ABSTRACT
The introduction of new vaccines is accompanied by a variety of challenges. Among these, very important ones concern the questions whether the public is willing to accept and willing to purchase the vaccine. Here we discuss factors associated with these questions in the context of vaccines that are becoming available against dengue virus infection. We reviewed published studies available from PubMed and Embase, conducting a meta-analysis when possible and narrative review when the data did not qualify for meta-analysis. We found that attitude toward vaccination and socioeconomic level had significant associations with dengue vaccine acceptance. In addition, socioeconomic level, knowledge, attitude and practice regarding dengue fever, having personally experienced dengue fever and vaccine price were associated with willingness to pay for dengue vaccine. To improve acceptance and willingness to pay for dengue vaccine, well-designed introduction programs that address the associated factors in a context-specific manner are essential.
KEYWORDS: dengue fever, dengue vaccine, dengue virus, public acceptance, willingness to pay
Introduction
Dengue fever (DF), caused by infection with one or more serotypes of dengue virus (DENV), is the most important mosquito-borne viral disease in humans. It is a major international public health concern, resulting in significant morbidity, mortality and economic cost.1 Almost 50% of the world's population live in areas that are at risk of DENV infection.2 Globally, the average cost per DF case is approximately US-$ 84.73, US-$ 70.10, US-$ 51.16 and US-$ 12.94 for fatal cases, cases admitted to hospital, ambulatory cases, and cases outside the healthcare sector, respectively.3 A total annual global aggregate cost of DF in 2013 was calculated as US-$ 8.9 billion (3.7–19.7 billion) or US-$ 1.56 per capita, with aggregate costs amounting to US-$ 4,093 million for non-fatal cases admitted to hospital, US-$ 2,987 million for ambulatory cases, US-$ 752 million for cases outside the healthcare sector, and US-$ 1,055 million for fatal cases.3 The global, annual disability adjusted life years lost by dengue amount to approximately 700,000.2 Thus, DENV infection and DF is a continuing global threat and significant economic burden.
In the absence of an antiviral therapy, fluid therapy is the only treatment for the management of DF.4 Vaccination is an effective method to prevent certain infectious diseases and is among the most important achievements of public health.5 However, DENV are difficult vaccine targets because they comprise 4 different serotypes. Nevertheless, an increasing number of publications on different phases of dengue vaccine (DV) studies have become available. For example, multiple phase II randomized controlled trials to evaluate the immunogenicity and safety of a DV from Sanofi Pasteur, the chimeric yellow fever-dengue virus tetravalent vaccine (CYD-TDV), have been conducted in Brazil, Colombia, Honduras, Mexico, Peru, Puerto Rico, Singapore and Thailand.6 A meta-analysis study to assess the safety of CYD-TDV found greater safety in the CYD-TDV group than in the placebo group.7 Currently, 2 phase III trials for CYD-TDV have been completed in Southeast Asia8 and Latin America,9 with reported efficacies of 56.5% and 60.8%, respectively. Assessments of long-term efficacy and safety have also been completed for CYD-TDV in Southeast Asia (Indonesia, Malaysia, Philippines, Thailand, Vietnam) and Latin America (Colombia, Brazil, Honduras, Mexico, Puerto Rico).10 In that study, which assessed the incidence of hospitalization for virologically confirmed DF over the first 25 months of 22,177 vaccinated participants and 11,089 controls, the efficacy of CYD-TDV against symptomatic DENV infection was 65.6% (95% CI: 60.7–69.9) for children under 9 y and 44.6% (95% CI: 31.6–55.0) for older participants.10
In any vaccination program, new vaccines such as the ones being developed and tested against DENV infection have the potential to be accompanied by a variety of challenges. A crucial question from several points of view is whether the public is willing to accept and purchase the vaccine. Here we discuss several factors associated with acceptance and willingness to pay (WTP) for DV, based on our analysis of publications that are indexed in Pubmed and Embase (cut-off date for updates: September 10, 2016).
