883) and Wuhan in 2011 (Genebank no: JX986738). The EV-A71 strains caused HA and HFMD was not formed cluster separately (Fig. 4b).
Figure 4.
Phylogenetic analysis of EV-A71, CV-A16, CV-A6 (A) Phylogenetic tree based on the VP1 alignment of CV-A6 circulated in Jiangsu 2013. (B) Phylogenetic tree based on the VP1 alignment of EV-A71 circulated in Jiangsu 2013. (C) Phylogenetic tree based on the VP1 alignment of CV-A16 circulated in Jiangsu 2013. The enterovirus strains circulated in Jiangsu 2013–2014 are leblled by ▴. The enterovirus strains circulated in Jiangsu 2012–2013 are leblled by •.This work was funded by National Science Project (2008BAI69B01) and National 11th Five Major Special Projects (2009ZX10004-804) Funding Program.
As shown in Fig. 4c, the CV-A16 strains of 2012–2013 and 2013–2014 belong to B2a sub-genotype. Genetic homogeneity between 2 y within the VP1 gene of these strains were 96.4%-99.9%. The strains from Jiangsu shared 75.8–76.3% nucleotide identity with the prototype strain G10 (Genebank no: CAU05876). The strains associated with HA and HFMD belonged to the same cluster (Fig. 4c).
Overall, the partial VP1 sequences (511bp) of CV-A6 were classified into 6 clusters (A-F). The strains in cluster F formed 2 branches FI ∼ II. The 2012–2013 strains belonged to FII branch, while strains from both the FI and FII branch co-circulated in 2013–2014. The Jiangsu strains shared 82.4–84.0% nucleotide identity with the prototype strain Gdula (Genebank no: AY421764). The CV-A6 strains caused HA and HFMD was not formed cluster separately (Fig. 4a).
Discussion
Phase III clinical trial of inactivated EV-A71 vaccine (Clinical Trial No. NTC01508247) was conducted in infants aged 6–35 months in Jiangsu Province during 2012–2013.13 In previous study, we carried out an active surveillance on 9,442 infants in clinical trials for HFMD.11 In the present study, the passive surveillance was used to monitor the HA and HFMD patients. The active surveillance system has higher sensitivity, which could monitor the HFMD patients who only had mild symptoms and did not need to be hospitalized. The passive surveillance system has lower sensitivity, which just monitors the patients in hospital. So, the passive surveillance focus on patients with severe clinical symptoms. In 2012–2013, 1425 HFMD patients were conformed in the active surveillance. In 2013–2014, 161 HFMD patients were monitored in the passive surveillance, only 11.3% (161/1425) of the total cases reported by the active surveillance system. The result was similar with the previous study.11
HFMD can be caused by many pathogens, including HEVs and non-HEVs. In Thailand, 50.0% HFMD cases was caused by HEVs. In Shenzhen, mainland China, 40.8% HFMD cases were caused by HEVs, and 59.2% HFMD cases were caused by non-HEVs.32 In the present study, 51.6% of HA patients and 47.8% of HFMD patients are caused by non-HEVs, which is similar with the previous studies. More researches should be carried out to identify pathogens in non-HEVs associated HFMD cases.