Factors associated with dengue vaccine acceptance
Vaccine acceptance is an important predictor of the actual acceptance of a given group or society toward the vaccine in question. Vaccine acceptance is also a social tool that has an important role for the start and continuation of the vaccination program. It is defined as the timely acceptance of all recommended vaccines according to the recommended schedule.11 So far studies on vaccine acceptance toward DV are rare. Until now, only 2 studies evaluating factors associated with DV acceptance have been published.12,13 They identified socioeconomic status (SES), knowledge, attitude and practice (KAP) regarding dengue, attitude toward vaccination, and personal experience of DF as factors that were correlated with DV acceptance. Our meta-analysis of the data from these 2 studies12,13 (with a total of 1151 participants) reveals that poor attitude toward vaccination (OR: 0.31, 95% CI: 0.13–0.71, P = 0.006) and low SES (OR: 0.57, 95% CI: 0.38–0.86, P = 0.007) are associated with low DV acceptance. Other factors had no association.
Attitude toward vaccination is an individual perspective toward a vaccination program and correlated with vaccination coverage rates.14 It is also associated with vaccine acceptance in other infectious diseases like influenza,15 measles,16 rubella,16 and human papillomavirus.17 These results suggest that a society with a good attitude toward vaccination tends to accept a vaccine. Low SES was closely associated with low DV acceptance among community members. Similar findings were obtained in the context of other infectious diseases including human papillomavirus,18,19 influenza,20 and cholera.21 To improve DV acceptance, DV introduction programs should target populations with low SES. In addition, to achieve high DV coverage in the future, the provision of partially or fully subsidized vaccines will be necessary.
Factors associated with willingness to pay for dengue vaccine
The data from previous studies13,22-24 did not qualify for meta-analysis, but their review yielded the following. The associations of 12 factors and WTP for DV were evaluated in studies conducted in Colombia,22 Indonesia,13,24 the Philippines,23 Thailand,22 and Vietnam.22 The factors included attitude toward vaccination, KAP regarding DF, knowledge regarding DENV, SES, personal experience of DF, knowing someone (or having a family member) who had had DF, price of DV, and previous vaccine purchase. Of these, preventive practice against dengue,22-24 attitude toward DF,24 knowledge of DF,13,22 knowledge of DENV,12 SES,13,23 personal experience with DF,22 and DV price23 were associated with WTP for DV. Other factors had no significant association with WTP for DV.
Willingness to pay for a vaccine is a parameter that reflects the demand of society for a vaccine. Studies on the WTP for DV are still very limited and the associated factors so far comparable with those identified in the context of other infectious diseases. For example, in the context of 3 hypothetical malaria vaccines in Nigeria, WTP was influenced by vaccine price, SES, and experienced illness among community members.25 A study in the Philippines showed that the WTP for a dog rabies vaccine was influenced by vaccine price, SES, and knowledge about rabies.26 Another study also found that vaccine price, knowledge, and SES were the predictors for WTP for vaccination, in this case against tick-borne encephalitis in Sweden.27 In the context of human papillomavirus vaccine, a study in Nigeria found that vaccine price and experienced illness influenced WTP.28 In Thailand, analyses of interviews with 2693 respondents demonstrated that attitude toward vaccination, knowledge, history of receiving vaccination, and educational level influenced the WTP for an influenza vaccine.29 Collectively, these findings reveal consistent factors associated with WTP for DV and other vaccines.
Correlations among associated factors
Our group has conducted a range of studies on the KAP regarding DF, attitude toward dengue vaccination, DV acceptance and WTP for DV.12,24,30,31 From these studies, we formulate the comprehensive correlation among continuous variables and variables that are correlated with DV acceptance and WTP for DV. Some factors associated with these are shown in Figure 1. However, a Confirmatory Factor Analysis is important to re-test this relationship model, and an Exploratory Factor Analysis with Principal Component Analysis could be important to construct alternative relationship models among variables. Nevertheless, our proposed model interestingly suggests that knowledge has no significant correlation with DV acceptance. Previous studies on dengue13 and other diseases32-36 also found that good knowledge regarding the disease was not associated with vaccine acceptance. Our model confirms that there is a robust correlation between attitude toward DF and DV acceptance. This is also supported by a study that had been conducted in Bandung, Indonesia.13
Figure 1.
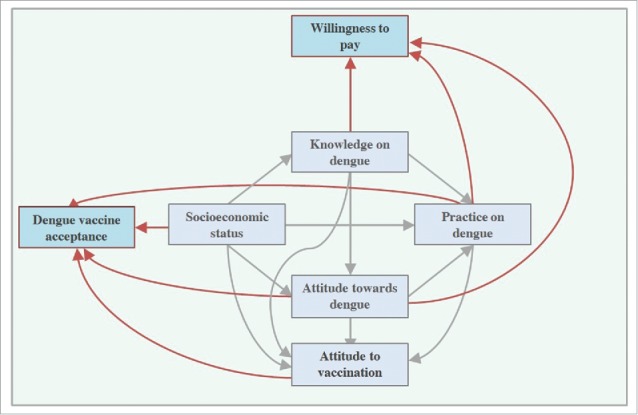
Correlation model between socioeconomic status, knowledge on dengue fever, attitude toward dengue fever, practice on dengue prevention, attitude toward vaccination, dengue vaccine acceptance and willingness to pay for dengue vaccine.
Designing dengue vaccine introduction programs
Our study indicates that increasing the understanding of a disease among community members without improving their attitude does not improve vaccine acceptance. Therefore, any dengue prevention program should be designed to not only increase the knowledge of community members on dengue but also to improve their attitude toward DF and vaccination. In addition, regarding WTP, our study reveals that the KAP regarding dengue are positively associated with the WTP for DV. Strategies that improve knowledge and positive attitude regarding dengue may therefore also improve the acceptance and the WTP for DV.
A study in Thailand found that the use of a health education video increased both knowledge and attitude toward vaccination, having a significant impact on acceptance and WTP for an influenza vaccine.29 Such strategy could be implemented in dengue hyper-endemic areas of Indonesia to increase the acceptance and WTP for DV among community members, especially parents. However, as this strategy might be costly, the information included should be selective. Based on our findings, information on DENV transmission and its prevention should be designed as the core information while that on specific signs and symptoms of DF could be supplementary.24 In addition, it will be essential to also include information that enables participants to improve their attitude and awareness, and to change their perception of DENV and DF in order to change the vaccination behavior.27
Such programs should be delivered by people who are trusted in the communities. In the context of Aceh, and Indonesia in general, healthcare professionals (nurses and doctors) and religious leaders are probably the most trusted individuals. Involving all of them should follow participation in program- and vaccine-specific training with subsequent recommendation or certification from trusted government authorities such as the Ministry of Health and the Provincial Health Office. Various studies have shown that trust is one of the most important factors for the success of any vaccination program,27,37-40 and that receiving vaccination advice from medical personnel was associated with a higher acceptance40 and better WTP for a vaccine.29 This reinforces the critical role of trusted individuals in the delivery of DV information and their involvement in future DV introduction programs. Furthermore, because decision-making processes regarding vaccination are complex and multidimensional, introduction programs should be able to (a) minimize specific barriers to vaccination such as fear of side effects; (b) moderate or adjust beliefs and expectations regarding the efficacy and usefulness of the vaccine, toward evidence-based levels; and (c) minimize any distrust and perceived religious barriers.