As far as we know, the study on enterovirus spetrum of HEVs-caused HA has not been reported in mainland China. In our study, in Jiangsu, mainland China, the most common HEVs serotypes in HA patients were EV-A71, CV-A16, CV-A24, E6 and CV-B5. In Taiwan during 1998–2005, CV-A16 and EV-A71 were the predominant serotypes in outpatients and outpatients with HFMD/HA. Each of these serotypes accounted for 23% of reports associated with an identified serotypes,7 which were similar with our results. EV-A71 and CV-A16 caused HA had also been reported in Japan.8,29 Notably, HA is associated with different serotypes of enteroviruses according to carried out in other regions. Members of HEV-A have been reported to be main viruses of HA, such as CAV2 in Taipei 2008,14 CAV5 in Korea 2009,15 CAV6 in France 2013.16 In addition, there have been reports of HA patients caused by CAV2, CAV4, CAV6, CAV8 and CAV10 in Japan during 2000–2005.17 In Thailand in 2012, CV-A8 was established as the most prevalent cause for HA followed by CV-A6.18
In the present study, all the clinical manifestations of HA/HFMD patients were recorded. Interestingly, the incidence of symptoms on respiratory system like sore throat in HA patients were significantly higher than that of HFMD patients. Moreover, the body temperature and the incidence of fever in HA patients were significantly higher than that of in HFMD patients. As for the HA patients, the most common symptom was in respiratory system except for the herpes or/and ulcer in buccalmucosa or isthmus of fauces. The different respiratory symptoms between HA and HFMD patients might have been related to the different infection pathogens, for the most common HEV serotypes are different between HA and HFMD group except for EV-A71 and CV-A16. The correlations between respiratory infections and enterovirus have been reported previously.19 From one study in Taiwan, about 50% hospitalized children with enterovirus infections appeared the clinical manifestations in respiratory system.20 Because enteroviruses are usually transmitted via the fecal-oral route and respiratory aerosols. It is thought that enterovirus initially proliferates in the distal bowel and upper respiratory tract. Enteroviruses can be found in the respiratory tract 1 to 2 weeks after infection and in feces for up to 11 weeks.21 As suggested in previous study, enterovirus detection should also be carried out for children with symptoms of respiratory system22.
The same enterovirus serotype could cause distinct disease spectrum and clinical manifestations. In Taiwan, clinical symptoms of CA6 infection changed from HA in 2009 to HFMD in 2010. Interestingly, the phylogenetic analysis of partial VP1 gene sequences showed no significant difference in the CA6 strains responsible for these diseases between 2009 and 2010.27 A previous study showed that 9.5% EV-A71 infected patients had HA and 50.5% EV-A71 infected patients had HFMD.30 In our study, 10 patients infected with EV-A71 were diagnosed as HA and 28 patients infected with EV-A71 were defined as HFMD. There is no significant difference between HFMD and HA patients in age, sex, circulating season, and nucleotide sequences of the infected virus. Therefore, the mechanisms for different clinical manifestations caused by EV-A71 might relate to the immune status of host, the route of infection, the dose of infection,23,24 which need to be investigated in further study.
It is common in HFMD patients co-infected with HEVs, but very few reported data on it. Our previous results showed that 20.8%-29.1% HFMD patients infected with non-EV-A71/CV-A16 were also co-infection.11 Present study showed that co-infection happened in 16.3% of HFMD patients infected with non-EV-A71/CV-A16. The clinical symptoms of patients co-infected with HEVs were not more severe than those of patients infected with single HEV. However, the HEVs co-infection could lead to the intra-type and inter-type recombination among those viruses,25 so attention might need to be paid on possible changes of the antigenicity, immunogenicity and pathogenicity of recombinant HEVs.26
All the EV-A71 caused HA and HFMD cases were in placebo group, which indicated that the EV71 vaccine provided good protection against EV71-associated HA and HFMD in infants and children. Moreover, the HFMD cases in placebo group were significant higher than that of vaccine group. But the HA and HFMD patients caused by non-EV-A71 enterovirus could not be protected by EV-A71 vaccine. Therefore, the EV-A71 vaccine could not be a once for all solution for the prevalence of HA/HFMD, and the development of a broad protecting enteroviruses vaccine is inevitable.
This extended follow-up study was carried out to investigate the epidemiological and etiological characteristics of HA and HFMD patients from the Phase III clinical trial of inactivated EV-A71 vaccine in Jiangsu Province. All the clinical manifestations of HA/HFMD patients were recorded. Moreover, the detection of multiple clinical specimens from the same patient would further elucidate the pathogen epidemiology, including co-infection of HEVs. These data provide a better understanding of the viral etiology of HA/HFMD in mainland China and will helpful for the prevention and management for the HA/HFMD epidemic.