Conclusions
Specific factors are associated with DV acceptance and WTP for DV. Attitude toward vaccination and SES are associated with both acceptance and WTP for DV. In addition, knowledge and practice regarding DF, having personally experienced DF, and DV price are also associated with the WTP for DV. To improve DV acceptance (and WTP), well-prepared introduction programs for DV that address the associated factors in a context-specific manner are essential to increase vaccine coverage.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- [1].Halstead SB. Dengue. Lancet 2007; 370(9599):1644-52; PMID:17993365; http://dx.doi.org/ 10.1016/S0140-6736(07)61687-0 [DOI] [PubMed] [Google Scholar]
- [2].Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: Past, present and future prospects. Clin Epidemiol 2013; 5:299-309; PMID:23990732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis 2016; 16(8):935-41; PMID:27091092; http://dx.doi.org/ 10.1016/S1473-3099(16)00146-8 [DOI] [PubMed] [Google Scholar]
- [4].WHO Dengue: guidelines for diagnosis, treatment, prevention and control – New edition. Geneva: World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR); 2009 [PubMed] [Google Scholar]
- [5].Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007-2012. Vaccine 2014; 32(19):2150-9; PMID:24598724; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.081 [DOI] [PubMed] [Google Scholar]
- [6].Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA et al.. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012; 380(9853):1559-67; PMID:22975340; http://dx.doi.org/ 10.1016/S0140-6736(12)61428-7 [DOI] [PubMed] [Google Scholar]
- [7].da Costa VG, Marques-Silva AC, Floriano VG, Moreli ML. Safety, immunogenicity and efficacy of a recombinant tetravalent dengue vaccine: A meta-analysis of randomized trials. Vaccine 2014; 32(39):4885-92; PMID:25045816; http://dx.doi.org/ 10.1016/j.vaccine.2014.07.008 [DOI] [PubMed] [Google Scholar]
- [8].Capeding MR, Tran NH, Hadinegoro SRS, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al.. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384(9951):1358-65; PMID:25018116; http://dx.doi.org/ 10.1016/S0140-6736(14)61060-6 [DOI] [PubMed] [Google Scholar]
- [9].Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO et al.. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372(2):113-23 [DOI] [PubMed] [Google Scholar]
- [10].Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373(13):1195-206; PMID:26214039; http://dx.doi.org/ 10.1056/NEJMoa1506223 [DOI] [PubMed] [Google Scholar]
- [11].Streefland P, Chowdhury AM, Ramos-Jimenez P. Patterns of vaccination acceptance. Soc Sci Med 1999; 49(12):1705-716; PMID:10574240; http://dx.doi.org/ 10.1016/S0277-9536(99)00239-7 [DOI] [PubMed] [Google Scholar]
- [12].Harapan H, Anwar S, Setiawan A, Sasmono R, Aceh Dengue Study . Dengue vaccine acceptance and associated factors in Indonesia: A community-based cross-sectional survey in Aceh. Vaccine 2016; 34:3670-5; PMID:27208588; http://dx.doi.org/ 10.1016/j.vaccine.2016.05.026 [DOI] [PubMed] [Google Scholar]
- [13].Hadisoemarto PF, Castro MC. Public acceptance and willingness-to-pay for a future dengue vaccine: a community-based survey in Bandung, Indonesia. PLoS Negl Trop Dis 2013; 7(9):e2427; PMID:24069482; http://dx.doi.org/ 10.1371/journal.pntd.0002427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jheeta M, Newell J. Childhood vaccination in Africa and Asia: The effects of parents' knowledge and attitudes. B World Health Organ 2008; 86(6):419-19; http://dx.doi.org/ 10.2471/BLT.07.047159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Peretti-Watel P, Raude J, Sagaon-Teyssier L, Constant A, Verger P, Beck F. Attitudes toward vaccination and the H1N1 vaccine: Poor people's unfounded fears or legitimate concerns of the elite? Soc Sci Med 2014; 109:10-8; PMID:24681239; http://dx.doi.org/ 10.1016/j.socscimed.2014.02.035 [DOI] [PubMed] [Google Scholar]