Our study has some limitations, however. First, this study only serves as an etiological surveillance of HA and HFMD patients in Jiangsu province. The surveillance data are not able to fully represent to the epidemic characteristics in mainland China. Second, the epidemic of EV-A71 in HA and HFMD patients might be underestimated for the herd immunity provided by EV-A71 vaccine inoculation.
In conclusion, we firstly reported the epidemiological and etiological characteristics of HA in Jinagsu, mainland China. EV-A71 and CV-A16 were the most common enterovirus in HA and HFMD. The EV-A71 vaccine can provide good protection against EV71-associated HFMD, but it cannot protect children from HFMD caused by other enteroviruses. Therefore, developing a multivalent vaccine will be helpful for the prevention and management for the HA/HFMD epidemic.
Materials and methods
Research objects
The study was approved by the institutional review broad of Jiangsu Provincial Center of Disease Control and Prevention, and done in accordance with the Declaration of Helsinki, Good Clinical Practice, and Chinese regulatory requirements. All guardians of participants provided written informed consent.
9422 healthy infants aged 6–35 months from 3 counties of Jiangsu Province, Donghai, Pizhou, and Baoying, were enrolled in the phase III clinical trial of EV-A71 vaccine. 5323 infants were in the vaccine group and 4099 infants were in the control group.
HA case was defined as well-characterized multiple herpes or/and ulcer formed in buccal mucosaor/and isthmus of fauces with presentation of fever, sore throat, and decreased appetite. No herpes on hand, foot and buttocks.7
HFMD was defined as having oral ulcers but chiefly on the buccal mucosa, tongue, hard and soft palate accompanied by typical vesicular rashes most commonly on the extensor surfaces of the hands, feet and/ or buttock. The patients with HFMD symptoms got herpes on buccal cosa or/and isthmus of fauces were also defined HFMD.13
HA and HFMD surveillance system
All the medical behaviors of participant in the clinical trial of EV-A71 vaccine were monitored. If any HA or HFMD symptoms were noticed, throat and rectal swabs were collected within 24h. HEVs detection kit (Jiangsu Melo Bioscience)11,13 was used for sample testing. If the test results were HEVs positive and EV-A71/CV-A16 negative, patients were followed up, and serial throat and rectal swabs were collected every 3 d until 2 consecutive samples showed negative. Totally, 1172 serial samples, including 586 throat swabs and 586 rectal swabs, were collected for the HEVs detection.
Laboratory testing
HEVs were detected according to the previously published method.15,16 In brief, 5′-UTR region of HEVs was first amplified. Based on the 5′-UTR testing results, specific enterovirus primer was designed for VP1 amplification. For samples with negative VP1 amplification, 3 universal primers of Enterovirus A, B, and C groups were used for amplification.
HEVs serotype and phylogenetic analysis
HEVs were serotyped by sequence comparison using BLAST.12 The HEV serotype was determined based on the previous study. If consecutive samples from the same patient showed the same testing result, a single HEV infection was determined; if samples of the same patient showed different results, it was classified as HEVs co-infection.11
Cluster W of MEGA5.0 was used for sequence alignment and the construction of phylogenetic trees. The analysis model of neighbor-joining and Kimura 2-parameter was applied, and the 1000 bootstrap was used as analysis parameters. Bootstrap values less than70% were hidden.
Statistics
Continuous variables were analyzed with the Student t test, and categorical data were compared with x2 tests. Significance was defined as p value less than 0.05. Data were analyzed with SAS (version 9·1).
Supplementary Material
Abbreviations
- HA
Herpangina
- HFMD
Hand Foot and Mouth Disease
- HEVs
Human Enterovirus
- HRV-A-C
Human Rhinoviruses A-C
- EV-A71
Enterovirus A 71
- CV-A16
Coxsackievirus A 16
- 5′-UTR
5-Untranslated Regions
- E
Echovirus
- NNIDRIS
Nationwide Notifible Infectious Diseases Reporting Information System
Disclosure of potential conflicts of interest
There are no potential conflicts of interest.
Funding
This work was supported by the National 12th Five Major Special Projects Funding Program (No.19 2012ZX10004701).
References
- [1].Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778-90; PMID:20961813 [DOI] [PubMed] [Google Scholar]
- [2].Nakayama T, Urano T, Osano M, Hayashi Y, Sekine S, Ando T, Makinom S. Outbreak of herpangina associated with Coxsackievirus B3 infection. Pediatr Infect Dis J 1989; 8:495-8; PMID:2549494 [DOI] [PubMed] [Google Scholar]
- [3].Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol 1999; 73:1941-8; PMID:9971773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nasri D, Bouslama L, Pillet S, Bourlet T, Aouni M, Pozzetto B. 2007. Basic rationale, current methods and future directions for molecular typing of human enterovirus. Expert Rev Mol Diagn 7:419-34; PMID:17620049 [DOI] [PubMed] [Google Scholar]
- [5].Enterovirus and rhinovirus species names have been changed to remove references to host species names. [approved Feb 2013, cited Feb 2016] http://www.picornaviridae.com/enterovirus/enterovirus.htm [Google Scholar]
- [6].Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, et al.. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis 2014;14:308-18; PMID:24485991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics 2007;120:e244-52; PMID:17671037 [DOI] [PubMed] [Google Scholar]
- [8].Sano T, Saito T, Kondo M, Watanabe S, Onoue Y, Konnai M, Sato Y, Orihara N. Enterovirus detection status of patients with herpangina and hand, foot and mouth disease in epidemic season 2007, Kanagawa Prefecture, Japan. Jpn J Infect Dis 2008;61:162-3; PMID:18362414 [PubMed] [Google Scholar]
- [9].Oliveira DB, Campos RK, Soares MS, Barros RB, Batista TC, Ferreira PC, Bonjardim CA, Trindade GS, Abrahão JS, Kroon EG. Outbreak of herpangina in the Brazilian Amazon in 2009 caused by Enterovirus B. Arch Virol 2014;159:1155-7; PMID:24197788 [DOI] [PubMed] [Google Scholar]
- [10].Lo SH, Huang YC, Huang CG, Tsao KC, Li WC, Hsieh YC, Chiu CH, Lin TY. Clinical and epidemiologic features of Coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect 2011;44:252-7; PMID:21524960 [DOI] [PubMed] [Google Scholar]
- [11].Yao X, Bian LL, Lu WW, Li JX, Mao QY, Wang YP, Gao F, Wu X, Ye Q, Xu M, et al.. Enterovirus spectrum from the active surveillance of hand foot and mouth disease patients under the clinical trial of inactivated Enterovirus A71 vaccine in Jiangsu, China, 2012–2013. J Med Virol 2015;87:2009-17; PMID:26010334 [DOI] [PubMed] [Google Scholar]
- [12].Bracho MA, González-Candelas F, Valero A, Córdoba J, Salazar A. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis 2011; 17:2223-31; PMID:22172227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al.. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381:2024-32; PMID:23726161 [DOI] [PubMed] [Google Scholar]
- [14].Lee MH, Huang LM, Wong WW, Wu TZ, Chiu TF, Chang LY. Molecular diagnosis and clinical presentations of enteroviral infections in Taipei during the 2008 epidemic. J Microbiol Immunol Infect 2011;44:178-83; PMID:21524611 [DOI] [PubMed] [Google Scholar]
- [15].Park K, Lee B, Baek K, Cheon D, Yeo S, Park J, Soh J, Cheon H, Yoon K, Choi Y. Enteroviruses isolated from herpangina and hand-foot-and-mouth disease in Korean children. Virol J 2012;9:205; PMID:22985487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].El Houmami N, Minodier P, Dubourg G, Martin-Laval A, Lafont E, Jouve JL, Charrel R, Raoult D, Fournier PE. An outbreak of Kingella kingae infections associated with hand, foot and mouth disease/herpangina virus outbreak in Marseille, France, 2013. Pediatr Infect Dis J 2015;34:246-50; PMID:25742075 [DOI] [PubMed] [Google Scholar]