- [16].Taddei C, Ceccherini V, Niccolai G, Porchia BR, Boccalini S, Levi M, Tiscione E, Santini MG, Baretti S, Bonanni P, Bechini A. Attitude toward immunization and risk perception of measles, rubella, mumps, varicella, and pertussis in health care workers working in 6 hospitals of Florence, Italy 2011. Hum Vacc Immunother 2014; 10(9):2612-22; http://dx.doi.org/ 10.4161/21645515.2014.970879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hopkins TG, Wood N. Female human papillomavirus (HPV) vaccination: Global uptake and the impact of attitudes. Vaccine 2013; 31(13):1673-9; PMID:23375978; http://dx.doi.org/ 10.1016/j.vaccine.2013.01.028 [DOI] [PubMed] [Google Scholar]
- [18].Schreiber SMS, Juul KE, Dehlendorff C, Kjaer SK. Socioeconomic predictors of human papillomavirus vaccination among girls in the Danish Childhood Immunization Program. J Adolescent Health 2015; 56(4):402-7; http://dx.doi.org/ 10.1016/j.jadohealth.2014.12.008 [DOI] [PubMed] [Google Scholar]
- [19].Jeudin P, Liveright E, del Carmen MG, Perkins RB. Race, ethnicity and income as factors for HPV vaccine acceptance and use. Hum Vacc Immunother 2013; 9(7):1413-20; http://dx.doi.org/ 10.4161/hv.24422 [DOI] [PubMed] [Google Scholar]
- [20].Kudale A, Purohit VS, Sundaram N, Schaetti C, Weiss MG. Socioeconomic, cultural and behavioural features of prior and anticipated influenza vaccine uptake in urban and rural Pune district, India: A mixed-methods case study. BMJ Open 2013; 3(2):e002573; PMID:23408082; http://dx.doi.org/ 10.1136/bmjopen-2013-002573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Merten S, Schaetti C, Manianga C, Lapika B, Chaignat C, Hutubessy R, Weiss MG. Local perceptions of cholera and anticipated vaccine acceptance in Katanga province, Democratic Republic of Congo. BMC Public Health 2013; 13:13; PMID:23297757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee JS, Mogasale V, Lim JK, Carabali M, Sirivichayakul C, Anh DD, Lee KS, Thiem VD, Limkittikul K, Tho le H. A multi-country study of the household willingness-to-pay for dengue vaccines: Household surveys in Vietnam, Thailand, and Colombia. PloS Negl Trop Dis 2015; 9(6):e0003810; PMID:26030922; http://dx.doi.org/ 10.1371/journal.pntd.0003810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Palanca-Tan R. The demand for a dengue vaccine: A contingent valuation survey in Metro Manila. Vaccine 2008; 26(7):914-23; PMID:18206277; http://dx.doi.org/ 10.1016/j.vaccine.2007.12.011 [DOI] [PubMed] [Google Scholar]
- [24].Harapan H, Anwar S, Bustaman A, et al.. Willingness to pay for a dengue vaccine and its associated determinants in Indonesia: A community-based, cross sectional survey in Aceh. Acta Trop 2016; 166:249-56; PMID: 27908746; http://dx.doi.org/20728765 10.1016/j.actatropica.2016.11.035 [DOI] [PubMed] [Google Scholar]
- [25].Udezi WA, Usifoh CO, Ihimekpen OO. Willingness to pay for three hypothetical malaria vaccines in Nigeria. Clin Ther 2010; 32(8):1533-44; PMID:20728765; http://dx.doi.org/ 10.1016/j.clinthera.2010.07.018 [DOI] [PubMed] [Google Scholar]
- [26].Birhane MG, Miranda ME, Dyer JL, Blanton JD, Recuenco S. Willingness to pay for dog rabies vaccine and registration in Ilocos Norte, Philippines (2012). PLoS Negl Trop Dis 2016; 10(3):e0004486; PMID:26999021; http://dx.doi.org/ 10.1371/journal.pntd.0004486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Slunge D. The willingness to pay for vaccination against tick-borne encephalitis and implications for public health policy: Evidence from Sweden. PLoS One 2015; 10(12):e0143875; PMID:26641491; http://dx.doi.org/ 10.1371/journal.pone.0143875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Umeh IB, Nduka SO, Ekwunife OI. Mothers' willingness to pay for HPV vaccines in Anambra state, Nigeria: A cross sectional contingent valuation study. Cost Eff Resour Alloc 2016; 14:8; PMID:27274335; http://dx.doi.org/ 10.1186/s12962-016-0057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Worasathit R, Wattana W, Okanurak K, Songthap A, Dhitavat J, Pitisuttithum P. Health education and factors influencing acceptance of and willingness to pay for influenza vaccination among older adults. BMC Geriatr 2015; 15:136; PMID:26503289; http://dx.doi.org/ 10.1186/s12877-015-0137-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harapan H, Anwar S, Bustaman A, Radiansyah A, Angraini P, Fasli R, Salwiyadi S, Bastian RA, Oktiviyari A, Akmal I. Knowledge, attitude, and practice regarding dengue infection among healthy inhabitants in Aceh, Indonesia 2016. Unpublished data [Google Scholar]
- [31].Harapan H, Anwar S, Bustaman A, Radiansyah A, Angraini P, Fasli R, Salwiyadi S, Bastian RA, Oktiviyari A, Akmal I, et al.. Modifiable determinants of attitude towards dengue vaccination among healthy inhabitants of Aceh, Indonesia: Findings from a community-based survey. Asia Pac J Trop Med 2016; 9(11):1115-1122; PMID: 27890375; http://dx.doi.org/16651301 10.1016/j.apjtm.2016.07.036 [DOI] [PubMed] [Google Scholar]
- [32].Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: A randomized intervention study of written information about HPV. Pediatrics 2006; 117(5):1486-93; PMID:16651301; http://dx.doi.org/ 10.1542/peds.2005-1381 [DOI] [PubMed] [Google Scholar]
- [33].Jaspers L, Budiningsih S, Wolterbeek R, Henderson FC, Peters AA. Parental acceptance of human papillomavirus (HPV) vaccination in Indonesia: A cross-sectional study. Vaccine 2011; 29(44):7785-93; PMID:21821079; http://dx.doi.org/ 10.1016/j.vaccine.2011.07.107 [DOI] [PubMed] [Google Scholar]
- [34].Khurana S, Sipsma HL, Caskey RN. HPV vaccine acceptance among adolescent males and their parents in two suburban pediatric practices. Vaccine 2015; 33(13):1620-4; PMID:25659275; http://dx.doi.org/ 10.1016/j.vaccine.2015.01.038 [DOI] [PubMed] [Google Scholar]
- [35].Khan AA, Varan AK, Esteves-Jaramillo A, Siddiqui M, Sultana S, Ali AS, Zaidi AK, Omer SB. Influenza vaccine acceptance among pregnant women in urban slum areas, Karachi, Pakistan. Vaccine 2015; 33(39):51039. [DOI] [PubMed] [Google Scholar]
- [36].Gottvall M, Grandahl M, Hoglund AT, Larsson M, Stenhammar C, Andrae B, Tydén T. Trust versus concerns-how parents reason when they accept HPV vaccination for their young daughter. Ups J Med Sci 2013; 118(4):263-70; PMID:23777602; http://dx.doi.org/ 10.3109/03009734.2013.809039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Quinn SC, Parmer J, Freimuth VS, Hilyard KM, Musa D, Kim KH. Exploring communication, trust in government, and vaccination intention later in the 2009 H1N1 pandemic: results of a national survey. Biosecur Bioterror 2013; 11(2):96-106; PMID:23617721; http://dx.doi.org/ 10.1089/bsp.2012.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gilles I, Bangerter A, Clemence A, Green EG, Krings F, C Staerklé, Wagner-Egger P. Trust in medical organizations predicts pandemic (H1N1) 2009 vaccination behavior and perceived efficacy of protection measures in the Swiss public. Eur J Epidemiol 2011; 26(3):203-10; PMID:21476079; http://dx.doi.org/ 10.1007/s10654-011-9577-2 [DOI] [PubMed] [Google Scholar]
- [39].Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: A critical review. Soc Sci Med 2014; 112:1-11; PMID:24788111; http://dx.doi.org/ 10.1016/j.socscimed.2014.04.018 [DOI] [PubMed] [Google Scholar]
- [40].Dube E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: An overview. Hum Vaccin Immunother 2013; 9(8):1763-73; PMID:23584253; http://dx.doi.org/ 10.4161/hv.24657 [DOI] [PMC free article] [PubMed] [Google Scholar]