- [17].Shima T, Saito T, Kondo M, Watanabe S, Mizuno K, Sato Y, Orihara N, Onoue Y, Nikkawa T. Enterovirus detection status from patients with herpangina and hand, foot and mouth disease in Kanagawa Prefecture, Japan. Jpn J Infect Dis 2007;60:63-4; PMID:17314432 [PubMed] [Google Scholar]
- [18].Puenpa J, Mauleekoonphairoj J, Linsuwanon P, Suwannakarn K, Chieochansin T, Korkong S, Theamboonlers A, Poovorawan Y. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One 2014;9:e98888; PMID:24887237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jennings LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of acute respiratory tract infections in children presenting to hospital: role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J 2004;23:1003-7; PMID:15545854 [DOI] [PubMed] [Google Scholar]
- [20].Jacques J, Moret H, Minette D, Lévêque N, Jovenin N, Deslée G, Lebargy F, Motte J, Andréoletti L. Epidemiological, molecular, and clinical features of enterovirus respiratory infections in French children between 1999 and 2005. J Clin Microbiol 2008;46:206-13; PMID:18003804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chung PW, Huang YC, Chang LY, Lin TY, Ning HC. Duration of enterovirus shedding in stool. J Microbiol Immunol Infect 2001;34:167-70; PMID:11605806 [PubMed] [Google Scholar]
- [22].Lin TY, Huang YC, Ning HC, Tsao KC. Surveillance of respiratory viral infections among pediatric outpatients in northern Taiwan. J Clin Virol 2004;30:81-5; PMID:15072759 [DOI] [PubMed] [Google Scholar]
- [23].Lee CJ, Huang YC, Yang S, Tsao KC, Chen CJ, Hsieh YC, Chiu CH, Lin TY. Clinical features of coxsackievirus A4, B3 and B4 infections in children. PLoS One 2014;9:e87391; PMID:24504149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yen FB, Chang LY, Kao CL, Lee PI, Chen CM, Lee CY, Shao PL, Wang SC, Lu CY, Huang LM. Coxsackieviruses infection in northern Taiwan–epidemiology and clinical characteristics. J Microbiol Immunol Infect 2009;42:38-46; PMID:19424557 [PubMed] [Google Scholar]
- [25].Oberste MS, Maher K, Pallansch MA. 2004. Evidence for frequent recombination within species human enterovirus B based on complete genomic sequences of all thirty-seven serotypes. J Virol 78:855-67; PMID:14694117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang YP, Lin TL, Hsu LC, Chen YJ, Tseng YH, Hsu CC, Fan WB, Yang JY, Chang FY, Wu HS. Genetic diversity and C2-like subgenogroup strains of enterovirus 71, Taiwan. Virol J 2008; 7:277-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bian L, Wang Y, Yao X, Mao Q, Xu M, Liang Z. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther 2015;13:1061-71; PMID:26112307 [DOI] [PubMed] [Google Scholar]
- [28].Park K, Lee B, Baek K, Cheon D, Yeo S, Park J, Soh J, Cheon H, Yoon K, Choi Y. Enteroviruses isolated from herpangina and hand-foot-and-mouth disease in Korean children. Virol J 2012;9:205; PMID:22985487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen SP, Huang YC, Li WC, Chiu CH, Huang CG, Tsao KC, Lin TY. Comparison of clinical features between coxsackievirus A2 and enterovirus 71 during the enterovirus outbreak in Taiwan, 2008: a children's hospital experience. J Microbiol Immunol Infect 2010;43:99-104; PMID:20457425 [DOI] [PubMed] [Google Scholar]
- [30].Liu CC, Tseng HW, Wang SM, Wang JR, Su IJ. An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. J Clinical Virology 2000;17:23-30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.